Abstract
Hydrogen sulfide (H2S), traditionally recognized as a toxic gas, has emerged as a critical regulator in many biological processes, including oxidative stress and cellular homeostasis. This review presents an exhaustive overview of the current understanding of H2S and its multifaceted role in mammalian cellular functioning and oxidative stress management. We delve into the biological sources and function of H2S, mechanisms underlying oxidative stress and cellular homeostasis, and the intricate relationships between these processes. We explore evidence from recent experimental and clinical studies, unraveling the intricate biochemical and molecular mechanisms dictating H2S’s roles in modulating oxidative stress responses and maintaining cellular homeostasis. The clinical implications and therapeutic potential of H2S in conditions characterized by oxidative stress dysregulation and disrupted homeostasis are discussed, highlighting the emerging significance of H2S in health and disease. Finally, this review underscores current challenges, controversies, and future directions in the field, emphasizing the need for further research to harness H2S’s potential as a therapeutic agent for diseases associated with oxidative stress and homeostatic imbalance. Through this review, we aim to emphasize H2S’s pivotal role in cellular function, encouraging further exploration into this burgeoning area of research.
1. Introduction
A captivating theme in modern biology is the ability of simple molecules to orchestrate complex physiological functions [1]. Hydrogen sulfide (H2S) [2], historically notorious merely as a hazardous, colorless, flammable gas with a characteristic rotten egg odor, is now understood to be a biological signaling molecule [3]. This shift in perception is due to the recognition of H2S as a significant player in diverse physiological and pathological processes [4]. This small molecule, considered as the third gasotransmitter alongside nitric oxide (NO) and carbon monoxide (CO), exerts a plethora of effects in mammalian physiology, such as vasodilation [5], vascular tone [6], modulating the inflammatory response [7,8], neurotransmission [9], antioxidant properties [10], apoptosis [11] cellular survival [12], regulating cellular metabolism [13], or acting as a cytoprotectant [14]. As a multifaceted molecule [15,16] with profound biological implications, H2S has increasingly been at the center of scientific scrutiny. This review aims to shed light on the emerging role of H2S in regulating oxidative stress and maintaining cellular homeostasis, which is crucial for maintaining proper cellular functioning and ensuring the organism’s survival [17].
It is essential to acknowledge that the intricate biological effects attributed to H2S are not directly induced by the gas itself. Rather, H2S triggers a complex array of oxidative modifications, prominently including persulfidation, disulfide formation, and polysulfide generation. These oxidative transformations operate as fundamental modulators of H2S’s versatile biological functions. The induction of such oxidative modifications is a prerequisite for H2S to proficiently engage in the regulation of cellular processes. Through these chemical transformations, H2S engages in selective interactions with specific biomolecules, including proteins, redox-sensitive molecules, and signaling entities. This interplay initiates an expansive spectrum of physiological responses. Consequently, the intricate and multifaceted biological ramifications of H2S emanate from its nuanced interplay with cellular components, facilitated by these oxidative modifications [18].
H2S is synthesized endogenously in mammalian tissues by the enzymatic degradation of sulfur-containing amino acids, primarily cysteine. Three primary enzymes are involved in this process, each differing in distribution, regulation, and function. These are cystathionine beta-synthase (CBS), cystathionine gamma-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST). These enzymes are strategically distributed in various mammalian tissues, emphasizing the widespread influence of H2S [19,20,21,22].
Cystathionine β-Synthase (CBS), a pyridoxal 5′-phosphate (PLP)-dependent enzyme, is primarily localized within the brain and central nervous system [23]. This enzyme takes center stage in the transsulfuration pathway, an essential biochemical pathway in the human body involving the interconversion of sulfur-containing amino acids, methionine, and cysteine [24]. CBS initiates the transsulfuration pathway by catalyzing its first step [25,26,27,28].
Cystathionine γ-Lyase (CSE), another PLP-dependent enzyme predominantly expressed in peripheral tissues, particularly in the liver, kidney, vascular smooth muscle, and endothelial cells, is a pyridoxal phosphate-dependent enzyme that catalyzes the final step in the transsulfuration pathway in the endogen production of H2S [29,30,31,32].
The 3-Mercaptopyruvate Sulfurtransferase (3-MST), in conjunction with cysteine aminotransferase (CAT), 3-MST, also produces H2S. The CAT/3-MST pathway is a significant source of H2S in the brain and vascular endothelial cells [33].
Many published articles cover the aspects related to H2S biosynthesis, enzymes involved, places of synthesis, and their roles in various body organs. Summarizing cutting-edge research from the past year, a novel pathway in mammals involves peroxisome-dependent DAO converting D-cysteine into 3MP, leading to H2S production in mitochondria. Additionally, H2S can be stored as bound sulfane sulfur in specific cysteine residues of target proteins, suggesting a role as a signaling molecule in the nervous system [34].
The biological functions of H2S are versatile and multidirectional, playing pivotal roles in vasodilation, neurotransmission, inflammation modulation, oxidative balance, cellular apoptosis, angiogenesis [35], glucose regulation, energy metabolism [36], and cellular survival and longevity [37]. But, all these biological functions are also part of cellular homeostasis [38,39].
H2S exhibits diverse physiological roles, acting as a potent vasodilator by activating ATP-sensitive potassium channels, leading to smooth muscle relaxation and consequent decreased blood pressure. Moreover, it promotes angiogenesis through upregulating VEGF and VEGFR-2, influencing wound healing, inflammation, and tumor growth [35,40].
In the central nervous system, H2S functions as a neuromodulator, modulating NMDA receptor responses and facilitating memory formation through hippocampal long-term potentiation. It also exerts anti-inflammatory effects by inhibiting NF-κB activation. Additionally, H2S displays cytoprotective effects by scavenging ROS and enhancing antioxidant enzyme activities, mitigating oxidative stress and cellular damage [10,41].
H2S is also involved in the regulation of glucose regulation and energy metabolism [13]. It enhances glucose uptake in peripheral tissues, contributing to overall glucose homeostasis. Furthermore, H2S plays a crucial role in mitochondrial function, influencing ATP synthesis and regulating metabolic energy production [42]. It modulates the activity of critical enzymes in glycolysis [43], gluconeogenesis, and the citric acid cycle, underscoring its role in maintaining metabolic homeostasis [44,45,46].
Oxidative stress denotes a physiological state characterized by an imbalance between the production of reactive oxygen species (ROS) and the system’s ability to neutralize these highly reactive molecules or repair the damage caused by them [47]. ROS are generated as natural by-products of oxygen metabolism and play a critical role in cell signaling and homeostasis [48]. However, when faced with environmental stress, ROS levels can drastically rise, resulting in significant damage to cell structures—a phenomenon known as oxidative stress [49]. Oxidative stress manifests as a cascade of destructive events affecting cellular proteins, lipids, and nucleic acids, impairing cellular functions and leading to various pathological conditions, including but not limited to cardiovascular disorders, neurodegenerative diseases, cancer, and diabetes [50].
The concentration-dependent dichotomy of H2S in cellular survival and apoptosis is concentration-dependent. Physiological levels of H2S confer cytoprotection, inhibiting cellular apoptosis or enhancing cellular resistance to oxidative stress, contributing to cellular longevity [51]. Conversely, H2S may induce apoptosis at higher concentrations, particularly in cancer cells, by impacting mitochondrial function [52]. H2S can cause the production of glutathione, a powerful antioxidant, thereby bolstering the cellular antioxidant defense system [53]. Furthermore, it can activate cellular stress response mechanisms, including the Nrf2 pathway, which plays a critical role in the protective mechanism against the harmful effects of oxidative stress [54,55,56].
Cellular homeostasis, on the other hand, encompasses the vast array of processes and mechanisms that cells employ to maintain the stability of their internal environment, even when faced with external changes [57]. It precisely regulates critical factors, such as pH, ion concentrations, temperature, nutrient supply, waste removal, and redox balance [58]. Disrupting any of these parameters can destabilize cellular homeostasis, contributing to cellular dysfunction, the initiation of pathological processes, and disease development [59,60].
With oxidative stress and cellular homeostasis playing central roles in cellular function and disease pathology, the intersection of these processes with H2S bioavailability and metabolism presents a fascinating study area [61]. This review comprehensively provides an overview of the complex relationship between H2S, oxidative stress, and cellular homeostasis. We aim to delve into the current understanding of the underlying biochemical and molecular mechanisms that dictate the role of H2S in these processes, drawing upon the latest research from in vitro, in vivo, and clinical studies (Figure 1).
