Oxidative Post-translational Protein Modifications upon Ischemia/Reperfusion Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Animal Experiments
2.2. Myocardial Infarction in Pig and Mouse Models
2.3. CMR Protocol and Analysis
2.4. Tissue Sample Preparation for Proteomics Analysis
2.5. Liquid Chromatography Tandem Mass Spectrometry Analysis
2.6. Peptide and Protein Identification
2.7. PTM Identification and Annotation
2.8. Quantification at the Peptide and Protein Levels
2.9. H2O2 Measurement
2.10. Statistical Analysis
3. Results
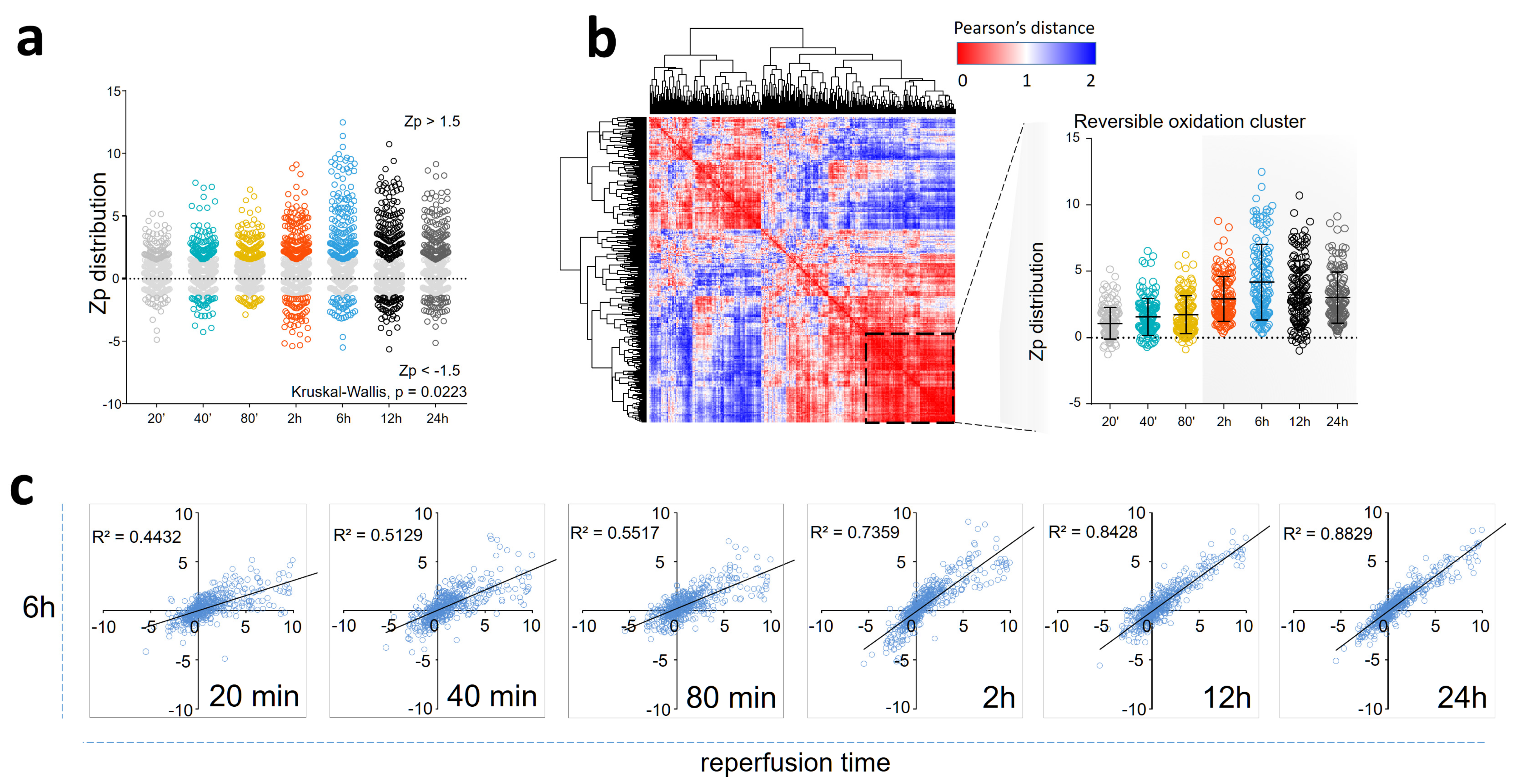
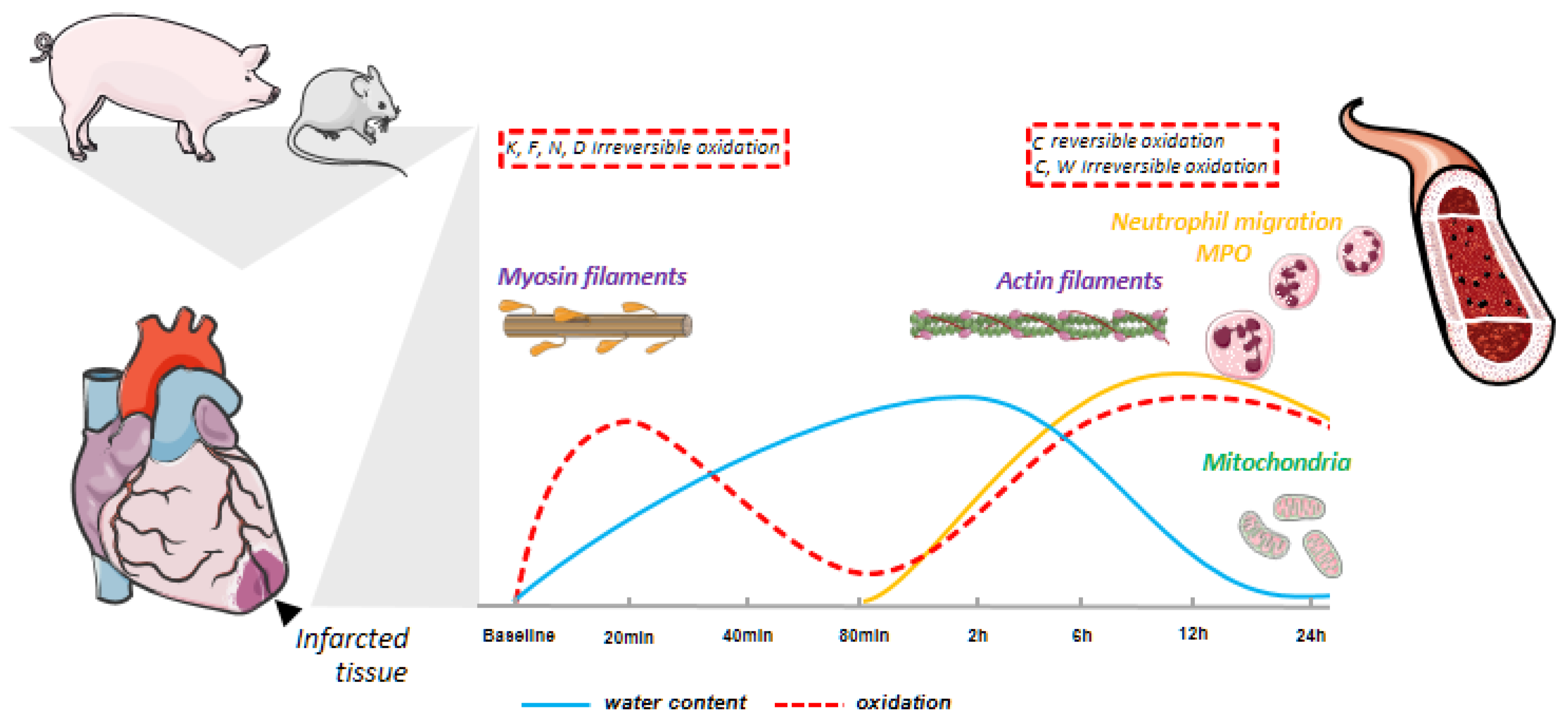
3.1. Myocardial Infarction Produces Reversible Oxidative Protein Damage after Six Hours of Reperfusion
3.2. Myocardial Infarction Produces Two Different Waves of Irreversible Oxidative Protein Damage after Reperfusion
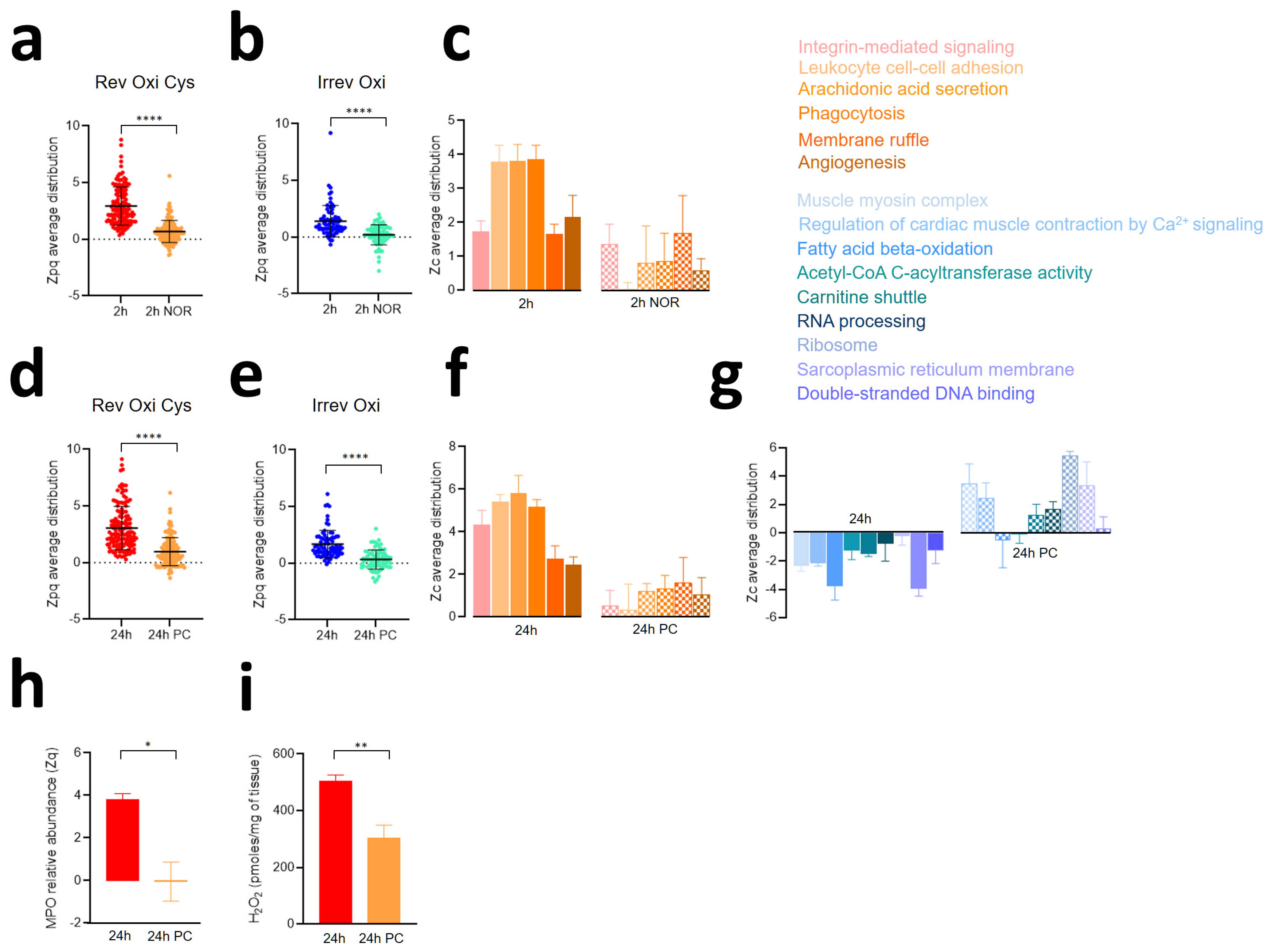
3.3. Protein Abundance Changes during the Second Wave of Oxidative Damage Support a Role for Neutrophil Infiltration
3.4. The Second Wave of Oxidative Protein Damage Is Caused by Reperfusion
3.5. Ischemic Preconditioning Reduces Oxidative Protein Damage, Inflammatory Reactions, and Contractile Dysfunction Caused by Ischemia-Reperfusion
3.6. The Biphasic Pattern of Oxidative Protein Damage and Protein Alterations Are Reproduced in a Mouse Model of Ischemia-Reperfusion
3.7. Neutrophil Depletion Lessens Oxidative Protein Damage and Mitochondrial Protein Decrease Caused by Ischemia-Reperfusion in the Mouse Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibanez, B.; Fuster, V.; Jimenez-Borreguero, J.; Badimon, J.J. Lethal myocardial reperfusion injury: A necessary evil? Int. J. Cardiol. 2011, 151, 3–11. [Google Scholar] [CrossRef]
- Ibanez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef]
- Ong, S.B.; Samangouei, P.; Kalkhoran, S.B.; Hausenloy, D.J. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J. Mol. Cell Cardiol. 2015, 78, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.J.; Halestrap, A.P. