Association Between Derivatives of Reactive Oxygen Metabolites and Hemodynamics in Children with Left-to-Right Shunt Congenital Heart Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definitions of CHD
2.3. Evaluation of CHD and Hemodynamics
2.4. Serum Measurements
2.5. Statistical Analyses
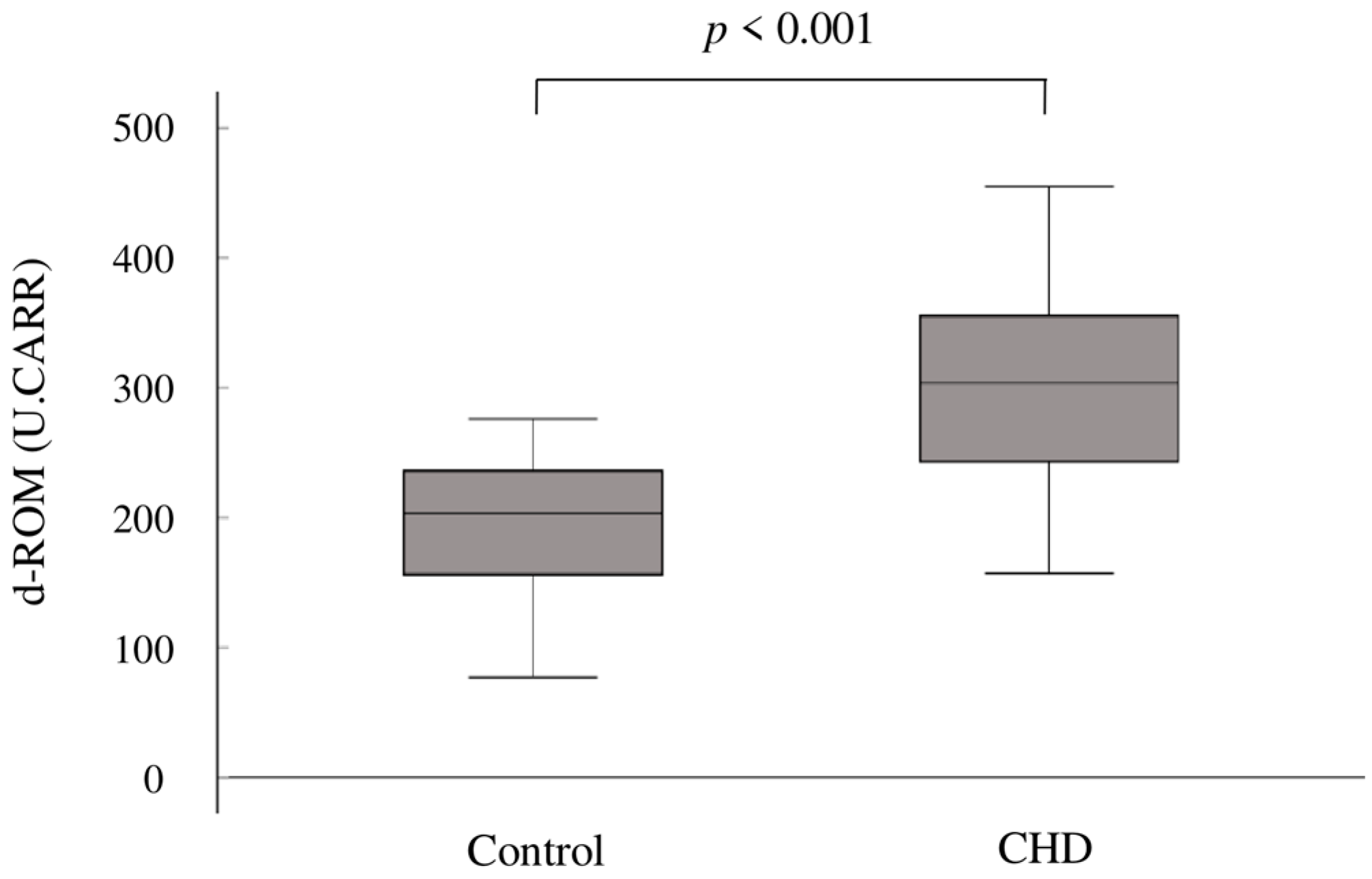
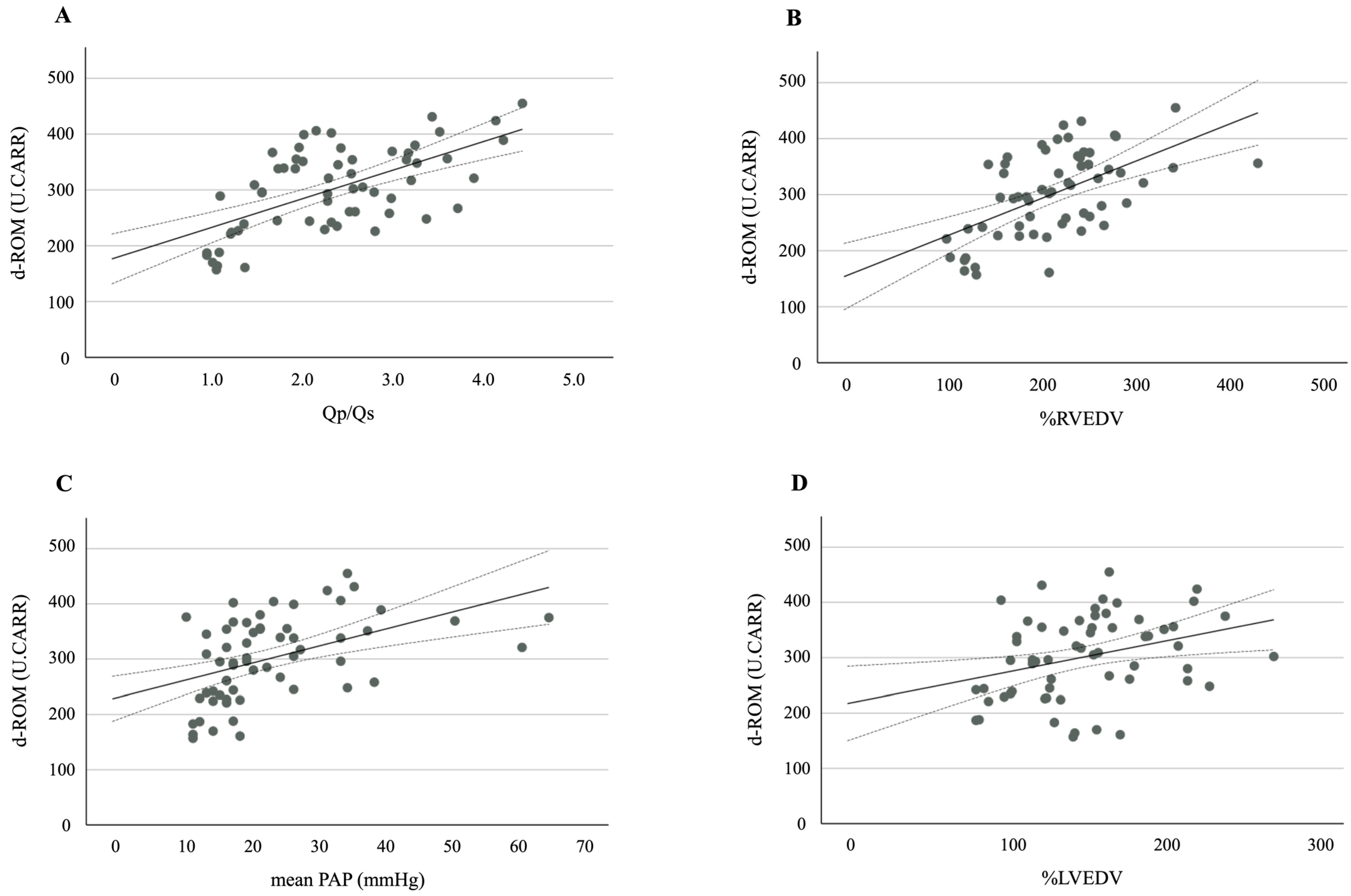
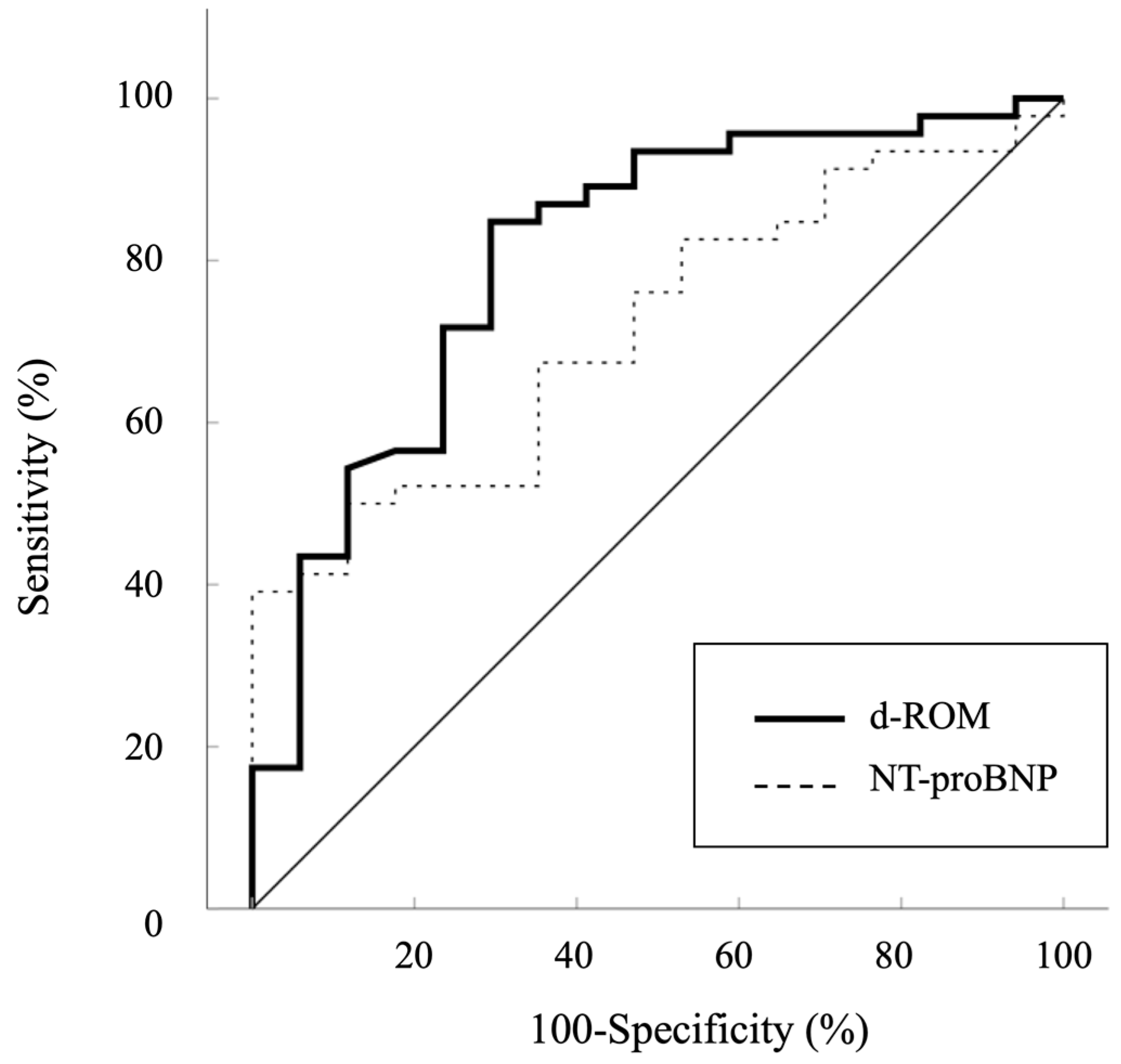
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef] [PubMed]
- Cesarone, M.R.; Belcaro, G.; Carratelli, M.; Cornelli, U.; De Sanctis, M.T.; Incandela, L.; Barsotti, A.; Terranova, R.; Nicolaides, A. A simple test to monitor oxidative stress. Int. Angiol. 1999, 18, 127–130. [Google Scholar] [PubMed]
- Iamele, L.; Fiocchi, R.; Vernocchi, A. Evaluation of an automated spectrophotometric assay for reactive oxygen metabolites in serum. Clin. Chem. Lab. Med. 2002, 40, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Brown, N.J.; Vaughan, D.E.; Harrison, D.G.; Mehta, J.L. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation 2004, 109 (Suppl. S1), IV6–IV19. [Google Scholar] [CrossRef]
- Pigazzani, F.; Gorni, D.; Dyar, K.A.; Pedrelli, M.; Kennedy, G.; Costantino, G.; Bruno, A.; Mackenzie, I.; MacDonald, T.M.; Tietge, U.J.F.; et al. The Prognostic Value of Derivatives-Reactive Oxygen Metabolites (d-ROMs) for Cardiovascular Disease Events and Mortality: A Review. Antioxidants 2022, 11, 1541. [Google Scholar] [CrossRef]
- Cheung, Y.-F.; O, K.; Woo, C.W.; Armstrong, S.; Siow, Y.L.; Chow, P.-C.; Cheung, E.W. Oxidative stress in children late after Kawasaki disease: Relationship with carotid atherosclerosis and stiffness. BMC Pediatr. 2008, 8, 20. [Google Scholar] [CrossRef]
