Tellurium-Doped Bioactive Glass Induces Ferroptosis in Osteosarcoma Cells Regardless of FSP1
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. Bioactive Glass Synthesis
2.3. Cytocompatibility Evaluation
2.4. Cell Viability
2.5. Real Time PCR (qPCR)
2.6. Western Blotting Analysis
2.7. Detection of Intracellular Fe2+
2.8. Immunofluorescence
2.9. Statistical Analysis
3. Results
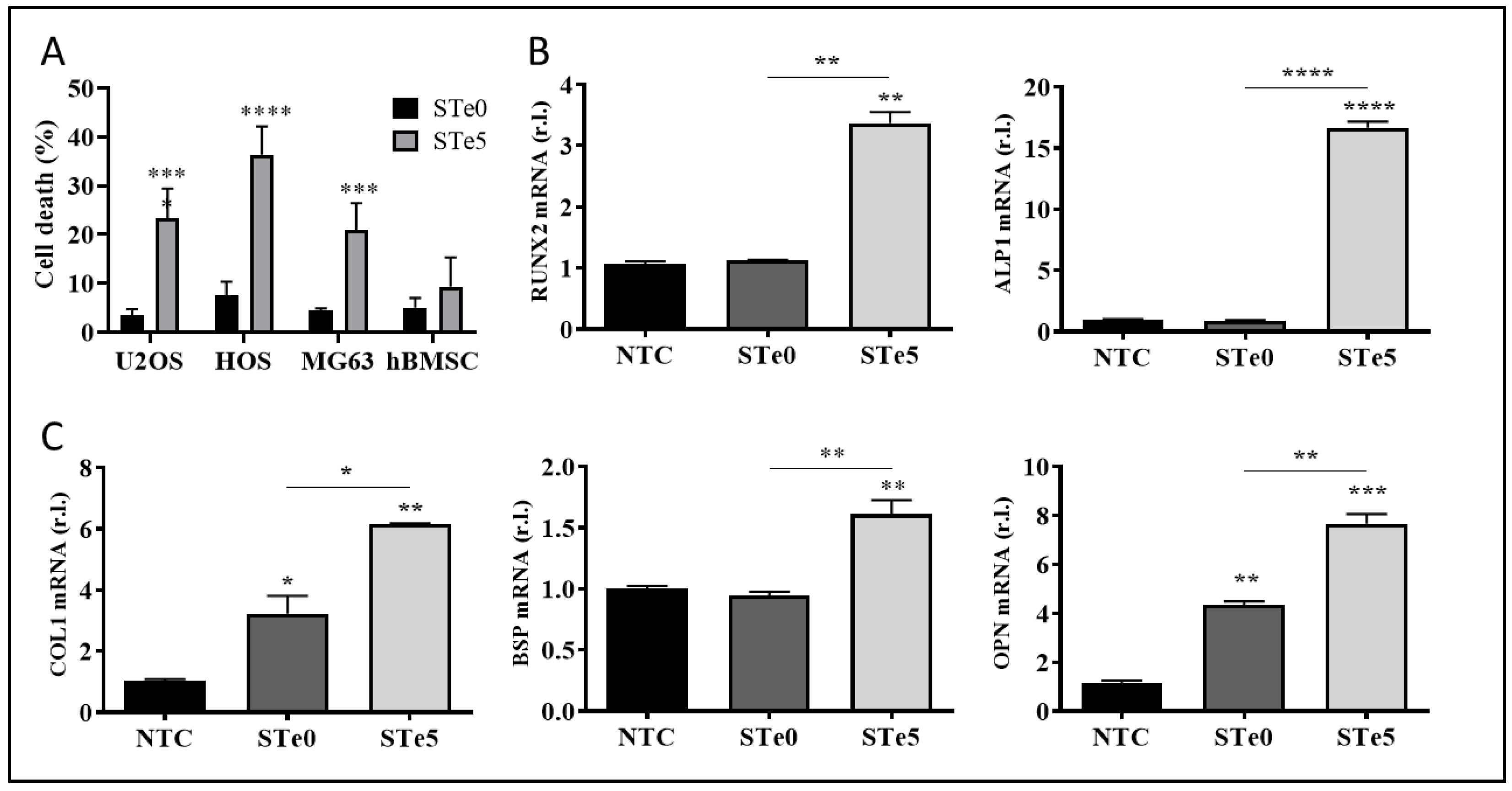
3.1. Selective Toxicity of Tellurium-Doped Bioactive Glass to Osteosarcoma Cells and Osteoinductive Effect on hBMSCs
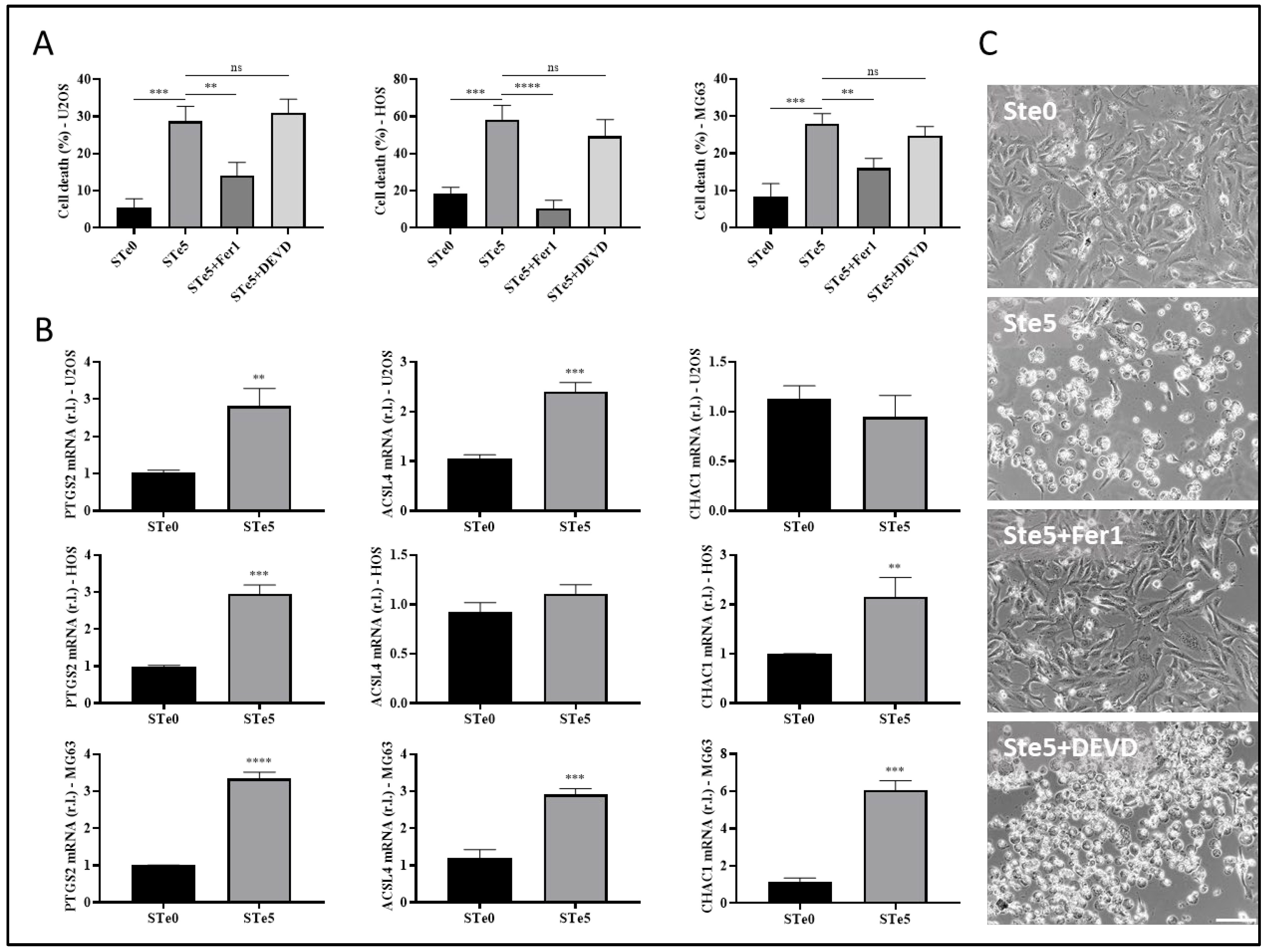
3.2. Tellurium-Doped Bioactive Glass Induces Ferroptosis-Mediated OS Cell Death
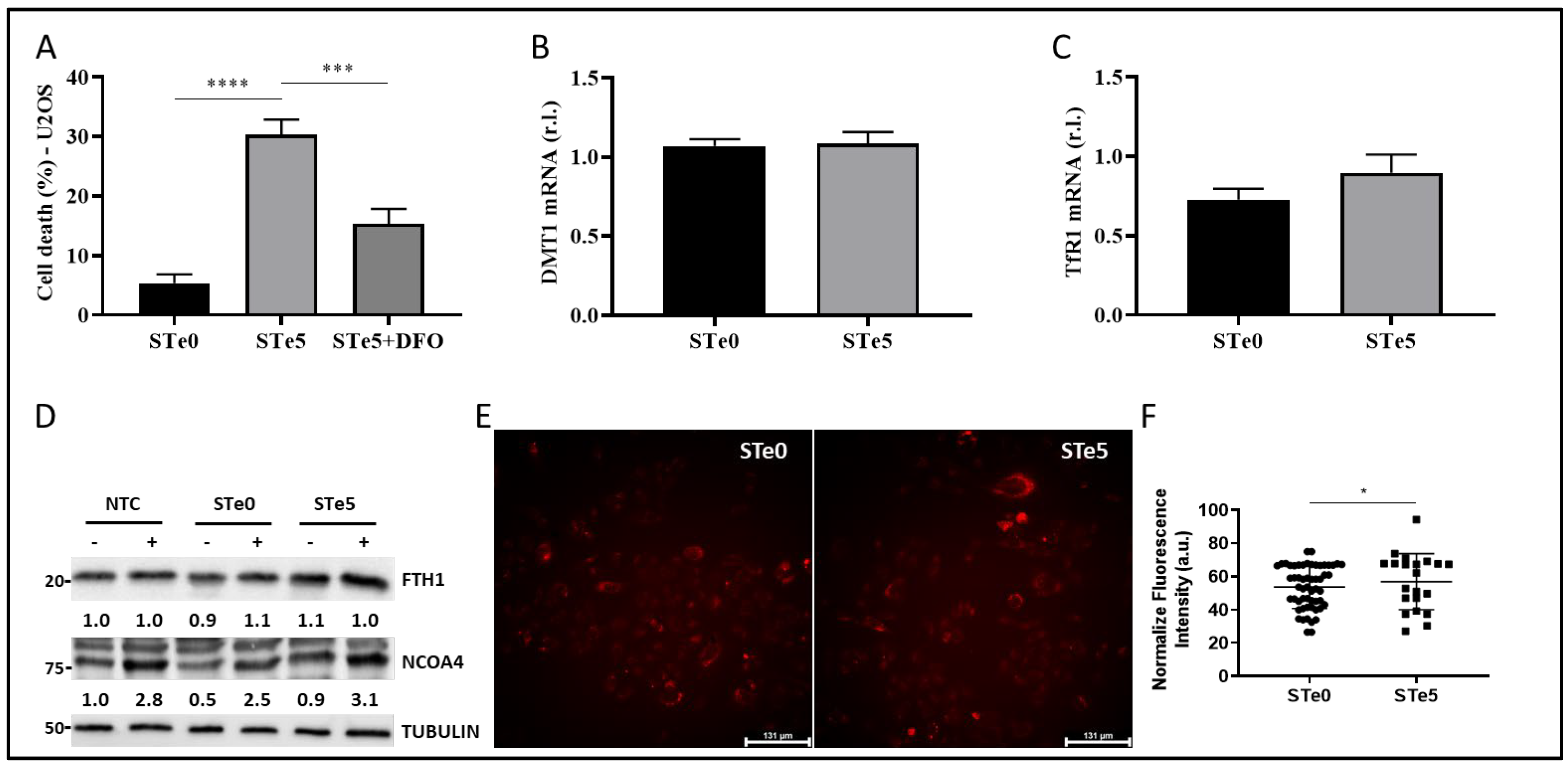
3.3. Role of Iron in Te-BAG-Induced Ferroptosis
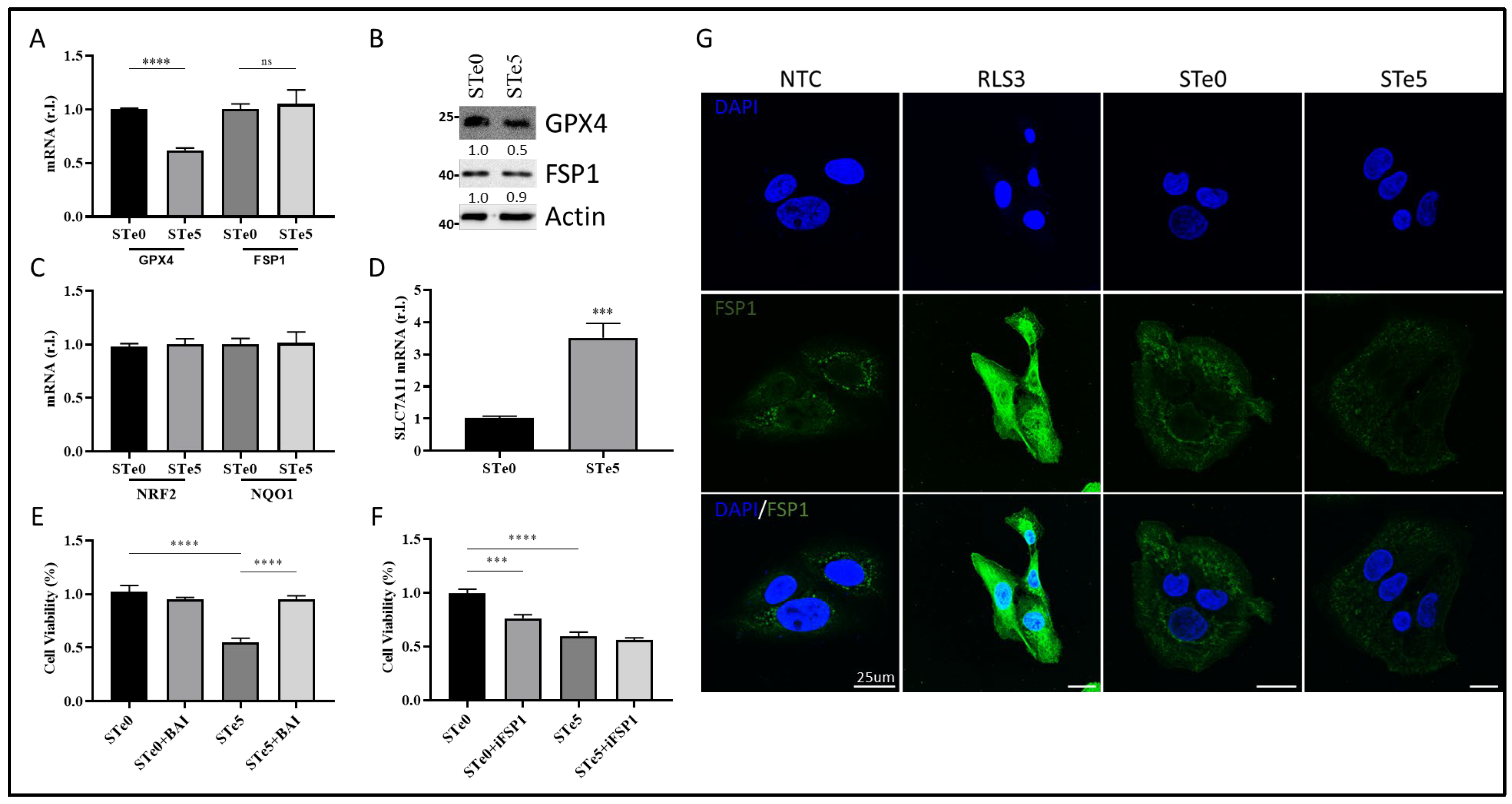
3.4. Te-BAG-Induced Ferroptosis Circumvents the Inhibitory Activity of FSP1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corazzari, M.; Collavin, L. Wild-type and mutant p53 in cancer-related ferroptosis. A matter of stress management? Front. Genet. 2023, 14, 1148192. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Saverio, V.; Monzani, R.; Ferrari, E.; Piacentini, M.; Corazzari, M. Ferroptosis: A new unexpected chance to treat metastatic melanoma? Cell Cycle 2020, 19, 2411–2425. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, H.; Graham, E.T.; Deik, A.A.; Eaton, J.K.; Wang, W.; Sandoval-Gomez, G.; Clish, C.B.; Doench, J.G.; Schreiber, S.L. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat. Chem. Biol. 2020, 16, 302–309. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, J.; Zhang, M.; Zhang, Y.; Ma, H.; Tran, N.T.; Chen, X.; Zhang, Y.; Chan, K.G.; Li, S. Exosomes drive ferroptosis by stimulating iron accumulation to inhibit bacterial infection in crustaceans. J. Biol. Chem. 2023, 299, 105463. [Google Scholar] [CrossRef]
