Changes in pH and Nitrite Nitrogen Induces an Imbalance in the Oxidative Defenses of the Spotted Babylon (Babylonia areolata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Behavioral Observation
2.4. Enzyme Activities Analysis
2.5. Statistical Analysis
3. Results
3.1. Effect of pH and Nitrite Nitrogen Stress on the Behavior of the Spotted Babylon
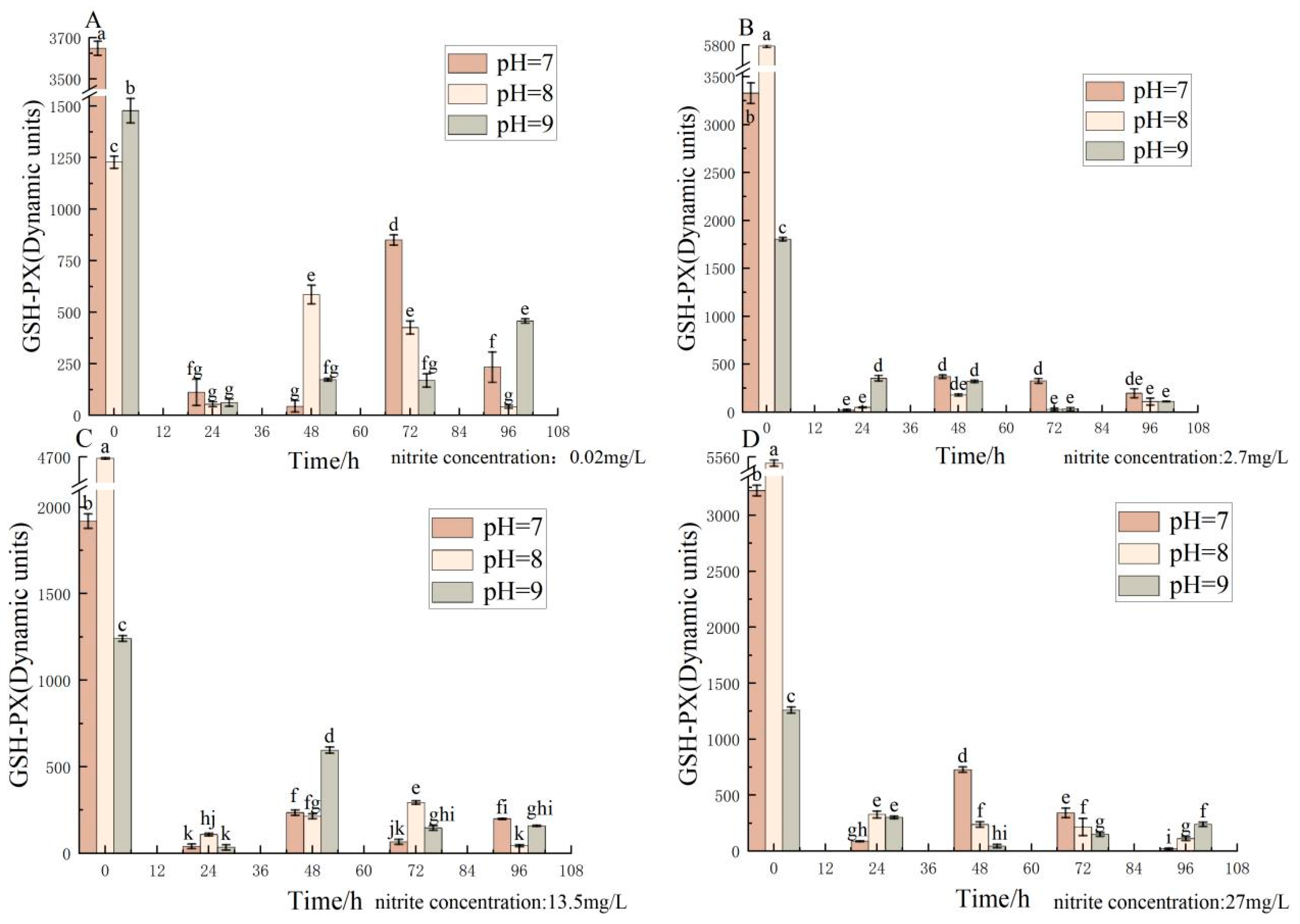
3.2. Effect of pH and Nitrite Nitrogen Stress on GSH-PX Activity of the Spotted Babylon
3.3. Effect of pH and Nitrite Nitrogen Stress on ACP Activity of the Spotted Babylon
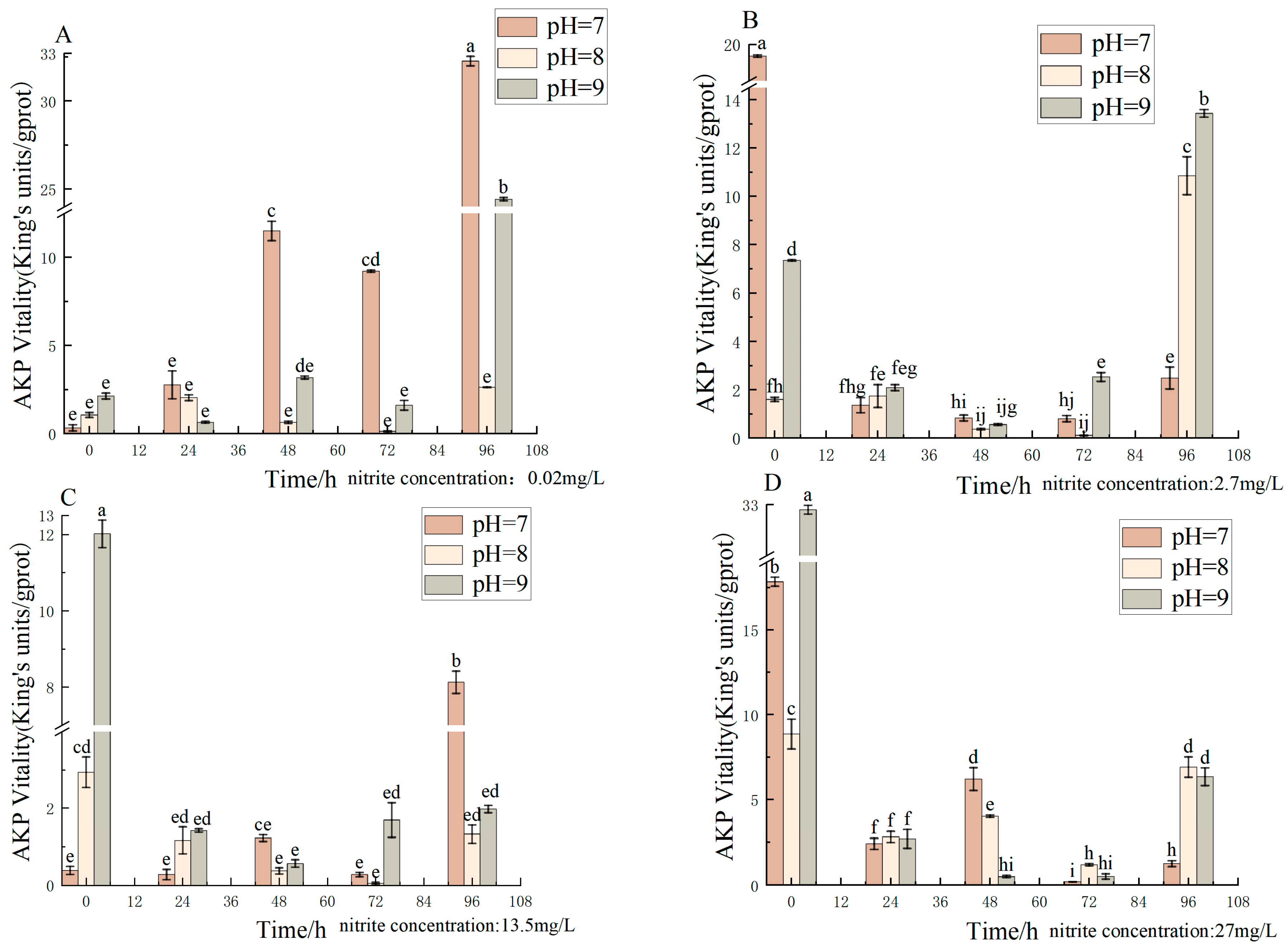
3.4. Effect of pH and Nitrite Nitrogen Stress on AKP Activity of the Spotted Babylon
3.5. Effect of pH and Nitrite Nitrogen Stress on POD Activity of the Spotted Babylon
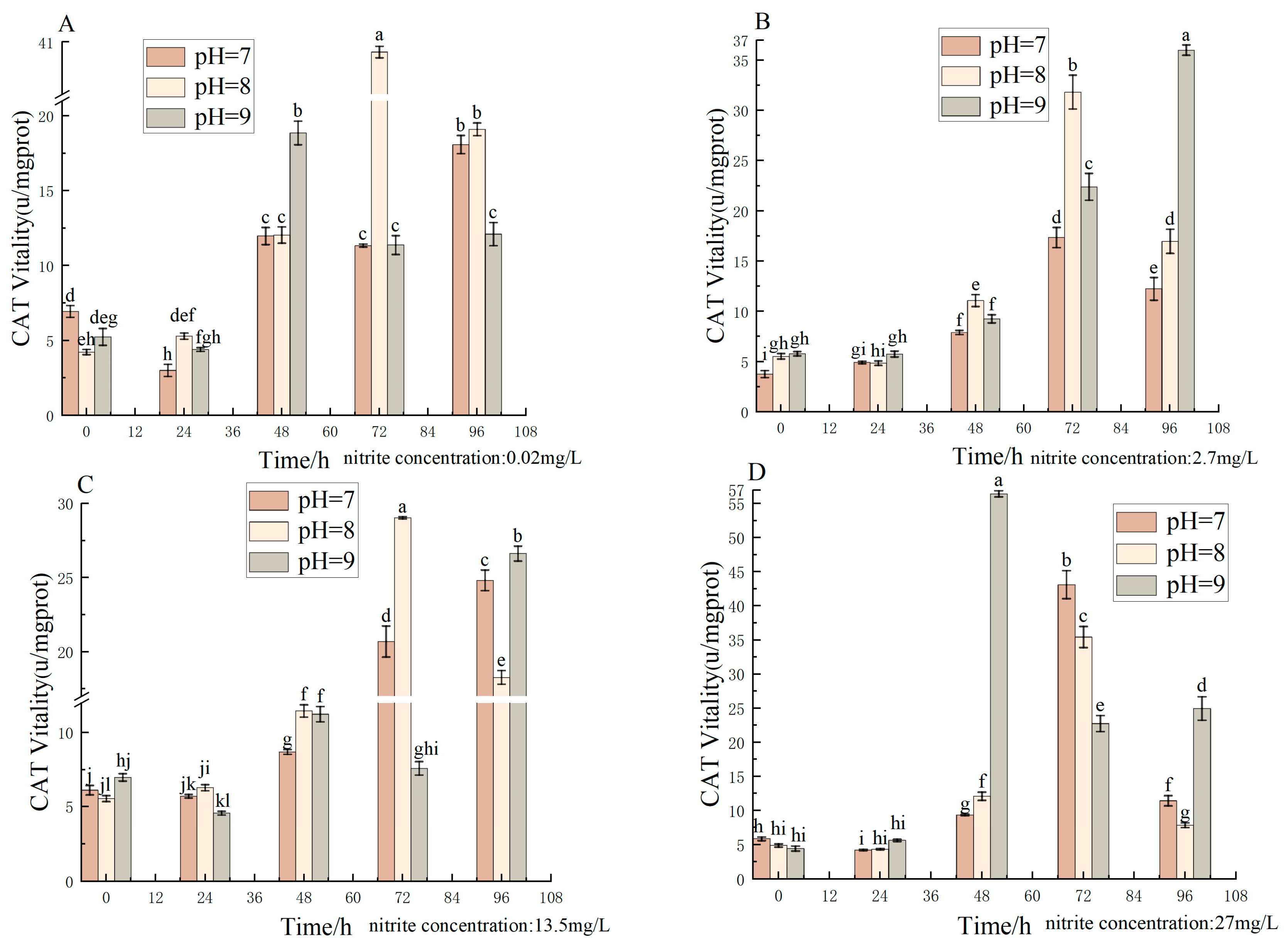
3.6. Effect of pH and Nitrite Nitrogen Stress on CAT Activity of the Spotted Babylon
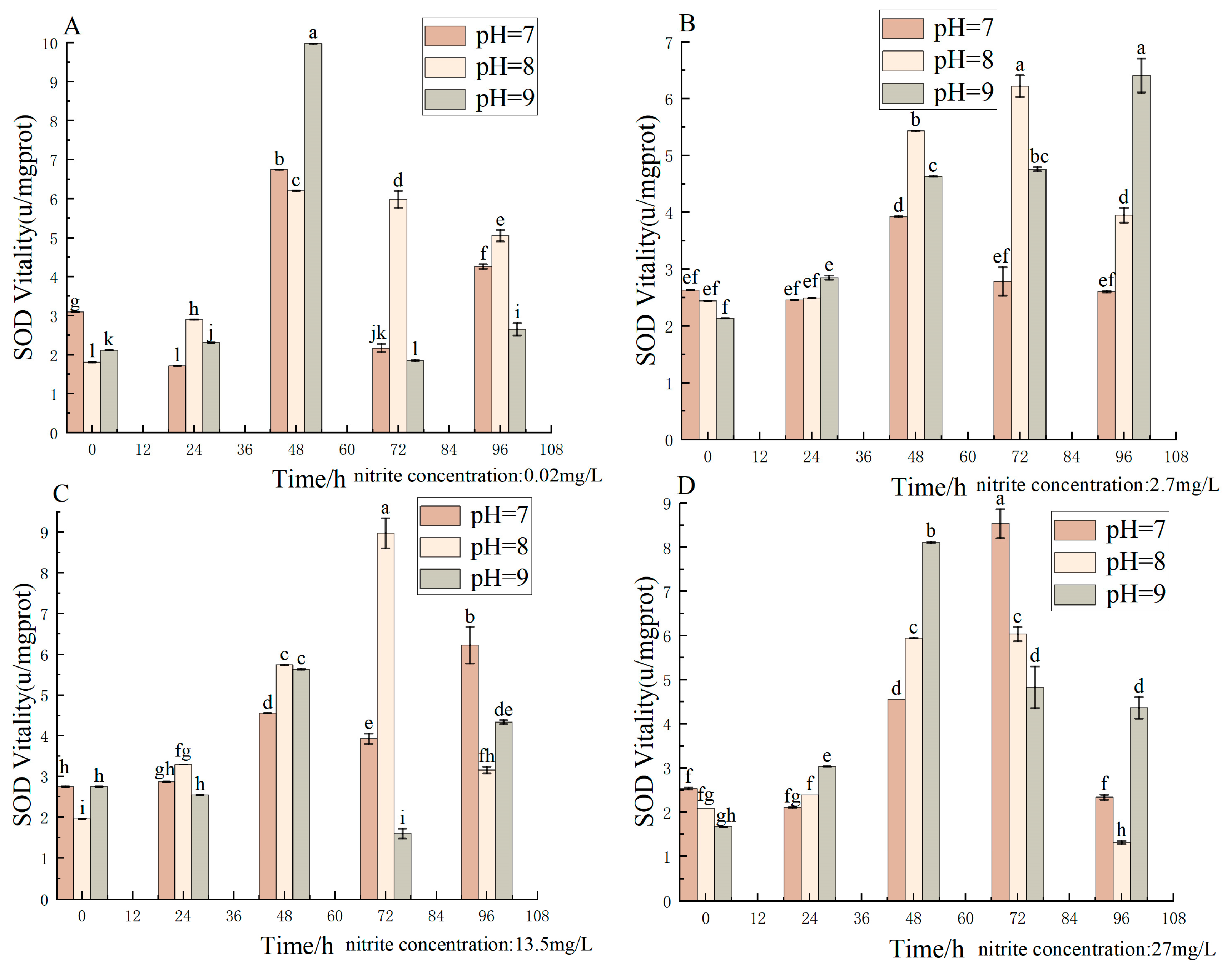
3.7. Effect of pH and Nitrite Nitrogen Stress on SOD Activity of the Spotted Babylon
4. Discussion
4.1. Behaviorial Changes of Spotted Babylons
4.2. Activity Change of Immunoenzymes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.C.; Liu, C.; Yang, Y.M.; Yang, Y.; Gu, Z.F.; Wang, A.M.; Liu, C.S. Effects of long-term exposure to ammonia on growth performance, immune response, and body biochemical composition of juvenile ivory shell, Babylonia. Aquaculture 2023, 562, 738857. [Google Scholar]
- Zhao, W.; Han, Q.; Yang, R.; Wen, W.G.; Deng, Z.H.; Li, H.; Zheng, Z.M.; Ma, Z.H.; Yu, G. Exposure to cadmium induced gut antibiotic resistance genes (ARGs) and microbiota alternations of Babylonia. Sci. Total Environ. 2023, 865, 161–243. [Google Scholar] [CrossRef]
- Gregory, T.D.; Nguyen, D.Q.D.; Nicholas, A.P.; Paul, C.; Southgate, P.C. Assessing potential for integrating sea grape (Caulerpa lentillifera) culture with sandfish (Holothuria scabra) and Babylon snail (Babylonia) co-culture. Aquaculture 2020, 522, 735153. [Google Scholar]
- Lü, W.; Zhong, M.; Fu, J.; Ke, S.; Gan, B.; Zhou, Y.; Shen, M.; Ke, C. Comparison and Optimal Prediction of Goptimal prediction of growth of Babylonia areolata and B. lutosa. Aquac. Rep. 2020, 18, 100425. [Google Scholar]
- Chen, C.-Z.; Li, P.; Liu, L.; Li, Z.-H. Exploring the interactions between the gut microbiome and the shifting surrounding aquatic environment in fisheries and aquaculture: A review. Environ. Res. 2022, 214, 114202. [Google Scholar]
