Abstract
Particulate matter (PM) with an aerodynamic diameter of ≤2.5 μm (PM2.5) significantly contributes to various disease-related respiratory inflammations. Armillaria mellea, recognized for its medicinal properties, could alleviate these respiratory ailments. However, its efficacy against PM2.5-induced inflammation remains elusive. In this study, we investigated whether A. mellea mycelia could mitigate PM2.5-induced respiratory inflammation and assessed the underlying mechanisms. Our results showed that A. mellea mycelia significantly reduced PM2.5-induced nitric oxide (NO) production and nuclear factor (NF)-κB activation in macrophages. Furthermore, A. mellea mycelia suppressed the expression of inflammatory mediators, indicating their potent antioxidant and anti-inflammatory properties. In murine models, A. mellea mycelia mitigated PM2.5-induced lung inflammation and cytokine secretion, restoring lung inflammatory status. Our results highlight the potential of A. mellea mycelia to treat PM2.5-induced respiratory inflammation. The antioxidant and anti-inflammatory effects of A. mellea mycelia demonstrated in vitro and in vivo hold promising potential for developing respiratory health improvement interventions upon PM2.5 exposure.
1. Introduction
Particulate matter (PM) with an aerodynamic diameter of ≤2.5 μm (PM2.5) is widely recognized for its role in inducing respiratory inflammation [1,2]. Primary PM2.5 sources include vehicle and industrial emissions, as well as wildfires [3]. Prolonged PM2.5 exposure is reportedly linked to an increased risk of respiratory diseases such as asthma, chronic obstructive pulmonary disease, and lung cancers [4,5,6]. Several countries maintain monitoring networks to assess environmental PM2.5 concentrations. Nevertheless, an annual escalation in PM2.5 pollution was registered, especially in regions with significant industrial development [7].
Exposure to PM2.5 could lead to immune cell infiltration and the activation of inflammatory pathways, particularly influencing the alveolar macrophage functions [8,9]. Such inflammatory pathway activation is linked to the oxidative stress response elicited by PM2.5 exposure in the respiratory tract [10]. Notably, oxidative stress assumes a pivotal role in the detrimental impacts and mechanisms of PM2.5 on the respiratory system, eliciting diverse injurious pathways that culminate in inflammation and cellular damage [11]. Since low-level exposure to PM2.5 poses certain public health risks [12], mitigating PM2.5-induced pulmonary inflammation is crucial for human health. Various natural products and their constituents have been investigated for their potential to alleviate PM2.5-induced pulmonary injury and enhance lung function. Suppressing oxidative stress provides benefits in alleviating PM2.5-induced systemic dysfunction [13,14,15]. For instance, Panax ginseng (black ginseng), a traditional herb, reportedly exerts beneficial effects against PM2.5-induced lung endothelial cell barrier disruption and reactive oxygen species (ROS) generation, thus attenuating pulmonary inflammation [16]. Securinega suffruticosa, a plant species native to Asia and Europe, mitigates PM2.5-induced lung and vascular inflammation by inhibiting ROS and downregulating the NOD-like receptor pyrin domain containing-3 (NLRP3) inflammasome signaling pathway [17]. Despite the identification of numerous herbal medicines with the potential to alleviate PM2.5-induced lung injury, their underlying mechanisms vary and require further investigation.
Armillaria mellea is a mushroom with recognized medicinal properties, including immunomodulatory, anti-inflammatory, and pulmonary protective attributes [18]. A. mellea reportedly exhibited antioxidant activity [19,20], potentially alleviating respiratory symptoms resulting from exposure to air pollution. Mycelia from medicinal mushrooms contain a variety of constituents that offer numerous biological benefits to human health [21]. However, the capacity of A. mellea mycelia to ameliorate PM2.5-induced respiratory inflammation remains unclear. In this study, we aimed to elucidate whether submerged cultured A. mellea mycelia can mitigate the adverse effects of PM2.5 on the airways and assess the underlying mechanisms for improving respiratory inflammation. Using rigorous experimental methodologies, including in vivo and in vitro assessments, we aimed to elucidate the therapeutic potential of A. mellea mycelia against PM2.5-induced respiratory ailments.
2. Materials and Methods
2.1. Cell Culture
RAW264.7 cells (ATCC TIB-71), a macrophage cell line, were maintained in Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% complement-inactivated fetal bovine serum (HyClone, Logan, UT, USA) at 37 °C in a 5% CO2 atmosphere [9].
2.2. Preparation of Ethanol Extracts of A. mellea Mycelia
The ethanol extracts of A. mellea mycelia were prepared according to previously established procedures [22]. For 10 d, A. mellea was cultured on potato dextrose agar slants at 25 °C. The A. mellea mycelia were then transferred into 1 L of synthetic culture medium (consisting of 2% glucose, 1% soybean powder, 0.1% yeast extract, and 0.1% peptone, pH 4.0) and were incubated at 25 °C for an additional 10 d with mild shaking. Subsequently, the fermentation process was scaled up in 20-ton fermenters for 10 d. The fermented broth was harvested, lyophilized, ground into a powder, and subjected to ethanolic extraction (using a ratio of 1:40 w/v in 95% ethanol). The ethanolic suspension was sonicated overnight and was then centrifuged at 15,000× g for 1 h. The resulting supernatant was subjected to vacuum concentration, which effectively removed ethanol and water from the final extract. The prepared extract was then condensed into a paste for use in subsequent experiments. We then performed high-performance liquid chromatography (HPLC) to identify and characterize the key components of A. mellea mycelia extract. The analysis revealed that the major constituents of A. mellea mycelia extract are Armillaridin (retention time: 19.6 min) and Melledonal C (retention time: 10.1 min), detected at a wavelength of 254 nm (Figure S1).
2.3. Preparation of PM2.5
Particulate matter with a diameter smaller than 2.5 μm (PM2.5) (RM8785) was purchased from the National Institute of Standards and Technology (Gaithersburg, MD, USA) [23]. PM2.5 was added to Dulbecco’s Modified Eagle Medium (DMEM) at specified concentrations for subsequent experiments, as described in our previous study [9].
2.4. Macrophage Phagocytosis Assay
The Phagocytosis Assay Kit (IgG FITC) from Cayman Chemical (Ann Arbor, MI, USA) was employed to assess the impact of PM2.5 on macrophage phagocytic activity, as described previously [9]. In brief, RAW264.7 cells were exposed to PM2.5 (30 μg/mL) for 24 h, followed by incubation with latex beads coated with fluorescent-labeled rabbit IgG for an additional 4 h. Subsequently, the cells were fixed using 4% paraformaldehyde, and flow cytometry (Becton Dickinson, San Jose, CA, USA) was utilized to analyze the phagocytic activity.
2.5. Western Blot Assay
The protein expression levels of iNOS, COX-2, and β-actin were assessed using a Western blot assay. RAW264.7 cells were treated with ethanol extracts of A. mellea mycelia, followed by exposure to PM2.5 (30 μg/mL) for 24 h. Cell lysates were prepared in 100 μL of RIPA buffer (Roch, Indianapolis, IN, USA) and subjected to Western blot analysis. The samples were separated by 10% SDS-PAGE and then transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Following blocking with 5% skim milk, the membranes were incubated with primary antibodies specific against inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (Abcam, Boston, MA, USA), respectively. The membrane was then probed with horseradish peroxidase (HRP)-conjugated secondary antibodies (Millipore). The protein of interest was visualized using ECL Western Blotting Detection Reagent (BIOMAN, Taipei, Taiwan) and analyzed with an Azure C400 system (Azure Biosystems, Dublin, CA, USA) [24].
2.6. Analysis of Nitric Oxide (NO) and Cytokine Production
RAW264.7 cells were treated with ethanol-extracted A. mellea mycelia and exposed to PM2.5 (30 μg/mL) for 24 h. The culture supernatant was collected for the determination of the NO concentration using Griess reagent (Sigma-Aldrich, St. Louis, MO, USA) [25]. The concentrations of a high mobility group box 1 (HMGB1), interleukin 1β (IL-1β), interferon γ-induced protein 10 (IP-10), and keratinocyte-derived cytokine (KC) were determined using a sandwich enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA) [26].
2.7. NF-κB Luciferase Activity Assay
RAW264.7 cells were transfected with an NF-κB-luciferase reporter construct and subsequently treated with ethanol-extracted A. mellea mycelia before exposure to PM2.5 (30 μg/mL). Following a 24 h incubation period, cell lysates were prepared for a luciferase assay using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA). Co-transfection with a β-galactosidase expression vector (Promega) was conducted to normalize the reporter gene assay [27].
2.8. Implementation of Animal Study
Male BALB/c mice (6 weeks old) were purchased from the National Laboratory Animal Center (Taipei, Taiwan) in adherence with the Animal Care and Use Guidelines for Chang Gung University. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC Approval No.: CGU108-145). The mice were divided into four groups (with six mice per group): (i) PBS (mock), (ii) PM2.5-exposed, (iii) A. mellea mycelial extract-treated, and (iv) a combination of PM2.5-exposed with A. mellea mycelial extract-treated. PM2.5 (25 μg per mouse) was administered by intratracheal instillation twice weekly for a total of eight times over four weeks (totaling 200 μg). PBS or A. mellea mycelial extract (1.5 mg per mouse) was administered through oral gavage once daily for a total duration of 27 days. On day 28, blood samples were collected from mouse tail veins using 25-gauge needles for all tested groups. Serum was isolated by centrifugation at 1000× g for 3 min at room temperature. The mice were euthanized, and bronchoalveolar lavage fluid (BALF) and lungs were isolated following established protocols, as previously described [9]. Briefly, the trachea was flushed with normal saline, and the collected fluid was centrifuged at 500× g for 5 min at 4 °C. Cytokine levels in the supernatants were then analyzed using ELISA.