- Kagan, V.E.; Mao, G.; Qu, F.; Angeli, J.P.; Doll, S.; Croix, C.S.; Dar, H.H.; Liu, B.; Tyurin, V.A.; Ritov, V.B.; et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017, 13, 81–90. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.H.; Kang, R.; Tang, D.L. Iron Metabolism in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 590226. [Google Scholar] [CrossRef]
- Zhou, B.R.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D.L. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.Y.; Zeng, Y.C.; Mo, X.L.; Hong, S.Q.; He, H.; Li, J.; Fatima, S.; Liu, Q.H. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. UK 2023, 13, 15515. [Google Scholar] [CrossRef]
- Tyurina, Y.Y.; Kapralov, A.A.; Tyurin, V.A.; Shurin, G.; Amoscato, A.A.; Rajasundaram, D.; Tian, H.; Bunimovich, Y.L.; Nefedova, Y.; Herrick, W.G.; et al. Redox phospholipidomics discovers pro-ferroptotic death signals in A375 melanoma cells in vitro and in vivo. Redox Biol. 2023, 61, 102650. [Google Scholar] [CrossRef] [PubMed]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Bio. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Ingold, I.; Berndt, C.; Schmitt, S.; Doll, S.; Poschmann, G.; Buday, K.; Roveri, A.; Peng, X.; Porto Freitas, F.; Seibt, T.; et al. Selenium Utilization by GPX4 Is Required to Prevent Hydroperoxide-Induced Ferroptosis. Cell 2018, 172, 409–422.e421. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Freitas, F.P.; Shah, R.; Aldrovandi, M.; da Silva, M.C.; Ingold, I.; Goya Grocin, A.; Xavier da Silva, T.N.; Panzilius, E.; Scheel, C.H.; et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 2019, 575, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Cotella, D.; Santoro, C.; Cora, D.; Barlev, N.A.; Piacentini, M.; Corazzari, M. Aldo-keto reductases protect metastatic melanoma from ER stress-independent ferroptosis. Cell Death Dis. 2019, 10, 902. [Google Scholar] [CrossRef]
- Dai, E.; Meng, L.; Kang, R.; Wang, X.; Tang, D. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 2020, 522, 415–421. [Google Scholar] [CrossRef]