- Li, H.; Cui, Z.G.; Cui, H.W.; Bai, Y.; Yin, Z.D.; Qu, K.M. Hazardous substances and their removal in recirculating aquaculture systems: A review. Aquaculture 2023, 569, 739399. [Google Scholar]
- Gregory, T.D.; Nguyen, D.Q.D.; Paul, C.S. Utilisation of organic matter from Babylon snail (Babylonia) culture sediments by cultured juvenile sandfish (Holothuria scabra). Aquac. Rep. 2020, 18, 100532. [Google Scholar]
- Ye, T.; Li, M.; Lin, Y.; Su, Z. An effective biological treatment method for marine aquaculture wastewater: Combined treatment of immobilized degradation bacteria modified by chitosan-based aerogel and macroalgae (Caulerpa lentillifera). Aquaculture 2023, 570, 739392. [Google Scholar]
- Luisa, P.R.; Luís, F.T. An overview of the Brazilian frog farming. Aquaculture 2022, 548, 737623. [Google Scholar]
- Zhang, J.M.; Fu, B.; Li, Y.C.; Sun, J.H.; Xie, J.; Wang, G.J.; Tian, J.J.; Jin, Z.Y.; Yu, E.M. The effect of nitrite nitrogen and nitrate treatment on growth performance, nutritional composition and flavor-associated metabolites of grass carp (Ctenopharyngodon idella). Aquaculture 2023, 562, 738784. [Google Scholar] [CrossRef]
- Sun, L.; Fang, L.; Cheng, Y.; Fu, H.; Wang, R.; Gu, Y.; Qiu, Y.B.; Sun, K.; Xu, H.; Lei, P. A strategy for nitrogen conversion in aquaculture water based on poly-γ-glutamic acid synthesis. Int. J. Biol. Macromol. 2023, 229, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.F.; Li, Y.H.; Chen, L.L.; Song, J.; Liu, Y. Ni-Fe oxide-PEDOT modified anode coupled with BAF treating ammonia and nitrite nitrogen in recirculating seawater of aquaculture system. Bioresource Technology 2021, 342, 126048. [Google Scholar] [CrossRef] [PubMed]
- John, E.M.; Krishnapriya, K.; Sankar, T.V. Treatment of ammonia and nitrite nitrogen in aquaculture wastewater by an assembled bacterial consortium. Aquaculture 2020, 526, 735390. [Google Scholar] [CrossRef]
- Scott, L.H.; Matthew, S.E.; Maya, S.V.; Jason, A.; Maxwell, D.R.; Michael, H.G. Integrated multi-trophic aquaculture mitigates the effects of ocean acidification: Seaweeds raise system pH and improve growth of juvenile abalone. Aquaculture 2022, 560, 738571. [Google Scholar]
- Hon, J.L.; Sharifah, R.; Pei, W.T.; Khor, W.; Hanafiah, F.; Nadiah, W.R.; Siti Izzah, A.H.; Suhairi, M.; Sabri, M.; Leong, S.L.; et al. Low water pH depressed growth and early development of giant freshwater prawn Macrobrachium rosenbergii larvae. Heliyon 2022, 7, e09989. [Google Scholar]