2.9. Immunohistochemistry (IHC) Analysis
Murine lung tissues were processed for hematoxylin–eosin (H&E) or immunohistochemistry (IHC) staining following established protocols, as detailed in prior work [9]. The lung sections were immunostained with antibodies targeting interleukin 1-beta (IL-1β) (ab9722, Abcam) and tumor necrosis factor-α (TNF-α) (ab6671, Abcam), respectively, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (D39-110, OriGene, Rockville, MD, USA), and developed using the Avidin-Biotin Complex Kit (ABC Kit, Vector Laboratories, Burlingame, CA, USA). Subsequently, the stained tissues were examined and analyzed using a microscope (AXIO IMAGER M2, Carl Zeiss, Oberkochen, Germany). Inflammatory cell infiltration in the lungs was assessed using the following criteria: score 0 for normal lung tissue, score 1 for mild inflammation, score 2 for moderate inflammation, and score 3 for severe inflammation.
2.10. Statistical Analysis
Statistical significance between the two groups was determined using Student’s t-test or the Mann–Whitney U test. Comparisons involving more than two groups were evaluated using one-way ANOVA with Tukey’s post hoc test. A p-value less than 0.05 was considered statistically significant. The figures were generated using Prism Program (v.9.0.0, GraphPad, San Diego, CA, USA).
3. Results
3.1. A. mellea Mycelia Mitigate PM2.5-Induced Nitric Oxide (NO) Production in Macrophages
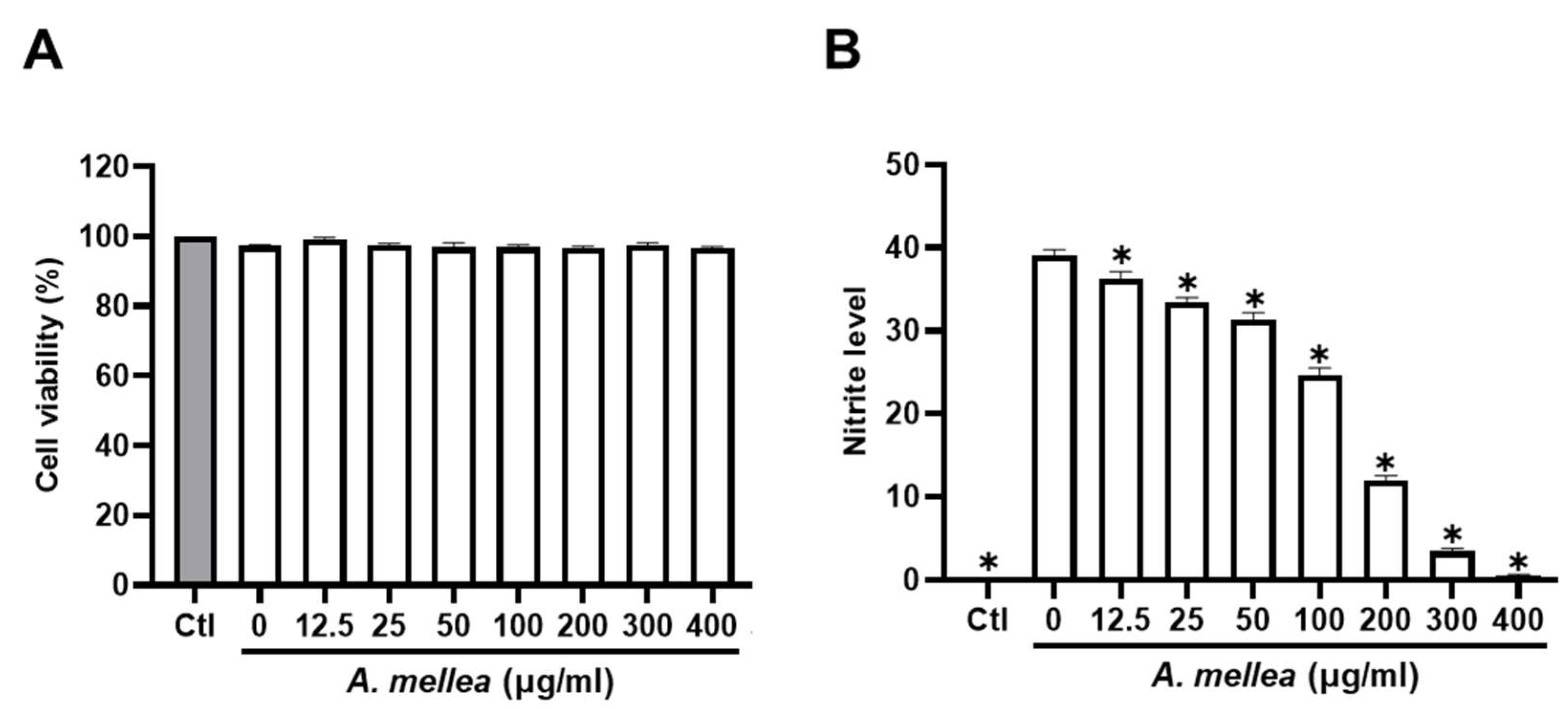
We initiated our investigation by assessing the impact of PM2.5 exposure on macrophage viability and NO production, exposing RAW264.7 macrophages to varying (0–30 μg/mL) PM2.5 concentrations for 24 h. Our results revealed that exposure to 30 μg/mL PM2.5 did not elicit significant changes in cell viability yet notably increased NO production (Figure S2). Consequently, we selected the PM2.5 concentration of 30 μg/mL for our subsequent experiments. To further elucidate the potential anti-inflammatory properties of the ethanol extracts of A. mellea mycelia, we assessed cytotoxicity and NO production using the macrophage models. As shown in Figure 1A, the A. mellea mycelial extract at 400 μg/mL did not significantly impact cell viability. The concentrations between 20 and 400 μg/mL significantly and concentration-dependently suppressed the PM2.5-induced NO production in macrophages (Figure 1B).
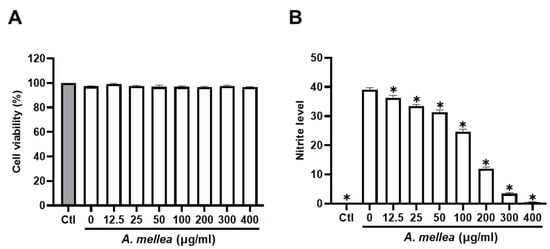
Figure 1.
A. mellea mycelia diminish PM2.5-induced nitric oxide production in macrophages. RAW264.7 cells were pretreated with varying concentrations of A. mellea mycelial extract (0–400 μg/mL) before exposure to PM2.5 (30 μg/mL) for 24 h. (A) Cell viability was evaluated using the MTT assay. (B) Nitric oxide production in the culture supernatant was quantified by measuring nitrite levels with Griess reagent. The data were presented as the mean ± standard deviation from three independent experiments. Statistical significance was determined using one-way ANOVA with Tukey’s post hoc test. *, p < 0.05 compared to A. mellea-untreated group.