- Gagliardi, M.; Saverio, V.; Rossin, F.; D’Eletto, M.; Corazzari, M. Transglutaminase 2 and Ferroptosis: A new liaison? Cell Death Discov. 2023, 9, 88. [Google Scholar] [CrossRef]
- Rroji, O.; Kumar, A.; Karuppagounder, S.S.; Ratan, R.R. Epigenetic regulators of neuronal ferroptosis identify novel therapeutics for neurological diseases: HDACs, transglutaminases, and HIF prolyl hydroxylases. Neurobiol. Dis. 2021, 147, 105145. [Google Scholar] [CrossRef]
- Simpson, E.; Brown, H.L. Understanding osteosarcomas. J. Am. Acad. Physician Assist. 2018, 31, 15–19. [Google Scholar] [CrossRef]
- Li, S.; Zhang, H.; Liu, J.; Shang, G. Targeted therapy for osteosarcoma: A review. J. Cancer Res. Clin. Oncol. 2023, 149, 6785–6797. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, X.; Jiang, Z.; Wang, H.; Yuan, H. Immune checkpoint inhibitors in osteosarcoma: A hopeful and challenging future. Front. Pharmacol. 2022, 13, 1031527. [Google Scholar] [CrossRef] [PubMed]
- Panczyszyn, E.; Saverio, V.; Monzani, R.; Gagliardi, M.; Petrovic, J.; Stojkovska, J.; Collavin, L.; Corazzari, M. FSP1 is a predictive biomarker of osteosarcoma cells’ susceptibility to ferroptotic cell death and a potential therapeutic target. Cell Death Discov. 2024, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Farooq, I.; Iqbal, K. A review of the effect of various ions on the properties and the clinical applications of novel bioactive glasses in medicine and dentistry. Saudi Dent. J. 2014, 26, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.; Ferreira, F.V.; Lopes, J.H.; Camilli, J.A.; Martin, R.A. Cancer Inhibition and In Vivo Osteointegration and Compatibility of Gallium-Doped Bioactive Glasses for Osteosarcoma Applications. ACS Appl. Mater. Interfaces 2022, 14, 45156–45166. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Miola, M.; Massera, J.; Cochis, A.; Kumar, A.; Rimondini, L.; Verne, E. Tellurium: A new active element for innovative multifunctional bioactive glasses. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111957. [Google Scholar] [CrossRef]
