Maintaining the Mitochondrial Quality Control System Was a Key Event of Tanshinone IIA against Deoxynivalenol-Induced Intestinal Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Drug Intervention
2.2. Cytotoxicity Measurement
2.3. Cell Redox State Measurement
2.4. MPTP Opening and MMP Measurement
2.5. RNA Extraction and Quantitative RT–PCR Analysis
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
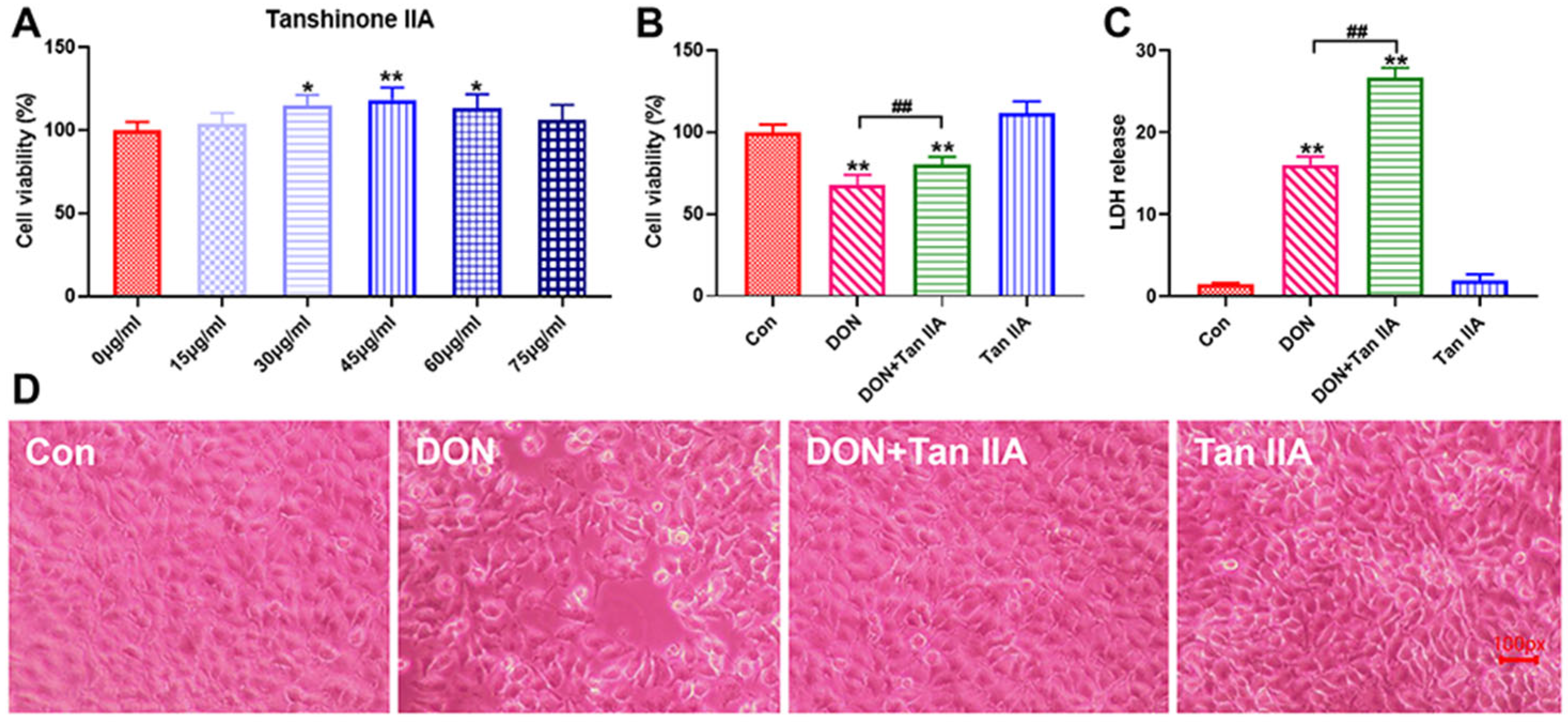
3.1. Tan IIA Alleviates the Cytotoxicity Caused by DON in IPEC-J2 Cells
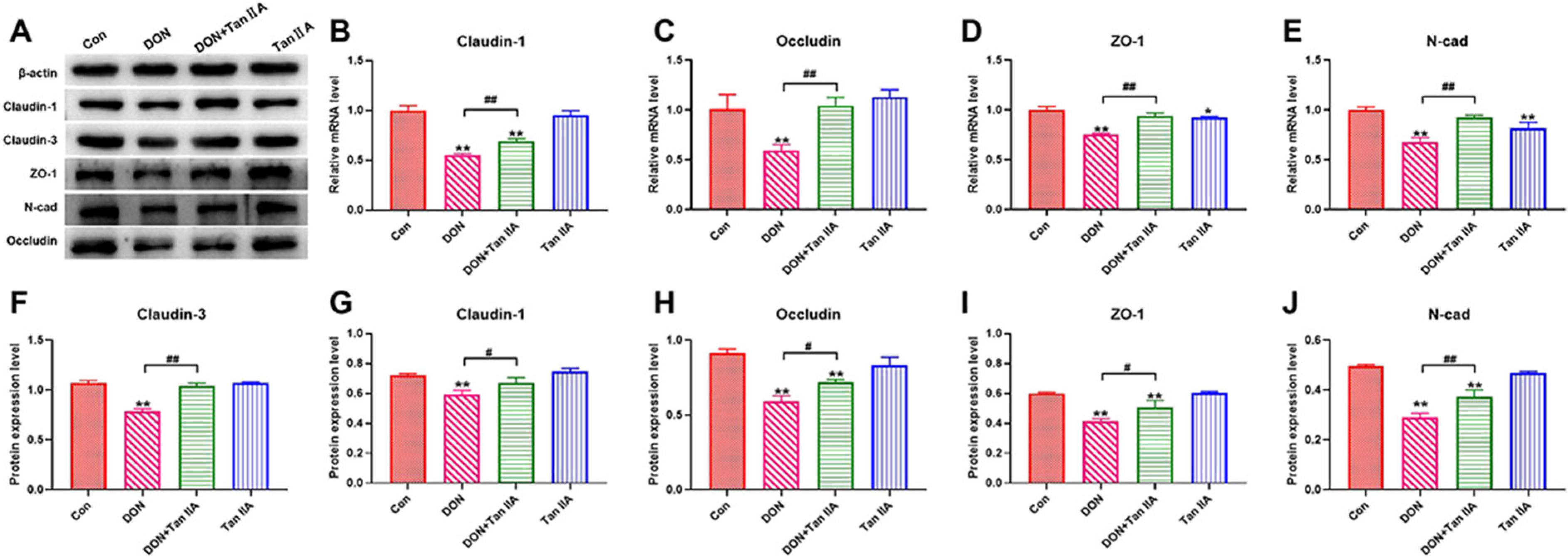
3.2. Tan IIA Alleviates the Barrier Function Impairment Caused by DON in IPEC-J2 Cells
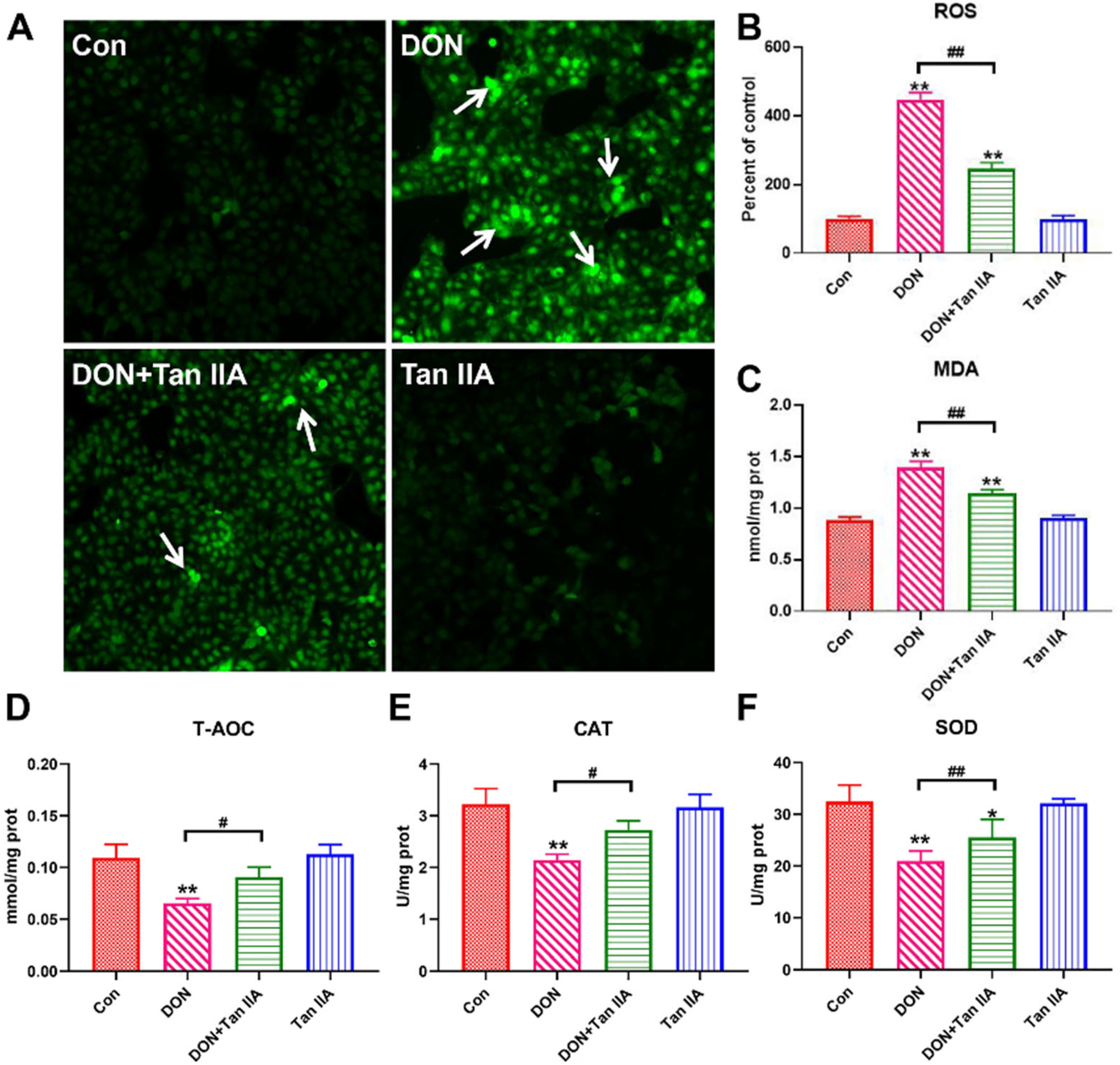
3.3. Tan IIA Alleviates the Oxidative Damage Caused by DON in IPEC-J2 Cells
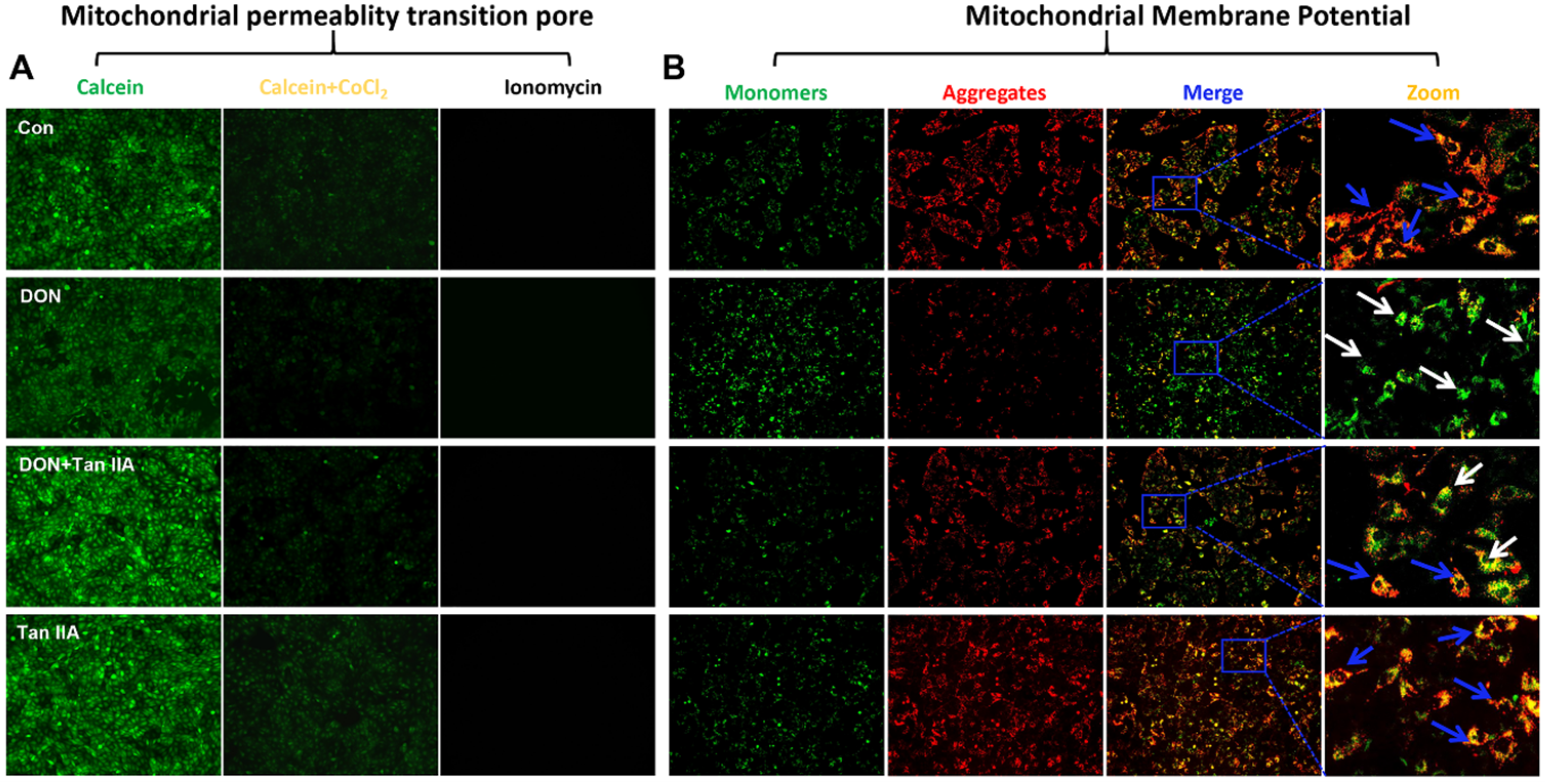
3.4. Tan IIA Alleviates the Mitochondrial Dysfunction Caused by DON in IPEC-J2 Cells
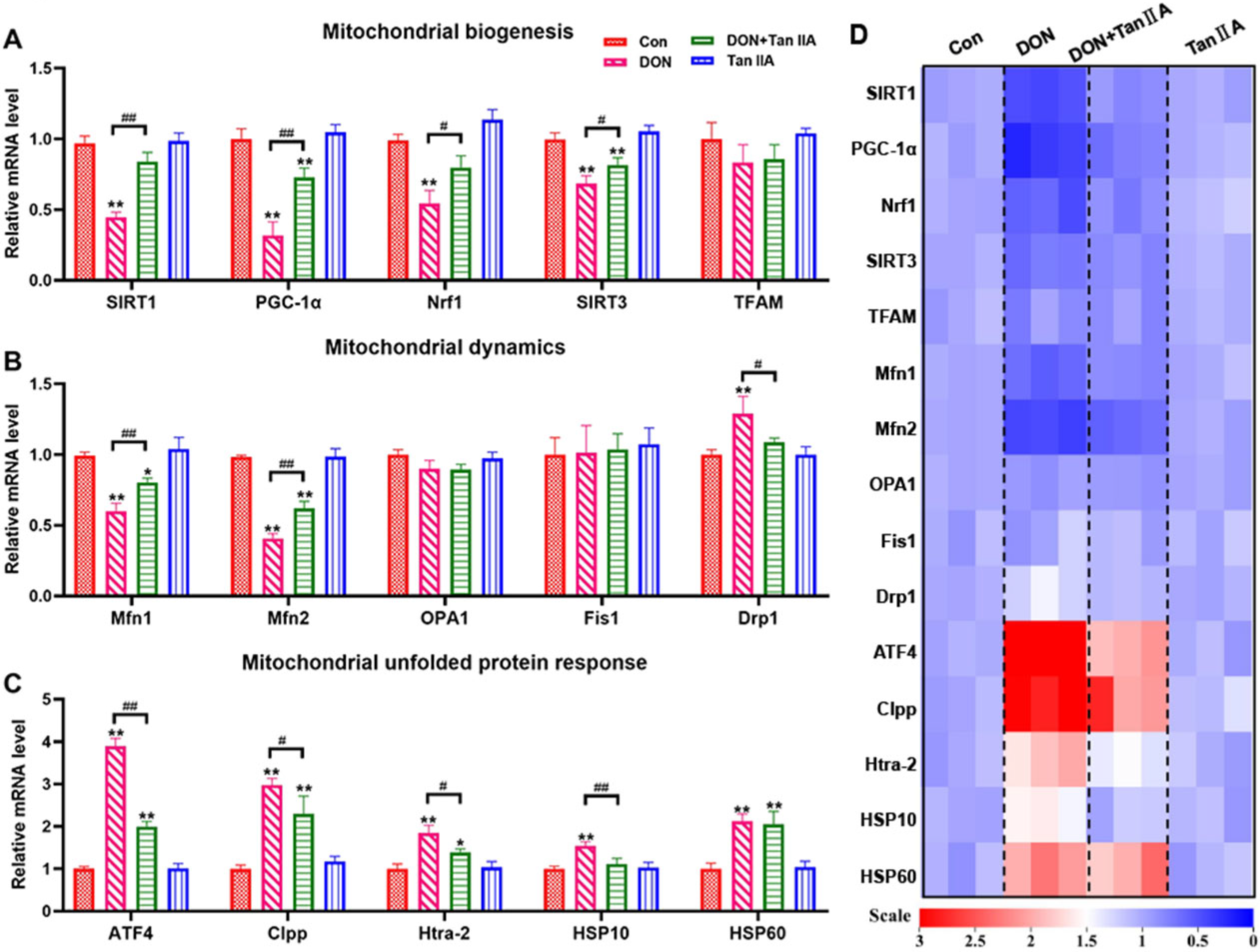
3.5. Tan IIA Alleviates the MQC Disorder Caused by DON in IPEC-J2 Cells
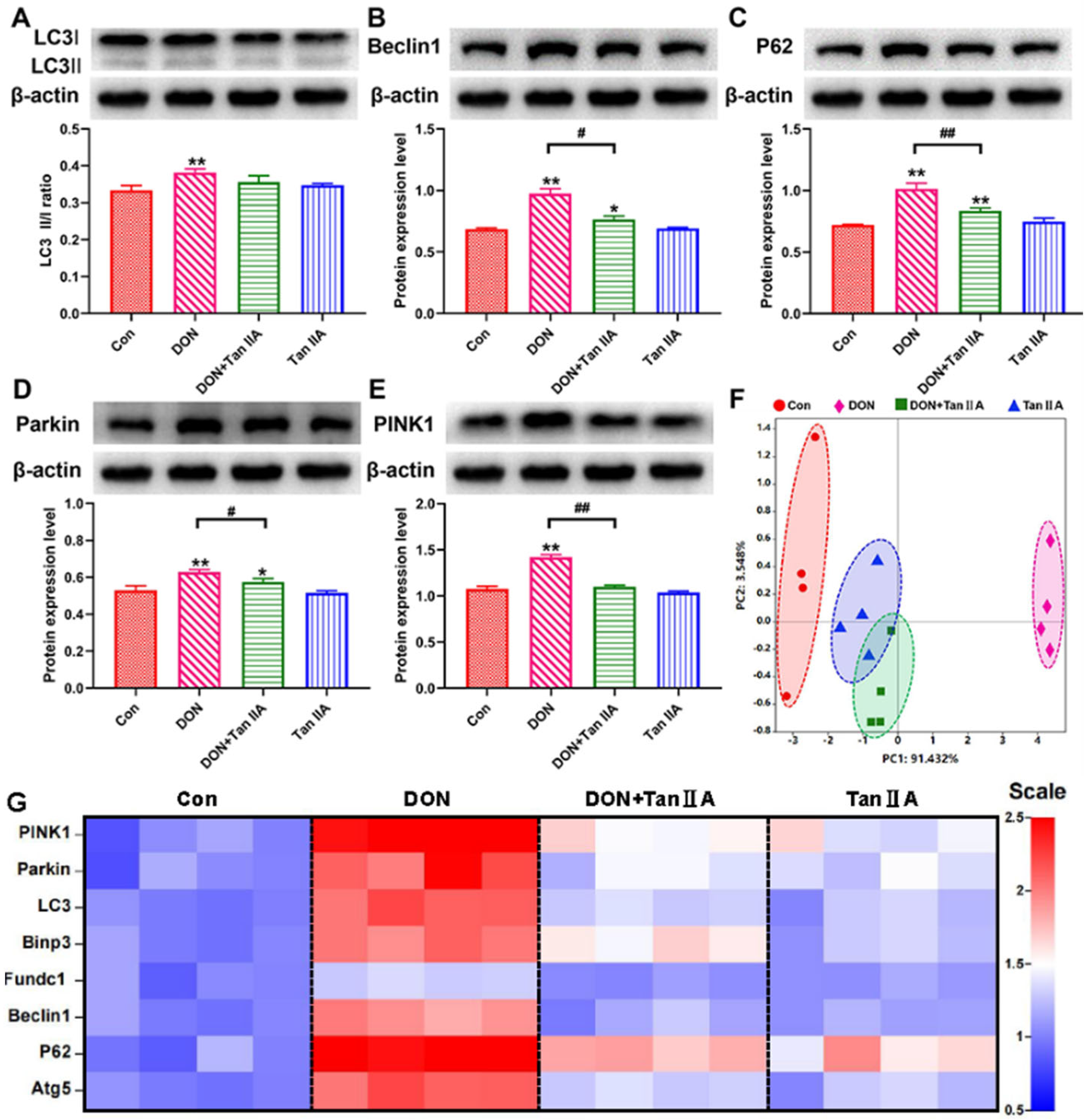
3.6. Tan IIA Alleviates IPEC-J2 Cell Mitophagy Caused by DON
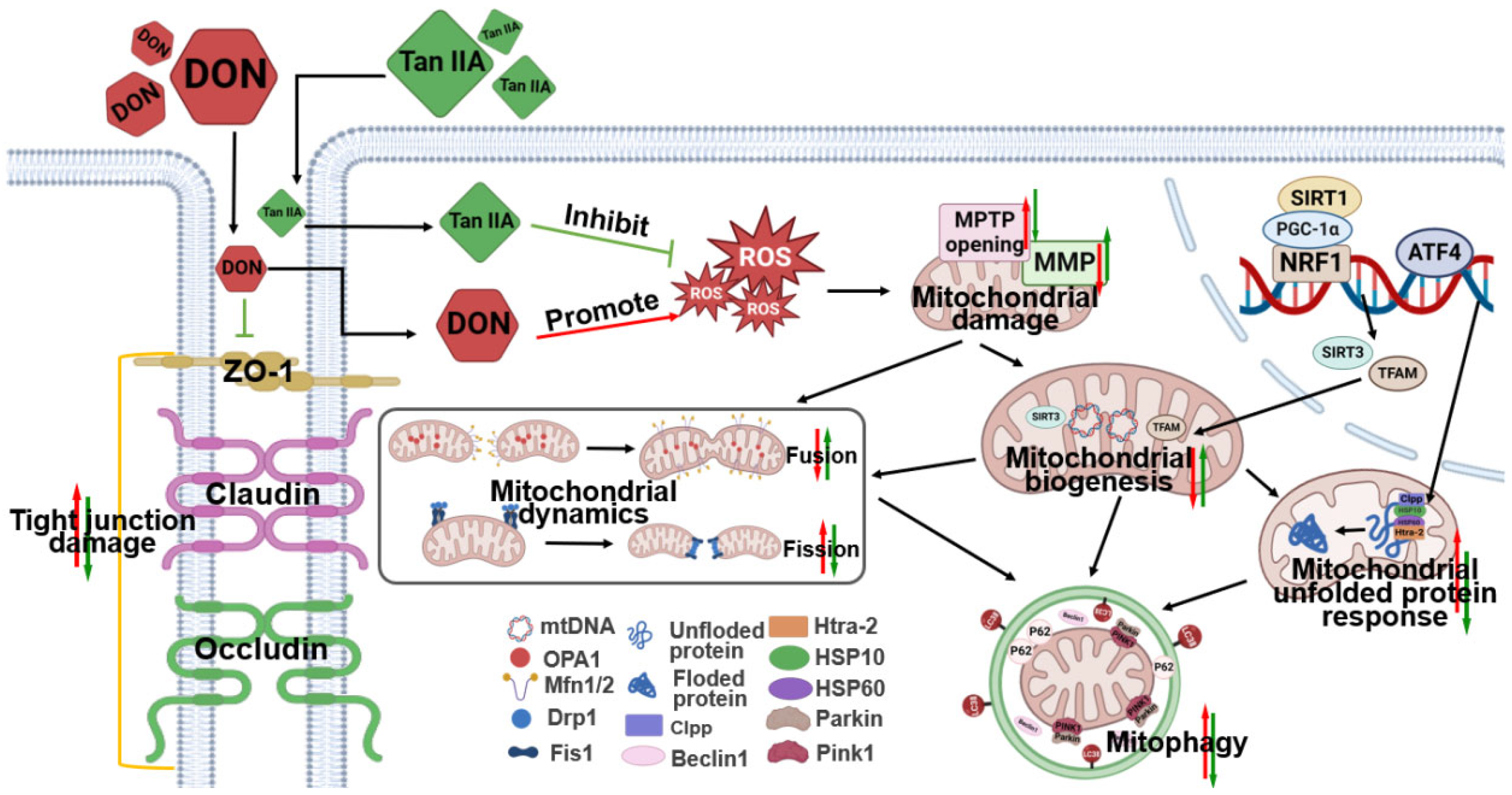
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Z.; Chen, F.; Wang, Y.; Wang, X.; Yang, X.; Zhang, C. Lycopene Maintains Mitochondrial Homeostasis to Counteract the Enterotoxicity of Deoxynivalenol. Antioxidants 2023, 12, 1958. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, T.; Chen, Y.; Chen, F.; Liu, Y.; Wang, Y.; Song, W.; Zhang, J.; Jiang, Y.; Wang, F.; et al. Deoxynivalenol induces testicular ferroptosis by regulating the Nrf2/System Xc(-)/GPX4 axis. Food Chem. Toxicol. 