- Lu, Z.-B.; Li, Y.-D.; Jiang, S.-G.; Yang, Q.-B.; Jiang, S.; Huang, J.-H.; Yang, L.-S.; Chen, X.; Zhou, F.-L. Transcriptome analysis of hepatopancreas in penaeus monodon under acute low pH stress. Fish Shellfish. Immunol. 2022, 131, 1166–1172. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Liu, Q.; Zhang, J.; Xiong, D. Changes in the intestine barrier function of Litopenaeus vannamei in response to pH stress. Fish Shellfish. Immunol. 2019, 88, 142–149. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Q.; Li, J.; Li, Z. Comparative proteomic profiling in Chinese shrimp Fenneropenaeus chinensis under low pH stress. Fish Shellfish. Immunol. 2022, 120, 526–535. [Google Scholar] [CrossRef]
- Steven, T.S.; Anne, Z.; Jelena, K.; Britt, K.M.; Roger, S.; Xavier, G.; Bendik, F.T. Effects of alkalinity on ammonia removal, carbon dioxide stripping, and system pH in semi-commercial scale water recirculating aquaculture systems operated with moving bed bioreactors. Aquac. Eng. 2015, 65, 46–54. [Google Scholar]
- Albina, P.; Durban, N.; Bertron, A.; Albrecht, A.; Robinet, J.C.; Erable, B. Nitrate and nitrite nitrogen bacterial reduction at alkaline pH and high nitrate concentrations, comparison of acetate versus dihydrogen as electron donors. J. Environ. Manag. 2021, 280, 111859. [Google Scholar] [CrossRef]
- Su, Q.X.; Huang, S.J.; Zhang, H.; Wei, Z.S.; Ng, H.Y. Abiotic transformations of sulfamethoxazole by hydroxylamine, nitrite nitrogen and nitric oxide during wastewater treatment: Kinetics, mechanisms and pH effects. J. Hazard. Mater. 2023, 444, 130328. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.D.; Yuan, X.; Zhao, W.Q.; Luo, X.B.; Li, F.B.; Liu, T.X. Chemodenitrification by Fe(II) and nitrite nitrogen: pH effect, mineralization and kinetic modeling. Chem. Geol. 2020, 541, 119586. [Google Scholar] [CrossRef]
- Yang, R.; Wu, K.C.; Yu, G.; Wen, W.G.; Chen, X.; Zhao, W.; Ye, L. Effects of culture model on growth and main environmental factors in snail Babylonia arelata. Fish. Sci. 2019, 38, 610–615. [Google Scholar]
- Tan, C.M.; Zhao, W.; Wu, K.C.; Zhang, Y.; Yang, R.; Wen, W.G.; Chen, X.; Yu, G. Effects of ammonia nitrogen stress on the activities of six immune enzymes of Babylonia areolata. Mar. Sci. 2019, 43, 8–15. [Google Scholar]