3.2. A. mellea Mycelia Alleviate PM2.5-Associated Inflammatory Mediator Effects in Macrophages
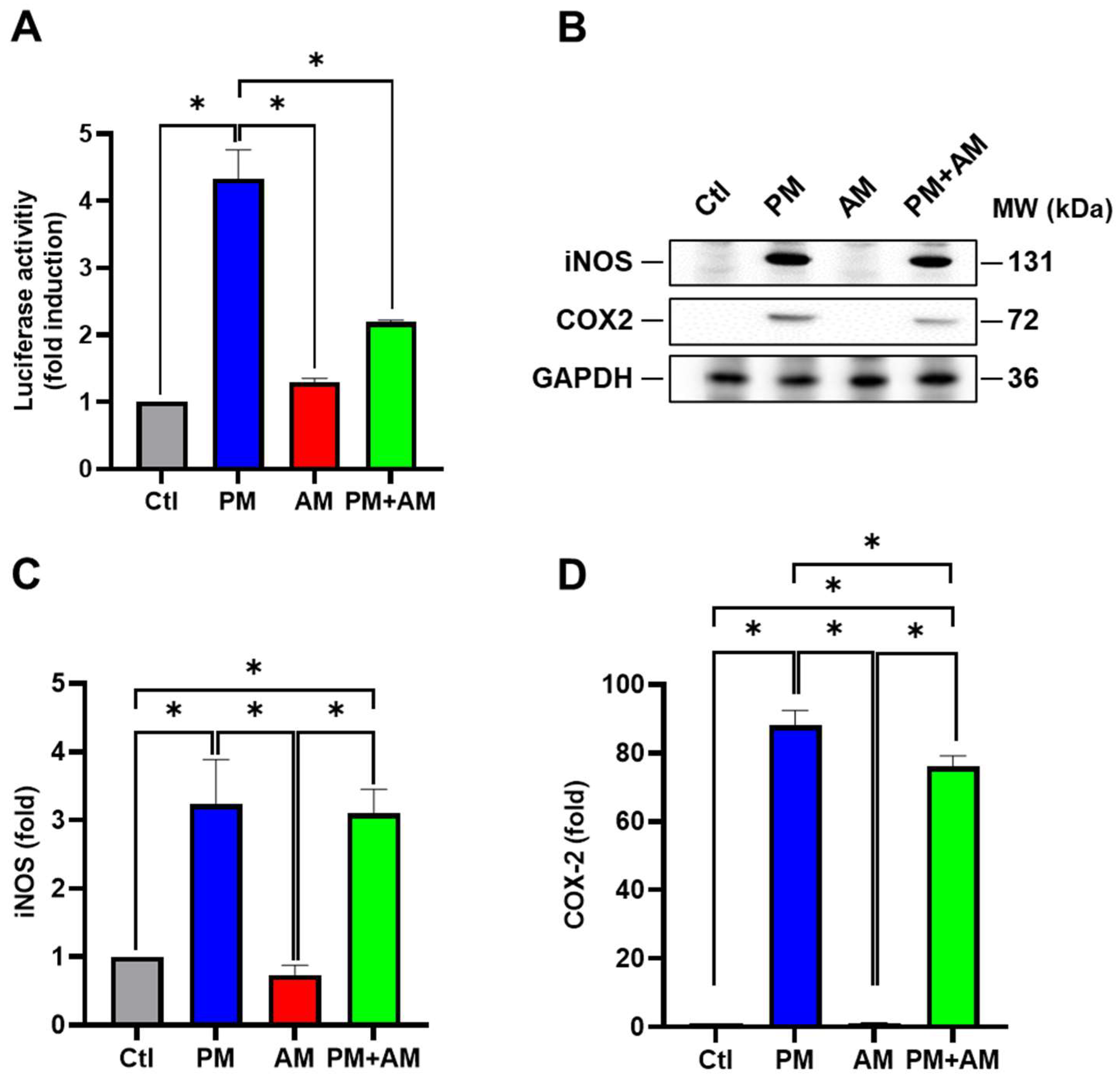
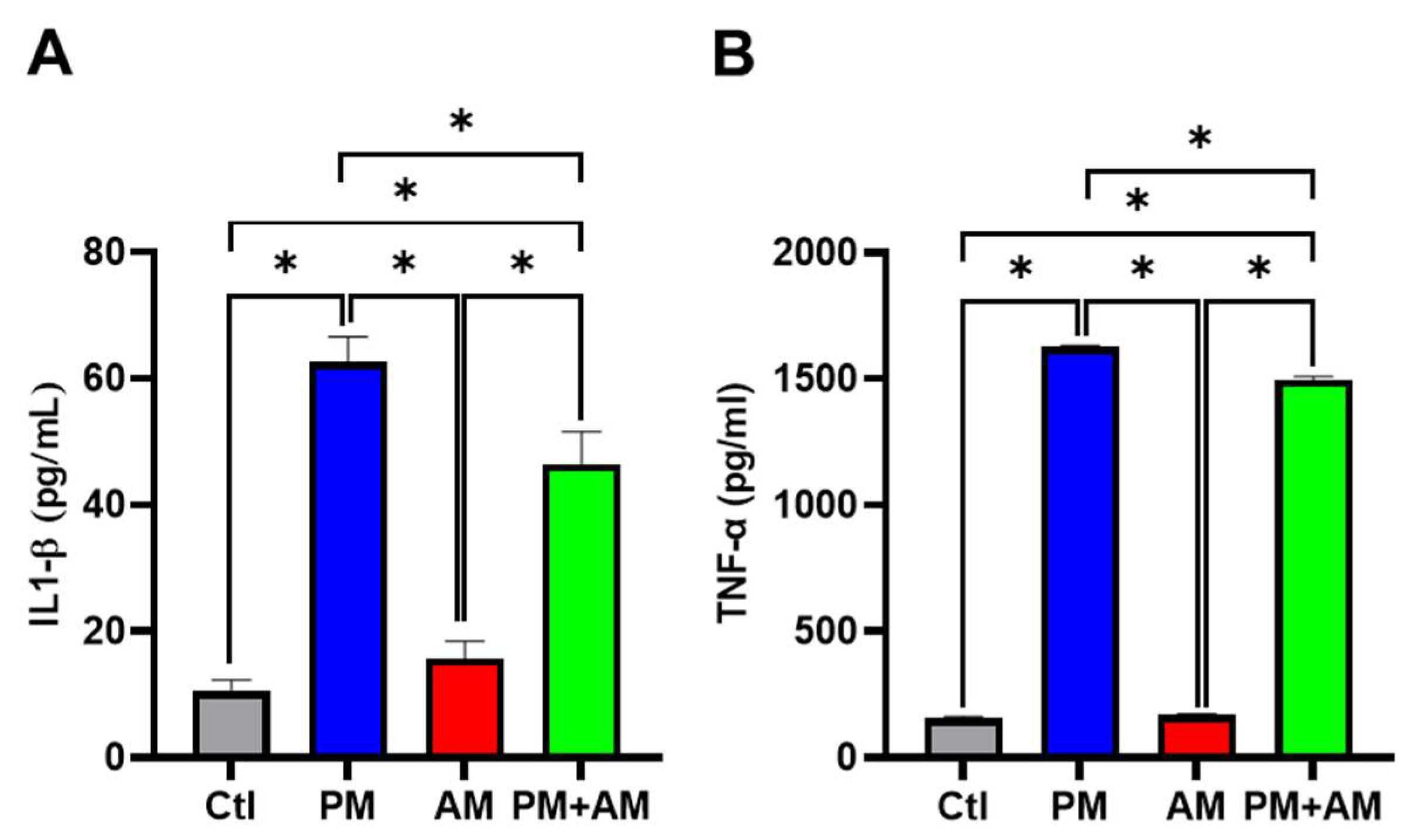
Next, we evaluated the potential PM2.5-induced NF-κB activation by A. mellea mycelia using an NF-κB luciferase activity assay. Our results demonstrated that treating macrophages with A. mellea mycelial extract resulted in reduced NF-κB activation (Figure 2A). Since NF-κB is a crucial transcription factor responsible for initiating iNOS and COX-2 expression, we further evaluated the corresponding protein expression levels using Western blotting (Figure 2B). The quantification results revealed a significant increase in iNOS (Figure 2C) and COX-2 (Figure 2D) protein levels in cells exposed to PM2.5, which were markedly decreased following treatment with A. mellea mycelial extract. In addition, we observed a notable suppression of the proinflammatory cytokines, IL-1β and TNF-α, in macrophages treated with the A. mellea mycelial extract (Figure 3). In contrast, the phagocytic activity of the macrophages remained unaffected by treatment with A. mellea mycelial extract (Figure S3). These findings strongly suggest that A. mellea mycelia possess potent properties for suppressing the PM2.5-induced production of inflammatory mediators.
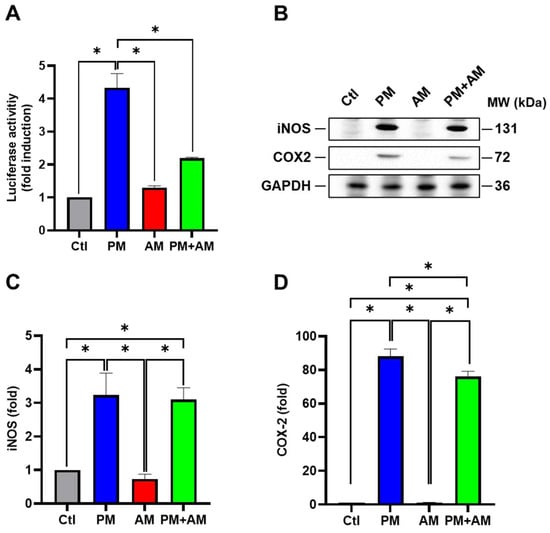
Figure 2.
A. mellea mycelia mitigate NF-κB activation upon PM2.5 exposure in macrophages. (A) RAW264.7 cells transfected with NF-κB luciferase reporter and β-galactosidase expression vector were treated with A. mellea mycelial extract (200 μg/mL) and concurrently exposed to PM2.5 (30 μg/mL) for 24 h. NF-κB luciferase activity was measured and normalized to the expression level of β-galactosidase. (B) Cell lysates were prepared to assess the expression levels of iNOS and COX-2 using a Western blot assay. Protein expression levels of (C) iNOS and (D) COX-2 were quantified and normalized to β-actin, respectively. The data were presented as means ± standard deviations obtained from three independent experiments. Statistical significance was determined using one-way ANOVA with Tukey’s post hoc test. *, p < 0.05.