- Ishikawa, T.; Seki, K. The association between oxidative stress and endothelial dysfunction in early childhood patients with Kawasaki disease. BMC Cardiovasc. Disord. 2018, 18, 30. [Google Scholar] [CrossRef]
- Ercan, S.; Cakmak, A.; Kösecik, M.; Erel, O. The oxidative state of children with cyanotic and acyanotic congenital heart disease. Anadolu Kardiyol. Derg. 2009, 9, 486–490. [Google Scholar] [PubMed]
- Pirinccioglu, A.G.; Alyan, O.; Kizil, G.; Kangin, M.; Beyazit, N. Evaluation of oxidative stress in children with congenital heart defects. Pediatr. Int. 2012, 54, 94–98. [Google Scholar] [CrossRef]
- Escobar-Diaz, M.C.; Pérez-Cruz, M.; Arráez, M.; Cascant-Vilaplana, M.M.; Albiach-Delgado, A.; Kuligowski, J.; Vento, M.; Masoller, N.; Gómez-Roig, M.D.; Gómez, O.; et al. Brain Oxygen Perfusion and Oxidative Stress Biomarkers in Fetuses with Congenital Heart Disease-A Retrospective, Case-Control Pilot Study. Antioxidants 2022, 11, 299. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Korones, S.B.; Berendes, H.W. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation 1971, 43, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.C. The ductus arteriosus in the neonatal period. J. Pediatr. 1957, 51, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, A.; Pospisil, U.; Salzer-Muhar, U.; Greber-Platzer, S.; Male, C. Predictors of spontaneous closure of isolated secundum atrial septal defect in children: A longitudinal study. Pediatrics 2006, 118, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, K.; Okamoto, M.; Kinoshita, N.; Izumi, S.; Owa, M.; Takao, S.; Sakakibara, H.; Nimura, Y. Clinical applications of a new type of real-time two-dimensional Doppler flow imaging system. Am. J. Cardiol. 1984, 54, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Sahn, D.J. Real-time two-dimensional Doppler echocardiographic flow mapping. Circulation 1985, 71, 849–853. [Google Scholar] [CrossRef]