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem. J. 1995, 307 Pt 1, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Patel, S. Danger-Associated Molecular Patterns (DAMPs): The Derivatives and Triggers of Inflammation. Curr. Allergy Asthma Rep. 2018, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Russo, I.; Frangogiannis, N.G. Inflammation as a therapeutic target in myocardial infarction: Learning from past failures to meet future challenges. Transl. Res. 2016, 167, 152–166. [Google Scholar] [CrossRef]
- Gu, S.X.; Blokhin, I.O.; Wilson, K.M.; Dhanesha, N.; Doddapattar, P.; Grumbach, I.M.; Chauhan, A.K.; Lentz, S.R. Protein methionine oxidation augments reperfusion injury in acute ischemic stroke. JCI Insight 2016, 1, e86460. [Google Scholar] [CrossRef]
- Whalen, D.A., Jr.; Hamilton, D.G.; Ganote, C.E.; Jennings, R.B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am. J. Pathol. 1974, 74, 381–397. [Google Scholar]
- Carlsson, M.; Ubachs, J.F.; Hedstrom, E.; Heiberg, E.; Jovinge, S.; Arheden, H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: Quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc. Imaging 2009, 2, 569–576. [Google Scholar] [CrossRef]
- Dall’Armellina, E.; Karia, N.; Lindsay, A.C.; Karamitsos, T.D.; Ferreira, V.; Robson, M.D.; Kellman, P.; Francis, J.M.; Forfar, C.; Prendergast, B.D.; et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ. Cardiovasc. Imaging 2011, 4, 228–236. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, R.; Sanchez-Gonzalez, J.; Aguero, J.; Garcia-Prieto, J.; Lopez-Martin, G.J.; Garcia-Ruiz, J.M.; Molina-Iracheta, A.; Rossello, X.; Fernandez-Friera, L.; Pizarro, G.; et al. Myocardial edema after ischemia/reperfusion is not stable and follows a bimodal pattern: Imaging and histological tissue characterization. J. Am. Coll. Cardiol. 2015, 65, 315–323. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, R.; Barreiro-Perez, M.; Martin-Garcia, A.; Sanchez-Gonzalez, J.; Aguero, J.; Galan-Arriola, C.; Garcia-Prieto, J.; Diaz-Pelaez, E.; Vara, P.; Martinez, I.; et al. Dynamic Edematous Response of the Human Heart to Myocardial Infarction: Implications for Assessing Myocardial Area at Risk and Salvage. Circulation 2017, 136, 1288–1300. [Google Scholar] [CrossRef]
