Enhancing of Rabbit Sperm Cryopreservation with Antioxidants Mito-Tempo and Berberine
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Semen Processing and Experimental Design
2.3. Measurement of Semen Quality Parameters
2.3.1. Reagents
2.3.2. Sperm Movement Characteristics
2.3.3. Flow Cytometry Analysis
Sperm Viability Assessment
Oxidative Stress Measurement
Mitochondrial Activity Evaluation
Acrosome Integrity Evaluation
Apoptosis Assessment
2.4. Statistical Analyses
2.5. Use of Generative AI for Language Editing
3. Results
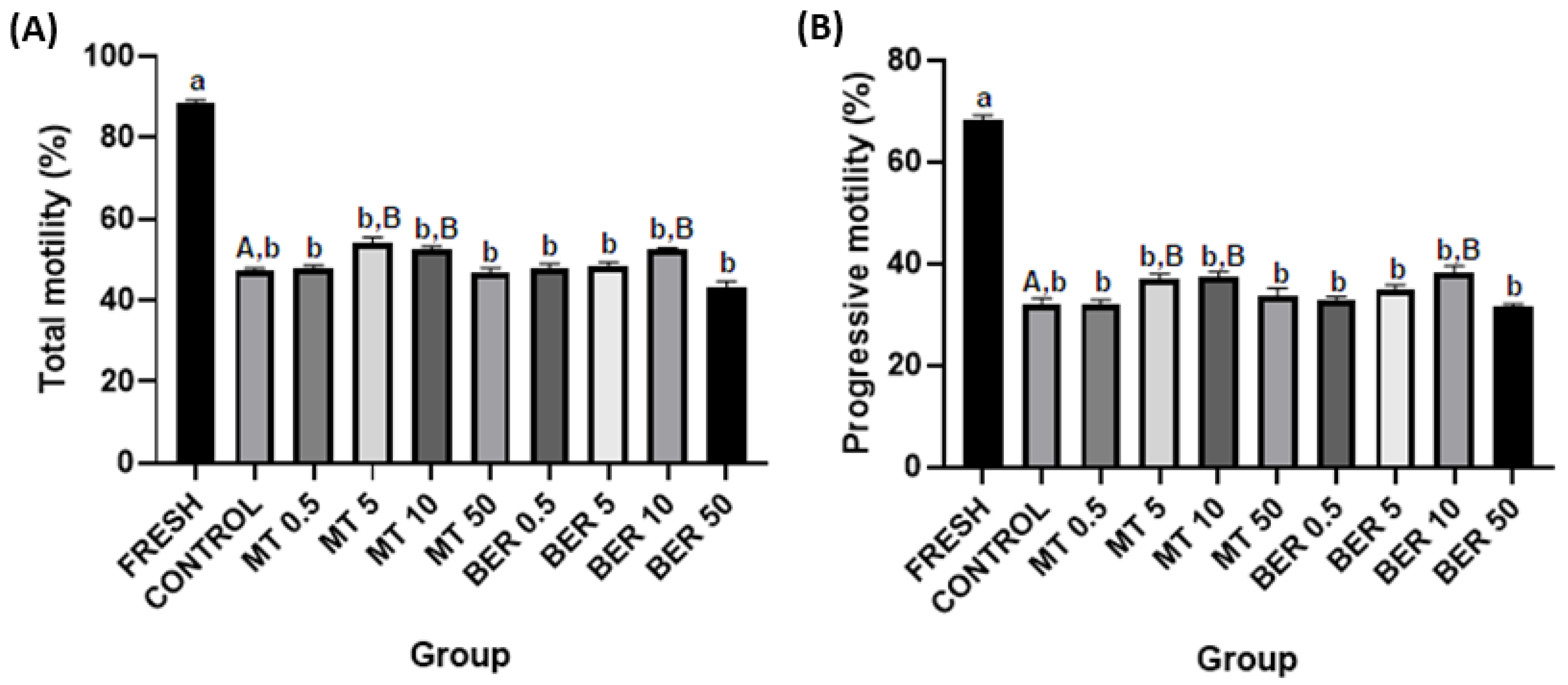
3.1. Effects of MT and BER on the Motility of Frozen-Thawed Sperm
3.2. Effects of MT and BER on the Viability of Frozen-Thawed Sperm
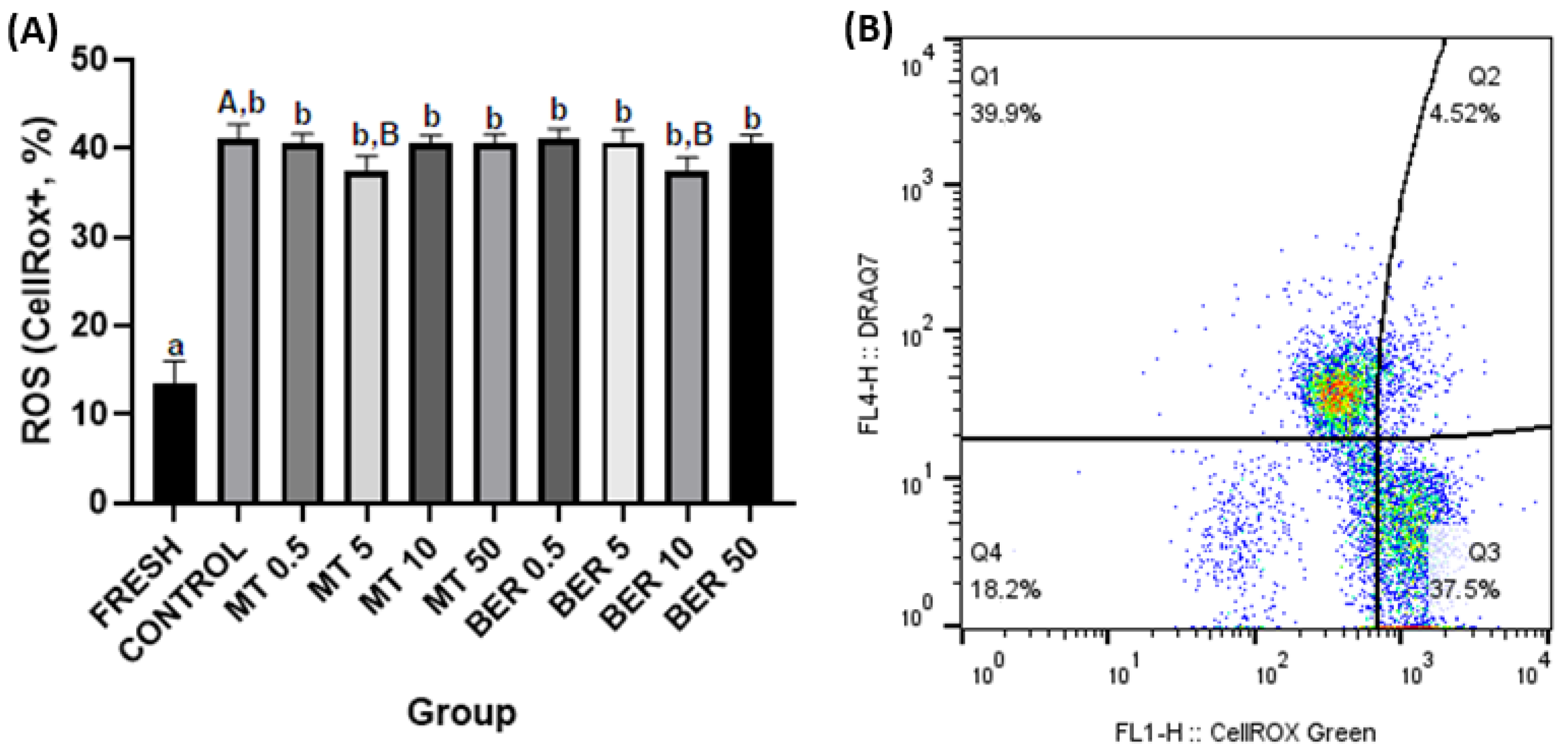
3.3. Effects of MT and BER on the ROS Generation of Frozen-Thawed Sperm
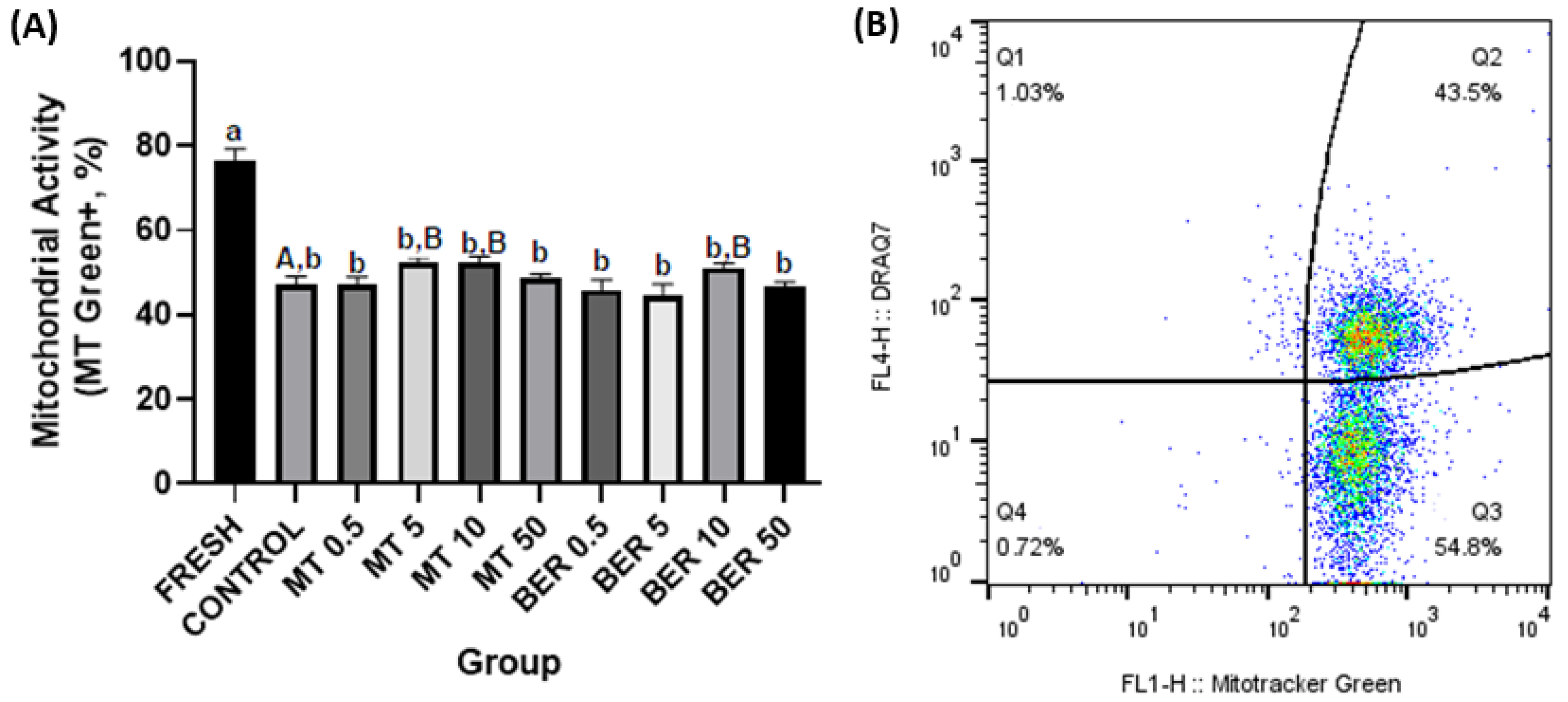
3.4. Effects of MT and BER on the Mitochondrial Activity of Frozen-Thawed Sperm
3.5. Effects of MT and BER on the Acrosome Integrity of Frozen-Thawed Sperm
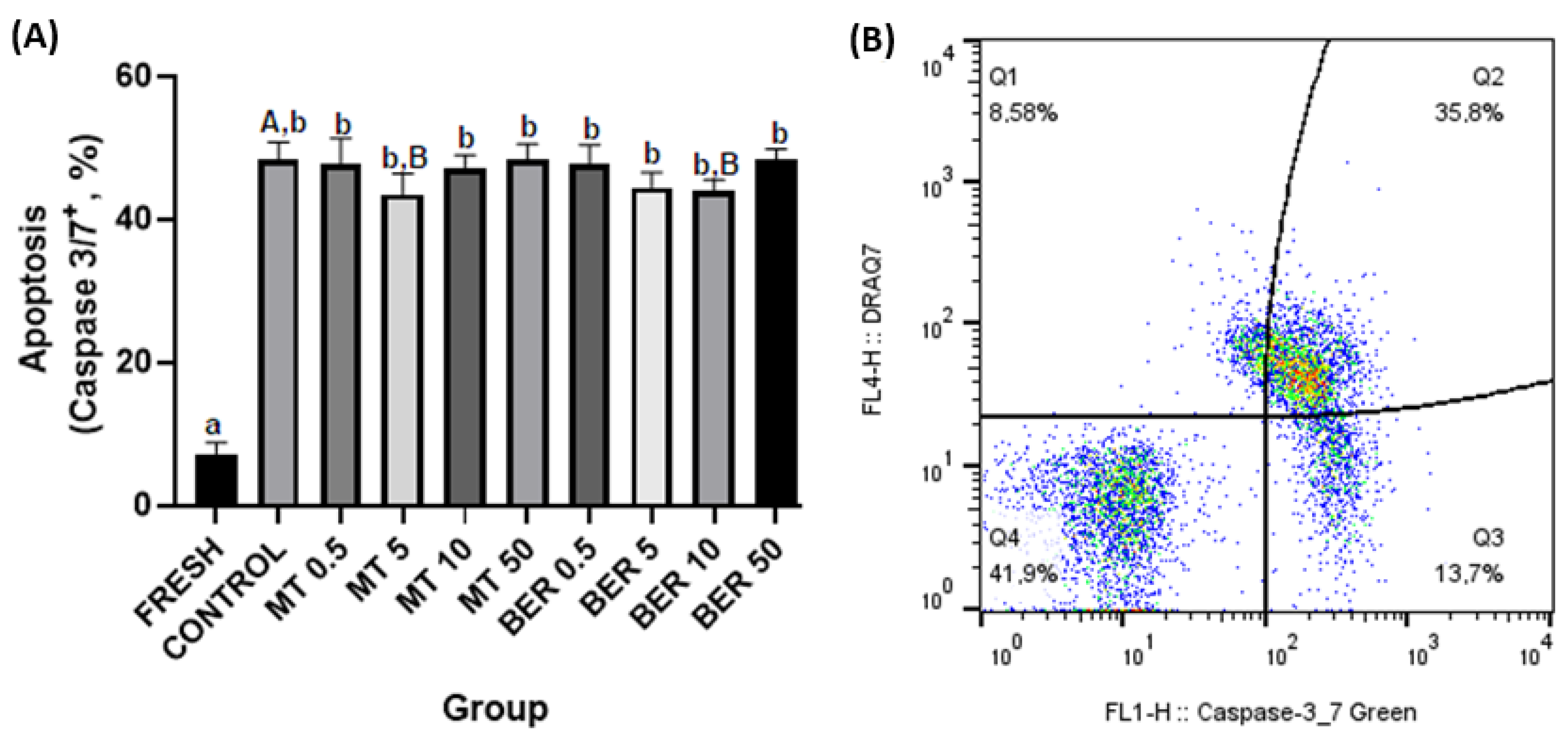
3.6. Effects of MT and BER on the Apoptotic-like Changes of Frozen-Thawed Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nishijima, K.; Kitajima, S.; Matsuhisa, F.; Niimi, M.; Wang, C.C.; Fan, J. Strategies for Highly Efficient Rabbit Sperm Cryopreservation. Animals 2021, 11, 1220. [Google Scholar] [CrossRef] [PubMed]
- Kodzik, N.; Ciereszko, A.; Judycka, S.; Słowińska, M.; Szczepkowska, B.; Świderska, B.; Dietrich, M.A. Cryoprotectant-specific alterations in the proteome of Siberian sturgeon spermatozoa induced by cryopreservation. Sci. Rep. 2024, 14, 17707. [Google Scholar] [CrossRef] [PubMed]
- Sion, B.; Janny, L.; Boucher, D.; Grizard, G. Annexin V binding to plasma membrane predicts the quality of human cryopreserved spermatozoa. Int. J. Androl. 2004, 27, 108–114. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef]