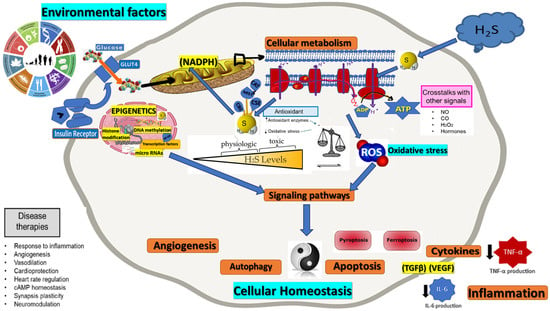
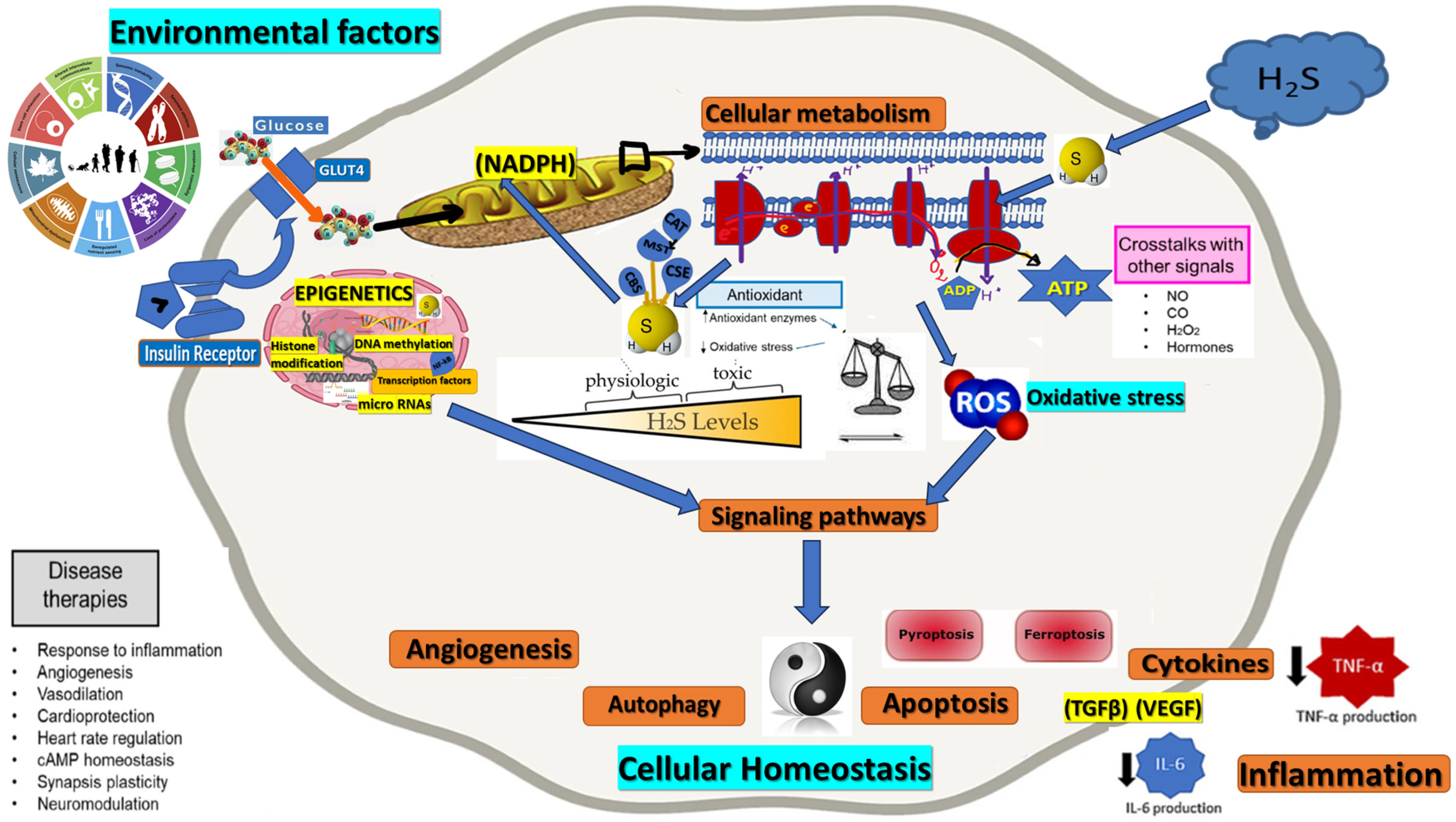
Figure 1.
Role of H2S in oxidative stress and cellular homeostasis intersection. Consequences regarding various physiological functions, including angiogenesis, autophagy, apoptosis, inflammation—cytokines level reduction (black arrows).
While previous reviews have discussed H2S’s involvement in vasodilation, neurotransmission, inflammation modulation, oxidative balance, apoptosis, angiogenesis, glucose regulation, energy metabolism, cellular survival, and longevity [35,36,37], our review uniquely ties these biological functions to the overarching concept of cellular homeostasis [38,39]. We explore further how H2S orchestrates multifaceted responses to maintain equilibrium within the cell, offering readers a comprehensive view of its role in various physiological processes.
In essence, the biological roles of H2S are integral to maintaining the complex orchestration of various physiological processes [62]. Any imbalance in its endogenous production or metabolism can lead to pathological conditions. This highlights the importance of ongoing research into the intricate biochemistry of H2S and its physiological and pathological roles in health and disease. As a regulator of oxidative stress and cellular homeostasis, H2S holds immense therapeutic potential for various conditions [41].
2. Methods
Recognizing the vast volume of the literature on H2S, we have conducted a systematic review, meticulously selecting and analyzing a subset of the most relevant and impactful articles. This allows us to distill key findings, present critical analyses, and offer insightful perspectives to the readers.
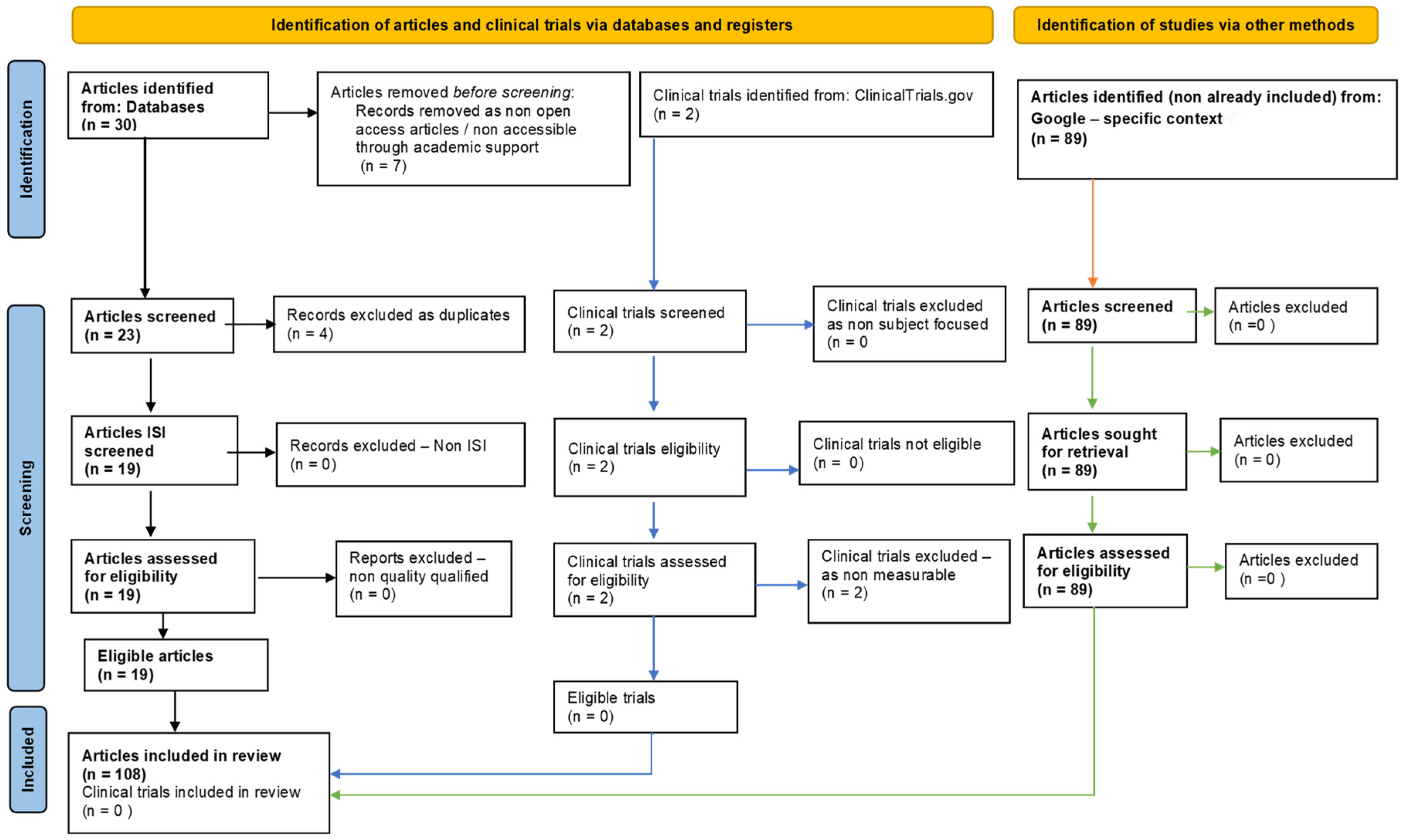
This one-year systematic review (23 July 2022–23 July 2023) followed PRISMA guidelines and is registered on PROSPERO, ID: 453191. To ensure comprehensive coverage of the relevant literature, we searched multiple databases, including PubMed, Scopus/Elsevier, Web of Science, and Google Search. The search strategy involved using specific keywords and medical subject headings (MeSH) related to hydrogen sulfide, oxidative stress; cellular homeostasis; and biochemical mechanisms. After removing non-eligible and duplicate references, our review included 108 relevant studies (Figure 2).
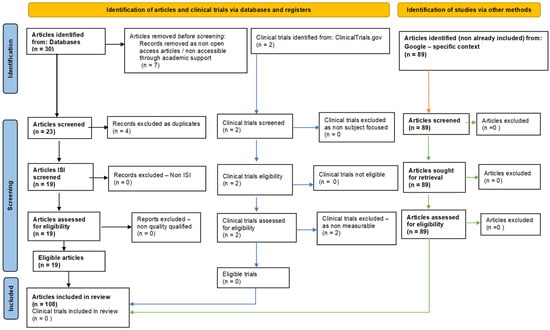
Figure 2.