2023, 175, 113730. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jiang, J.; Mu, P.; Lin, R.; Wen, J.; Deng, Y. Toxicokinetics and metabolism of deoxynivalenol in animals and humans. Arch. Toxicol. 2022, 96, 2639–2654. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Srivastava, S.; Dewangan, J.; Divakar, A.; Kumar Rath, S. Global occurrence of deoxynivalenol in food commodities and exposure risk assessment in humans in the last decade: A survey. Crit. Rev. Food Sci. Nutr. 2020, 60, 1346–1374. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Nepovimova, E.; Wu, Q.; Wu, W.; Kuca, K. Deoxynivalenol upregulates hypoxia-inducible factor-1α to promote an “immune evasion” process by activating STAT3 signaling. Food Chem. Toxicol. 2023, 179, 113975. [Google Scholar] [CrossRef]
- Jia, B.; Lin, H.; Yu, S.; Liu, N.; Yu, D.; Wu, A. Mycotoxin deoxynivalenol-induced intestinal flora disorders, dysfunction and organ damage in broilers and pigs. J. Hazard. Mater. 2023, 451, 131172. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Ni, H.M.; Williams, J.A.; Ding, W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef]
- Ng, M.Y.W.; Wai, T.; Simonsen, A. Quality control of the mitochondrion. Dev. Cell 2021, 56, 881–905. [Google Scholar] [CrossRef]
- Eldeeb, M.A.; Thomas, R.A.; Ragheb, M.A.; Fallahi, A.; Fon, E.A. Mitochondrial quality control in health and in Parkinson’s disease. Physiol. Rev. 2022, 102, 1721–1755. [Google Scholar] [CrossRef]
- An, H.; Zhou, B.; Ji, X. Mitochondrial quality control in acute ischemic stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2021, 41, 3157–3170. [Google Scholar] [CrossRef]
