Telomere Maintenance Is Associated with Type 2 Diabetes Remission in Response to a Long-Term Dietary Intervention without Non-Weight Loss in Patients with Coronary Heart Disease: From the CORDIOPREV Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Diabetes Remission Criteria
2.3. Dietary Intervention
2.4. Laboratory Measurements
2.5. DNA Isolation from Blood Samples
2.6. Quantitative PCR Analysis of Telomere Length
2.7. Telomerase Activity Assay
2.8. Measurement of Insulin Resistance and Beta-Cell Function Indexes
2.9. Statistical Analyses
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Type 2 Diabetes Remission Is Associated with Longer Telomeres
3.3. Accelerated Telomere Shortening in the Non-Responders Group
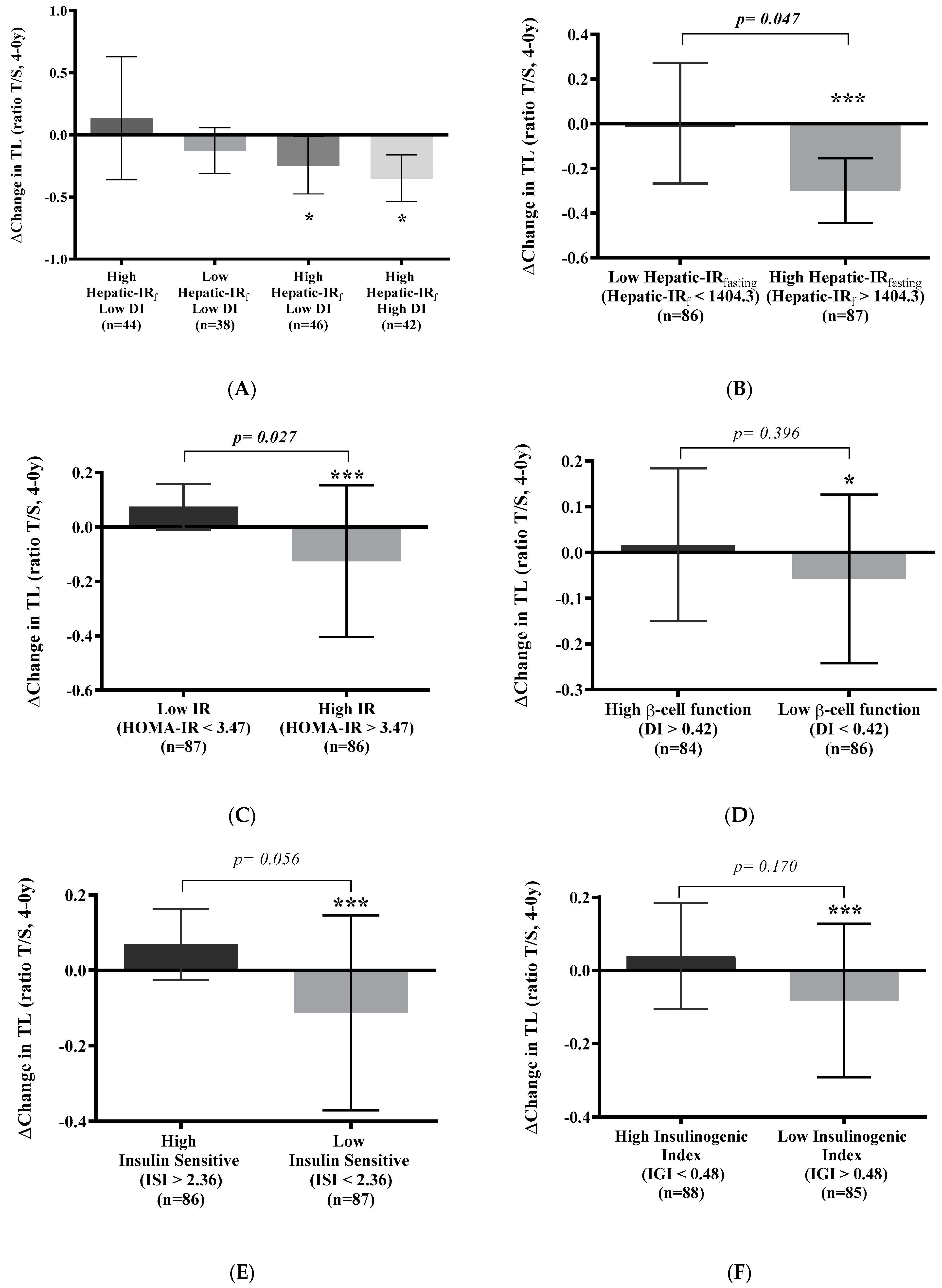
3.4. Telomere Shortening Is Related to Insulin Resistance
3.5. Relationship between Change in Telomere Length and Plasma Oxidative Stress Parameter
3.6. Increased Telomerase Activity in the Responders Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.X.; Ma, X.N.; Guan, C.H.; Li, Y.D.; Mauricio, D.; Fu, S.B. Cardiovascular Disease in Type 2 Diabetes Mellitus: Progress toward Personalized Management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Al-Mrabeh, A.; Sattar, N. Understanding the Mechanisms of Reversal of Type 2 Diabetes. Lancet Diabetes Endocrinol. 2019, 7, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary Care-Led Weight Management for Remission of Type 2 Diabetes (DiRECT): An Open-Label, Cluster-Randomised Trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason, K.; Lönroth, H.; et al. Association of Bariatric Surgery with Long-Term Remission of Type 2 Diabetes and with Microvascular and Macrovascular Complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Roncero-Ramos, I.; Gutierrez-Mariscal, F.M.; Gomez-Delgado, F.; Villasanta-Gonzalez, A.; Torres-Peña, J.D.; Cruz-Ares, S.D.L.; Rangel-Zuñiga, O.A.; Luque, R.M.; Ordovas, J.M.; Delgado-Lista, J.; et al. Beta Cell Functionality and Hepatic Insulin Resistance Are Major Contributors to Type 2 Diabetes Remission and Starting Pharmacological Therapy: From CORDIOPREV Randomized Controlled Trial. Transl. Res. 2021, 238, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; Cardelo, M.P.; de la Cruz, S.; Alcala-Diaz, J.F.; Roncero-Ramos, I.; Guler, I.; Vals-Delgado, C.; López-Moreno, A.; Luque, R.M.; Delgado-Lista, J.; et al. Reduction in Circulating Advanced Glycation End Products by Mediterranean Diet Is Associated with Increased Likelihood of Type 2 Diabetes Remission in Patients with Coronary Heart Disease: From the Cordioprev Study. Mol. Nutr. Food Res. 2021, 65, 1901290. [Google Scholar] [CrossRef]
- Rangel-Zuñiga, O.A.; Vals-Delgado, C.; Alcala-Diaz, J.F.; Quintana-Navarro, G.M.; Krylova, Y.; Leon-Acuña, A.; Luque, R.M.; Gomez-Delgado, F.; Delgado-Lista, J.; Ordovas, J.M.; et al. A Set of MiRNAs Predicts T2DM Remission in Patients with Coronary Heart Disease: From the CORDIOPREV Study. Mol. Ther. Nucleic Acids 2021, 23, 255–263. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Cao, L.; Sun, Y.; Qiu, Y.; Zhang, Y.; Cao, R.; Covasa, M.; Zhong, L. Association between Telomere Length and Diabetes Mellitus: A Meta-Analysis. J. Int. Med. Res. 2016, 44, 1156–1173. [Google Scholar] [CrossRef]