Adapted PRISMA flow diagram, customized for our study.
The criteria for selecting articles were determined based on their alignment with our review’s focus, i.e., the impact of H2S on oxidative stress modulation and cellular homeostasis. Inclusion criteria encompassed studies that directly investigated H2S’s roles within these contexts, involving both in vitro and in vivo experimental approaches. We also considered clinical studies with implications for translational applications. Exclusion criteria were applied to studies that were not directly relevant to our focus, including those that solely discussed the general biochemistry of H2S without delving into its roles in oxidative stress or cellular homeostasis.
To minimize bias, we employed a multistep screening process, involving initial title and abstract screening, followed by full-text assessment, performed independently by all the three authors of this article. Any discrepancies were resolved through consensus discussion. Additionally, to address potential bias, we assessed the diversity of study designs, methodologies, and outcome measures across the selected articles. Our aim was to ensure a balanced representation of different perspectives within the literature and to avoid undue emphasis on a particular subset of studies.
The initial search, performed by the first author in the International Databases, identified a total of 30 articles. After eliminating duplicates, the remaining 19 full-text articles of the selected records were subsequently evaluated to determine their eligibility based on predefined inclusion and exclusion criteria. The 19 articles were analyzed to assess their relevance to our research question by all the authors, and the analysis is included in Tables 1 and 2, based on their link to oxidative stress (Table 2) or to homeostasis (Table 2).
Additionally, to support the ideas expressed in this review, bibliographical references were identified by other methods—Google contextual search, restricted to last-year time.
The selection of predominantly review articles in this systematic review was influenced by the specific focus on summarizing and analyzing recent advancements in the understanding of hydrogen sulfide’s role in oxidative stress and cellular homeostasis.
During the search process, we did encounter a limited number of original research papers directly addressing the role of hydrogen sulfide in oxidative stress and cellular homeostasis. However, these original research papers often focused on specific aspects of the topic, such as molecular mechanisms or specific pathways, rather than providing a comprehensive overview of the entire field. Additionally, review articles often incorporated insights from multiple original research studies, enhancing the synthesis of information. While original research papers provided invaluable insights, the review articles offered a consolidated perspective by summarizing and critically evaluating findings from various studies, including both original research and experimental work.
It is worth noting that our focus on recent advancements within the past year further contributed to the prominence of review articles, as they are more likely to provide up-to-date summaries of the latest research findings. While we acknowledge the significance of original research papers, this systematic review aims to offer a comprehensive understanding of the current landscape through the lens of very recent data.
3. Results
3.1. Hydrogen Sulfide and Oxidative Stress—Last-Year Data
The intricate relationship between hydrogen sulfide (H2S) and oxidative stress is an area of extensive interest in molecular biology, biochemistry, and medicine [63]. Oxidative stress, characterized by an imbalance between the generation of reactive oxygen species (ROS) and the ability of the biological system to detoxify these reactive intermediates, has significant implications for cellular function, tissue integrity, and the pathogenesis of numerous diseases [64,65].
Emerging as the third endogenous gaseous signaling molecule or ‘gasotransmitter’ following nitric oxide (NO) and carbon monoxide (CO), H2S has attracted substantial attention due to its potential role in mitigating oxidative stress. Uniquely, H2S displays both direct and indirect antioxidant capacities, a bifunctional characteristic that underscores its critical position within the antioxidant defense machinery [66,67,68].
H2S’s direct antioxidant capacity is attributable to its chemical properties, which allow it to neutralize ROS. For example, H2S can react with superoxide anions (O2−) to produce a hydrosulfide anion (HS−) and hydrogen peroxide (H2O2). This reaction reduces the concentration of O2−, a potent ROS [69,70]. Furthermore, H2S can also directly neutralize H2O2, forming water (H2O) and elemental sulfur, disarming the potential harm these reactive species pose [71].
Beyond its role as a direct ROS scavenger, H2S also displays indirect antioxidant properties, bolstering the body’s endogenous antioxidant defense systems, achieved by upregulating key antioxidant enzymes, including superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) [72,73]. These enzymes, pivotal for maintaining redox homeostasis, can neutralize ROS, thus limiting oxidative damage [74]. Through signaling mechanisms not yet fully elucidated, H2S has been demonstrated to induce the expression of these enzymes, thereby enhancing the cellular capacity to counteract ROS and reduce oxidative stress [75].
H2S’s protective influence extends to the cellular mitochondria, critical organelles for energy production and cellular metabolism [76]. Under physiological conditions, H2S can support mitochondrial function and cellular energy metabolism by participating in electron transport and ATP synthesis [77]. Conversely, under conditions of oxidative stress, H2S adopts a cytoprotective role. It attenuates mitochondrial ROS production, promotes mitochondrial biogenesis, and blocks the opening of mitochondrial permeability transition pores (mPTP). Under oxidative insults, these actions significantly preserve mitochondrial integrity, function, and cell survival [78,79].
The interplay between H2S and other gasotransmitters, such as NO, also impacts oxidative stress. H2S can react with NO to form a new compound, nitroxyl (HNO). Interestingly, HNO exhibits potent antioxidant activity and may contribute to cellular antioxidant defense. This suggests that the interaction between H2S and NO may represent an additional mechanism through which H2S exerts its antioxidant effects [80].
Despite these seemingly beneficial effects, the impact of H2S on oxidative stress is not unequivocally protective. Indeed, H2S’s outcomes are highly concentration-dependent [81,82]. Physiological concentrations of H2S are generally associated with cytoprotection against oxidative stress [83]. However, elevated H2S levels can contribute to oxidative stress, especially under pathophysiological conditions [10]. This paradoxical effect is primarily due to the ability of high concentrations of H2S to inhibit cytochrome c oxidase, a specific enzyme in the mitochondrial electron transport chain. Inhibition of this enzyme impedes electron transport, which can culminate in elevated ROS production and exacerbated oxidative stress [84].
This dichotomous role of H2S in oxidative stress is reminiscent of the ‘Janus-faced’ characteristic many biological molecules exhibit [85]. The context-dependent nature of H2S’s biological actions adds complexity to our understanding of this gasotransmitter’s role in health and disease. A comprehensive exploration of the nuances in H2S signaling and identifying the precise tipping point where H2S transitions from a cytoprotectant to a cytotoxic agent is essential to fully realize the therapeutic potential of H2S in oxidative stress-associated conditions. Advancing research technologies unravel the mysteries surrounding H2S and its interplay with oxidative stress (Table 1).

Table 1.
Synthetic Data on Hydrogen Sulfide and Oxidative Stress.
Table 1.
Synthetic Data on Hydrogen Sulfide and Oxidative Stress.
| Ref. | Title | Synthetic Data on Hydrogen Sulfide and Oxidative Stress | Outcomes |
|---|---|---|---|
| [8] | Effect of Hydrogen Sulfide on Essential Functions of Polymorphonuclear Leukocytes | Distinct from information available until 2022, this article emphasizes the dual nature of H2S in inflammation, acting both as a pro-inflammatory and anti-inflammatory molecule. It also elaborates on the complex interactions of H2S with various signaling pathways, its effects on different organ systems, and its potential therapeutic applications. The detailed exploration of H2S’s role in renal disease, including its interaction with uremic toxins and its impact on oxidative stress, offers a novel perspective that contributes to the understanding of H2S’s function in human health and disease. | The article elucidates the dual role of hydrogen sulfide (H2S) in inflammation, its complex interactions with signaling pathways, and its potential therapeutic applications, particularly in renal disease. |
| [10] | Advances of H2S in Regulating Neurodegenerative Diseases by Preserving Mitochondria Function | A comprehensive insight into the multifaceted role of H2S in neurodegenerative diseases, emphasizing its neuroprotective properties. It delves into the specific mechanisms through which H2S modulates mitochondrial activity, produces reactive sulfur species, and modifies proteins through sulfhydration. The article emphasizes the potential therapeutic applications of H2S in regulating neurodegenerative diseases through anti-oxidative, anti-inflammatory, anti-apoptotic, and S-sulfhydration. | The article reveals hydrogen sulfide’s dual role as a neuromodulator and neuroprotectant, offering new therapeutic avenues in neurodegenerative diseases. |