- Abdelmaksoud, N.M.; Abulsoud, A.I.; Abdelghany, T.M.; Elshaer, S.S.; Rizk, S.M.; Senousy, M.A. Mitochondrial remodeling in colorectal cancer initiation, progression, metastasis, and therapy: A review. Pathol. Res. Pract. 2023, 246, 154509. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Xu, C.; An, P.; Luo, Y.; Jiao, L.; Luo, J.; Li, Y. Mitochondrial Dysfunction and Therapeutic Perspectives in Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 16053. [Google Scholar] [CrossRef] [PubMed]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Jackson, D.N.; Theiss, A.L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 2020, 11, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lin, C.J.; Hu, G.; Wang, M.C. ‘Inside Out’–A dialogue between mitochondria and bacteria. FEBS J. 2019, 286, 630–641. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Duan, L. The role of microbiota-mitochondria crosstalk in pathogenesis and therapy of intestinal diseases. Pharmacol. Res. 2022, 186, 106530. [Google Scholar] [CrossRef]
- Doguer, C.; Ha, J.H.; Collins, J.F. Intersection of Iron and Copper Metabolism in the Mammalian Intestine and Liver. Compr. Physiol. 2018, 8, 1433–1461. [Google Scholar] [CrossRef]
- Yoo, W.; Zieba, J.K.; Foegeding, N.J.; Torres, T.P.; Shelton, C.D.; Shealy, N.G. High-fat diet-induced colonocyte dysfunction escalates microbiota-derived trimethylamine N-oxide. Science 2021, 373, 813–818. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Huang, T.; Lei, Z.; Liu, F.; An, G.; Wen, T. Tanshinone IIA Protects against Dextran Sulfate Sodium- (DSS-) Induced Colitis in Mice by Modulation of Neutrophil Infiltration and Activation. Oxidative Med. Cell. Longev. 