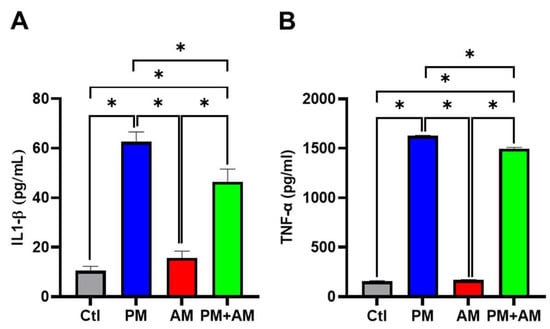
Figure 3.
Armillaria mellea mycelia reduce PM2.5-induced inflammatory mediators in macrophages. RAW264.7 cells were pretreated with A. mellea mycelial extract (200 μg/mL) before exposure to PM2.5 (30 μg/mL) for 24 h. The secretion levels of (A) IL-1β and (B) TNF-α in the culture supernatant were quantified using an ELISA. The data were expressed as the mean ± standard deviation obtained from three independent experiments. Statistical significance was assessed using one-way ANOVA with Tukey’s post hoc test. *, p < 0.05.
3.3. A. mellea Mycelia Relieve Pulmonary Inflammation in Long-Term PM2.5 Exposure in Murine Models
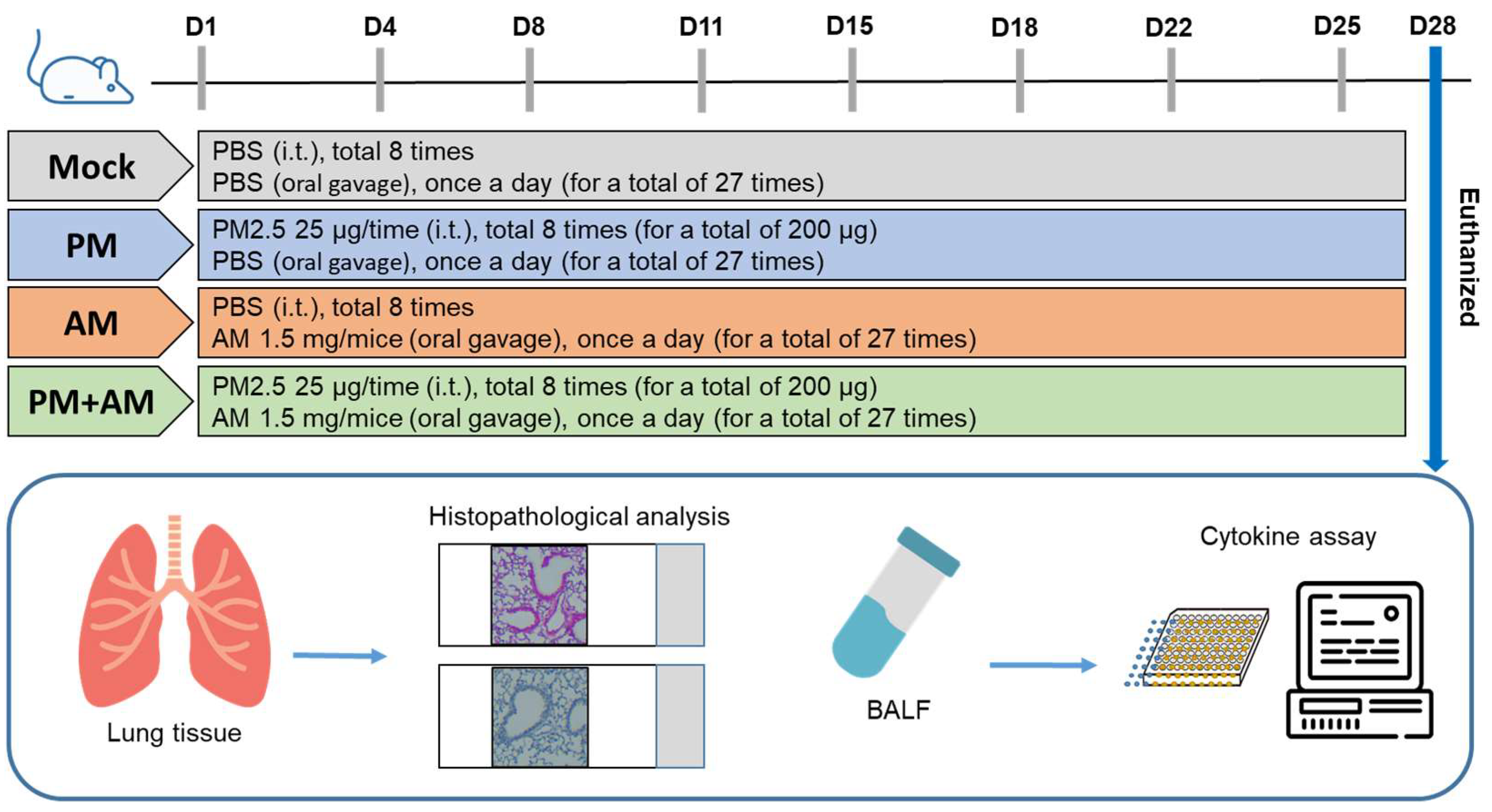
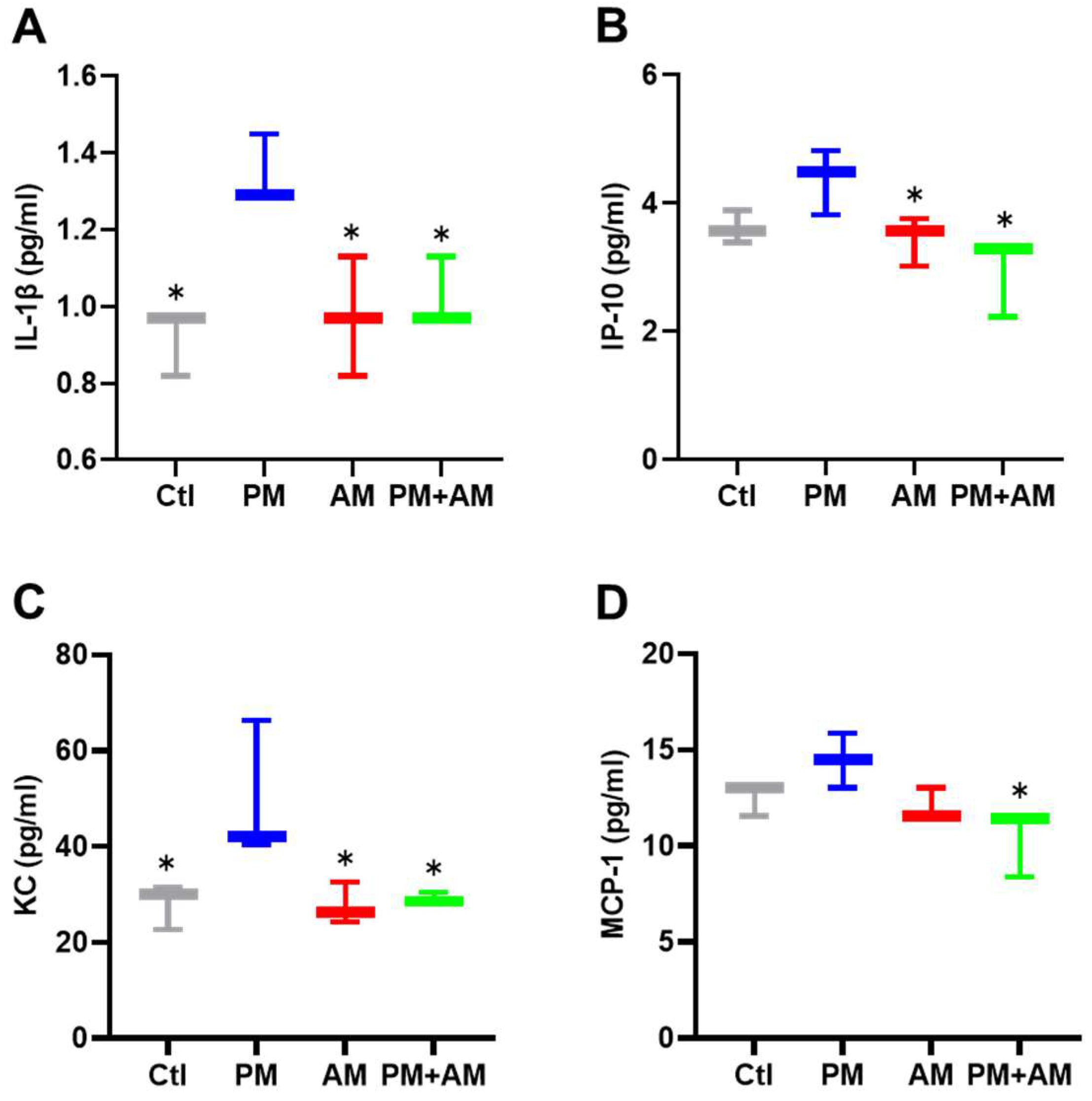
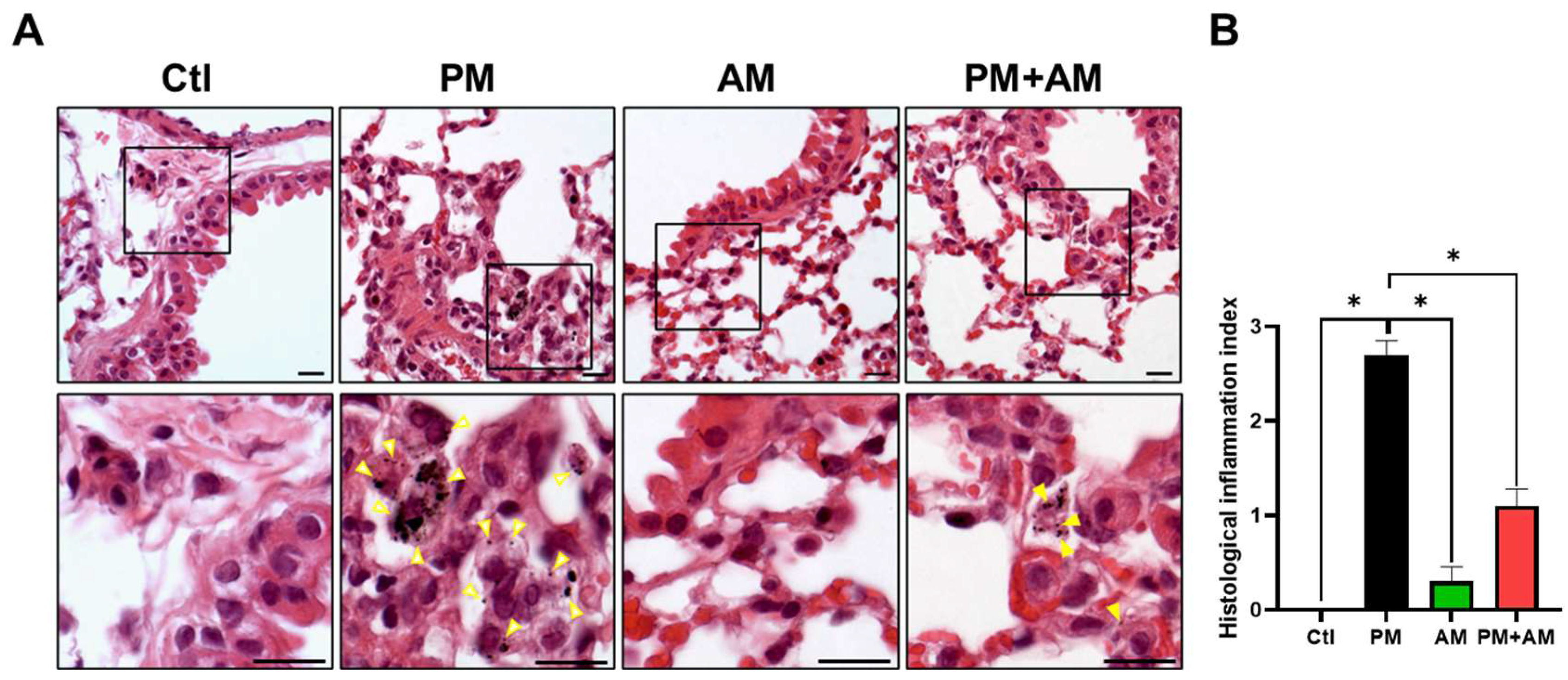
To evaluate the potential of A. mellea mycelial extract to ameliorate PM2.5-induced inflammation and pathogenesis in the host’s respiratory tract, we established a panel of long-term PM2.5-exposed murine models (Figure 4). We divided the mice into four groups as follows: (i) untreated mock, (ii) PM2.5-exposed, (iii) A. mellea mycelial extract-treated, and (iv) PM2.5-exposed + A. mellea mycelial extract-treated. We exposed the mice to PM2.5 for 25 d (totaling 200 μg) and simultaneously administered A. mellea mycelial extract (1.5 mg/mouse) once daily for 27 d. On day 28, we euthanized the mice and harvested their bronchoalveolar lavage fluid (BALF) for cytokine analysis. We registered significantly reduced IL-1β, IP-10, KC, and MCP-1 secretion levels in the A. mellea mycelial extract-treated mice before PM2.5 exposure compared to those exposed to PM2.5 alone (Figure 5). Subsequently, we prepared lung tissue samples for H&E staining to assess the number of infiltrated inflammatory cells. The lung tissues isolated from the untreated mock mice exhibited healthy alveoli and bronchi with minimal inflammatory cell infiltration (Figure 6). Following exposure to PM2.5, which accumulated in the cells, the bronchial wall thickened, and inflammatory cell infiltration significantly increased compared to that in the healthy mice. Conversely, when we exposed the mice to PM2.5 and concurrently treated them with A. mellea mycelial extract, the treatment effectively prevented the development of an inflammatory response in their lungs. Thus, our study demonstrated that A. mellea mycelial extract could mitigate PM2.5-induced inflammation and pathogenesis in the murine respiratory tract.