- Graham, T.P., Jr.; Jarmakani, J.M.; Atwood, G.F.; Canent, R.V., Jr. Right ventricular volume determinations in children. Normal values and observations with volume or pressure overload. Circulation 1973, 47, 144–153. [Google Scholar] [CrossRef]
- Dodge, H.T.; Sheehan, F.H. Quantitative contrast angiography for assessment of ventricular performance in heart disease. J. Am. Coll. Cardiol. 1983, 1, 73–81. [Google Scholar] [CrossRef]
- Nakazawa, M.; Marks, R.A.; Isabel-Jones, J.; Jarmakani, J.M. Right and left ventricular volume characteristics in children with pulmonary stenosis and intact ventricular septum. Circulation 1976, 53, 884–890. [Google Scholar] [CrossRef]
- Kujiraoka, T.; Satoh, Y.; Ayaori, M.; Shiraishi, Y.; Arai-Nakaya, Y.; Hakuno, D.; Yada, H.; Kuwada, N.; Endo, S.; Isoda, K.; et al. Hepatic extracellular signal-regulated kinase 2 suppresses endoplasmic reticulum stress and protects from oxidative stress and endothelial dysfunction. J. Am. Heart Assoc. 2013, 2, e000361. [Google Scholar] [CrossRef]
- Vanreusel, I.; Vermeulen, D.; Goovaerts, I.; Stoop, T.; Ectors, B.; Cornelis, J.; Hens, W.; de Bliek, E.; Heuten, H.; Van Craenenbroeck, E.M.; et al. Circulating Reactive Oxygen Species in Adults with Congenital Heart Disease. Antioxidants 2022, 11, 2369. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Schulz, E.; Jansen, T.; Wenzel, P.; Daiber, A.; Münzel, T. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 2008, 10, 1115–1126. [Google Scholar] [CrossRef]