2016, 2016, 7916763. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Lin, Z.; Ke, L.; Shi, P.; Li, S.; Huang, L.; Lin, X.; Yao, H. Recent Research Progress (2015–2021) and Perspectives on the Pharmacological Effects and Mechanisms of Tanshinone IIA. Front. Pharmacol. 2021, 12, 778847. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lu, Q.; Chen, H.W.; Feng, J.; Wan, L.; Zhou, D.X. Protective effect of sodium tanshinone IIA sulfonate on injury of small intestine in rats with sepsis and its mechanism. Chin. J. Integr. Med. 2012, 18, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.; Lu, N.; Guo, Y.; Fan, X. Tanshinone IIA ameliorates trinitrobenzene sulfonic acid (TNBS)-induced murine colitis. Dig. Dis. Sci. 2008, 53, 421–428. [Google Scholar] [CrossRef]
- Wang, D.; Sun, F.; Lu, C.; Chen, P.; Wang, Z.; Qiu, Y.; Mu, H.; Miao, Z.; Duan, J. Inulin based glutathione-responsive delivery system for colon cancer treatment. Int. J. Biol. Macromol. 2018, 111, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Qian, J.X.; Wei, Y.; Guo, Q.; Jin, J.; Sun, X.; Liu, S.L.; Xu, C.F.; Zhang, G.X. Tanshinone IIA Sodium Sulfonate Attenuates LPS-Induced Intestinal Injury in Mice. Gastroenterol. Res. Pract. 2018, 2018, 9867150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, F.; Wang, Y.; Zhang, K.; Yang, X.; Wang, X. Tanshinone IIA protects intestinal epithelial cells from deoxynivalenol-induced pyroptosis. Ecotoxicol. Environ. Saf. 2024, 269, 115743. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Chen, Y.; Fan, J.; Zhang, C.; He, X.; Yang, X. JNK molecule is a toxic target for IPEC-J2 cell barrier damage induced by T-2 toxin. Ecotoxicol. Environ. Saf. 2023, 263, 115247. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, Y.; Liu, G.; Shao, Q.; Niu, X.; Tai, W.; Shen, T.; Fan, M.; Chen, M.; Lei, L.; et al. Narirutin activates TFEB (transcription factor EB) to protect against Acetaminophen-induced liver injury by targeting PPP3/calcineurin. Autophagy 2023, 19, 2240–2256. [Google Scholar] [CrossRef]
- Song, Y.; Yang, Y.; Zeng, W.; Loor, J.J.; Jiang, Q.; Peng, Z.; Li, Y.; Jiang, S.; Feng, X.; Du, X.; et al. β-Hydroxybutyrate impairs neutrophil migration distance through activation of a protein kinase C and myosin light chain 2 signaling pathway in ketotic cows. J. Dairy Sci. 2022, 105, 761–771. [Google Scholar] [CrossRef]
- Liu, L.; Gao, H.; Wen, T.; Gu, T.; Zhang, S.; Yuan, Z. Tanshinone IIA attenuates AOM/DSS-induced colorectal tumorigenesis in mice via inhibition of intestinal inflammation. Pharm. Biol. 2021, 59, 89–96. [Google Scholar] [CrossRef]
- Wang, J.; Kong, L.; Guo, R.B.; He, S.Y.; Liu, X.Z.; Zhang, L.; Liu, Y.; Yu, Y.; Li, X.T.; Cheng, L. Multifunctional icariin and tanshinone IIA co-delivery liposomes with potential application for Alzheimer’s disease. Drug Deliv. 2022, 29, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, D.; Gajendran, B.; Hu, Q.; Zhang, J.; Wang, S.; Han, M.; Xu, Y.; Shen, X. Tanshinone IIA ameliorates experimental diabetic cardiomyopathy by inhibiting endoplasmic reticulum stress in cardiomyocytes via SIRT1. Phytother. Res. 2023, 37, 3543–3558. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Khan, F.B.; Safdari, H.A.; Almatroudi, A.; Alzohairy, M.A.; Safdari, M.; Amirizadeh, M.; Rehman, S.; Equbal, M.J.; Hoque, M. Prospective therapeutic potential of Tanshinone IIA: An updated overview. Pharmacol. Res. 2021, 164, 105364. [Google Scholar] [CrossRef] [PubMed]
- Rusetskaya, N.Y.; Loginova, N.Y.; Pokrovskaya, E.P.; Chesovskikh, Y.S.; Titova, L.E. Redox regulation of the NLRP3-mediated inflammation and pyroptosis. Biomed. Khim. 2023, 69, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Ballard, J.W.O.; Towarnicki, S.G. Mitochondria, the gut microbiome and ROS. Cell. Signal. 2020, 75, 109737. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Chen, Z.; Yu, Y.; Shi, H.; Yu, Y.; Wang, Y.; Chen, R.; Ge, J. Mitochondrial calpain-1 activates NLRP3 inflammasome by cleaving ATP5A1 and inducing mitochondrial ROS in CVB3-induced myocarditis. Basic Res. Cardiol. 2022, 117, 40. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells by promoting ROS production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.; Sun, J.; Gong, Q.; Ma, H.; Kan, X.; Cao, Y.; Wang, J.; Fu, S. Butyrate alleviates oxidative stress by regulating NRF2 nuclear accumulation and H3K9/14 acetylation via GPR109A in bovine mammary epithelial cells and mammary glands. Free Radic Biol. Med. 2020, 152, 728–742. [Google Scholar] [CrossRef]
- Wu, J.; Lu, Z.; Jiang, D.; Guo, Y.; Qiao, H.; Zhang, Y.; Zhu, T.; Cai, Y.; Zhang, X.; Zhanghao, K.; et al. Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale. Cell 2021, 184, 3318–3332.e3317. [Google Scholar] [CrossRef]