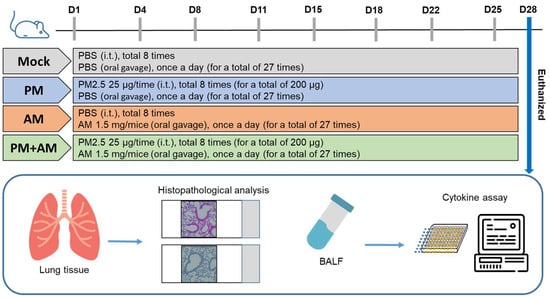
Figure 4.
Experimental design and establishment of murine models to evaluate the biological effects of A. mellea. Mice received intratracheal administration of PM2.5 from day 1 to day 25, totaling 200 μg. Simultaneously, mice were treated with A. mellea mycelial extract (1.5 mg/mouse) once daily for a total of 27 days. Upon euthanasia of the mice on day 28, bronchoalveolar lavage fluid (BALF) was collected for cytokine analysis, and lung tissues were prepared for histopathological examination.
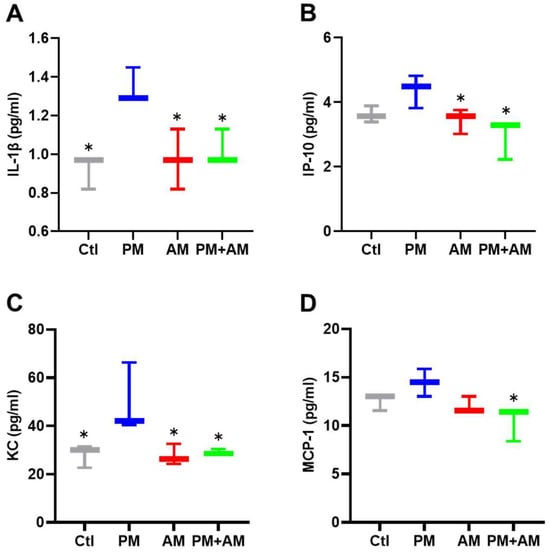
Figure 5.
A. mellea mycelia alleviate PM2.5-induced inflammatory mediators in mice. Mice were administered PM2.5 and treated with A. mellea mycelial extract according to the experimental design in Figure 4. Following euthanasia, BALF collected from the mice was subjected to an ELISA to measure the concentrations of (A) IL-1β, (B) IP-10, (C) KC, and (D) MCP-1. The data are presented as the mean ± standard deviation obtained from each treatment group. Statistical significance was determined using the Mann–Whitney U test. *, p < 0.05.
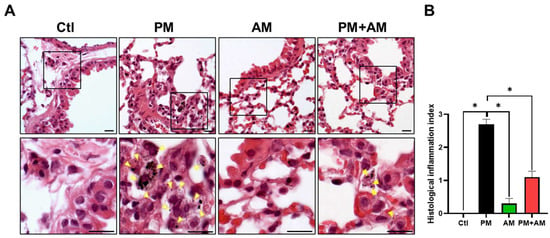
Figure 6.
A. mellea mycelia attenuate PM2.5-induced lung tissue inflammation in murine models. Mice were administered PM2.5 and treated with A. mellea mycelial extract following the protocol outlined in Figure 4. (A) Upon euthanasia, lung tissue sections were subjected to H&E staining. Magnified images are displayed beneath each corresponding cropped area. The yellow arrowheads indicate macrophages with phagocytosed PM2.5. Scale bars, 10 μm. (B) Inflammatory cell infiltration in the lungs was assessed using a scoring system adapted from a previous study [9]: score 0, normal lung tissue; score 1, slight inflammation; score 2, moderate inflammation; score 3, severe inflammation. Statistical significance was determined using the Mann–Whitney U test. *, p < 0.05.