- Binek, A.; Fernandez-Jimenez, R.; Jorge, I.; Camafeita, E.; Lopez, J.A.; Bagwan, N.; Galan-Arriola, C.; Pun, A.; Aguero, J.; Fuster, V.; et al. Proteomic footprint of myocardial ischemia/reperfusion injury: Longitudinal study of the at-risk and remote regions in the pig model. Sci. Rep. 2017, 7, 12343. [Google Scholar] [CrossRef]
- Chick, J.M.; Kolippakkam, D.; Nusinow, D.P.; Zhai, B.; Rad, R.; Huttlin, E.L.; Gygi, S.P. An ultra-tolerant database search reveals that a myriad of modified peptides contributes to unassigned spectra in shotgun proteomics. Nat. Biotechnol. 2015, 33, 743–749. [Google Scholar] [CrossRef]
- Kong, A.T.; Leprevost, F.V.; Avtonomov, D.M.; Mellacheruvu, D.; Nesvizhskii, A.I. MSFragger: Ultrafast and comprehensive peptide identification in mass spectrometry–based proteomics. Nat. Methods 2017, 14, 513. [Google Scholar] [CrossRef]
- Yu, F.; Teo, G.C.; Kong, A.T.; Haynes, S.E.; Avtonomov, D.M.; Geiszler, D.J.; Nesvizhskii, A.I. Identification of modified peptides using localization-aware open search. Nat. Commun. 2020, 11, 4065. [Google Scholar] [CrossRef]
- Bagwan, N.; Bonzon-Kulichenko, E.; Calvo, E.; Lechuga-Vieco, A.V.; Michalakopoulos, S.; Trevisan-Herraz, M.; Ezkurdia, I.; Rodriguez, J.M.; Magni, R.; Latorre-Pellicer, A.; et al. Comprehensive Quantification of the Modified Proteome Reveals Oxidative Heart Damage in Mitochondrial Heteroplasmy. Cell Rep. 2018, 23, 3685–3697.e4. [Google Scholar] [CrossRef]
- Garcia-Marques, F.; Trevisan-Herraz, M.; Martinez-Martinez, S.; Camafeita, E.; Jorge, I.; Lopez, J.A.; Mendez-Barbero, N.; Mendez-Ferrer, S.; Del Pozo, M.A.; Ibanez, B.; et al. A Novel Systems-Biology Algorithm for the Analysis of Coordinated Protein Responses Using Quantitative Proteomics. Mol. Cell Proteom. 2016, 15, 1740–1760. [Google Scholar] [CrossRef]
- Bonzon-Kulichenko, E.; Camafeita, E.; Lopez, J.A.; Gomez-Serrano, M.; Jorge, I.; Calvo, E.; Nunez, E.; Trevisan-Herraz, M.; Bagwan, N.; Barcena, J.A.; et al. Improved integrative analysis of the thiol redox proteome using filter-aided sample preparation. J. Proteom. 2020, 214, 103624. [Google Scholar] [CrossRef]