| [14] | SOD1 is an essential H2S detoxifying enzyme | Contrary to the prevailing knowledge until 2022, the paper uncovers the role of superoxide dismutase [Cu-Zn] (SOD1) as an efficient H2S oxidase, essential in limiting cytotoxicity from both endogenous and exogenous sulfide. It highlights SOD1’s ability to convert H2S to sulfate under limiting sulfide conditions rapidly and its role in forming per- and polysulfides, which induce cellular thiol oxidation. | The article reveals SOD1’s role as an H2S oxidase, essential in limiting H2S cytotoxicity, and regulating reactive sulfur species, enriching our understanding of H2S detoxification. |
| [43] | Generation and Physiology of Hydrogen Sulfide and Reactive Sulfur Species in Bacteria | The article presents a nuanced understanding of hydrogen sulfide’s (H2S) role in the oxidative stress response, highlighting its combinatorial redox action with hydrogen peroxide (H2O2) in mediating cytotoxicity and its contrasting protective effect against ROS-mediated killing. This complex interplay, including the formation of sulfheme iron complexes and the impact on catalases, adds to the existing knowledge by elucidating the multifaceted roles of H2S in oxidative stress and immunometabolism. | The article reveals hydrogen sulfide’s dual role in oxidative stress, mediating cytotoxicity and offering protection against ROS-mediated killing, enhancing understanding of immunometabolism. |
| [86] | Hydrogen Sulfide: A Gaseous Mediator and Its Key Role in Programmed Cell Death, Oxidative Stress, Inflammation, and Pulmonary Disease | The article advances the understanding of hydrogen sulfide (H2S) in oxidative stress, emphasizing its dual role as an antioxidant and a pro-oxidant. It highlights the complex mechanisms of H2S in quenching free radicals and its potential therapeutic targeting in pulmonary diseases, adding nuance to existing knowledge. | Insights into the tightly controlled metabolism of H2S in mammals, achieved through physiological enzymes catalyzed reactions. |
| [87] | Hydrogen sulfide: A new therapeutic target in vascular diseases | Emphasizes the intricate relationship between H2S and oxidative stress in regulating blood pressure. It details the mechanisms by which H2S acts as a vasorelaxant agent, its interaction with nitric oxide (NO), and the effects of various H2S donors in treating HBP. The potential of H2S as a therapeutic target for hypertension, including its role in inhibiting inflammation, suppressing vascular smooth muscle cell proliferation, and mitigating oxidative stress, thereby contributing to the understanding of H2S’s multifaceted role in cardiovascular health. | The article reveals hydrogen sulfide’s multifaceted role in regulating hypertension, emphasizing its potential as a therapeutic target in cardiovascular health. |
| [88] | Hydrogen Sulphide-Based Therapeutics for Neurological Conditions: Perspectives and Challenges | A comprehensive insight into the catabolism of H2S and its role in oxidative stress within the brain. Specifically, it highlights the interplay between enzymes, like sulfide quinone oxidoreductase (SQR) and its homolog SQRDL, along with neuroglobin, in the metabolism of H2S in the brain. Emphasizes the protective effect of H2S against oxidative stress by enhancing the synthesis of glutathione (GSH) and directly scavenging ROS. Explores the potential therapeutic applications of H2S donors in various neurological conditions, including Parkinson’s and Alzheimer’s diseases, offering a more refined understanding of H2S’s multifaceted role. | The article elucidates the complex role of hydrogen sulfide (H2S) in neuroprotection, highlighting its potential therapeutic applications in neurodegenerative disorders and emphasizing the need for further research to understand its multifaceted functions. |
| [89] | H2S regulation of ferroptosis attenuates sepsis-induced cardiomyopathy | The article presents new insights into the role of sodium hydrosulfide (NaHS) in alleviating sepsis-induced cardiomyopathy (SIC). Specifically, it demonstrates that NaHS mitigates oxidative stress and lipid peroxidation in cardiomyocytes, highlighting its potential as a therapeutic target for SIC. This adds to understanding NaHS’s anti-inflammatory, anti-oxidative stress, and anti-apoptotic properties and its regulation of pathways involved in sepsis multiorgan injury. | The study reveals that NaHS alleviates sepsis-induced cardiomyopathy by reducing oxidative stress and lipid peroxidation, suggesting therapeutic potential. |
| [90] | Sulfur content in foods and beverages and its role in human and animal metabolism: A scoping review of recent studies | The text highlights recent insights into sulfur dioxide’s physiological and toxicological roles (SO2) and its derivatives, emphasizing their complex effects on oxidative stress, gastrointestinal health, and food preservation. It contrasts previous understanding by detailing SO2’s potential preventive role in colitis, its impact on the gut microbiome, and its intricate interaction with various biological pathways. | The article reveals new insights into SO2’s roles in oxidative stress, colitis prevention, and gut microbiome interaction. |
| [91] | Dose-Dependent Effect of Hydrogen Sulfide in Cyclophosphamide-Induced Hepatotoxicity in Rats | This study introduces new insights into the role of hydrogen sulfide (H2S) in mitigating cyclophosphamide (CP)-induced hepatotoxicity. It emphasizes the protective effects of NaHS, an H2S donor, against liver damage caused by CP, a chemotherapeutic agent. The research highlights how H2S attenuates oxidative stress in the liver, including regulating critical enzymes and interactions with nitric oxide (NO). This adds to the existing knowledge by elucidating the potential therapeutic applications of H2S in preventing drug-induced liver injury. | The study reveals hydrogen sulfide’s potential in mitigating cyclophosphamide-induced hepatotoxicity, highlighting therapeutic applications in preventing drug-induced liver injury. |
3.2. Hydrogen Sulfide and Cellular Homeostasis—Last-Year Data
H2S has garnered considerable attention as an endogenous gaseous signaling molecule that is a critical regulator of cellular homeostasis. Cell homeostasis refers to maintaining a stable, constant cell condition governed by a complex network of regulatory mechanisms [92,93]. H2S, acting as a gasotransmitter, has been increasingly involved in numerous physiological processes integral to maintaining cellular homeostasis [10,94].
To begin with, the role of H2S in cellular bioenergetics is significant. It is widely known that the cell’s primary powerhouse, the mitochondria, governs energy production via oxidative phosphorylation, producing ATP, the cell’s energy currency. H2S has been shown to modulate mitochondrial function at several levels. One of the ways it does this is by influencing the function of cytochrome c oxidase, an element of the mitochondrial electron transport chain, thereby affecting ATP production. By acting on the mitochondria, H2S regulates reactive oxygen species (ROS) production, linking its role to oxidative stress and cellular redox homeostasis [11,95,96].
The role of H2S in cellular homeostasis extends to metabolic regulation. H2S has been connected with glucose homeostasis, contributing to insulin secretion and sensitivity. It modulates the activity of several enzymes involved in the citric acid cycle, glycolysis, and gluconeogenesis, thereby influencing overall energy metabolism. It can enhance glucose uptake in peripheral tissues and has been involved in lipid metabolism, linking its role to metabolic disorders [97].
H2S also plays a pivotal role in regulating cellular signal transduction. It can modulate the activity of a wide range of proteins through a process known as persulfidation, wherein a sulfur atom is added to the protein, affecting its function. This post-translational modification can impact various signaling pathways and has been linked to autophagy, inflammation, and cellular stress response [13,83].
The role of H2S in cellular proliferation and apoptosis further exemplifies its importance in cellular homeostasis. It can inhibit apoptosis and promote cell survival at physiological concentrations, while at higher concentrations, it can promote apoptosis. The dichotomy in the effects of H2S on cell survival and apoptosis is critical to processes, such as tissue repair and regeneration and the response to cellular stress. H2S achieves this through several mechanisms, including the modulation of mitochondrial function, the regulation of apoptotic proteins, such as Bcl-2 and Bax, and the modulation of caspase activity [63,94,98].
H2S has also emerged as a critical regulator in cellular homeostasis through epigenetic mechanisms. Through its role in modulating transcription factors, chromatin remodeling, and methylation patterns, H2S intricately controls cellular functions [99,100].
Given its involvement in these diverse cellular processes, it is unsurprising that dysregulation of H2S production or metabolism has been linked to various pathological conditions. A decrease in H2S levels has been reported in several diseases, including cardiovascular disease, neurodegenerative disorders, and kidney disease [101]. On the other hand, excessive production of H2S has been linked to conditions, such as Down syndrome [102] and cancer [103,104], underscoring the importance of a delicate balance in H2S homeostasis and also the role of H2S in general cellular homeostasis (Table 2).

Table 2.
Synthetic Data on Hydrogen Sulfide and Cellular Homeostasis.
Table 2.
Synthetic Data on Hydrogen Sulfide and Cellular Homeostasis.