- Cheng, F.; Luk, A.O.; Shi, M.; Huang, C.; Jiang, G.; Yang, A.; Wu, H.; Lim, C.K.P.; Tam, C.H.T.; Fan, B.; et al. Shortened Leukocyte Telomere Length Is Associated With Glycemic Progression in Type 2 Diabetes: A Prospective and Mendelian Randomization Analysis. Diabetes Care 2022, 45, 701–709. [Google Scholar] [CrossRef]
- Cheng, F.; Luk, A.O.; Tam, C.H.T.; Fan, B.; Wu, H.; Yang, A.; Lau, E.S.H.; Ng, A.C.W.; Lim, C.K.P.; Lee, H.M.; et al. Shortened Relative Leukocyte Telomere Length Is Associated with Prevalent and Incident Cardiovascular Complications in Type 2 Diabetes: Analysis from the Hong Kong Diabetes Register. Diabetes Care 2020, 43, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Chang, E.; Kashefi-Aazam, M.; Rogaev, E.I.; Piatyszek, M.A.; Shay, J.W.; Harley, C.B. Telomere Shortening Is Associated with Cell Division In Vitro and In Vivo. Exp. Cell Res. 1995, 220, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Gavia-García, G.; Rosado-Pérez, J.; Arista-Ugalde, T.L.; Aguiñiga-Sánchez, I.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Telomere Length and Oxidative Stress and Its Relation with Metabolic Syndrome Components in the Aging. Biology 2021, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rodríguez, A.; Marti, A.; Zalba, G.; Martínez-gonzález, M.A.; Marti, A. Higher Adherence to an Empirically-Derived Mediterranean Dietary Pattern Is Positively Associated with Telomere Length: The SUN Project. Br. J. Nutr. 2021, 126, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rodríguez, A.; Zazpe, I.; Alonso-Pedrero, L.; Zalba, G.; Guillen-Grima, F.; Martinez-Gonzalez, M.A.; Marti, A. Association between Diet Quality Indexes and the Risk of Short Telomeres in an Elderly Population of the SUN Project. Clin. Nutr. 2020, 39, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- García-Calzón, S.; Martínez-González, M.A.; Razquin, C.; Arós, F.; Lapetra, J.; Martínez, J.A.; Zalba, G.; Marti, A. Mediterranean Diet and Telomere Length in High Cardiovascular Risk Subjects from the PREDIMED-NAVARRA Study. Clin. Nutr. 2016, 35, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rodríguez, A.; Morell-Azanza, L.; Martín-Calvo, N.; Zalba, G.; Chueca, M.; Azcona-Sanjulian, M.C.; Marti, A. Association between Favourable Changes in Objectively Measured Physical Activity and Telomere Length after a Lifestyle Intervention in Paediatric Patients with Abdominal Obesity. Appl. Physiol. Nutr. Metab. 2021, 46, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rodríguez, A.; Morell-Azanza, L.; Zalba, G.; Zazpe, I.; Azcona-SanJulián, M.C.; Marti, A. Associations of Telomere Length with Two Dietary Quality Indices after a Lifestyle Intervention in Children with Abdominal Obesity: A Randomized Controlled Trial. Pediatr. Obes. 2020, 15, e12661. [Google Scholar] [CrossRef]
- Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Alcala-Diaz, J.F.; Perez-Caballero, A.I.; Gomez-Delgado, F.; Fuentes, F.; Quintana-Navarro, G.; Lopez-Segura, F.; Ortiz-Morales, A.M.; et al. CORonary Diet Intervention with Olive Oil and Cardiovascular PREVention Study (the CORDIOPREV Study): Rationale, Methods, and Baseline Characteristics A Clinical Trial Comparing the Efficacy of a Mediterranean Diet Rich in Olive Oil versus a Low-Fat Diet on Cardiovascular Disease in Coronary Patients. Am. Heart J. 2016, 177, 42–50. [Google Scholar] [CrossRef]
- American Diabetes Association. American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, 81–90. [Google Scholar] [CrossRef]
- Buse, J.B.; Caprio, S.; Cefalu, W.T.; Ceriello, A.; Del Prato, S.; Inzucchi, S.E.; McLaughlin, S.; Phillips, G.L.; Robertson, R.P.; Rubino, F.; et al. How Do We Define Cure of Diabetes? Diabetes Care 2009, 32, 2133–2135. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Peña, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-Term Secondary Prevention of Cardiovascular Disease with a Mediterranean Diet and a Low-Fat Diet (CORDIOPREV): A Randomised Controlled Trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Morales, A.M.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Corina, A.; Quintana-Navarro, G.; Cardelo, M.P.; Yubero-Serrano, E.; Malagon, M.M.; Delgado-Lista, J.; Ordovas, J.M.; et al. Biological Senescence Risk Score. A Practical Tool to Predict Biological Senescence Status. Eur. J. Clin. Investig. 2020, 50, e13305. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Dykes, D.; Polesky, H. A Simple Salting out Procedure for Extracting DNA from Human Nucleated Cells. Nucleic Acids Res. 1988, 16, 1215. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere Measurement by Quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Piatyszek, M.; Prowse, K.; Harley, C.; West, M.; Ho, P.; Coviello, G.; Wright, W.; Weinrich, S.; Shay, J. Specific Assocation of Human Telomerase Activity with Immortal Cells and Cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R. Insulin Sensitivity Indices Obtained From Oral Glucose Tolerance Testing. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Tang, W.; Fu, Q.; Zhang, Q.; Sun, M.; Gao, Y.; Liu, X.; Qian, L.; Shan, S.; Yang, T. The Association between Serum Uric Acid and Residual β -Cell Function in Type 2 Diabetes. J. Diabetes Res. 2014, 2014, 709691. [Google Scholar] [CrossRef]
- Ma, D.; Yu, Y.; Yu, X.; Zhang, M.; Yang, Y. The Changes of Leukocyte Telomere Length and Telomerase Activity after Sitagliptin Intervention in Newly Diagnosed Type 2 Diabetes. Diabetes Metab. Res. Rev. 2015, 31, 256–261. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, L.; Cui, X.; Feng, L.; Zhao, X.; He, S.; Ping, F.; Li, W.; Li, Y. Influence of Diet on Leukocyte Telomere Length, Markers of Inflammation and Oxidative Stress in Individuals with Varied Glucose Tolerance: A Chinese Population Study. Nutr. J. 2016, 15, 39. [Google Scholar] [CrossRef]
- Zhao, H.; Han, L.; Chang, D.; Ye, Y.; Shen, J.; Daniel, C.R.; Gu, J.; Chow, W.-H.; Wu, X. Social-Demographics, Health Behaviors, and Telomere Length in the Mexican American Mano a Mano Cohort. Oncotarget 2017, 8, 96553–96567. [Google Scholar] [CrossRef] [PubMed]