- Wang, A.W.; Zhang, H.; Ikemoto, I.; Anderson, D.J.; Loughlin, K.R. Reactive oxygen species generation by seminal cells during cryopreservation. Urology 1997, 49, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Cao, Z.; Liu, H.; Khan, A.; Rahman, S.U.; Khan, M.Z.; Sathanawongs, A.; Zhang, Y. Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance OMICS to assess sperm cryo-tolerance in farm animals. Front. Vet. Sci. 2021, 8, 609180. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Chatterjee, S.; Sirard, M.A.; Gagnon, C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol. Reprod. Dev. 2000, 55, 282–288. [Google Scholar] [CrossRef]
- Lasso, J.L.; Noiles, E.E.; Alvarez, J.G.; Storey, B.T. Mechanism of superoxide dismutase loss from human sperm cells during cryopreservation. J. Androl. 1994, 15, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.A. Oxidative stress, osmotic stress, and apoptosis: Impacts on sperm function and preservation in the horse. Anim. Reprod. Sci. 2008, 107, 257–267. [Google Scholar] [CrossRef]
- Gadea, J.; Molla, M.; Selles, E.; Marco, M.A.; Garcia-Vazquez, F.A.; Gardon, J.C. Reduced glutathione content in human sperm is decreased after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology 2011, 62, 40–46. [Google Scholar] [CrossRef]
- Amidi, F.; Pazhohan, A.; Nashtaei, M.S.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank. 2016, 17, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, L.; Hernández-García, D.; Schnabel, D.; Salas-Vidal, E.; Castro-Obregón, S. Function of reactive oxygen species during animal development: Passive or active? Dev. Biol. 2008, 320, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Reactive oxygen species in male reproduction: A boon or a bane? Andrologia 2021, 53, e13577. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.Y.; Naveed, M.I.; Raza, S.; Fang, X.; Roy, P.K.; Bang, S.; Tanga, B.M.; Saadeldin, I.M.; Lee, S.; Cho, J. Role of antioxidants in fertility preservation of sperm—A narrative review. Anim. Biosci. 