| Ref. | Title | Synthetic Data on Hydrogen Sulfide and Cellular Homeostasis | Outcomes |
|---|---|---|---|
| [11] | Hydrogen Sulfide (H2S) Signaling as a Protective Mechanism against Endogenous and Exogenous Neurotoxicants | H2S mediates redox homeostasis, inflammatory response, mitochondrial functions, and synaptic transmission. New findings on H2S’s regulation of SIRT1 expression, its interaction with CREB, and its involvement in reducing homocysteine (Hcy)-induced endoplasmic reticulum stress (ERS), thus extending previous knowledge on its neuroprotective effects. | New insights detail hydrogen sulfide’s role in neuroprotection, redox homeostasis, SIRT1 regulation, and the reduction in Hcy-induced ERS. |
| [13] | From Gasotransmitter to Immunomodulator: The Emerging Role of Hydrogen Sulfide in Macrophage Biology | The paper emphasizes H2S as a potent inflammatory mediator, modulating macrophage activities, such as migration, phagocytosis, and cytokine production. Additionally, the article underscores H2S’s involvement in maintaining GSH levels, thus contributing to cellular redox homeostasis. | Recent findings emphasize H2S as an inflammatory mediator in macrophages, contributing to cellular homeostasis. |
| [63] | Recent Development of the Molecular and Cellular Mechanisms of Hydrogen Sulfide Gasotransmitter | The article highlights novel insights into H2S’s role in cellular homeostasis, emphasizing the interactions between H2S and other gasotransmitters, like NO and CO. It particularly illustrates how H2S can persulfidate specific enzymes, impacting various signaling pathways, and reveals new findings on H2S in the regulation of endocytosis, autophagy, and renal sodium homeostasis dysfunction. This information expands our understanding of H2S’s multifaceted roles in cellular processes. | H2S regulates endocytosis, autophagy, and renal sodium homeostasis dysfunction. |
| [83] | Protein persulfidation: Rewiring the hydrogen sulfide signaling in cell stress response | The article emphasizes the process of persulfidation as a biological switch in the cell stress response. The explanation of H2S’s biphasic model, where its levels operate within an optimal concentration range for health, represents an advancement in understanding its dynamic regulation of biological homeostasis, potentially opening novel therapeutic avenues. | Persulfidation’s significance in stress response and the revelation of H2S’s biphasic model |
| [94] | The Impact of Drugs on Hydrogen Sulfide Homeostasis in Mammals | This review highlights a novel aspect of H2S’s role in cellular homeostasis by emphasizing its interaction with commonly prescribed pharmacological drugs. Specifically, it catalogs the impact of these drugs on H2S production in mammalian cells and tissues, providing new insights into their influence on various physiological and pathological conditions. | The review reveals the impact of pharmacological drugs on hydrogen sulfide production in mammalian systems. |
| [96] | Mitochondria in endothelial cells angiogenesis and function: current understanding and future perspectives | The text highlights novel insights into H2S’s role in endothelial cell (EC) angiogenesis and mitochondrial function. Specifically, it elucidates the dual role of H2S in mitochondrial metabolism, showing its function enhancement at low concentrations and inhibition at high concentrations. | Explanation of H2S’s impact on specific enzymes and signaling pathways related to angiogenesis. |
| [97] | H2S- and Redox-State-Mediated PTP1B S-Sulfhydration in Insulin Signaling | This article introduces new insights into the role of H2S in cellular homeostasis, specifically in the context of insulin signaling. Furthermore, the report reveals the differential effects of insulin and metformin on H2S and GSH levels in different cell lines, thereby enriching our understanding of the role of H2S in insulin signaling and redox regulation. | It emphasizes the significance of PTP1B S-sulfhydration mediated by H2S and the redox state in insulin response and regulation. |
| [98] | Hydrogen sulfide plays an important role by regulating endoplasmic reticulum stress in myocardial diseases | This article introduces the emerging understanding of H2S’s role in regulating endoplasmic reticulum (ER) stress in myocardial diseases, a subject not widely explored until recently. The text emphasizes the novel findings on H2S’s involvement in physiological and pathological processes related to ER stress. | H2S’s involvement in physiological and pathological processes related to ER stress. |
| [99] | Epigenetic regulation of macrophage polarization in wound healing | The article explores the intricate role of H2S in the polarization and function of macrophages in cardiac pathophysiology, including atherosclerosis. Specifically, it highlights the inhibition of cystathionine γ-lyase (CSE) expression and H2S production in macrophages by homocysteine (Hcy) and the contribution of the dysregulated CSE-H2S signaling pathway to atherosclerosis pathogenesis. | Dysregulation of the CSE- H2S signaling pathway in macrophages contributes to atherosclerosis pathogenesis, with homocysteine (Hcy) affecting macrophage polarization. |
3.3. H2S Therapeutic Potential
H2S research has rapidly expanded from a basic biological understanding to clinical and translational science, demonstrating immense therapeutic potential. As elaborated in previous sections, the nuanced roles of H2S in oxidative stress and cellular homeostasis make it an attractive molecule for therapeutic targeting, especially in conditions characterized by oxidative stress, inflammation, and metabolic imbalance.
From the cardiovascular perspective, the vasodilatory properties of H2S have driven exploration into its potential for treating hypertension and other cardiovascular diseases. Drugs that release H2S or enhance endogenous H2S production could potentially be employed to ameliorate hypertension and atherosclerosis and reduce the risk of heart attacks. Also, the anti-inflammatory properties of H2S suggest the potential to treat various inflammatory conditions, ranging from autoimmune diseases to systemic inflammation seen in sepsis [22].
In neurodegenerative diseases, like Alzheimer’s and Parkinson’s, which involve oxidative stress and neuronal death, H2S’s antioxidative and anti-apoptotic properties could be leveraged to slow disease progression. Preclinical studies using H2S donors have shown promising results in models of neurodegeneration [105,106].
The capacity of H2S to regulate glucose uptake and energy metabolism might be harnessed for managing metabolic diseases, such as diabetes [107,108]. Modulating H2S levels could improve glucose homeostasis and insulin sensitivity, potentially serving as an adjunct to existing therapies [44].
Despite the therapeutic potential, the journey from bench to bedside is laden with challenges. First, the concentration-dependent dual effects of H2S—protective at physiological levels and toxic at high concentrations—necessitate precise control over therapeutic dosing. Developing methods to precisely modulate H2S levels in specific tissues or cell types without systemic side effects remains a significant challenge.
Moreover, while we have a broad understanding of the diverse roles of H2S, the underlying molecular and biochemical mechanisms remain less understood. For instance, the post-translational modification known as persulfidation, pivotal in H2S biology, needs further exploration. A more profound understanding of these mechanisms is vital for developing targeted therapies.
Controversies exist, too, adding to the complexity. Some studies have reported contradictory results, likely due to differences in experimental models, techniques, and conditions. A consensus on the standard methodologies for measuring H2S levels and their effects is urgently required to harmonize these disparities and advance the field.
Looking toward the future, there is a need for more in-depth studies elucidating the interplay between H2S, oxidative stress, and cellular homeostasis. Large-scale, well-controlled clinical trials are necessary to assess the safety and efficacy of H2S-based therapies. Innovative strategies for targeted H2S delivery at desired locations and concentrations will be crucial for practical therapeutic applications.
4. Discussion
In this comprehensive one-year review, we have ventured into the intricate roles of hydrogen sulfide (H2S) in maintaining cellular homeostasis and orchestrating the regulation of oxidative stress. By synthesizing the recent literature, we have unearthed fresh insights into the multifaceted functions of H2S and its profound implications for both physiological equilibrium and pathological perturbations.
Our endeavor bridges the chasm between nascent research and established knowledge surrounding H2S, underscoring its dynamic participation in maintaining cellular equilibrium and modulating oxidative stress. While earlier reviews laid essential groundwork, our approach stands apart in several pivotal ways. We meticulously curated and exhaustively analyzed articles published over the past year, thus capturing the latest strides and emerging trends in H2S research. This focused timeline facilitated the presentation of cutting-edge discoveries that augment the ongoing discourse on H2S’s multifarious roles.
H2S has gained unprecedented attention as a signaling molecule [96] in recent years. Its pivotal roles across animals and plants [107,108] in diverse cellular processes have triggered intensive exploration. Divergent as animals and plants may be in terms of physiology, metabolism, and anatomy, their pathways intersect when it comes to hydrogen sulfide’s indispensable roles. Redox signaling, ion channel regulation, and stress response mechanisms, which H2S masterfully orchestrates, resonate profoundly in both these biological domains. Furthermore, H2S displays therapeutic potential in both realms, as it mitigates cardiovascular disorders in animals and enhances stress tolerance in plants, emphasizing its cross-species therapeutic applicability [11,95,96].
Emerging research spotlights H2S’s intricate interaction with lipid peroxidation, a process involving oxidative lipid degradation. As a regulator of lipid peroxidation, H2S exhibits both protective and regulatory effects, influencing the expression and activity of enzymes pivotal in lipid peroxidation pathways. Notably, our exploration delves into the connection between H2S and ferroptosis—a recently identified form of regulated cell death marked by iron-dependent lipid peroxide accumulation. H2S’s multifaceted role in modulating lipid peroxidation and cellular redox balance positions it as a potent inhibitor of ferroptosis. Its capacity to scavenge lipid peroxides and ROS and promote the expression of antioxidant enzymes, such as glutathione peroxidase 4 (GPX4), highlights its regulatory prowess in thwarting ferroptotic processes [10,41].
Our review casts light on H2S’s intricate involvement in the aging process. With its multifaceted roles in cellular signaling, antioxidant defense, and metabolic regulation, H2S significantly impacts pathways linked to aging [69,70]. By scavenging ROS and inhibiting lipid peroxidation, H2S safeguards cellular constituents, such as DNA, proteins, and lipids, from oxidative damage—fundamental to aging-associated oxidative stress mitigation. Moreover, its anti-inflammatory effects contribute to attenuating the inflammatory state often linked to healthier aging [85].
Mitochondrial dysfunction is a hallmark of aging, and here, H2S’s protective function shines. It preserves mitochondrial integrity by sustaining electron transport chain activity, reducing ROS production, and fostering mitochondrial biogenesis. These mechanisms collectively bolster cellular energy production and overall health [76].
Proper proteostasis, or protein homeostasis, is paramount for averting the accumulation of misfolded proteins—a hallmark of aging-related ailments. H2S plays a pivotal role in maintaining proteostasis through the modulation of heat shock proteins (HSPs) and proteasomal degradation mechanisms. Additionally, H2S’s influence on cellular senescence, a state of irreversible growth arrest linked to aging, is evident. By affecting the expression of senescence-associated markers and promoting the clearance of senescent cells through autophagy, H2S emerges as a potent modulator of cellular senescence [85].
Our review serves as an impetus for future explorations by pinpointing areas ripe for further investigation. The elucidation of H2S’s interactions with antioxidant defense systems, a cellular redox state, and pivotal signaling cascades furnishes a foundational framework for mechanistic dissections. This knowledge frontier beckons researchers to unravel the precise molecular mechanisms governing H2S’s protective effects. Furthermore, the interplay between H2S and other antioxidants, such as coenzyme Q10, unfurls exciting avenues for collaborative research.
5. Conclusions
H2S plays a multifaceted role in maintaining cellular homeostasis, impacting cellular bioenergetics, redox balance, signal transduction, cell survival and apoptosis, and metabolic regulation. Despite the significant efforts to understand the role of H2S in cellular homeostasis, several questions remain. For instance, how is H2S homeostasis maintained at the cellular level? What are the specific targets of H2S in the cell? Further exploring these questions could pave the way for developing therapeutic strategies to modulate H2S levels to treat various diseases [42].