- Miola, M.; Verne, E. Bioactive and Antibacterial Glass Powders Doped with Copper by Ion-Exchange in Aqueous Solutions. Materials 2016, 9, 405. [Google Scholar] [CrossRef]
- Miola, M.; Brovarone, C.V.; Maina, G.; Rossi, F.; Bergandi, L.; Ghigo, D.; Saracino, S.; Maggiora, M.; Canuto, R.A.; Muzio, G.; et al. In vitro study of manganese-doped bioactive glasses for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 107–118. [Google Scholar] [CrossRef]
- Verne, E.; Di Nunzio, S.; Bosetti, M.; Appendino, P.; Brovarone, C.V.; Maina, G.; Cannas, M. Surface characterization of silver-doped bioactive glass. Biomaterials 2005, 26, 5111–5119. [Google Scholar] [CrossRef]
- Zhong, C.L.; Qin, B.Y.; Xie, X.Y.; Bai, Y. Antioxidant and Antimicrobial Activity of Tellurium Dioxide Nanoparticles Sols. J. Nano Res. 2013, 25, 8–15. [Google Scholar] [CrossRef]
- Kovrlija, I.; Panczyszyn, E.; Demir, O.; Laizane, M.; Corazzari, M.; Locs, J.; Loca, D. Doxorubicin loaded octacalcium phosphate particles as controlled release drug delivery systems: Physico-chemical characterization, in vitro drug release and evaluation of cell death pathway. Int. J. Pharm. 2024, 653, 123932. [Google Scholar] [CrossRef] [PubMed]
- Monzani, R.; Gagliardi, M.; Clemente, N.; Saverio, V.; Panczyszyn, E.; Santoro, C.; Yissachar, N.; Visciglia, A.; Pane, M.; Amoruso, A.; et al. The Gut-Ex-Vivo System (GEVS) Is a Dynamic and Versatile Tool for the Study of DNBS-Induced IBD in BALB/C and C57BL/6 Mice, Highlighting the Protective Role of Probiotics. Biology 2022, 11, 1574. [Google Scholar] [CrossRef] [PubMed]