3.4. A. mellea Mycelia Improve PM2.5-Induced Pulmonary Inflammation
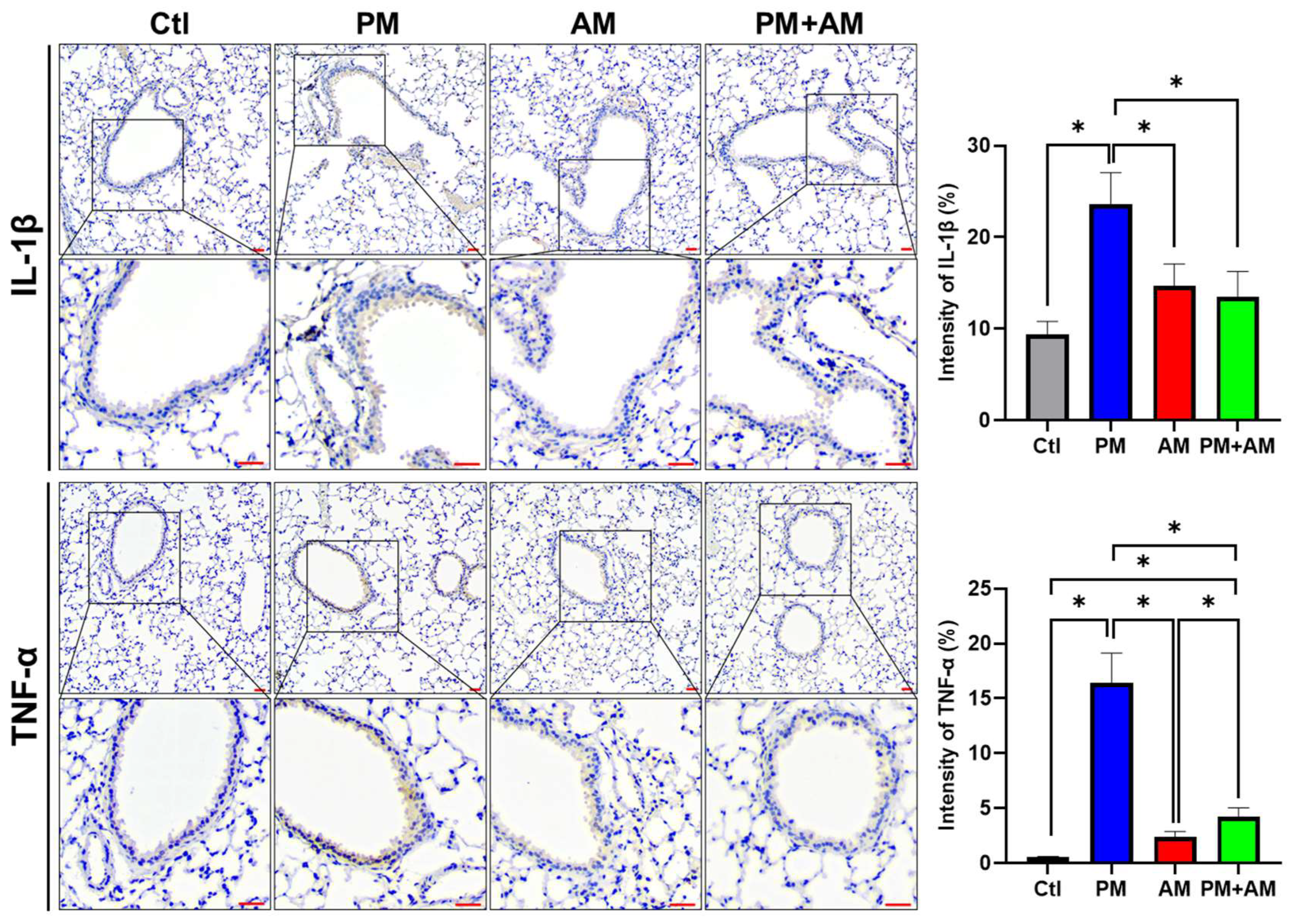
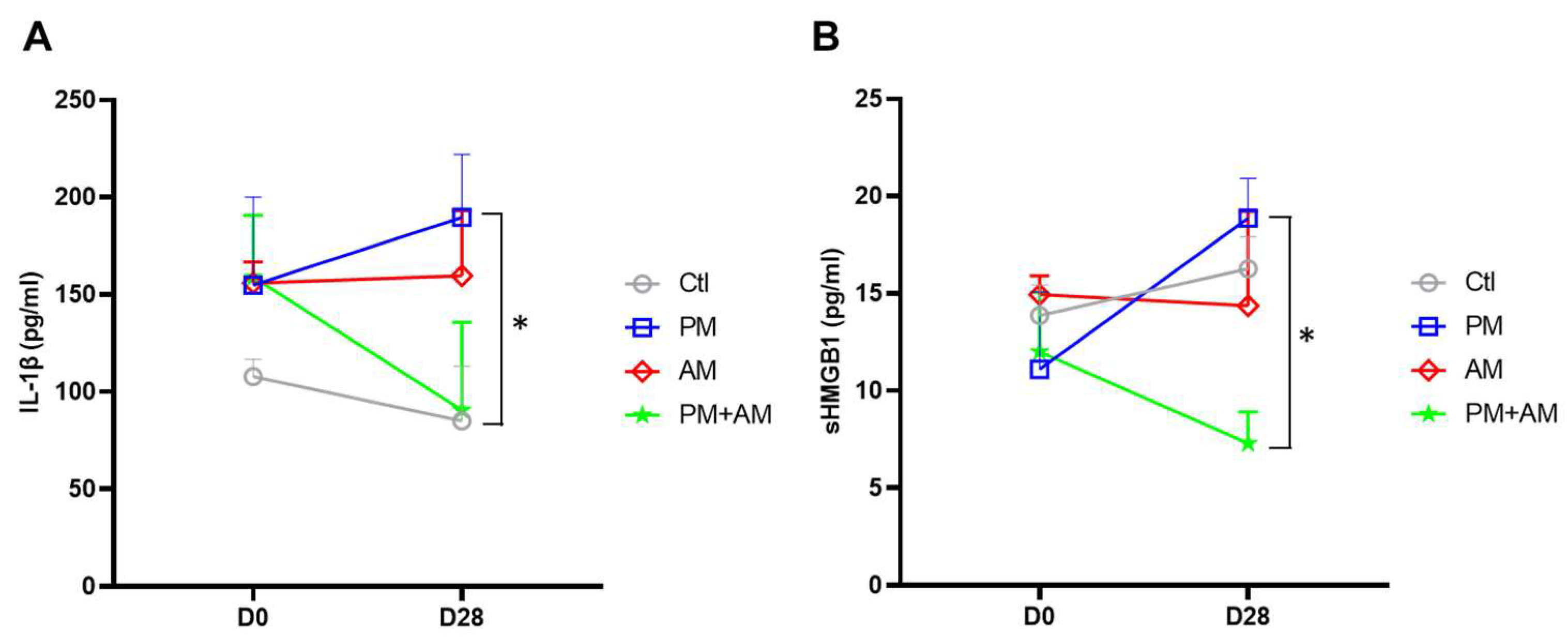
To investigate whether A. mellea mycelial extract would mitigate PM2.5-induced lung inflammation, we subjected murine lung tissue samples to IHC analysis. Lung tissues isolated from the PM2.5-exposed mice exhibited a pronounced expression of proinflammatory cytokines (IL-1β and TNF-α). In the PM2.5-exposed mice treated with A. mellea mycelial extract, the production of these cytokines was remarkably reduced compared to that in the healthy group (Figure 7). In addition, we investigated the proinflammatory cytokine levels in the murine serum, revealing significantly increased IL-1β and sHMGB1 levels in the serum of PM2.5-exposed mice compared to their day-0 levels (Figure 8). In contrast, mice treated with A. mellea mycelial extract for 28 d exhibited prominently reduced IL-1β and sHMGB1 secretion levels compared to those of the PM2.5-exposed group. These results demonstrate the efficacy of A. mellea mycelial extract in mitigating PM2.5-induced inflammation and the related respiratory system pathogenesis, indicated by the reduced cytokine secretion and lung inflammatory status restoration in long-term PM2.5-exposed models.
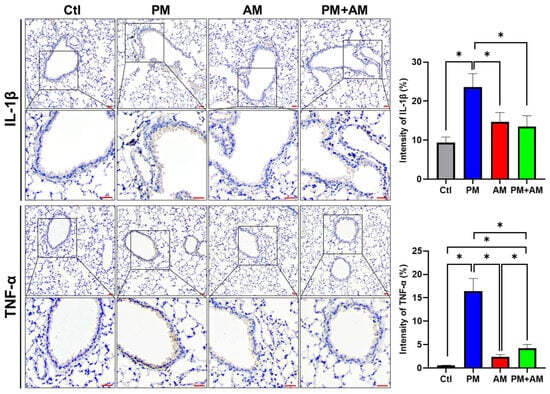
Figure 7.
A. mellea mycelia decrease the expression of IL-1β and TNF-α in PM2.5-exposed lung tissues. Mice were administered PM2.5 and A. mellea mycelial extract following the protocol outlined in Figure 4. Murine lung tissues were prepared for immunohistochemical (IHC) staining to assess the expression levels of IL-1β and TNF-α, respectively. Magnified images are displayed beneath each corresponding cropped area. Scale bars: 200 μm. The intensity of IL-1β and TNF-α expression in gastric tissues, as detected by IHC staining, is shown in the right panels. Statistical significance was determined using the Mann–Whitney U test. *, p < 0.05.
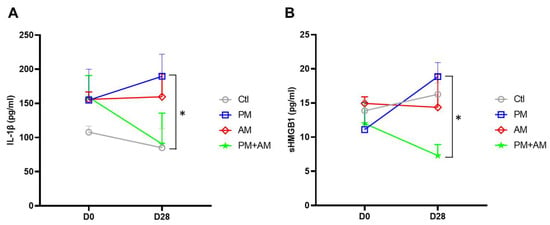
Figure 8.