One of the central threads that emerged from this review is the profound effect of H2S on the redox status of cells. H2S exhibits antioxidant properties, can induce the production of glutathione, and activates the Nrf2 pathway, all contributing to the regulation of oxidative stress and the maintenance of cellular redox balance [11].
Moreover, the significance of H2S in cellular energy metabolism was elucidated. H2S interacts with multiple enzymatic components of energy metabolism pathways, regulates glucose uptake, and maintains mitochondrial function, thus playing a critical role in overall energy homeostasis [96].
The review also highlights the controversies and challenges within this rapidly advancing field. Despite extensive studies, the precise mechanisms underlying the biological effects of H2S remain to be fully elucidated. Notably, the post-translational modification known as persulfidation, through which H2S exerts influence, necessitates further exploration. The delicate balance of H2S homeostasis in the cell and the dichotomy of its protective and deleterious effects warrant deeper understanding.
In conclusion, the study of H2S represents a frontier in our understanding of gasotransmitters, oxidative stress, and cellular homeostasis. The complexity and breadth of H2S influence in biological systems make it an attractive target for future research and therapeutic developments. We hope this review will spark a further investigation into this critical molecule, ultimately paving the way for new therapeutic approaches for various diseases where oxidative stress and disturbances in cellular homeostasis are implicated.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodrigues, L.M.; Gregório, J.; Wehrwein, E. Contemporary views on the future of physiology—A report from the 2019 P-MIG focus group. Front. Physiol. 2023, 14, 1176146. [Google Scholar] [CrossRef]
- Munteanu, C.; Rotariu, M.; Turnea, M.-A.; Anghelescu, A.; Albadi, I.; Dogaru, G.; Silișteanu, S.C.; Ionescu, E.V.; Firan, F.C.; Ionescu, A.M.; et al. Topical Reappraisal of Molecular Pharmacological Approaches to Endothelial Dysfunction in Diabetes Mellitus Angiopathy. Curr. Issues Mol. Biol. 2022, 44, 3378–3397. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Munteanu, D.; Onose, G. Hydrogen sulfide (H2S)—Therapeutic relevance in rehabilitation and balneotherapy Systematic literature review and meta-analysis based on the PRISMA paradig. Balneo PRM Res. J. 2021, 12, 176–195. [Google Scholar] [CrossRef]
- Panthi, S.; Chung, H.J.; Jung, J.; Jeong, N.Y. Physiological importance of hydrogen sulfide: Emerging potent neuroprotector and neuromodulator. Oxidative Med. Cell. Longev. 2016, 2016, 9049782. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Qian, L.L.; Wang, R.X. Hydrogen Sulfide-Induced Vasodilation: The Involvement of Vascular Potassium Channels. Front. Pharmacol. 2022, 13, 911704. [Google Scholar] [CrossRef]
- Huerta de la Cruz, S.; Medina-Terol, G.J.; Tapia-Martínez, J.A.; Silva-Velasco, D.L.; Beltran-Ornelas, J.H.; Sánchez-López, A.; Sancho, M.; Centurión, D. Hydrogen sulfide as a neuromodulator of the vascular tone. Eur. J. Pharmacol. 2023, 940, 175455. [Google Scholar] [CrossRef]
- Munteanu, C.; Rotariu, M.; Turnea, M.; Dogaru, G.; Popescu, C.; Spînu, A.; Andone, I.; Postoiu, R.; Ionescu, E.; Oprea, C.; et al. Recent Advances in Molecular Research on Hydrogen Sulfide (H2S) Role in Diabetes Mellitus (DM)—A Systematic Review. Int. J. Mol. Sci. 2022, 23, 6720. [Google Scholar] [CrossRef]
- Farahat, S.; Kherkheulidze, S.; Nopp, S.; Kainz, A.; Borriello, M.; Perna, A.F.; Cohen, G. Effect of Hydrogen Sulfide on Essential Functions of Polymorphonuclear Leukocytes. Toxins 2023, 15, 198. [Google Scholar] [CrossRef]
- Seydi, E.; Irandoost, Z.; Khansari, M.G.; Naserzadeh, P.; Tanbakosazan, F.; Pourahmad, J. Toxicity of Hydrogen Sulfide on Rat Brain Neurons. Drug Res. 2022, 72, 197–202. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Q. Advances of H2S in Regulating Neurodegenerative Diseases by Preserving Mitochondria Function. Antioxidants 2023, 12, 652. [Google Scholar] [CrossRef]
- Aschner, M.; Skalny, A.V.; Ke, T.; da Rocha, J.B.; Paoliello, M.M.; Santamaria, A.; Bornhorst, J.; Rongzhu, L.; Svistunov, A.A.; Djordevic, A.B.; et al. Hydrogen Sulfide (H2S) Signaling as a Protective Mechanism against Endogenous and Exogenous Neurotoxicants. Curr. Neuropharmacol. 2022, 20, 1908–1924. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xia, H.; Sharp, T.E., 3rd; Lapenna, K.B.; Elrod, J.W.; Casin, K.M.; Liu, K.; Calvert, J.W.; Chau, V.Q.; Salloum, F.N.; et al. Mitochondrial H2S Regulates BCAA Catabolism in Heart Failure. Circ. Res. 2022, 131, 222–235. [Google Scholar] [CrossRef]
- Cornwell, A.; Badiei, A. From Gasotransmitter to Immunomodulator: The Emerging Role of Hydrogen Sulfide in Macrophage Biology. Antioxidants 2023, 12, 935. [Google Scholar] [CrossRef] [PubMed]
- Switzera, C.H.; Kasamatsu, S.; Ihara, H.; Eaton, P. SOD1 is an essential H2S detoxifying enzyme. Proc. Natl. Acad. Sci. USA 2023, 120, e2205044120. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Rathod, D.C.; Vaidya, S.M.; Hopp, M.-T.; Kühl, T.; Imhof, D. Shapes and Patterns of Heme-Binding Motifs in Mammalian Heme-Binding Proteins. Biomolecules 2023, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Bătrînu, M.G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Chen, C.-J.; Cheng, M.-C.; Hsu, C.-N.; Tain, Y.-L. Sulfur-Containing Amino Acids, Hydrogen Sulfide, and Sulfur Compounds on Kidney Health and Disease. Metabolites 2023, 13, 688. [Google Scholar] [CrossRef]
- Li, X.; Jiang, K.; Ruan, Y.; Zhao, S.; Zhao, Y.; He, Y.; Wang, Z.; Wei, J.; Li, Q.; Yang, C.; et al. Hydrogen Sulfide and Its Donors: Keys to Unlock the Chains of Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 12202. [Google Scholar] [CrossRef]
- Yang, Y.L.; Zhang, K.; Zhou, Z.T.; Jiang, Z.L.; Liu, Y.; Zhang, Y.X.; Liu, Z.H.; Ji, X.Y.; Wu, D.D. The Role of Hydrogen Sulfide in the Development and Progression of Lung Cancer. Molecules 2022, 27, 9005. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shackelford, R.E.; Shen, X.; Dominic, P.; Kevil, C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2023, 20, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Jing, M.R.; Cai, C.B.; Zhu, S.G.; Zhang, C.J.; Wang, Q.M.; Zhai, Y.K.; Ji, X.Y.; Wu, D.D. Role of hydrogen sulphide in physiological and pathological angiogenesis. Cell Prolif. 2023, 56, e13374. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Dawoud, A.; Zeinelabdeen, Y.A.; Kiriacos, C.J.; Daniel, K.A.; Eltahtawy, O.; Abdelhalim, M.M.; Braoudaki, M.; Youness, R.A. Molecular Engines, Therapeutic Targets, and Challenges in Pediatric Brain Tumors: A Special Emphasis on Hydrogen Sulfide and RNA-Based Nano-Delivery. Cancers 2022, 14, 5244. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, M.; Zuhra, K.; Kopec, J.; Hutchin, A.; Szabo, C.; Majtan, T. H2S biogenesis by cystathionine beta-synthase: Mechanism of inhibition by aminooxyacetic acid and unexpected role of serine. Cell. Mol. Life Sci. 2022, 79, 438. [Google Scholar] [CrossRef]
- Czikora, Á.; Erdélyi, K.; Ditrói, T.; Szántó, N.; Jurányi, E.P.; Szanyi, S.; Tóvári, J.; Strausz, T.; Nagy, P. Cystathionine β-synthase overexpression drives metastatic dissemination in pancreatic ductal adenocarcinoma via inducing epithelial-to-mesenchymal transformation of cancer cells. Redox Biol. 2022, 57, 102505. [Google Scholar] [CrossRef]
- Paul, B.D.; Pieper, A.A. Protective Roles of Hydrogen Sulfide in Alzheimer’s Disease and Traumatic Brain Injury. Antioxidants 2023, 12, 1095. [Google Scholar] [CrossRef]
- Hatami, N.; Büttner, C.; Bock, F.; Simfors, S.; Musial, G.; Reis, A.; Cursiefen, C.; Clahsen, T. Cystathionine β-synthase as novel endogenous regulator of lymphangiogenesis via modulating VEGF receptor 2 and 3. Commun. Biol. 2022, 5, 950. [Google Scholar] [CrossRef]
- Cornwell, A.; Fedotova, S.; Cowan, S.; Badiei, A. Cystathionine γ-lyase and hydrogen sulfide modulates glucose transporter Glut1 expression via NF-κB and PI3k/Akt in macrophages during inflammation. PLoS ONE 2022, 17, e0278910. [Google Scholar] [CrossRef]