2023, 36, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, M.; Daghigh-Kia, H.; Najafi, A.; Mehdipour, Z.; Mohammadi, H. Protective effect of rosiglitazone on microscopic and oxidative stress parameters of ram sperm after freeze-thawing. Sci. Rep. 2022, 12, 13981. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Colacurci, N.; Rocco, L. Protective effects of curcumin on the outcome of cryopreservation in human sperm. Reprod. Sci. 2021, 28, 2895–2905. [Google Scholar] [CrossRef]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. Biofactors 2022, 48, 414–423. [Google Scholar] [CrossRef]
- Moslemi, M.K.; Tavanbakhsh, S. Selenium–vitamin E supplementation in infertile men: Effects on semen parameters and pregnancy rate. Int. J. Gen. Med. 2011, 4, 99–104. [Google Scholar] [CrossRef]
- Azawi, O.I.; Hussein, E.K. Effect of vitamins C or E supplementation to Tris diluent on the semen quality of Awassi rams preserved at 5 °C. Vet. Res. Forum 2013, 4, 157–160. [Google Scholar]
- Mistry, H.D.; Pipkin, F.B.; Redman, C.W.G.; Poston, L. Selenium in reproductive health. Am. J. Obstet. Gynecol. 2012, 206, 21–30. [Google Scholar] [CrossRef]
- Alsalman, A.R.S.; Almashhedy, L.A.; Alta’ee, A.H.; Hadwan, M.H. Effect of zinc supplementation on urate pathway enzymes in spermatozoa and seminal plasma of asthenozoospermic patients: A randomized controlled trial. Int. J. Fertil. Steril. 2020, 13, 315–323. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative stress and male infertility: The protective role of antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Najafi, A.; Mohammadi, H.; Sharifi, S.D.; Rahimi, A. Apigenin supplementation substantially improves rooster sperm freezability and post-thaw function. Sci. Rep. 2024, 14, 4527. [Google Scholar] [CrossRef]
- Iovine, C.; Mottola, F.; Santonastaso, M.; Finelli, R.; Agarwal, A.; Rocco, L. In vitro ameliorative effects of ellagic acid on vitality, motility, and DNA quality in human spermatozoa. Mol. Reprod. Dev. 2021, 88, 167–174. [Google Scholar] [CrossRef]