- Guo, Z.H.; Wang, Q.Y. Study on the Toxicity of nitrite nitrogen Nitrogen to Babylonia areolata. Mar. Fish. Res. 2006, 43, 88–92. [Google Scholar]
- Deng, R.X.; Huang, X.M.; Zhao, W.; Deng, Z.H.; Chen, H.D.; Wen, W.G.; Ma, Z.H. Effects of pH Acute Stress on the Behavior and Immune Enzyme Activity of Babylonia areolata. Fish. Mod. 2022, 49, 84–90. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Do, T.T.H.; Le TH, G.; Sovan, L.; Vu, N.U.; Nguyen, T.P. Effects of nitrite nitrogen at different temperatures on physiological parameters and growth in clown knifefish (Chitala ornata, Gray 1831). Aquaculture 2020, 521, 735060. [Google Scholar]
- Yusnita, A.T.; Ros, S.R.; Suhaini, M.; Rabi’atul, A.Z.; Sharifah, R.; Mazlan, A.G.; Hua, T.N.; Hon, J.L. Environmental changes affecting physiological responses and growth of hybrid grouper—The interactive impact of low pH and temperature. Environ. Pollut. 2021, 271, 116375. [Google Scholar]
- Lu, X.; Wang, Z.Y.; Duan, H.R.; Wu, Z.P.; Hu, S.H.; Ye, L.; Yuan, Z.G.; Zheng, M. Significant production of nitric oxide by aerobic nitrite nitrogen reduction at acidic pH. Water Res. 2023, 230, 119542. [Google Scholar] [CrossRef]
- Liu, Y.T.; Wang, H.M.; Wu, L.F.; Han, J.; Sui, B.Y.; Meng, L.G.; Xu, Y.X.; Lu, S.W.; Wang, H.Y.; Peng, J.F. Intestinal changes associated with nitrite nitrogen exposure in Bufo gargarizans larvae: Histological damage, immune response, and microbiota dysbiosis. Aquat. Toxicol. 2022, 249, 106228. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.P.; Du, C.M.; Huang, H.Q.; Gu, H.J.; Dong, X.W.; Hu, Y.H. TCS response regulator OmpR plays a major role in stress resistance, antibiotic resistance, motility, and virulence in Edwardsiella piscicida. Aquaculture 2022, 559, 738441. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhu, L.L.; Zhao, X.J.; Cheng, Y.X. Effects of salinity stress on osmotic pressure, free amino acids, and im-mune-associated parameters of the juvenile Chinese mitten crab, Eriocheir sinensis. Aquaculture 2022, 549, 737776. [Google Scholar] [CrossRef]
- Qi, C.L.; Wang, X.D.; Han, F.L.; Chen, X.F.; Li, E.C.; Zhang, M.L.; Qin, J.G.; Chen, L.Q. Dietary arginine alleviates the oxidative stress, inflammation and immunosuppression of juvenile Chinese mitten crab Eriocheir sinensis under high pH stress. Aquac. Rep. 2021, 19, 100619. [Google Scholar] [CrossRef]