A. mellea mycelia mitigate PM2.5-induced inflammation in mice. Mice were administered PM2.5 and treated with A. mellea mycelial extract according to the protocol described in Figure 4. After euthanizing the mice, serum samples were collected on day 0 and day 28, then subjected to ELISA to measure the concentrations of (A) IL-1β and (B) sHMGB1. Statistical significance was assessed using the Mann–Whitney U test. *, p < 0.05.
4. Discussion
In this study, we investigated the potential of A. mellea mycelia to alleviate PM2.5-induced inflammation and respiratory pathogenesis. Moreover, by exploring the involved molecular mechanisms, we gained a deeper insight into how A. mellea mycelia mitigate PM2.5-induced respiratory inflammation and restore pulmonary function. Our results demonstrated that PM2.5 exposure impaired macrophage functions and exacerbated respiratory inflammation, which closely mirrors real-life environmental exposure scenarios. Building on this well-established platform, we employed these in vitro and in vivo models to assess the effects of A. mellea mycelia in alleviating PM2.5-induced macrophage inflammation. Our results emphasize the preventive potential of A. mellea mycelia in addressing PM2.5-related pulmonary pathogenesis.
A. mellea, renowned for its medicinal components such as polysaccharides and sesquiterpenes, exhibits a broad spectrum of biological activities. Polysaccharides isolated from A. mellea have been extensively studied and have shown notable health benefits, including immunoregulatory [28], anti-tumor [29], anti-inflammatory [30], antioxidant [31], neuroprotective [32], and lung-protective [18] properties. We used ethanol extracts of A. mellea mycelia in this study, assuming their richness in sesquiterpenes, as described in our recent publication [22]. Furthermore, the sesquiterpenes in A. mellea have been studied for their anti-tumor [33] and anti-depressant [34] effects. In conjunction with the aforementioned findings, the multifaceted biological activities of A. mellea mycelial sesquiterpenes support their potential as valuable therapeutic agent sources for addressing various health conditions.
A. mellea has been valued for its medicinal properties for centuries. In traditional medicine, such fungal extracts and preparations have been used to treat various health concerns, including respiratory ailments, gastrointestinal disorders, and neurodegenerative diseases [18,35,36]. However, A. mellea is a natural product with a complex chemical composition, and its efficacy may vary depending on its geographical origin, growth conditions, and extraction methods [19,20]. The standardization of A. mellea preparations and rigorous quality control measures are essential for ensuring the consistency and the reproducibility of therapeutic outcomes.
In this study, we established a macrophage model to investigate the effects of A. mellea mycelial extract on PM2.5-induced inflammation. Our findings revealed that A. mellea mycelial extract efficiently reduced PM2.5-triggered NO production in macrophages. We observed a concentration-dependent decrease in NO levels in A. mellea mycelial extract-treated macrophages, indicating the capacity of this extract to modulate the inflammatory responses initiated by PM2.5 exposure. Furthermore, A. mellea mycelial extract diminished NF-κB activation, leading to the downregulation of key inflammatory mediators such as iNOS and COX-2. Our findings are consistent with those of previous studies [30,35,37], demonstrating the antioxidant and anti-inflammatory properties of A. mellea mycelial extract, including the repression of NO production, the suppression of NF-κB signaling, and the subsequent inhibition of proinflammatory cytokine production. These results highlight the potential of A. mellea mycelial extract to mitigate PM2.5-induced inflammation at the molecular level.
In the murine models of long-term PM2.5 exposure, the administration of A. mellea mycelial extract significantly alleviated pulmonary inflammation. The reduced secretion levels of proinflammatory cytokines (IL-1β, IP-10, KC, and MCP-1) in BALF indicate the anti-inflammatory effects of A. mellea mycelial extract in vivo. Our histological analysis further corroborated these findings, demonstrating reduced inflammatory cell infiltration in the lungs of the A. mellea mycelia-treated mice compared to those in the PM2.5-exposed mice. Although the murine models of long-term PM2.5 exposure provide valuable insights into the efficacy of A. mellea mycelial extract in alleviating pulmonary inflammation, these models may not fully recapitulate the complexity of PM2.5 exposure-induced human respiratory diseases and the biological effects of A. mellea, especially in its broader roles in immune regulation, anti-inflammatory responses, and antioxidative stress. In this study, we focused on macrophages as the primary assay platform; however, we acknowledge that further research is essential to explore A. mellea’s interactions with other cell types and its overall health impacts. Further disease-specific clinical studies in humans should be conducted to validate our findings in a translational context.
5. Conclusions
In summary, our study illustrates the potent antioxidant and anti-inflammatory properties of A. mellea mycelia in managing PM2.5-induced inflammation and respiratory pathogenesis. In macrophage models, A. mellea mycelia effectively reduced NO production and suppressed NF-κB activation, thereby reducing inflammatory mediator expression. Moreover, in long-term PM2.5-exposed murine models, A. mellea mycelia significantly attenuated the secretion of proinflammatory cytokines and restored lung inflammatory status. The outcomes of our study hold promising potential for developing novel interventions aimed at mitigating the adverse effects of PM2.5 on respiratory health, thereby providing invaluable insights into improving human well-being.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox13111381/s1, Figure S1. HPLC profiles of A. mellea mycelial extract. Figure S2. Assessment of PM2.5-induced nitric oxide production in macrophages. Figure S3. Effect of A. mellea mycelia on macrophage phagocytosis.
Author Contributions
Y.-P.H.: performed the experiments, analyzed the data, and wrote the manuscript. Y.-T.H.: performed the animal study and analyzed the data. H.-Y.W.: performed the animal study and analyzed the data. L.-F.C.: performed the experiments and analyzed the data. Y.-S.T.: performed the experiments and analyzed the data. Y.-M.J.: performed the experiments and analyzed the data. W.-P.C.: performed the experiments and analyzed the data. T.-W.L.: performed the experiments and analyzed the data. C.-C.C.: conceived and designed the experiments and wrote the manuscript. C.-H.L.: wrote the manuscript and reviewed the final version of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Science and Technology Council (112-2320-B-182-036-MY3 and 112-2320-B-182-042-MY3), Chang Gung Memorial Hospital (CMRPD1L0321, CMRPD1M0491-2, CMRPD1P0161-2, and CORPD1M0021-3), Grape King Bio Research Foundation (BR-AE041), and Research Center for Emerging Viral Infections from the Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Taiwan Ministry of Education, and Tomorrow Medical Foundation.