- Grba, J.; Kuželová, L.; Makarevich, A.; Baláži, A.; Dragin, S.; Tekic, D.; Chrenek, P. The effect of ellagic acid on rabbit sperm in vitro parameters after cryopreservation. Czech J. Anim. Sci. 2024, 69, 110–117. [Google Scholar] [CrossRef]
- Zarei, F.; Daghigh Kia, H.; Masoudi, R.; Moghaddam, G.; Ebrahimi, M. Supplementation of ram’s semen extender with Mito-TEMPO I: Improvement in quality parameters and reproductive performance of cooled-stored semen. Cryobiology 2021, 98, 215–218. [Google Scholar] [CrossRef]
- Elkhawagah, A.R.; Ricci, A.; Bertero, A.; Poletto, M.L.; Nervo, T.; Donato, G.G.; Vincenti, L.; Martino, N.A. Supplementation with MitoTEMPO before cryopreservation improves sperm quality and fertility potential of Piedmontese beef bull semen. Front. Vet. Sci. 2024, 11, 1376057. [Google Scholar] [CrossRef]
- Trnka, J.; Blaikie, F.H.; Smith, R.A.J.; Murphy, M.P. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic. Biol. Med. 2008, 44, 1406–1419. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Bai, H.; Liu, J.; Li, J.; Wu, B. Mitochondria-targeted antioxidant MitoTEMPO improves the post-thaw sperm quality. Cryobiology 2018, 80, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, X.; Li, J.; Xia, Q.; Gao, J.; Wu, B. Mito-Tempo alleviates cryodamage by regulating intracellular oxidative metabolism in spermatozoa from asthenozoospermic patients. Cryobiology 2019, 91, 18–22. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, S.K.; Katiyar, R.; Rautela, R.; Bisla, A.; Ngou, A.A.; Pande, M.; Srivastava, N.; Bhure, S.K. Effect of Mito-Tempo incorporated semen extender on physico-morphological attributes and functional membrane integrity of frozen-thawed buffalo spermatozoa. Cryoletters 2021, 42, 111–119. [Google Scholar] [PubMed]
- Masoudi, R.; Asadzadeh, N.; Sharafi, M. Effects of freezing extender supplementation with mitochondria-targeted antioxidant Mito-TEMPO on frozen-thawed rooster semen quality and reproductive performance. Anim. Reprod. Sci. 2021, 225, 106671. [Google Scholar] [CrossRef] [PubMed]