- Xian, J.A.; Wang, A.L.; Chen, X.D.; Gou, N.N.; Miao, Y.T.; Liao, S.A.; Ye, C.X. Cytotoxicity of nitrite nitrogen on haemocytes of the tiger shrimp, Penaeus monodon, using flow cytometric analysis. Aquaculture 2011, 317, 240–244. [Google Scholar] [CrossRef]
- Ding, Z.F. Oxidative stress responses of the crayfish Procambrus clarkii to Spiroplasma eriocheiris challenge. Aquac. Rep. 2022, 25, 101219. [Google Scholar] [CrossRef]
- Hou, T.L.; Liu, H.L.; Li, C.T. Traditional Chinese herb formulas in diet enhance the non-specific immune responses of yellow catfish (Pelteobagrus fulvidraco) and resistance against Aeromonas hydrophila. Fish Shellfish. Immunol. 2022, 131, 631–636. [Google Scholar] [CrossRef]
- Wang, Z.H.; Fan, X.Y.; Zhen, L.L.; Guo, Y.; Ren, Y.; Li, Q. Comparative analysis for immune response of coelomic fluid from coelom and polian vesicle in Apostichopus japonicus to Vibrio splendidus infection. Fish Shellfish. Immunol. Rep. 2023, 4, 100074. [Google Scholar] [CrossRef]
- Ren, Y.C.; Men, X.H.; Yu, Y.; Li, B.; Zhou, Y.G.; Zhao, C.Y. Effects of transportation stress on antioxidation, immunity capacity and hypoxia tolerance of rainbow trout (Oncorhynchus mykiss). Aquac. Rep. 2022, 22, 100940. [Google Scholar] [CrossRef]
- Xu, Z.H.; Joe, M.R.; Xie, D.D.; Lu, W.J.; Ren, X.C.; Yuan, J.J.; Mao, L.C. The oxidative stress and antioxidant responses of Litopenaeus vannamei to low temperature and air exposure. Fish Shellfish. Immunol. 2018, 72, 564–571. [Google Scholar] [CrossRef]
- Pan, S.M.; Yan, X.B.; Li, A.; Suo, X.X.; Liu, H.; Tan, B.P.; Huang, W.B.; Yang, Y.Z.; Zhang, H.T.; Dong, X.H. Impacts of tea poly-phenols on growth, antioxidant capacity and immunity in juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) fed high-lipid diets. Fish Shellfish. Immunol. 2022, 128, 348–359. [Google Scholar] [CrossRef]
- Xian, J.A.; Zhang, X.X.; Wang, A.L.; Li, J.T.; Zheng, P.H.; Lu, Y.P.; Wang, D.M.; Ye, J.M. Oxidative burst activity in haemocytes of the freshwater prawn Macrobrachium rosenbergii. Fish Shellfish. Immunol. 2018, 73, 272–278. [Google Scholar] [CrossRef]
- Wang, L.; Nan Wu, N.; Zhang, Y.; Wang, G.L.; Pu, S.Y.; Guan, T.Y.; Zhu, C.K.; Wang, H.; Li, J.L. Effects of copper on non-specific immunity and antioxidant in the oriental river prawn (Macrobrachium nipponense). Ecotoxicol. Environ. Saf. 2022, 236, 113465. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Liu, Z.Q.; Liu, C.; Liu, R.Y.; Yang, C.Y.; Wang, L.L.; Song, L.S. Cortisol modulates glucose metabolism and oxidative response after acute high temperature stress in Pacific oyster Crassostrea gigas. Fish Shellfish. Immunol. 2022, 126, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Mi, K.H.; Xue, W.; Wei, W.Z.; Yang, H. Acute BPA exposure-induced oxidative stress, depressed immune genes expression and damage of hepatopancreas in red swamp crayfish Procambarus clarkii. Fish Shellfish. Immunol. 2020, 103, 95–102. [Google Scholar] [CrossRef]
- Jiang, Q.; Ao, S.Q.; Ji, P.; Zhou, Y.F.; Tang, H.Y.; Zhou, L.Y.; Zhang, X.J. Assessment of deltamethrin toxicity in Macrobrachium nipponense based on histopathology, oxidative stress and immunity damage. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 246, 109040. [Google Scholar] [CrossRef]
- Wu, L.L.; Wang, Y.N.; Han, M.M.; Song, Z.C.; Song, C.B.; Xu, S.H.; Li, J.; Wang, Y.F.; Li, X.; Yue, X.L. Growth, stress and non-specific immune responses of turbot (Scophthalmus maximus) larvae exposed to different light spectra. Aquaculture 2022, 520, 734950. [Google Scholar] [CrossRef]
- Ma, H.; Wei, P.P.; Li, X.; Liu, S.T.; Tian, Y.; Zhang, Q.; Liu, Y. Effects of photoperiod on growth, digestive, metabolic and non-special immunity enzymes of Takifugu rubripes larvae. Aquaculture 2021, 542, 736840. [Google Scholar] [CrossRef]
- Afsharnasab, M.; Kakoolaki, S.; Mohammadidost, M. Immunity enhancement with administration of Gracilaria corticata and Saccharomyces cerevisiae compared to gamma irradiation in expose to WSSV in shrimp, in juvenile Litopenaeus vannamei: A comparative study. Fish Shellfish. Immunol. 2016, 56, 21–33. [Google Scholar] [CrossRef]



| Reverse-back Rate/% | Behavior | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | 96 h | 0 h | 24 h | 48 h | 72 h | 96 h | ||
| pH = 7.0 | 0.02 mg/L | 0 | 0 | 0 | 0 | 2 ± 0.6 | Normal | Normal | Normal | slow | reverse back |
| 2.7 mg/L | 0 | 0 | 0 | 2 ± 0.2 | 4 ± 0.2 | Normal | Normal | slow | reverse back | reverse back | |
| 13.5 mg/L | 0 | 0 | 0 | 4 ± 0.3 | 6 ± 0.9 | Normal | Normal | slow | reverse back | reverse back | |
| 27 mg/L | 0 | 0 | 0 | 5 ± 0.9 | 8 ± 0.4 | Normal | Normal | slow | reverse back | reverse back | |
| pH = 8.0 | 0.02 mg/L | 0 | 0 | 0 | 0 | 0 | Normal | Normal | Normal | Normal | slow |
| 2.7 mg/L | 0 | 0 | 0 | 0 | 1 ± 0.2 | Normal | Normal | Normal | slow | reverse back | |