Institutional Review Board Statement
This study does not involve the use of human subjects. The mouse experiments were performed in accordance with the Animal Care and Use Guidelines for Chang Gung University under a protocol approved by the Institutional Animal Care Use Committee (IACUC Approval No.: CGU108-145).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are contained within the article. The original contributions presented in the study were included in the main article and the Supplementary Material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to thank the editor and reviewers for their editorial assistance and valuable comments. The authors sincerely thank the Microscopy Center at Chang Gung University for their technical assistance. The authors deeply thank Chieh-Ying Kuo for her invaluable assistance in conducting the murine model experiments.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Co-authors You-Shan Tsai, Yih-Min Jiang, Wan-Ping Chen, and Ting-Wei Lin belong to Grape King Bio Ltd. However, the funders/company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdorster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Particle toxicology and health-where are we? Part. Fibre Toxicol. 2019, 16, 19. [Google Scholar] [CrossRef]
- McDuffie, E.E.; Martin, R.V.; Spadaro, J.V.; Burnett, R.; Smith, S.J.; O’Rourke, P.; Hammer, M.S.; van Donkelaar, A.; Bindle, L.; Shah, V.; et al. Source sector and fuel contributions to ambient PM2.5 and attributable mortality across multiple spatial scales. Nat. Commun. 2021, 12, 3594. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Zhang, Z.; Lau, A.K.H.; Lin, C.Q.; Chuang, Y.C.; Chan, J.; Jiang, W.K.; Tam, T.; Yeoh, E.K.; Chan, T.C.; et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: A longitudinal, cohort study. Lancet Planet Health 2018, 2, e114–e125. [Google Scholar] [CrossRef] [PubMed]
- Gehring, U.; Wijga, A.H.; Koppelman, G.H.; Vonk, J.M.; Smit, H.A.; Brunekreef, B. Air pollution and the development of asthma from birth until young adulthood. Eur. Respir. J. 2020, 56, 2000147. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Oftedal, B.; Chen, J.; Rodopoulou, S.; Renzi, M.; Atkinson, R.W.; Bauwelinck, M.; Klompmaker, J.O.; Mehta, A.; Vienneau, D.; et al. Long-term exposure to low ambient air pollution concentrations and mortality among 28 million people: Results from seven large european cohorts within the ELAPSE project. Lancet Planet. Health 2022, 6, e9–e18. [Google Scholar] [CrossRef]
- Xin, D.; Xin, L. The impact of economic policy uncertainty on PM2.5 pollution-evidence from 25 countries. Environ. Sci. Pollut. Res. Int. 2022, 29, 38126–38142. [Google Scholar] [CrossRef]
- Zhang, J.; Zeng, X.; Li, Y.; Zhao, W.; Chen, Z.; Du, Q.; Zhou, F.; Ji, N.; Huang, M. Exposure to Ambient Particles Alters the Evolution of Macrophage Phenotype and Amplifies the Inducible Release of Eotaxin-1 in Allergen-Sensitized Mice. J. Biomed. Nanotechnol. 2019, 15, 382–395. [Google Scholar] [CrossRef]
- Chen, Y.W.; Huang, M.Z.; Chen, C.L.; Kuo, C.Y.; Yang, C.Y.; Chiang-Ni, C.; Chen, Y.M.; Hsieh, C.M.; Wu, H.Y.; Kuo, M.L.; et al. PM2.5 impairs macrophage functions to exacerbate pneumococcus-induced pulmonary pathogenesis. Part. Fibre Toxicol. 2020, 17, 37. [Google Scholar] [CrossRef]
- Su, R.; Jin, X.; Zhang, W.; Li, Z.; Liu, X.; Ren, J. Particulate matter exposure induces the autophagy of macrophages via oxidative stress-mediated PI3K/AKT/mTOR pathway. Chemosphere 2017, 167, 444–453. [Google Scholar] [CrossRef]
- Hou, T.; Zhu, L.; Wang, Y.; Peng, L. Oxidative stress is the pivot for PM2.5-induced lung injury. Food Chem. Toxicol. 2024, 184, 114362. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Braun, D.; Christidis, T.; Cork, M.; Rodopoulou, S.; Samoli, E.; Stafoggia, M.; Wolf, K.; Wu, X.; Yuchi, W.; et al. Long-Term Exposure to Low-level PM2.5 and Mortality: Investigation of Heterogeneity by Harmonizing Analyses in Large Cohort Studies in Canada, United States, and Europe. Environ. Health Perspect. 2023, 131, 127003. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Huang, T.H.; Chi, M.C.; Guo, S.E.; Lee, C.W.; Hwang, S.L.; Shi, C.S. N-acetylcysteine alleviates fine particulate matter (PM2.5)-induced lung injury by attenuation of ROS-mediated recruitment of neutrophils and Ly6C(high) monocytes and lung inflammation. Ecotoxicol. Environ. Saf. 2022, 239, 113632. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tang, Y.; Sun, J.; Feng, J.; Chen, L.; Chen, H.; Zeng, S.; Chen, C.; Li, X.; Zhu, H.; et al. Flavone protects HBE cells from DNA double-strand breaks caused by PM2.5. Hum. Cell 2018, 31, 116–126. [Google Scholar] [CrossRef]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Moon, J.H.; Kim, M.J.; Lee, H.L.; Jeong, H.R.; Kim, J.C.; Heo, H.J. Powdered Green Tea (Matcha) Attenuates the Cognitive Dysfunction via the Regulation of Systemic Inflammation in Chronic PM2.5-Exposed BALB/C Mice. Antioxidants 2021, 10, 1932. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory Effects of Black Ginseng on Particulate Matter-Induced Pulmonary Injury. Am. J. Chin. Med. 2019, 47, 1237–1251. [Google Scholar] [CrossRef]
- Han, B.H.; Jang, S.H.; Jang, Y.J.; Na, S.W.; Yoon, J.J.; Moon, H.G.; Kim, S.Y.; Seo, C.S.; Lee, H.S.; Lee, Y.M.; et al. Diesel vehicles-derived PM2.5 induces lung and cardiovascular injury attenuates by Securiniga suffruticosa: Involvement of NF-kappaB-mediated NLRP3 inflammasome activation pathway. Biomed. Pharmacother. 2023, 162, 114637. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Ren, L.; Dai, X.; Zhao, J.; Gao, C.; Zhang, S.; Dong, J.; Zhao, Z.; Li, Y.; et al. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Armillaria mellea (Vahl) P. Kumm.: A review. Int. J. Biol. Macromol. 2024, 259, 129175. [Google Scholar] [CrossRef]
- Kostic, M.; Smiljkovic, M.; Petrovic, J.; Glamoclija, J.; Barros, L.; Ferreira, I.; Ciric, A.; Sokovic, M. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef]
- Erbiai, E.H.; da Silva, L.P.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Maouni, A. Chemical Composition, Bioactive Compounds, and Antioxidant Activity of Two Wild Edible Mushrooms Armillaria mellea and Macrolepiota procera from Two Countries (Morocco and Portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef] [PubMed]
- Li, I.C.; Lin, T.W.; Lee, T.Y.; Lo, Y.; Jiang, Y.M.; Kuo, Y.H.; Chen, C.C.; Chang, F.C. Oral Administration of Armillaria mellea Mycelia Promotes Non-Rapid Eye Movement and Rapid Eye Movement Sleep in Rats. J. Fungi 2021, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Klouda, G.A.; Filliben, J.J.; Parish, H.J.; Chow, J.C.; Watson, J.G.; Cary, R.A. Reference material 8785: Air particulate matter on filter media. Aerosol Sci Technol. 2005, 39, 173–183. [Google Scholar] [CrossRef]
- Lin, H.J.; Jiang, Z.P.; Lo, H.R.; Feng, C.L.; Chen, C.J.; Yang, C.Y.; Huang, M.Z.; Wu, H.Y.; Chen, Y.A.; Chen, Y.; et al. Coalescence of RAGE in Lipid Rafts in Response to Cytolethal Distending Toxin-Induced Inflammation. Front. Immunol. 2019, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.D.; Kou, Y.Y.; Liao, C.Y.; Li, C.H.; Huang, S.P.; Cheng, Y.W.; Liao, W.C.; Chen, H.X.; Wu, P.L.; Kang, J.J.; et al. Zinc oxide nanoparticles impair bacterial clearance by macrophages. Nanomedicine 2014, 9, 1327–1339. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tsai, W.H.; Wu, H.Y.; Chen, C.Y.; Yeh, W.L.; Chen, Y.H.; Hsu, H.Y.; Chen, W.W.; Chen, Y.W.; Chang, W.W.; et al. Probiotic Lactobacillus spp. Act against Helicobacter pylori-induced Inflammation. J. Clin. Med. 2019, 8, 90. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, T.L.; Huang, M.Z.; Li, S.W.; Wu, H.Y.; Chiu, Y.F.; Yang, C.Y.; Chiu, C.H.; Lai, C.H. Gut Commensal Parabacteroides goldsteinii MTS01 Alters Gut Microbiota Composition and Reduces Cholesterol to Mitigate Helicobacter pylori-Induced Pathogenesis. Front Immunol. 2022, 13, 916848. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, H.; Zhang, X.; Tong, H.; Liu, J. Structural elucidation and immunological activity of a polysaccharide from the fruiting body of Armillaria mellea. Bioresour. Technol. 2009, 100, 1860–1863. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, J.; Lang, Y.; Yao, L.; Xu, H.; Shi, H.; Xu, S. A polysaccharide from Armillaria mellea exhibits strong in vitro anticancer activity via apoptosis-involved mechanisms. Int. J. Biol. Macromol. 2012, 51, 663–667. [Google Scholar] [CrossRef]
- Chang, C.W.; Lur, H.S.; Lu, M.K.; Cheng, J.J. Sulfated polysaccharides of Armillariella mellea and their anti-inflammatory activities via NF-κB suppression. Food Res. Int. 2013, 54, 239–245. [Google Scholar] [CrossRef]
- Prasad, R.; Varshney, V.K.; Harsh, N.S.; Kumar, M. Antioxidant Capacity and Total Phenolics Content of the Fruiting Bodies and Submerged Cultured Mycelia of Sixteen Higher Basidiomycetes Mushrooms from India. Int. J. Med. Mushrooms 2015, 17, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Kuo, Y.H.; Cheng, J.J.; Sung, P.J.; Ni, C.L.; Chen, C.C.; Shen, C.C. Three New Sesquiterpene Aryl Esters from the Mycelium of Armillaria mellea. Molecules 2015, 20, 9994–10003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Du, Y.; Liu, X.; Sun, X.; Cai, E.; Zhu, H.; Zhao, Y. Study on antidepressant-like effect of protoilludane sesquiterpenoid aromatic esters from Armillaria mellea. Nat. Prod. Res. 2021, 35, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Zhu, S.; Cheng, P.; Lu, Z.M.; Xu, H.Y.; Shi, J.S.; Xu, Z.H. Bioassay-guided fractionation of ethyl acetate extract from Armillaria mellea attenuates inflammatory response in lipopolysaccharide (LPS) stimulated BV-2 microglia. Phytomedicine 2017, 26, 55–61. [Google Scholar] [CrossRef]
- Yao, L.; Lv, J.; Duan, C.; An, X.; Zhang, C.; Li, D.; Li, C.; Liu, S. Armillaria mellea fermentation liquor ameliorates p-chlorophenylalanine-induced insomnia associated with the modulation of serotonergic system and gut microbiota in rats. J. Food Biochem. 2022, 46, e14075. [Google Scholar] [CrossRef]
- Chang, C.C.; Cheng, J.J.; Lee, I.J.; Lu, M.K. Purification, structural elucidation, and anti-inflammatory activity of xylosyl galactofucan from Armillaria mellea. Int. J. Biol. Macromol. 2018, 114, 584–591. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).