- Barfourooshi, H.A.; Esmaeilkhanian, S.; Dadashpour Davachi, A.; Asadzadeh, N.; Masoudi, R. Effect of Mito-TEMPO on post-thawed semen quality in goats. Iran. J. Vet. Med. 2023, 17, 393–400. [Google Scholar] [CrossRef]
- Hassan, H.A.; Banchi, P.; Domain, G.; Vanderheyden, L.; Prochowska, S.; Nizański, W.; Van Soom, A. Mito-Tempo improves acrosome integrity of frozen-thawed epididymal spermatozoa in tomcats. Front. Vet. Sci. 2023, 10, 1170347. [Google Scholar] [CrossRef] [PubMed]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Tillhon, M.; Guamán Ortiz, L.M.; Lombardi, P.; Scovassi, A.I. Berberine: New perspectives for old remedies. Biochem. Pharmacol. 2012, 84, 1260–1267. [Google Scholar] [CrossRef]
- Abudureheman, B.; Zhou, X.; Shu, X.; Chai, Z.; Xu, Y.; Li, S.; Tian, J.; Pan, H.; Ye, X. Evaluation of biochemical properties, antioxidant activities, and phenolic content of two wild-grown Berberis fruits: Berberis nummularia and Berberis atrocarpa. Foods 2022, 11, 2569. [Google Scholar] [CrossRef]
- Golubev, D.; Platonova, E.; Zemskaya, N.; Shevchenko, O.; Shaposhnikov, M.; Nekrasova, P.; Patov, S.; Ibragimova, U.; Valuisky, N.; Borisov, A.; et al. Berberis vulgaris L. extract supplementation exerts regulatory effects on the lifespan and healthspan of Drosophila through its antioxidant activity depending on the sex. Biogerontology 2024, 25, 507–528. [Google Scholar] [CrossRef]
- Tvrdá, E.; Greifová, H.; Ivanič, P.; Lukáč, N. In vitro effects of berberine on the vitality and oxidative profile of bovine spermatozoa. Int. J. Anim. Vet. Sci. 2019, 13, 244–249. [Google Scholar] [CrossRef]
- Vašíček, J.; Baláži, A.; Svoradová, A.; Vozaf, J.; Dujíčková, L.; Makarevich, A.V.; Bauer, M.; Chrenek, P. Comprehensive flow-cytometric quality assessment of ram sperm intended for gene banking using standard and novel fertility biomarkers. Int. J. Mol. Sci. 2022, 23, 5920. [Google Scholar] [CrossRef]