- Wu, D.; Tan, B.; Sun, Y.; Hu, Q. Cystathionine γ lyase S-sulfhydrates Drp1 to ameliorate heart dysfunction. Redox Biol. 2022, 58, 102519. [Google Scholar] [CrossRef]
- Thanki, K.K.; Johnson, P.; Higgins, E.J.; Maskey, M.; Phillips, C.N.; Dash, S.; Almenas, F.A.; Govar, A.A.; Tian, B.; Villéger, R.; et al. Deletion of cystathionine-γ-lyase in bone marrow-derived cells promotes colitis-associated carcinogenesis. Redox Biol. 2022, 55, 102417. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cui, C.; Cui, C.; Chen, Z.; Zhang, H.; Cui, Q.; Xu, G.; Fan, J.; Han, Y.; Tang, L.; et al. Hepatocellular cystathionine γ lyase/hydrogen sulfide attenuates nonalcoholic fatty liver disease by activating farnesoid X receptor. Hepatology 2022, 76, 1794–1810. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.; Dobariya, P.; Bellamkonda, H.; More, S.S. Role of 3-Mercaptopyruvate Sulfurtransferase (3-MST) in Physiology and Disease. Antioxidants 2023, 12, 603. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366–1367. [Google Scholar] [CrossRef]
- Robert, B.; Subramaniam, S. Gasotransmitter-Induced Therapeutic Angiogenesis: A Biomaterial Prospective. ACS Omega 2022, 7, 45849–45866. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K. Mitochondrial and metabolic features of salugenesis and the healing cycle. Mitochondrion 2023, 70, 131–163. [Google Scholar] [CrossRef]
- Rodkin, S.; Nwosu, C.; Sannikov, A.; Tyurin, A.; Chulkov, V.S.; Raevskaya, M.; Ermakov, A.; Kirichenko, E.; Gasanov, M. The Role of Gasotransmitter-Dependent Signaling Mechanisms in Apoptotic Cell Death in Cardiovascular, Rheumatic, Kidney, and Neurodegenerative Diseases and Mental Disorders. Int. J. Mol. Sci. 2023, 24, 6014. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Fekete, M.; Szarvas, Z.; Fazekas-Pongor, V.; Feher, A.; Csipo, T.; Forrai, J.; Dosa, N.; Peterfi, A.; Lehoczki, A.; Tarantini, S.; et al. Nutrition Strategies Promoting Healthy Aging: From Improvement of Cardiovascular and Brain Health to Prevention of Age-Associated Diseases. Nutrients 2023, 15, 47. [Google Scholar] [CrossRef]
- Cheng, Z.; Kishore, R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox Biol. 2020, 37, 101704. [Google Scholar] [CrossRef]
- Rodkin, S.; Nwosu, C.; Sannikov, A.; Raevskaya, M.; Tushev, A.; Vasilieva, I.; Gasanov, M. The Role of Hydrogen Sulfide in Regulation of Cell Death following Neurotrauma and Related Neurodegenerative and Psychiatric Diseases. Int. J. Mol. Sci. 2023, 24, 10742. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Li, K.; Qin, Y.Z.; Zhou, J.J.; Li, T.; Qian, L.; Yang, C.Y.; Ji, X.Y.; Wu, D.D. Recent advances in the role of endogenous hydrogen sulphide in cancer cells. Cell Prolif. 2023, 56, e13449. [Google Scholar] [CrossRef]
- Han, S.; Li, Y.; Gao, H. Generation and Physiology of Hydrogen Sulfide and Reactive Sulfur Species in Bacteria. Antioxidants 2022, 11, 2487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, B.; Ma, D.; Wang, L.; Duan, W. Hydrogen sulfide, adipose tissue and diabetes mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Moore, J.; Devarie-Baez, N.O.; Lewis, J.; Wu, H.; Shukla, K.; Lopez, E.I.S.; Vitvitsky, V.; Key, C.C.C.; Porosnicu, M.; et al. Redox integration of signaling and metabolism in a head and neck cancer model of radiation resistance using COSMRO. Front. Oncol. 2023, 12, 946320. [Google Scholar] [CrossRef]
- Miljkovic, J.L.; Burger, N.; Gawel, J.M.; Mulvey, J.F.; Norman, A.A.I.; Nishimura, T.; Tsujihata, Y.; Logan, A.; Sauchanka, O.; Caldwell, S.T.; et al. Rapid and selective generation of H2S within mitochondria protects against cardiac ischemia-reperfusion injury. Redox Biol. 2022, 55, 102429. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Andrés Juan, C.; Manuel Pérez de la Lastra, J.; Plou, F.J.; Pérez-Lebeña, E.; Reinbothe, S. Molecular Sciences The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Gusev, E.Y.; Zotova, N.V. Cellular Stress and General Pathological Processes. Curr. Pharm. Des. 2019, 25, 251–297. [Google Scholar] [CrossRef]
- Xiao, Q.; Ying, J.; Xiang, L.; Zhang, C. The biologic effect of hydrogen sulfide and its function in various diseases. Medicine 2018, 97, e13065. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulfide, an endogenous stimulator of mitochondrial function in cancer cells. Cells 2021, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen sulfide in physiology and pathogenesis of bacteria and viruses. IUBMB Life 2018, 70, 393–410. [Google Scholar] [CrossRef]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of hydrogen sulfide in nrf2-and sirtuin-dependent maintenance of cellular redox balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef] [PubMed]
- Ling, K.; Zhou, W.; Guo, Y.; Hu, G.; Chu, J.; Xie, F.; Li, Y.; Wang, W. H2S attenuates oxidative stress via Nrf2/NF-κB signaling to regulate restenosis after percutaneous transluminal angioplasty. Exp. Biol. Med. 2021, 246, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.; Raftari, M.; Cezario, R.; Salma, R.; Godoy, P.; Emami, S.N.; Haghdoost, S. Roles of Oxidative Stress and Nrf2 Signaling in Pathogenic and Non-Pathogenic Cells: A Possible General Mechanism of Resistance to Therapy. Antioxidants 2023, 12, 1371. [Google Scholar] [CrossRef]
- Ramsay, D.S.; Woods, S.C. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol. Rev. 2014, 121, 225–247. [Google Scholar] [CrossRef]
- Billman, G.E. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front. Physiol. 2020, 11, 200. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef]
- Altaany, Z.; Alkaraki, A.; Abo Alrob, O.; Taani, O.; Khatatbeh, M. The Interplay of Exogenous and Endogenous Hydrogen Sulfide (H2S) in Maintaining Redox Homeostasis in Individuals with Low Ferritin Levels. Appl. Sci. 2023, 13, 6621. [Google Scholar] [CrossRef]
- Kimura, H. The physiological role of hydrogen sulfide and beyond. Nitric Oxide Biol. Chem. 2014, 41, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mesfin, F.M.; Hunter, C.E.; Olson, K.R.; Shelley, W.C.; Brokaw, J.P.; Manohar, K.; Markel, T.A. Recent Development of the Molecular and Cellular Mechanisms of Hydrogen Sulfide Gasotransmitter. Antioxidants 2022, 11, 1788. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Mladenov, M.; Lubomirov, L.; Grisk, O.; Avtanski, D.; Mitrokhin, V.; Sazdova, I.; Keremidarska-Markova, M.; Danailova, Y.; Nikolaev, G.; Konakchieva, R.; et al. Oxidative Stress, Reductive Stress and Antioxidants in Vascular Pathogenesis and Aging. Antioxidants 2023, 12, 1126. [Google Scholar] [CrossRef]
- Huang, Y.; Omorou, M.; Gao, M.; Mu, C.; Xu, W.; Xu, H. Hydrogen sulfide and its donors for the treatment of cerebral ischaemia-reperfusion injury: A comprehensive review. Biomed. Pharmacother. 2023, 161, 114506. [Google Scholar] [CrossRef]
- Domán, A.; Dóka, É.; Garai, D.; Bogdándi, V.; Balla, G.; Balla, J.; Nagy, P. Interactions of reactive sulfur species with metalloproteins. Redox Biol. 2023, 60, 102617. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y. The role of sulfur compounds in chronic obstructive pulmonary disease. Front. Mol. Biosci. 2022, 9, 928287. [Google Scholar] [CrossRef]
- Marini, E.; Rolando, B.; Sodano, F.; Blua, F.; Concina, G.; Guglielmo, S.; Lazzarato, L.; Chegaev, K. Comparative Study of Different H2S Donors as Vasodilators and Attenuators of Superoxide-Induced Endothelial Damage. Antioxidants 2023, 12, 344. [Google Scholar] [CrossRef]
- Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants 2023, 12, 860. [Google Scholar] [CrossRef]
- Bergstedt, J.H.; Skov, P.V.; Letelier-Gordo, C.O. Efficacy of H2O2 on the removal kinetics of H2S in saltwater aquaculture systems, and the role of O2 and NO3−. Water Res. 2022, 222, 118892. [Google Scholar] [CrossRef] [PubMed]