- Bonfigli, A.R.; Spazzafumo, L.; Prattichizzo, F.; Bonafè, M.; Mensà, E.; Micolucci, L.; Giuliani, A.; Fabbietti, P.; Testa, R.; Boemi, M.; et al. Leukocyte Telomere Length and Mortality Risk in Patients with Type 2 Diabetes. Oncotarget 2016, 7, 50835–50844. [Google Scholar] [CrossRef] [PubMed]
- Hovatta, I.; de Mello, V.D.F.; Kananen, L.; Lindström, J.; Eriksson, J.G.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Peltonen, M.; Tuomilehto, J.; Uusitupa, M. Leukocyte Telomere Length in the Finnish Diabetes Prevention Study. PLoS ONE 2012, 7, e34948. [Google Scholar] [CrossRef] [PubMed]
- Canudas, S.; Hernández-Alonso, P.; Galié, S.; Muralidharan, J.; Morell-Azanza, L.; Zalba, G.; García-Gavilán, J.; Martí, A.; Salas-Salvadó, J.; Bulló, M. Pistachio Consumption Modulates DNA Oxidation and Genes Related Totelomere Maintenance: A Crossover Randomized Clinical Trial. Am. J. Clin. Nutr. 2019, 109, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.; Vasu, V.; Griffin, D. Telomere Biology and Human Phenotype. Cells 2019, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Carroll, L.; Joglekar, M.V.; Januszewski, A.S.; Wong, K.K.; Hardikar, A.A.; Jenkins, A.J.; Ma, R.C.W. Diabetes, Metabolic Disease, and Telomere Length. Lancet Diabetes Endocrinol. 2021, 9, 117–126. [Google Scholar] [CrossRef]
- Korkiakoski, A.; Käräjämäki, A.J.; Ronkainen, J.; Auvinen, J.; Hannuksela, J.; Kesäniemi, Y.A.; Ukkola, O. Nonalcoholic Fatty Liver Disease and Its Prognosis Associates with Shorter Leucocyte Telomeres in a 21-Year Follow-up Study. Scand. J. Clin. Lab. Investig. 2022, 82, 173–180. [Google Scholar] [CrossRef]
- Demissie, S.; Levy, D.; Benjamin, E.J.; Cupples, L.A.; Gardner, J.P.; Herbert, A.; Kimura, M.; Larson, M.G.; Meigs, J.B.; Keaney, J.F.; et al. Insulin Resistance, Oxidative Stress, Hypertension, and Leukocyte Telomere Length in Men from the Framingham Heart Study. Aging Cell 2006, 5, 325–330. [Google Scholar] [CrossRef]
- Gardner, J.P.; Li, S.; Srinivasan, S.R.; Chen, W.; Kimura, M.; Lu, X.; Berenson, G.S.; Aviv, A. Rise in Insulin Resistance Is Associated with Escalated Telomere Attrition. Circulation 2005, 111, 2171–2177. [Google Scholar] [CrossRef]
- Rattan, R.; Lamare, A.A.; Jena, S.; Sahoo, N.; Mandal, M.K. Association of Serum Telomerase Activity in Type 2 Diabetes Mellitus Patients with or without Microalbuminuria. J. Evid. Based Med. Healthc. 2021, 8, 667–671. [Google Scholar] [CrossRef]
- AlDehaini, D.M.B.; Al-Bustan, S.A.; Ali, M.E.; Malalla, Z.H.A.; Sater, M.; Giha, H.A. Shortening of the Leucocytes’ Telomeres Length in T2DM Independent of Age and Telomerase Activity. Acta Diabetol. 2020, 57, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-gavilán, J.; Bulló, M.; Salas-salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576–601. [Google Scholar] [CrossRef] [PubMed]





| Responders (n = 69) | Non-Responders (n = 104) | |
|---|---|---|
| Males/Females (n) | 58/11 | 86/18 |
| Low-fat diet/Mediterranean diet (n) | 37/32 | 60/44 |
| Age (years) | 60 (58 to 62) | 59 (57 to 61) |
| Height (m) | 1.64 (1.62 to 1.66) | 1.66 (1.65 to 1.67) |