- Pagliarini, V.; Giglio, P.; Bernardoni, P.; De Zio, D.; Fimia, G.M.; Piacentini, M.; Corazzari, M. Downregulation of E2F1 during ER stress is required to induce apoptosis. J. Cell Sci. 2015, 128, 1166–1179. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, J.M.; Leveque, P.; Gallez, B.; Vasquez, C.C.; Buc Calderon, P. Tellurite-induced oxidative stress leads to cell death of murine hepatocarcinoma cells. Biometals 2010, 23, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Baniwal, S.K.; Shah, P.K.; Shi, Y.; Haduong, J.H.; Declerck, Y.A.; Gabet, Y.; Frenkel, B. Runx2 promotes both osteoblastogenesis and novel osteoclastogenic signals in ST2 mesenchymal progenitor cells. Osteoporos. Int. 2012, 23, 1399–1413. [Google Scholar] [CrossRef]
- Wang, X.; Hua, P.; He, C.; Chen, M. Non-apoptotic cell death-based cancer therapy: Molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharm. Sin. B 2022, 12, 3567–3593. [Google Scholar] [CrossRef]
- Ji, P.; Yu, L.; Guo, W.C.; Mei, H.J.; Wang, X.J.; Chen, H.; Fang, S.; Yang, J. Doxorubicin Inhibits Proliferation of Osteosarcoma Cells Through Upregulation of the Notch Signaling Pathway. Oncol. Res. 2014, 22, 185–191. [Google Scholar] [CrossRef]
- Solania, A.; Gonzalez-Paez, G.E.; Wolan, D.W. Selective and Rapid Cell-Permeable Inhibitor of Human Caspase-3. ACS Chem. Biol. 2019, 14, 2463–2470. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Y.; Wang, S.; Hu, S.; Wang, Y.; Zhou, W.; Chen, Y.; Li, Q.; Zhu, L.; Yang, H.; et al. Melatonin alleviates septic ARDS by inhibiting NCOA4-mediated ferritinophagy in alveolar macrophages. Cell Death Discov. 2024, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.J.; Xu, D.; Liang, M.X.; Wo, G.Q.; Chen, W.Q.; Tang, J.H.; Zhang, W. Pitavastatin induces autophagy-dependent ferroptosis in MDA-MB-231 cells via the mevalonate pathway. Heliyon 2024, 10, e27084. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Deng, R.R.; Xiang, D.X. Naodesheng Pills Ameliorate Cerebral Ischemia Reperfusion-Induced Ferroptosis via Inhibition of the ERK1/2 Signaling Pathway. Drug Des. Devel Ther. 2024, 18, 1499–1514. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Antonioli, M.; Fusco, C.; Liu, Y.; Mari, M.; Orhon, I.; Refolo, G.; Germani, F.; Corazzari, M.; Romagnoli, A.; et al. Autophagy induction in atrophic muscle cells requires ULK1 activation by TRIM32 through unanchored K63-linked polyubiquitin chains. Sci. Adv. 2019, 5, eaau8857. [Google Scholar] [CrossRef]
- Cao, W.; Wang, L.; Xu, H.P. Selenium/tellurium containing polymer materials in nanobiotechnology. Nano Today 2015, 10, 717–736. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, T.; Qiu, Y.; Lin, Y.; Yao, Y.; Lian, W.; Lin, L.; Song, J.; Yang, H. An inorganic prodrug, tellurium nanowires with enhanced ROS generation and GSH depletion for selective cancer therapy. Chem. Sci. 2019, 10, 7068–7075. [Google Scholar] [CrossRef]
- Xie, Y.C.; Song, X.X.; Sun, X.F.; Huang, J.; Zhong, M.Z.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D.L. Identification of baicalein as a ferroptosis inhibitor by natural product library screening. Biochem. Biophys. Res. Commun. 2016, 473, 775–780. [Google Scholar] [CrossRef]
- Lovat, P.E.; Di Sano, F.; Corazzari, M.; Fazi, B.; Donnorso, R.P.; Pearson, A.D.; Hall, A.G.; Redfern, C.P.; Piacentini, M. Gangliosides link the acidic sphingomyelinase-mediated induction of ceramide to 12-lipoxygenase-dependent apoptosis of neuroblastoma in response to fenretinide. J. Natl. Cancer Inst. 2004, 96, 1288–1299. [Google Scholar] [CrossRef]
- Xavier da Silva, T.N.; Schulte, C.; Alves, A.N.; Maric, H.M.; Friedmann Angeli, J.P. Molecular characterization of AIFM2/FSP1 inhibition by iFSP1-like molecules. Cell Death Dis. 2023, 14, 281. [Google Scholar] [CrossRef]
- Tschuck, J.; Theilacker, L.; Rothenaigner, I.; Weiss, S.A.I.; Akdogan, B.; Lam, V.T.; Muller, C.; Graf, R.; Brandner, S.; Putz, C.; et al. Farnesoid X receptor activation by bile acids suppresses lipid peroxidation and ferroptosis. Nat. Commun. 2023, 14, 6908. [Google Scholar] [CrossRef] [PubMed]