- Putman, A.K.; Contreras, G.A.; Mottillo, E.P. Thermogenic Adipose Redox Mechanisms: Potential Targets for Metabolic Disease Therapies. Antioxidants 2023, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Benchoam, D.; Semelak, J.A.; Cuevasanta, E.; Mastrogiovanni, M.; Grassano, J.S.; Ferrer-Sueta, G.; Zeida, A.; Trujillo, M.; Möller, M.N.; Estrin, D.A.; et al. Acidity and nucleophilic reactivity of glutathione persulfide. J. Biol. Chem. 2020, 295, 15466–15481. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Wang, Z.; Li, B.; Zhu, H. Modulation of redox homeostasis: A strategy to overcome cancer drug resistance. Front. Pharmacol. 2023, 14, 1156538. [Google Scholar] [CrossRef] [PubMed]
- Verhaegen, D.; Smits, K.; Osório, N.; Caseiro, A. Oxidative Stress in Relation to Aging and Exercise. Encyclopedia 2022, 2, 1545–1558. [Google Scholar] [CrossRef]
- Pinho, S.A.; Anjo, S.I.; Cunha-Oliveira, T. Metabolic Priming as a Tool in Redox and Mitochondrial Theragnostics. Antioxidants 2023, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.T.; Huynh, T.D.; Wang, C.S.; Lai, K.H.; Lin, Z.C.; Lin, W.N.; Chen, Y.L.; Peng, T.Y.; Wu, H.C.; Lee, I.T. The Potential Implications of Hydrogen Sulfide in Aging and Age-Related Diseases through the Lens of Mitohormesis. Antioxidants 2022, 11, 1619. [Google Scholar] [CrossRef]
- Chavda, V.; Lu, B. Reverse Electron Transport at Mitochondrial Complex I in Ischemic Stroke, Aging, and Age-Related Diseases. Antioxidants 2023, 12, 895. [Google Scholar] [CrossRef]
- Papiri, G.; D’Andreamatteo, G.; Cacchiò, G.; Alia, S.; Silvestrini, M.; Paci, C.; Luzzi, S.; Vignini, A. Multiple Sclerosis: Inflammatory and Neuroglial Aspects. Curr. Issues Mol. Biol. 2023, 45, 1443–1470. [Google Scholar] [CrossRef]
- Locascio, A.; Annona, G.; Caccavale, F.; D’Aniello, S.; Agnisola, C.; Palumbo, A. Nitric Oxide Function and Nitric Oxide Synthase Evolution in Aquatic Chordates. Int. J. Mol. Sci. 2023, 24, 11182. [Google Scholar] [CrossRef]
- Kim, D.S.; Pessah, I.N.; Santana, C.M.; Purnell, B.S.; Li, R.; Buchanan, G.F.; Rumbeiha, W.K. Investigations into hydrogen sulfide-induced suppression of neuronal activity in vivo and calcium dysregulation in vitro. Toxicol. Sci. 2023, 192, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Santana Maldonado, C.; Weir, A.; Rumbeiha, W.K. A comprehensive review of treatments for hydrogen sulfide poisoning: Past, present, and future. Toxicol. Mech. Methods 2023, 33, 183–196. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, Z.; Huang, Z.; Duan, X.; Wang, Y.; Cao, J.; Li, L.; He, K.; Nice, E.C.; He, W.; et al. Protein persulfidation: Rewiring the hydrogen sulfide signaling in cell stress response. Biochem. Pharmacol. 2023, 209, 115444. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, X.; Feng, J.; Zhu, S. Biological Functions of Hydrogen Sulfide in Plants. Int. J. Mol. Sci. 2022, 23, 15107. [Google Scholar] [CrossRef] [PubMed]
- Bresgen, N.; Kovacs, M.; Lahnsteiner, A.; Felder, T.K.; Rinnerthaler, M. The Janus-Faced Role of Lipid Droplets in Aging: Insights from the Cellular Perspective. Biomolecules 2023, 13, 912. [Google Scholar] [CrossRef]
- Zhu, Z.; Lian, X.; Bhatia, M. Hydrogen Sulfide: A Gaseous Mediator and Its Key Role in Programmed Cell Death, Oxidative Stress, Inflammation and Pulmonary Disease. Antioxidants 2022, 11, 2162. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, Q.; Li, X.; Wei, R.; Ge, T.; Zheng, X.; Li, B.; Liu, K.; Cui, R. Hydrogen sulfide: A new therapeutic target in vascular diseases. Front. Endocrinol. 2022, 13, 934231. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.H.; Iqbal, M.; Manhoosh, B.; Gholampoor, N.; Ma, D.; Marwah, M.; Sanchez-Aranguren, L. Hydrogen Sulphide-Based Therapeutics for Neurological Conditions: Perspectives and Challenges. Neurochem. Res. 2023, 48, 1981–1996. [Google Scholar] [CrossRef]
- Cao, G.; Zeng, Y.; Zhao, Y.; Lin, L.; Luo, X.; Guo, L.; Zhang, Y.; Cheng, Q. H2S regulation of ferroptosis attenuates sepsis-induced cardiomyopathy. Mol. Med. Rep. 2022, 26, 335. [Google Scholar] [CrossRef]
- Dordevic, D.; Capikova, J.; Dordevic, S.; Tremlová, B.; Gajdács, M.; Kushkevych, I. Sulfur content in foods and beverages and its role in human and animal metabolism: A scoping review of recent studies. Heliyon 2023, 9, e15452. [Google Scholar] [CrossRef]
- Ozatik, F.Y.; Ozatik, O.; Teksen, Y.; Kocak, H.; Ari, N.S.; Cengelli Unel, C. Dose-Dependent Effect of Hydrogen Sulfide in Cyclophosphamide-Induced Hepatotoxicity in Rats. Turk. J. Gastroenterol. 2023, 34, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Beckett, E.A.H.; Gaganis, V.; Bakker, A.J.; Towstoless, M.; Hayes, A.; Hryciw, D.H.; Lexis, L.; Tangalakis, K. Unpacking the homeostasis core concept in physiology: An Australian perspective. Adv. Physiol. Educ. 2023, 47, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Rotariu, M.; Turnea, M.; Ionescu, A.M.; Popescu, C.; Spinu, A.; Ionescu, E.V.; Oprea, C.; Țucmeanu, R.E.; Tătăranu, L.G.; et al. Main Cations and Cellular Biology of Traumatic Spinal Cord Injury. Cells 2022, 11, 250. [Google Scholar] [CrossRef]
- Alsaeedi, A.; Welham, S.; Rose, P.; Zhu, Y. The Impact of Drugs on Hydrogen Sulfide Homeostasis in Mammals. Antioxidants 2023, 12, 908. [Google Scholar] [CrossRef]
- Richardson, R.B.; Mailloux, R.J. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants 2023, 12, 674. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yao, J.; Wang, Z.; Xu, J. Mitochondria in endothelial cells angiogenesis and function: Current understanding and future perspectives. J. Transl. Med. 2023, 21, 441. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, W.; Lee, D. H2S- and Redox-State-Mediated PTP1B S-Sulfhydration in Insulin Signaling. Int. J. Mol. Sci. 2023, 24, 2898. [Google Scholar] [CrossRef]
- Zhao, H.; Fu, X.; Zhang, Y.; Yang, Y.; Wang, H. Hydrogen sulfide plays an important role by regulating endoplasmic reticulum stress in myocardial diseases. Front. Pharmacol. 2023, 14, 1172147. [Google Scholar] [CrossRef]
- Chen, C.; Liu, T.; Tang, Y.; Luo, G.; Liang, G.; He, W. Epigenetic regulation of macrophage polarization in wound healing. Burn. Trauma 2023, 11, tkac057. [Google Scholar] [CrossRef]
- Dogaru, B.G.; Munteanu, C. The Role of Hydrogen Sulfide (H2S) in Epigenetic Regulation of Neurodegenerative Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12555. [Google Scholar] [CrossRef]
- Piragine, E.; Malanima, M.A.; Lucenteforte, E.; Martelli, A.; Calderone, V. Circulating Levels of Hydrogen Sulfide (H2S) in Patients with Age-Related Diseases: A Systematic Review and Meta-Analysis. Biomolecules 2023, 13, 1023. [Google Scholar] [CrossRef] [PubMed]
- Panagaki, T.; Randi, E.B.; Augsburger, F.; Szabo, C. Overproduction of H2S, generated by CBS, inhibits mitochondrial Complex IV and suppresses oxidative phosphorylation in down syndrome. Proc. Natl. Acad. Sci. USA 2019, 116, 18769–18771. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hessler, W.; Henary, M. H2S Sensors: Synthesis, Optical Properties, and Selected Biomedical Applications under Visible and NIR Light. Molecules 2023, 28, 1295. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yu, Y.; Zhu, L.; Lai, N.; Zhang, L.; Guo, Y.; Lin, X.; Yang, D.; Ren, N.; Zhu, Z.; et al. Implications of hydrogen sulfide in colorectal cancer: Mechanistic insights and diagnostic and therapeutic strategies. Redox Biol. 2023, 59, 102601. [Google Scholar] [CrossRef] [PubMed]
- Kaziród, K.; Myszka, M.; Dulak, J.; Łoboda, A. Hydrogen sulfide as a therapeutic option for the treatment of Duchenne muscular dystrophy and other muscle-related diseases. Cell. Mol. Life Sci. 2022, 79, 608. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.P.; Xie, W.; Christopher Kwon, Y.I.; Juckel, N.; Xie, J.; Dronamraju, V.R.; Vince, R.; Lee, M.K.; More, S.S. Sulfanegen stimulates 3-mercaptopyruvate sulfurtransferase activity and ameliorates Alzheimer’s disease pathology and oxidative stress in vivo. Redox Biol. 2022, 57, 102484. [Google Scholar] [CrossRef]
- Akpoveso, O.O.P.; Ubah, E.E.; Obasanmi, G. Antioxidant Phytochemicals as Potential Therapy for Diabetic Complications. Antioxidants 2023, 12, 123. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, W.; Yin, L.; Shi, Z.; Luan, J.; Chen, L.; Liu, L. The Potential Roles of Post-Translational Modifications of PPARγ in Treating Diabetes. Biomolecules 2022, 12, 1832. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).