| Weight (kg) | 80.4 (77.7 to 83.0) | 88.8 (85.9 to 91.6) |
| Body mass index (kg/m2) | 29.8 (29.0 to 30.7) | 32.1 (31.2 to 33.0) |
| Waist circumference (cm) | 101 (99 to 103) | 108 (106 to 110) |
| HDL-cholesterol (mmol/L) | 43 (40 to 46) | 41 (39 to 43) |
| LDL-cholesterol (mmol/L) | 88 (82 to 93) | 94 (89 to 100) |
| C-reactive protein (nmol/L) | 2.75 (2.29 to 3.22) | 2.95 (2.51 to 3.40) |
| Triglycerides (mmol/L) | 133 (114 to 152) | 149 (136 to 162) |
| HbA1c (%; mmol/mol) | 6.53 (6.36 to 6.71); 48 | 6.82 (6.65 to 6.98); 51 |
| Glucose (mmol/L) | 5.5 (5.3 to 5.7) | 6.6 (6.3 to 6.9) |
| Insulin (nmol/L) | 9.4 (7.7 to 11.0) | 13.4 (11.1 to 15.7) |
| HOMA-IR | 3.50 (2.36 to 4.37) | 4.79 (4.14 to 5.45) |
| ISI | 3.14 (2.73 to 3.56) | 2.39 (2.15 to 2.64) |
| IGI | 0.70 (0.30 to 1.10) | 0.68 (0.40 to 0.97) |
| Hepatic-IRfasting | 1435 (1083 to 1786) | 1951 (1685 to 2217) |
| DI | 0.68 (0.55 to 0.81) | 0.43 (0.38 to 0.48) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda-Rodriguez, A.; Alcala-Diaz, J.F.; Rangel-Zuñiga, O.A.; Arenas-de Larriva, A.P.; Gutierrez-Mariscal, F.M.; Torres-Peña, J.D.; Mora-Ortiz, M.; Romero-Cabrera, J.L.; Luque, R.M.; Ordovas, J.M.; et al. Telomere Maintenance Is Associated with Type 2 Diabetes Remission in Response to a Long-Term Dietary Intervention without Non-Weight Loss in Patients with Coronary Heart Disease: From the CORDIOPREV Randomized Controlled Trial. Antioxidants 2024, 13, 125. https://doi.org/10.3390/antiox13010125
Ojeda-Rodriguez A, Alcala-Diaz JF, Rangel-Zuñiga OA, Arenas-de Larriva AP, Gutierrez-Mariscal FM, Torres-Peña JD, Mora-Ortiz M, Romero-Cabrera JL, Luque RM, Ordovas JM, et al. Telomere Maintenance Is Associated with Type 2 Diabetes Remission in Response to a Long-Term Dietary Intervention without Non-Weight Loss in Patients with Coronary Heart Disease: From the CORDIOPREV Randomized Controlled Trial. Antioxidants. 2024; 13(1):125. https://doi.org/10.3390/antiox13010125
Chicago/Turabian StyleOjeda-Rodriguez, Ana, Juan F. Alcala-Diaz, Oriol Alberto Rangel-Zuñiga, Antonio P. Arenas-de Larriva, Francisco M. Gutierrez-Mariscal, Jose D. Torres-Peña, Marina Mora-Ortiz, Juan L. Romero-Cabrera, Raul M. Luque, Jose M. Ordovas, and et al. 2024. "Telomere Maintenance Is Associated with Type 2 Diabetes Remission in Response to a Long-Term Dietary Intervention without Non-Weight Loss in Patients with Coronary Heart Disease: From the CORDIOPREV Randomized Controlled Trial" Antioxidants 13, no. 1: 125. https://doi.org/10.3390/antiox13010125
APA StyleOjeda-Rodriguez, A., Alcala-Diaz, J. F., Rangel-Zuñiga, O. A., Arenas-de Larriva, A. P., Gutierrez-Mariscal, F. M., Torres-Peña, J. D., Mora-Ortiz, M., Romero-Cabrera, J. L., Luque, R. M., Ordovas, J. M., Perez-Martinez, P., Delgado-Lista, J., Yubero-Serrano, E. M., & Lopez-Miranda, J. (2024). Telomere Maintenance Is Associated with Type 2 Diabetes Remission in Response to a Long-Term Dietary Intervention without Non-Weight Loss in Patients with Coronary Heart Disease: From the CORDIOPREV Randomized Controlled Trial. Antioxidants, 13(1), 125. https://doi.org/10.3390/antiox13010125