| 13.5 mg/L | 0 | 0 | 0 | 0 | 3 ± 0.2 | normal | Normal | Normal | slow | reverse back | |
| 27 mg/L | 0 | 0 | 0 | 3 ± 0.2 | 5 ± 0.1 | Normal | Normal | Normal | reverse back | reverse back | |
| pH = 9.0 | 0.02 mg/L | 0 | 0 | 0 | 0 | 3 ± 0.7 | Normal | Normal | Normal | slow | reverse back |
| 2.7 mg/L | 0 | 0 | 0 | 3 ± 0.2 | 5± 0.1 | Normal | Normal | slow | slow | reverse back | |
| 13.5 mg/L | 0 | 0 | 0 | 4 ± 0.7 | 7 ± 1.2 | Normal | Normal | slow | reverse back | reverse back | |
| 27 mg/L | 0 | 0 | 0 | 6 ± 0.9 | 9 ± 1.3 | Normal | Normal | slow | reverse back | reverse back | |
| Source | df | Mean Square | F | Sig. |
|---|---|---|---|---|
| Corrected Model | 58 | 5,377,369.576 | 791.063 | 0.001 |
| Intercept | 1 | 99,742,175.04 | 14,673.039 | 0.001 |
| Time | 4 | 53,430,113.16 | 7860.087 | 0.001 |
| pH | 2 | 4,996,513.484 | 735.035 | 0.001 |
| NaNO2 | 3 | 591,197.699 | 86.971 | 0.001 |
| Time × pH | 8 | 5,024,023.875 | 739.083 | 0.001 |
| Time × NaNO2 | 12 | 1,013,173.161 | 149.048 | 0.001 |
| pH × NaNO2 | 6 | 1,146,681.894 | 168.688 | 0.001 |
| Time × pH × NaNO2 | 23 | 1,246,782.462 | 183.414 | 0.001 |
| Error | 121 | 6797.649 |
| Source | df | Mean Square | F | Sig. |
|---|---|---|---|---|
| Corrected Model | 58 | 148.324 | 183.276 | 0.001 |
| Intercept | 1 | 11,255.171 | 13,907.373 | 0.001 |
| Time | 4 | 1251.402 | 1546.286 | 0.001 |
| pH | 2 | 66.525 | 82.202 | 0.001 |
| NaNO2 | 3 | 58.397 | 72.158 | 0.001 |
| Time × pH | 8 | 56.297 | 69.563 | 0.001 |
| Time × NaNO2 | 12 | 82.259 | 101.642 | 0.001 |
| pH × NaNO2 | 6 | 64.737 | 79.992 | 0.001 |
| Time × pH × NaNO2 | 23 | 60.275 | 74.478 | 0.001 |
| Error | 121 | 0.809 |
| Source | df | Mean Square | F | Sig. |
|---|---|---|---|---|
| Corrected Model | 58 | 553.398 | 131.221 | 0.001 |
| Intercept | 1 | 7056.206 | 1673.155 | 0.001 |
| Time | 4 | 1410.927 | 334.557 | 0.001 |
| pH | 2 | 670.532 | 158.995 | 0.001 |
| NaNO2 | 3 | 697.56 | 165.404 | 0.001 |
| Time × pH | 8 | 195.971 | 46.468 | 0.001 |
| Time × NaNO2 | 12 | 969.969 | 229.997 | 0.001 |
| Ph × NaNO2 | 6 | 319.657 | 75.796 | 0.001 |
| Time × pH × NaNO2 | 23 | 354.439 | 84.044 | 0.001 |
| Error | 121 | 4.217 |
| Source | df | Mean Square | F | Sig. |
|---|---|---|---|---|
| Corrected Model | 58 | 12.96 | 61.064 | 0.001 |
| Intercept | 1 | 623.051 | 2935.726 | 0.001 |
| Time | 4 | 96.425 | 454.34 | 0.001 |
| pH | 2 | 14.306 | 67.406 | 0.001 |
| NaNO2 | 3 | 2.291 | 10.793 | 0.001 |