- You, J.; Wu, J.; Zhang, Q.; Ye, Y.; Wang, S.; Huang, J.; Liu, H.; Wang, X.; Zhang, W.; Bu, L.; et al. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H552–H562. [Google Scholar] [CrossRef]
- Oka, T.; Akazawa, H.; Naito, A.T.; Komuro, I. Angiogenesis and cardiac hypertrophy: Maintenance of cardiac function and causative roles in heart failure. Circ. Res. 2014, 114, 565–571. [Google Scholar] [CrossRef]
- Masutani, S.; Saiki, H.; Kurishima, C.; Ishido, H.; Tamura, M.; Senzaki, H. Heart failure with preserved ejection fraction in children: Hormonal imbalance between aldosterone and brain natriuretic peptide. Circ. J. 2013, 77, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Zink, S.; Singer, H. B-type natriuretic peptide in paediatric patients with congenital heart disease. Eur. Heart J. 2006, 27, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Raman, S.V.; Hoffman, T.M.; Hayes, J.; Daniels, C.J. Serum markers of systemic right ventricular function and exercise performance. Pediatr. Cardiol. 2008, 29, 641–648. [Google Scholar] [CrossRef]
- Kunii, Y.; Kamada, M.; Ohtsuki, S.; Araki, T.; Kataoka, K.; Kageyama, M.; Nakagawa, N.; Seino, Y. Plasma brain natriuretic peptide and the evaluation of volume overload in infants and children with congenital heart disease. Acta Medica Okayama 2003, 57, 191–197. [Google Scholar]
- Kavga, M.; Varlamis, G.; Giannopoulos, A.; Papadopoulou-Legbelou, K.; Varlamis, S.; Bompotis, G.; Koulourida, V.; Nikolaides, N. Correlation of plasma B-type natriuretic peptide with shunt volume in children with congenital heart disease involving left-to-right shunt. Hell. J. Cardiol. 2013, 54, 192–198. [Google Scholar]