- Martinez-Acedo, P.; Nunez, E.; Gomez, F.J.; Moreno, M.; Ramos, E.; Izquierdo-Alvarez, A.; Miro-Casas, E.; Mesa, R.; Rodriguez, P.; Martinez-Ruiz, A.; et al. A novel strategy for global analysis of the dynamic thiol redox proteome. Mol. Cell Proteom. 2012, 11, 800–813. [Google Scholar] [CrossRef]
- Garcia-Prieto, J.; Garcia-Ruiz, J.M.; Sanz-Rosa, D.; Pun, A.; Garcia-Alvarez, A.; Davidson, S.M.; Fernandez-Friera, L.; Nuno-Ayala, M.; Fernandez-Jimenez, R.; Bernal, J.A.; et al. beta3 adrenergic receptor selective stimulation during ischemia/reperfusion improves cardiac function in translational models through inhibition of mPTP opening in cardiomyocytes. Basic Res. Cardiol. 2014, 109, 422. [Google Scholar] [CrossRef]
- García-Prieto, J.; Villena-Gutiérrez, R.; Gómez, M.; Bernardo, E.; Pun-García, A.; García-Lunar, I.; Crainiciuc, G.; Fernández-Jiménez, R.; Sreeramkumar, V.; Bourio-Martínez, R.; et al. Neutrophil stunning by metoprolol reduces infarct size. Nat. Commun. 2017, 8, 14780. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, R.; Galan-Arriola, C.; Sanchez-Gonzalez, J.; Aguero, J.; Lopez-Martin, G.J.; Gomez-Talavera, S.; Garcia-Prieto, J.; Benn, A.; Molina-Iracheta, A.; Barreiro-Perez, M.; et al. Effect of Ischemia Duration and Protective Interventions on the Temporal Dynamics of Tissue Composition after Myocardial Infarction. Circ. Res. 2017, 121, 439–450. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, R.; Garcia-Prieto, J.; Sanchez-Gonzalez, J.; Aguero, J.; Lopez-Martin, G.J.; Galan-Arriola, C.; Molina-Iracheta, A.; Doohan, R.; Fuster, V.; Ibanez, B. Pathophysiology Underlying the Bimodal Edema Phenomenon After Myocardial Ischemia/Reperfusion. J. Am. Coll. Cardiol. 2015, 66, 816–828. [Google Scholar] [CrossRef]
- Clemente-Moragon, A.; Gomez, M.; Villena-Gutierrez, R.; Lalama, D.V.; Garcia-Prieto, J.; Martinez, F.; Sanchez-Cabo, F.; Fuster, V.; Oliver, E.; Ibanez, B. Metoprolol exerts a non-class effect against ischaemia-reperfusion injury by abrogating exacerbated inflammation. Eur. Heart J. 2020, 41, 4425–4440. [Google Scholar] [CrossRef]