- Creecy, A.; Damrath, J.G.; Wallace, J.M. Control of Bone Matrix Properties by Osteocytes. Front. Endocrinol. 2020, 11, 578477. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Peng, Y.; Li, Y.; Kpegah, J.; Chen, S. Multifunctional inorganic biomaterials: New weapons targeting osteosarcoma. Front. Mol. Biosci. 2022, 9, 1105540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Zhao, N.; Wang, C.; Kamar, S.; Zhou, Y.; He, Z.; Yang, J.; Sun, B.; Shi, X.; et al. Progress in the chemotherapeutic treatment of osteosarcoma. Oncol. Lett. 2018, 16, 6228–6237. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, J.; Feng, H. Targeting iron metabolism in osteosarcoma. Discov. Oncol. 2023, 14, 31. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, R.; Wang, W.; Wang, C.; Zhang, N.; Shao, X.; He, Q.; Ying, M. Advances in targeted therapy for osteosarcoma based on molecular classification. Pharmacol. Res. 2021, 169, 105684. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Q.; Peng, Y.; Dong, Z.; Tang, L.; Su, X.; Liu, L.; Chen, C.; Ramalingam, M.; Cheng, L. Identification of Small-Molecule Inhibitors for Osteosarcoma Targeted Therapy: Synchronizing In Silico, In Vitro, and In Vivo Analyses. Front. Bioeng. Biotechnol. 2022, 10, 921107. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Ritter, J.; Bielack, S.S. Osteosarcoma. Ann. Oncol. 2010, 7, vii320–vii325. [Google Scholar] [CrossRef]
- de Azevedo, J.W.V.; de Medeiros Fernandes, T.A.A.; Fernandes, J.V., Jr.; de Azevedo, J.C.V.; Lanza, D.C.F.; Bezerra, C.M.; Andrade, V.S.; de Araujo, J.M.G.; Fernandes, J.V. Biology and pathogenesis of human osteosarcoma. Oncol. Lett. 2020, 19, 1099–1116. [Google Scholar] [CrossRef]
| % mol | ||
|---|---|---|
| STe0 | STe5 | |
| SiO2 | 48.6 | 43.6 |
| Na2O | 16.7 | 16.7 |
| CaO | 34.2 | 34.2 |
| P2O5 | 0.5 | 0.5 |
| TeO2 | 0.0 | 5.0 |
| Name/Gene ID | Sequence |
|---|---|
| alp [250] | GAGTATGAGAGTGACGAGAAAG/GAAGTGGGAGTGCTTGTATC |
| bsp [3381] | CAGAAGAGGAGGAGGAAGAA/CCCAGTGTTGTAGCAGAAAG |