- Fang, L.; Bai, C.; Chen, Y.; Dai, J.; Xiang, Y.; Ji, X.; Huang, C.; Dong, Q. Inhibition of ROS production through mitochondria-targeted antioxidant and mitochondrial uncoupling increases post-thaw sperm viability in yellow catfish. Cryobiology 2014, 69, 386–393. [Google Scholar] [CrossRef]
- Chelewani, A.P.; Takahashi, E.; Nishimura, T.; Fujimoto, T. Optimizing the post-thaw quality of cryopreserved masu salmon (Oncorhynchus masou) sperm: Evaluating the effects of antioxidant-supplemented extender. Aquaculture 2024, 593, 741332. [Google Scholar] [CrossRef]
- Len, J.S.; Koh, W.S.D.; Tan, S.-X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39, BSR20191601. [Google Scholar] [CrossRef]
- Niżański, W.; Partyka, A.; Prochowska, S. Evaluation of spermatozoal function—Useful tools or just science. Reprod. Domest. Anim. 2016, 51 (Suppl. S1), 37–45. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; du Plessis, S.S. Utility of antioxidants during assisted reproductive techniques: An evidence-based review. Reprod. Biol. Endocrinol. 2014, 12, 112. [Google Scholar] [CrossRef]
- Berean, D.I.; Bogdan, L.M.; Cimpean, R. Advancements in Understanding and Enhancing Antioxidant-Mediated Sperm Cryopreservation in Small Ruminants: Challenges and Perspectives. Antioxidants 2024, 13, 624. [Google Scholar] [CrossRef]
- Yi, X.; Qiu, Y.; Tang, X.; Lei, Y.; Pan, Y.; Raza, S.H.A.; Althobaiti, N.A.; Albalawi, A.E.; Al Abdulmonem, W.; Makhlof, R.T.M.; et al. Effect of Five Different Antioxidants on the Effectiveness of Goat Semen Cryopreservation. Reprod. Sci. 2024, 31, 1958–1972. [Google Scholar] [CrossRef]
- Chakraborty, S.; Saha, S. Understanding sperm motility mechanisms and the implication of sperm surface molecules in promoting motility. Middle East Fertil. Soc. J. 2022, 27, 4. [Google Scholar] [CrossRef]