- Chen, S.; Lu, Z.; Jia, H.; Yang, B.; Liu, C.; Yang, Y.; Zhang, S.; Wang, Z.; Yang, L.; Li, S.; et al. Hepatocyte-specific Mas activation enhances lipophagy and fatty acid oxidation to protect against acetaminophen-induced hepatotoxicity in mice. J. Hepatol. 2023, 78, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.J.; Wang, X.Q. Deoxynivalenol induces intestinal injury: Insights from oxidative stress and intestinal stem cells. Environ. Sci. Pollut. Res. Int. 2023, 30, 48676–48685. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. The toxicity mechanisms of DON to humans and animals and potential biological treatment strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 790–812. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Cheng, Y.; Pang, T.; Kuai, Y.; An, Y.; Wu, K.; Li, Y.; Lai, M.; Wang, B.; Wang, S. Sodium butyrate alleviates deoxynivalenol-induced porcine intestinal barrier disruption by promoting mitochondrial homeostasis via PCK2 signaling. J. Hazard. Mater. 2023, 459, 132013. [Google Scholar] [CrossRef]
- Chang, X.; Li, Y.; Cai, C.; Wu, F.; He, J.; Zhang, Y.; Zhong, J.; Tan, Y.; Liu, R.; Zhu, H.; et al. Mitochondrial quality control mechanisms as molecular targets in diabetic heart. Metabolism 2022, 137, 155313. [Google Scholar] [CrossRef]
- Ji, X.; Tang, Z.; Zhang, F.; Zhou, F.; Wu, Y.; Wu, D. Dietary taurine supplementation counteracts deoxynivalenol-induced liver injury via alleviating oxidative stress, mitochondrial dysfunction, apoptosis, and inflammation in piglets. Ecotoxicol. Environ. Saf. 2023, 253, 114705. [Google Scholar] [CrossRef]