| col1 [1277] | GGATTCCAGTTCGAGTATGG/CAGTGGTAGGTGATGTTCTG |
| dmt1 [4891] | GCTGTCTTCCAAGATGTAGAG/GGATGGGTATGAGAGCAAAG |
| fsp1 [84883] | CCTGCCCTTCTCTCATCTTA/GTCCTCATAGGCCTGGATAG |
| gpx4 [2879] | AGCTCTTCTGGGAAGTAGAC/CCTCCCTGTACCACATCTAT |
| l34 [6164] | GTCCCCGAACCCTGGTAATAGA/GGCCCTGCTGACATGTTTCTT |
| nqo1 [1728] | GGATGAGACACCACTGTATTT/CTCCTCATCCTGTACCTCTT |
| nrf2 [4780] | CCTGCCCTTCTCTCATCTTA/GTCCTCATAGGCCTGGATAG |
| opn [6696] | CCCATCTCAGAAGCAGAATC/TGGCTTTCGTTGGACTTAC |
| ptgs2 [5743] | GCCTGGTCTGATGATGTATG/GTATTAGCCTGCTTGTCTGG |
| runx2 [860] | GAATGCCTCTGCTGTTATGA/GAAGACGGTTATGGTCAAGG |
| slc7a11 [23657] | CTGGGTTTCTTGTCCCATATAA/GTTGCCCTTTCCCTCTATTC |
| tfr1 [7037] | GTGAGGGATCTGAACCAATAC/TGGAAGTAGCACGGAAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pańczyszyn, E.; Lallukka, M.; Gagliardi, M.; Saverio, V.; Monzani, R.; Miola, M.; Verné, E.; Corazzari, M. Tellurium-Doped Bioactive Glass Induces Ferroptosis in Osteosarcoma Cells Regardless of FSP1. Antioxidants 2024, 13, 1327. https://doi.org/10.3390/antiox13111327
Pańczyszyn E, Lallukka M, Gagliardi M, Saverio V, Monzani R, Miola M, Verné E, Corazzari M. Tellurium-Doped Bioactive Glass Induces Ferroptosis in Osteosarcoma Cells Regardless of FSP1. Antioxidants. 2024; 13(11):1327. https://doi.org/10.3390/antiox13111327
Chicago/Turabian StylePańczyszyn, Elżbieta, Mari Lallukka, Mara Gagliardi, Valentina Saverio, Romina Monzani, Marta Miola, Enrica Verné, and Marco Corazzari. 2024. "Tellurium-Doped Bioactive Glass Induces Ferroptosis in Osteosarcoma Cells Regardless of FSP1" Antioxidants 13, no. 11: 1327. https://doi.org/10.3390/antiox13111327
APA StylePańczyszyn, E., Lallukka, M., Gagliardi, M., Saverio, V., Monzani, R., Miola, M., Verné, E., & Corazzari, M. (2024). Tellurium-Doped Bioactive Glass Induces Ferroptosis in Osteosarcoma Cells Regardless of FSP1. Antioxidants, 13(11), 1327. https://doi.org/10.3390/antiox13111327