- Martinez-Bartolome, S.; Navarro, P.; Martin-Maroto, F.; Lopez-Ferrer, D.; Ramos-Fernandez, A.; Villar, M.; Garcia-Ruiz, J.P.; Vazquez, J. Properties of average score distributions of SEQUEST: The probability ratio method. Mol. Cell Proteom. 2008, 7, 1135–1145. [Google Scholar] [CrossRef]
- Bonzon-Kulichenko, E.; Garcia-Marques, F.; Trevisan-Herraz, M.; Vazquez, J. Revisiting peptide identification by high-accuracy mass spectrometry: Problems associated with the use of narrow mass precursor windows. J. Proteome Res. 2015, 14, 700–710. [Google Scholar] [CrossRef]
- Navarro, P.; Vazquez, J. A refined method to calculate false discovery rates for peptide identification using decoy databases. J. Proteome Res. 2009, 8, 1792–1796. [Google Scholar] [CrossRef]
- Hulstaert, N.; Shofstahl, J.; Sachsenberg, T.; Walzer, M.; Barsnes, H.; Martens, L.; Perez-Riverol, Y. ThermoRawFileParser: Modular, Scalable, and Cross-Platform RAW File Conversion. J. Proteome Res. 2020, 19, 537–542. [Google Scholar] [CrossRef]
- Creasy, D.M.; Cottrell, J.S. Unimod: Protein modifications for mass spectrometry. Proteomics 2004, 4, 1534–1536. [Google Scholar] [CrossRef]
- Trevisan-Herraz, M.; Bagwan, N.; García-Marqués, F.; Rodriguez, J.M.; Jorge, I.; Ezkurdia, I.; Bonzon-Kulichenko, E.; Vázquez, J. SanXoT: A modular and versatile package for the quantitative analysis of high-throughput proteomics experiments. Bioinformatics 2019, 35, 1594–1596. [Google Scholar] [CrossRef]
- Navarro, P.; Trevisan-Herraz, M.; Bonzon-Kulichenko, E.; Nunez, E.; Martinez-Acedo, P.; Perez-Hernandez, D.; Jorge, I.; Mesa, R.; Calvo, E.; Carrascal, M.; et al. General statistical framework for quantitative proteomics by stable isotope labeling. J. Proteome Res. 2014, 13, 1234–1247. [Google Scholar] [CrossRef]
- da Huang, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, S. A Bayesian extension of the hypergeometric test for functional enrichment analysis. Biometrics 2014, 70, 84–94. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Mundt, A.K.A.F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.5. 2017. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 29 December 2023).