- Verstegen, J.; Iguer-Ouada, M.; Onclin, K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 2002, 57, 149–179. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Pesini, E.; Díez-Sánchez, C.; López-Pérez, M.J.; Enríquez, J.A. The role of the mitochondrion in sperm function: Is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr. Top. Dev. Biol. 2007, 77, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, T.; Liu, J. Effect of berberine on human sperm parameters in vitro. Transl. Androl. Urol. 2016, 5 (Suppl. S1), AB229. [Google Scholar] [CrossRef][Green Version]
- Liang, H.L.; Sedlic, F.; Bosnjak, Z.; Nilakantan, V. SOD1 and MitoTEMPO partially prevent mitochondrial permeability transition pore opening, necrosis, and mitochondrial apoptosis after ATP depletion recovery. Free Radic. Biol. Med. 2010, 49, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, M. Mitochondria-targeted antioxidant MitoTEMPO protects mitochondrial function against amyloid beta toxicity in primary cultured mouse neurons. Biochem. Biophys. Res. Commun. 2016, 478, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Smaili, S.; Hsu, Y.T.; Sanders, K.; Russell, C.; Youle, R.J. Bax translocation to mitochondria subsequent to a rapid loss of mitochondrial membrane potential. Cell Death Differ. 2001, 8, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Grycová, L.; Dostál, J.; Marek, R. Quaternary protoberberine alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, T.; Liu, C.; Wang, X.-Y.; Zhang, J.-Q.; Wu, F.; Lin, G.; Ma, Y.-M.; Ma, B.-L. Mitochondrial membrane potential played crucial roles in the accumulation of berberine in HepG2 cells. Biosci. Rep. 2019, 39, BSR20190477. [Google Scholar] [CrossRef]
- Pereira, G.C.; Branco, A.F.; Matos, J.A.; Pereira, S.L.; Parke, D.; Perkins, E.L.; Serafim, T.L.; Sardao, V.A.; Santos, M.S.; Moreno, A.J.; et al. Mitochondrially targeted effects of berberine [Natural Yellow 18, 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a)quinolizinium] on K1735-M2 mouse melanoma cells: Comparison with direct effects on isolated mitochondrial fractions. J. Pharmacol. Exp. Ther. 2007, 323, 636–649. [Google Scholar] [CrossRef]