- Li, X.; Zheng, Y.; Long, Y.; Zhang, X.; Zhang, L.; Tian, D.; Zhou, D.; Lv, Q. Effect of iloprost on biomarkers in patients with congenital heart disease-pulmonary arterial hypertension. Clin. Exp. Pharmacol. Physiol. 2017, 44, 914–923. [Google Scholar] [CrossRef]
- Aggarwal, S.; Gross, C.; Fineman, J.R.; Black, S.M. Oxidative stress and the development of endothelial dysfunction in congenital heart disease with increased pulmonary blood flow: Lessons from the neonatal lamb. Trends Cardiovasc. Med. 2010, 20, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.S.; Augusto, V.S.; Silveira, A.P.C.; Jordão, A.A.; Baddini-Martinez, J.; Neto, O.P.; Rodrigues, A.J.; Evora, P.R.B. Oxidative-stress biomarkers in patients with pulmonary hypertension. Pulm. Circ. 2013, 3, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, T.; Xu, X.; Wang, M.; Zhong, L.; Yang, Y.; Zhai, Z.; Xiao, F.; Wang, C. Oxidative stress and nitric oxide signaling related biomarkers in patients with pulmonary hypertension: A case control study. BMC Pulm. Med. 2015, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Vanreusel, I.; Taeymans, J.; Van Craenenbroeck, E.; Segers, V.F.; Van Berendoncks, A.; Briedé, J.J.; Hens, W. Elevated oxidative stress in patients with congenital heart disease and the effect of cyanosis: A meta-analysis. Free. Radic. Res. 2023, 57, 470–486. [Google Scholar] [CrossRef]
- Impellizzeri, P.; Nascimben, F.; Di Fabrizio, D.; Antonuccio, P.; Antonelli, E.; Peri, F.M.; Calabrese, U.; Arena, S.; Romeo, C. Pathogenesis of Congenital Malformations: Possible Role of Oxidative Stress. Am. J. Perinatol. 2022, 39, 816–823. [Google Scholar] [CrossRef]

| Control (n = 60) | CHD (n = 60) | p Value | |
|---|---|---|---|
| Age (months) | 6.0 (3.0–48.8) | 8.5 (2.8–53.5) | 0.710 |
| Sex (male/female) | 30/30 | 30/30 | 1.000 |