| Time × pH | 8 | 11.654 | 54.913 | 0.001 |
| Time × NaNO2 | 12 | 6.459 | 30.432 | 0.001 |
| pH × NaNO2 | 6 | 4.465 | 21.039 | 0.001 |
| Time × pH × NaNO2 | 23 | 5.4 | 25.446 | 0.001 |
| Error | 121 | 0.212 |
| Source | df | Mean Square | F | Sig. |
|---|---|---|---|---|
| Corrected Model | 58 | 394.751 | 432.259 | 0.001 |
| Intercept | 1 | 34,471.143 | 37,746.486 | 0.001 |
| Time | 4 | 2721.773 | 2980.388 | 0.001 |
| pH | 2 | 251.208 | 275.078 | 0.001 |
| NaNO2 | 3 | 244.356 | 267.574 | 0.001 |
| Time × pH | 8 | 311.775 | 341.398 | 0.001 |
| Time × NaNO2 | 12 | 256.935 | 281.348 | 0.001 |
| pH × NaNO2 | 6 | 268.576 | 294.096 | 0.001 |
| Time × pH × NaNO2 | 23 | 156.744 | 171.637 | 0.001 |
| Error | 121 | 0.913 |
| Source | df | Mean Square | F | Sig. |
|---|---|---|---|---|
| Corrected Model | 58 | 41.465 | 491.993 | 0.001 |
| Intercept | 1 | 3315.377 | 39,337.969 | 0.001 |
| Time | 4 | 169.983 | 2016.898 | 0.001 |
| pH | 2 | 37.784 | 448.323 | 0.001 |
| NaNO2 | 3 | 32.664 | 387.568 | 0.001 |
| Time × pH | 8 | 40.713 | 483.067 | 0.001 |
| Time × NaNO2 | 12 | 33.487 | 397.338 | 0.001 |
| pH × NaNO2 | 6 | 43.742 | 519.018 | 0.001 |
| Time × pH × NaNO2 | 23 | 26.555 | 315.08 | 0.001 |
| Error | 121 | 0.084 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, R.; Yang, R.; Fu, Z.; Zhao, W.; Li, M.; Yu, G.; Ma, Z.; Zong, H. Changes in pH and Nitrite Nitrogen Induces an Imbalance in the Oxidative Defenses of the Spotted Babylon (Babylonia areolata). Antioxidants 2023, 12, 1659. https://doi.org/10.3390/antiox12091659
Ding R, Yang R, Fu Z, Zhao W, Li M, Yu G, Ma Z, Zong H. Changes in pH and Nitrite Nitrogen Induces an Imbalance in the Oxidative Defenses of the Spotted Babylon (Babylonia areolata). Antioxidants. 2023; 12(9):1659. https://doi.org/10.3390/antiox12091659
Chicago/Turabian StyleDing, Ruixia, Rui Yang, Zhengyi Fu, Wang Zhao, Minghao Li, Gang Yu, Zhenhua Ma, and Humin Zong. 2023. "Changes in pH and Nitrite Nitrogen Induces an Imbalance in the Oxidative Defenses of the Spotted Babylon (Babylonia areolata)" Antioxidants 12, no. 9: 1659. https://doi.org/10.3390/antiox12091659
APA StyleDing, R., Yang, R., Fu, Z., Zhao, W., Li, M., Yu, G., Ma, Z., & Zong, H. (2023). Changes in pH and Nitrite Nitrogen Induces an Imbalance in the Oxidative Defenses of the Spotted Babylon (Babylonia areolata). Antioxidants, 12(9), 1659. https://doi.org/10.3390/antiox12091659