- Ye, J.; Le, J.; Sun, Y. Berberine improves mitochondrial function in colon epithelial cells to protect L-cells from necrosis in preservation of GLP-1 secretion. Diabetes 2018, 67 (Suppl. S1), 2451-PUB. [Google Scholar] [CrossRef]
- Saleh, S.R.; Attia, R.; Ghareeb, D.A. The ameliorating effect of berberine-rich fraction against gossypol-induced testicular inflammation and oxidative stress. Oxid. Med. Cell. Longev. 2018, 2018, 1056173. [Google Scholar] [CrossRef]
- Bailey, J.L.; Blodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon minireview. J. Androl. 2000, 21, 1–7. [Google Scholar] [CrossRef]
- Palacín, I.; Santolaria, P.; Alquezar-Baeta, C.; Soler, C.; Yániz, J. Relationship of sperm plasma membrane and acrosomal integrities with sperm morphometry in Bos taurus. Asian J. Androl. 2020, 22, 578–582. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuželová, L.; Svoradová, A.; Baláži, A.; Vašíček, J.; Langraf, V.; Kolesárová, A.; Sláma, P.; Chrenek, P. Enhancing of Rabbit Sperm Cryopreservation with Antioxidants Mito-Tempo and Berberine. Antioxidants 2024, 13, 1360. https://doi.org/10.3390/antiox13111360
Kuželová L, Svoradová A, Baláži A, Vašíček J, Langraf V, Kolesárová A, Sláma P, Chrenek P. Enhancing of Rabbit Sperm Cryopreservation with Antioxidants Mito-Tempo and Berberine. Antioxidants. 2024; 13(11):1360. https://doi.org/10.3390/antiox13111360
Chicago/Turabian StyleKuželová, Lenka, Andrea Svoradová, Andrej Baláži, Jaromír Vašíček, Vladimír Langraf, Adriana Kolesárová, Petr Sláma, and Peter Chrenek. 2024. "Enhancing of Rabbit Sperm Cryopreservation with Antioxidants Mito-Tempo and Berberine" Antioxidants 13, no. 11: 1360. https://doi.org/10.3390/antiox13111360
APA StyleKuželová, L., Svoradová, A., Baláži, A., Vašíček, J., Langraf, V., Kolesárová, A., Sláma, P., & Chrenek, P. (2024). Enhancing of Rabbit Sperm Cryopreservation with Antioxidants Mito-Tempo and Berberine. Antioxidants, 13(11), 1360. https://doi.org/10.3390/antiox13111360