| Height (cm) | 70.4 (60.2–108.6) | 68.8 (57.6–105.3) | 0.558 |
| Weight (kg) | 7.6 (5.8–17.0) | 7.4 (4.8–16.4) | 0.707 |
| BMI (kg/m2) | 15.7 (14.4–17.1) | 14.8 (14.1–16.4) | 0.496 |
| HR (beats/min) | 118 (93–124) | 120 (104–130) | 0.191 |
| Systolic BP (mm Hg) | 82 (78–95) | 81 (76–90) | 0.177 |
| Diastolic BP (mm Hg) | 47 (42–56) | 46.0 (41–52) | 0.165 |
| CHD (n = 60) | |
|---|---|
| SO2 in aorta (%) | 97.8 ± 2.3 |
| SO2 in PA (%) | 85.6 ± 5.4 |
| Qp/Qs | 2.33 ± 0.86 |
| mPAP (mmHg) | 24 ± 11 |
| %RVEDV | 176 ± 50 |
| %LVEDV | 152 ± 44 |
| d-ROM (U.CARR) | 302 ± 75 |
| NT-proBNP (pg/mL) | 380 * (98–1066) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, T.; Masui, D.; Uchiyama, H. Association Between Derivatives of Reactive Oxygen Metabolites and Hemodynamics in Children with Left-to-Right Shunt Congenital Heart Disease. Antioxidants 2024, 13, 1294. https://doi.org/10.3390/antiox13111294
Ishikawa T, Masui D, Uchiyama H. Association Between Derivatives of Reactive Oxygen Metabolites and Hemodynamics in Children with Left-to-Right Shunt Congenital Heart Disease. Antioxidants. 2024; 13(11):1294. https://doi.org/10.3390/antiox13111294
Chicago/Turabian StyleIshikawa, Takamichi, Daisuke Masui, and Hiroki Uchiyama. 2024. "Association Between Derivatives of Reactive Oxygen Metabolites and Hemodynamics in Children with Left-to-Right Shunt Congenital Heart Disease" Antioxidants 13, no. 11: 1294. https://doi.org/10.3390/antiox13111294
APA StyleIshikawa, T., Masui, D., & Uchiyama, H. (2024). Association Between Derivatives of Reactive Oxygen Metabolites and Hemodynamics in Children with Left-to-Right Shunt Congenital Heart Disease. Antioxidants, 13(11), 1294. https://doi.org/10.3390/antiox13111294