- Simko, T.W.A.V. R Package “Corrplot”: Visualization of a Correlation Matrix, version 0.84. 2017. Available online: https://github.com/taiyun/corrplot (accessed on 29 December 2023).
- Ruiz-Meana, M.; Nunez, E.; Miro-Casas, E.; Martinez-Acedo, P.; Barba, I.; Rodriguez-Sinovas, A.; Inserte, J.; Fernandez-Sanz, C.; Hernando, V.; Vazquez, J.; et al. Ischemic preconditioning protects cardiomyocyte mitochondria through mechanisms independent of cytosol. J. Mol. Cell Cardiol. 2014, 68, 79–88. [Google Scholar] [CrossRef]
- Rizo-Tellez, S.A.; Sekheri, M.; Filep, J.G. Myeloperoxidase: Regulation of Neutrophil Function and Target for Therapy. Antioxidants 2022, 11, 2302. [Google Scholar] [CrossRef]
- Talman, V.; Teppo, J.; Poho, P.; Movahedi, P.; Vaikkinen, A.; Karhu, S.T.; Trost, K.; Suvitaival, T.; Heikkonen, J.; Pahikkala, T.; et al. Molecular Atlas of Postnatal Mouse Heart Development. J. Am. Heart Assoc. 2018, 7, e010378. [Google Scholar] [CrossRef]
- Horckmans, M.; Ring, L.; Duchene, J.; Santovito, D.; Schloss, M.J.; Drechsler, M.; Weber, C.; Soehnlein, O.; Steffens, S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 2017, 38, 187–197. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Cho, N.; Satkunendrarajah, K.; Austin, J.W.; Wang, J.; Fehlings, M.G. Immunoglobulin G (IgG) attenuates neuroinflammation and improves neurobehavioral recovery after cervical spinal cord injury. J. Neuroinflamm. 2012, 9, 224. [Google Scholar] [CrossRef]
- Martin, T.P.; MacDonald, E.A.; Elbassioni, A.A.M.; O’Toole, D.; Zaeri, A.A.I.; Nicklin, S.A.; Gray, G.A.; Loughrey, C.M. Preclinical models of myocardial infarction: From mechanism to translation. Br. J. Pharmacol. 2022, 179, 770–791. [Google Scholar] [CrossRef] [PubMed]
- Kevin, L.G.; Camara, A.K.; Riess, M.L.; Novalija, E.; Stowe, D.F. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H566–H574. [Google Scholar] [CrossRef]
- Napankangas, J.P.; Liimatta, E.V.; Joensuu, P.; Bergmann, U.; Ylitalo, K.; Hassinen, I.E. Superoxide production during ischemia-reperfusion in the perfused rat heart: A comparison of two methods of measurement. J. Mol. Cell. Cardiol. 2012, 53, 906–915. [Google Scholar] [CrossRef]
- Tatarkova, Z.; Aplan, P.; Matejovicova, M.; Lehotsky, J.; Dobrota, D.; Flameng, W. Effect of ischemia and reperfusion on protein oxidation in isolated rabbit hearts. Physiol. Res. 2005, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Bovo, E.; Mazurek, S.R.; Zima, A.V. Oxidation of ryanodine receptor following ischemia/reperfusion increases propensity of Ca2+ waves during β-adrenergic receptor stimulation. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H1032–H1040. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Methner, C.; Nadtochiy, S.M.; Logan, A.; Pell, V.R.; Ding, S.; James, A.M.; Cochemé, H.M.; Reinhold, J.; Lilley, K.S.; et al. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat. Med. 2013, 19, 753–759. [Google Scholar] [CrossRef]
- Wu, B.; Meng, K.; Ji, Q.; Cheng, M.; Yu, K.; Zhao, X.; Tony, H.; Liu, Y.; Zhou, Y.; Chang, C.; et al. Interleukin-37 ameliorates myocardial ischaemia/reperfusion injury in mice. Clin. Exp. Immunol. 2014, 176, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Kuroda, J.; Ago, T.; Zhai, P.; Ikeda, Y.; Oka, S.; Fong, G.H.; Tian, R.; Sadoshima, J. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1alpha and upregulation of peroxisome proliferator-activated receptor-alpha. Circ. Res. 2013, 112, 1135–1149. [Google Scholar] [CrossRef]
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407. [Google Scholar] [CrossRef]