- Ma, K.; Bai, Y.; Li, J.; Ren, Z.; Li, J.; Zhang, J.; Shan, A. Lactobacillus rhamnosus GG ameliorates deoxynivalenol-induced kidney oxidative damage and mitochondrial injury in weaned piglets. Food Funct. 2022, 13, 3905–3916. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, C.; Song, B.; Wang, L.; Xiao, H.; Jiang, Z. Resveratrol Ameliorates Intestinal Damage Challenged With Deoxynivalenol Through Mitophagy in vitro and in vivo. Front. Vet. Sci. 2021, 8, 807301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, Y.; Zhang, X.; Zhang, K.; Chen, F.; Fan, J.; Wang, X.; Yang, X. Maintaining the Mitochondrial Quality Control System Was a Key Event of Tanshinone IIA against Deoxynivalenol-Induced Intestinal Toxicity. Antioxidants 2024, 13, 121. https://doi.org/10.3390/antiox13010121
Zhang C, Wang Y, Zhang X, Zhang K, Chen F, Fan J, Wang X, Yang X. Maintaining the Mitochondrial Quality Control System Was a Key Event of Tanshinone IIA against Deoxynivalenol-Induced Intestinal Toxicity. Antioxidants. 2024; 13(1):121. https://doi.org/10.3390/antiox13010121
Chicago/Turabian StyleZhang, Cong, Youshuang Wang, Xinyu Zhang, Kefei Zhang, Fengjuan Chen, Jiayan Fan, Xuebing Wang, and Xu Yang. 2024. "Maintaining the Mitochondrial Quality Control System Was a Key Event of Tanshinone IIA against Deoxynivalenol-Induced Intestinal Toxicity" Antioxidants 13, no. 1: 121. https://doi.org/10.3390/antiox13010121
APA StyleZhang, C., Wang, Y., Zhang, X., Zhang, K., Chen, F., Fan, J., Wang, X., & Yang, X. (2024). Maintaining the Mitochondrial Quality Control System Was a Key Event of Tanshinone IIA against Deoxynivalenol-Induced Intestinal Toxicity. Antioxidants, 13(1), 121. https://doi.org/10.3390/antiox13010121