- Zweier, J.L. Measurement of superoxide-derived free radicals in the reperfused heart. Evidence for a free radical mechanism of reperfusion injury. J. Biol. Chem. 1988, 263, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.B.; Davies, M.J.; Hearse, D.J.; Slater, T.F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ. Res. 1987, 61, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Chen, C.L.; Pfeiffer, D.R.; Zweier, J.L. Mitochondrial complex II in the post-ischemic heart: Oxidative injury and the role of protein S-glutathionylation. J. Biol. Chem. 2007, 282, 32640–32654. [Google Scholar] [CrossRef]
- Kang, P.T.; Chen, C.L.; Lin, P.; Zhang, L.; Zweier, J.L.; Chen, Y.R. Mitochondrial complex I in the post-ischemic heart: Reperfusion-mediated oxidative injury and protein cysteine sulfonation. J. Mol. Cell Cardiol. 2018, 121, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Kohr, M.J.; Aponte, A.; Sun, J.; Gucek, M.; Steenbergen, C.; Murphy, E. Measurement of S-nitrosylation occupancy in the myocardium with cysteine-reactive tandem mass tags: Short communication. Circ. Res. 2012, 111, 1308–1312. [Google Scholar] [CrossRef]
- Kohr, M.J.; Sun, J.; Aponte, A.; Wang, G.; Gucek, M.; Murphy, E.; Steenbergen, C. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ. Res. 2011, 108, 418–426. [Google Scholar] [CrossRef]
- Kumar, V.; Kleffmann, T.; Hampton, M.B.; Cannell, M.B.; Winterbourn, C.C. Redox proteomics of thiol proteins in mouse heart during ischemia/reperfusion using ICAT reagents and mass spectrometry. Free Radic. Biol. Med. 2013, 58, 109–117. [Google Scholar] [CrossRef]
- Rookyard, A.W.; Paulech, J.; Thyssen, S.; Liddy, K.A.; Puckeridge, M.; Li, D.K.; White, M.Y.; Cordwell, S.J. A Global Profile of Reversible and Irreversible Cysteine Redox Post-Translational Modifications During Myocardial Ischemia/Reperfusion Injury and Antioxidant Intervention. Antioxid. Redox Signal 2021, 34, 11–31. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, Y.J.; Gu, S.S.; Tan, J.L.; Paul, C.; Wang, Y.G.; Yang, H.T. Degradation of cardiac myosin light chain kinase by matrix metalloproteinase-2 contributes to myocardial contractile dysfunction during ischemia/reperfusion. J. Mol. Cell Cardiol. 2014, 77, 102–112. [Google Scholar] [CrossRef]
- Jones, S.P.; Zachara, N.E.; Ngoh, G.A.; Hill, B.G.; Teshima, Y.; Bhatnagar, A.; Hart, G.W.; Marban, E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation 2008, 117, 1172–1182. [Google Scholar] [CrossRef]
- Labugger, R.; Organ, L.; Collier, C.; Atar, D.; Van Eyk, J.E. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation 2000, 102, 1221–1226. [Google Scholar] [CrossRef]
- Ma, J.; Liu, T.; Wei, A.C.; Banerjee, P.; O’Rourke, B.; Hart, G.W. O-GlcNAcomic Profiling Identifies Widespread O-Linked beta-N-Acetylglucosamine Modification (O-GlcNAcylation) in Oxidative Phosphorylation System Regulating Cardiac Mitochondrial Function. J. Biol. Chem. 2015, 290, 29141–29153. [Google Scholar] [CrossRef]
- Parker, B.L.; Palmisano, G.; Edwards, A.V.; White, M.Y.; Engholm-Keller, K.; Lee, A.; Scott, N.E.; Kolarich, D.; Hambly, B.D.; Packer, N.H.; et al. Quantitative N-linked glycoproteomics of myocardial ischemia and reperfusion injury reveals early remodeling in the extracellular environment. Mol. Cell Proteom. 2011, 10, M110.006833. [Google Scholar] [CrossRef]
- Parker, B.L.; Shepherd, N.E.; Trefely, S.; Hoffman, N.J.; White, M.Y.; Engholm-Keller, K.; Hambly, B.D.; Larsen, M.R.; James, D.E.; Cordwell, S.J. Structural basis for phosphorylation and lysine acetylation cross-talk in a kinase motif associated with myocardial ischemia and cardioprotection. J. Biol. Chem. 2014, 289, 25890–25906. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Correa, G.A.; Jin, W.; Wang, Z.; Zhong, X.; Gao, W.D.; Dias, W.B.; Vecoli, C.; Hart, G.W.; Murphy, A.M. O-linked GlcNAc modification of cardiac myofilament proteins: A novel regulator of myocardial contractile function. Circ. Res. 2008, 103, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, C.; Zong, N.C.; Liem, D.A.; Cadeiras, M.; Scruggs, S.B.; Yu, H.; Kim, A.K.; Yang, P.; Deng, M.; et al. Regulation of acetylation restores proteolytic function of diseased myocardium in mouse and human. Mol. Cell Proteom. 2013, 12, 3793–3802. [Google Scholar] [CrossRef]
- White, M.Y.; Hambly, B.D.; Jeremy, R.W.; Cordwell, S.J. Ischemia-specific phosphorylation and myofilament translocation of heat shock protein 27 precedes alpha B-crystallin and occurs independently of reactive oxygen species in rabbit myocardium. J. Mol. Cell Cardiol. 2006, 40, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.A.S.; Emter, C.A. Large Animal Models of Heart Failure: A Translational Bridge to Clinical Success. JACC Basic. Transl. Sci. 2020, 5, 840–856. [Google Scholar] [CrossRef]
- Lunney, J.K. Advances in swine biomedical model genomics. Int. J. Biol. Sci. 2007, 3, 179–184. [Google Scholar] [CrossRef]
- Cook, N.L.; Pattison, D.I.; Davies, M.J. Myeloperoxidase-derived oxidants rapidly oxidize and disrupt zinc-cysteine/histidine clusters in proteins. Free Radic. Biol. Med. 2012, 53, 2072–2080. [Google Scholar] [CrossRef]
- Friedrichs, K.; Baldus, S.; Klinke, A. Fibrosis in Atrial Fibrillation—Role of Reactive Species and MPO. Front. Physiol. 2012, 3, 214. [Google Scholar] [CrossRef] [PubMed]
- Garai, D.; Rios-Gonzalez, B.B.; Furtmuller, P.G.; Fukuto, J.M.; Xian, M.; Lopez-Garriga, J.; Obinger, C.; Nagy, P. Mechanisms of myeloperoxidase catalyzed oxidation of H2S by H2O2 or O2 to produce potent protein Cys-polysulfide-inducing species. Free Radic. Biol. Med. 2017, 113, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Ehrenshaft, M.; Deterding, L.J.; Mason, R.P. Tripping up Trp: Modification of protein tryptophan residues by reactive oxygen species, modes of detection, and biological consequences. Free Radic. Biol. Med. 2015, 89, 220–228. [Google Scholar] [CrossRef]
- Jackowski, C.; Christe, A.; Sonnenschein, M.; Aghayev, E.; Thali, M.J. Postmortem unenhanced magnetic resonance imaging of myocardial infarction in correlation to histological infarction age characterization. Eur. Heart J. 2006, 27, 2459–2467. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binek, A.; Castans, C.; Jorge, I.; Bagwan, N.; Rodríguez, J.M.; Fernández-Jiménez, R.; Galán-Arriola, C.; Oliver, E.; Gómez, M.; Clemente-Moragón, A.; et al. Oxidative Post-translational Protein Modifications upon Ischemia/Reperfusion Injury. Antioxidants 2024, 13, 106. https://doi.org/10.3390/antiox13010106
Binek A, Castans C, Jorge I, Bagwan N, Rodríguez JM, Fernández-Jiménez R, Galán-Arriola C, Oliver E, Gómez M, Clemente-Moragón A, et al. Oxidative Post-translational Protein Modifications upon Ischemia/Reperfusion Injury. Antioxidants. 2024; 13(1):106. https://doi.org/10.3390/antiox13010106
Chicago/Turabian StyleBinek, Aleksandra, Celia Castans, Inmaculada Jorge, Navratan Bagwan, José Manuel Rodríguez, Rodrigo Fernández-Jiménez, Carlos Galán-Arriola, Eduardo Oliver, Mónica Gómez, Agustín Clemente-Moragón, and et al. 2024. "Oxidative Post-translational Protein Modifications upon Ischemia/Reperfusion Injury" Antioxidants 13, no. 1: 106. https://doi.org/10.3390/antiox13010106
APA StyleBinek, A., Castans, C., Jorge, I., Bagwan, N., Rodríguez, J. M., Fernández-Jiménez, R., Galán-Arriola, C., Oliver, E., Gómez, M., Clemente-Moragón, A., Ibanez, B., Camafeita, E., & Vázquez, J. (2024). Oxidative Post-translational Protein Modifications upon Ischemia/Reperfusion Injury. Antioxidants, 13(1), 106. https://doi.org/10.3390/antiox13010106






