Individualised Exercise Training Enhances Antioxidant Buffering Capacity in Idiopathic Pulmonary Fibrosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility
2.3. Exercise Intervention
2.4. Measurements
2.4.1. Control Participants
2.4.2. IPF Participants
2.5. Outcomes
2.6. Redox Biomarker Analyses
2.7. Statistical Analysis
3. Results
3.1. Baseline Demographics
3.2. Baseline CPET in Control and IPF Patients
3.3. Systemic Redox Status in Control and IPF Patients at Baseline
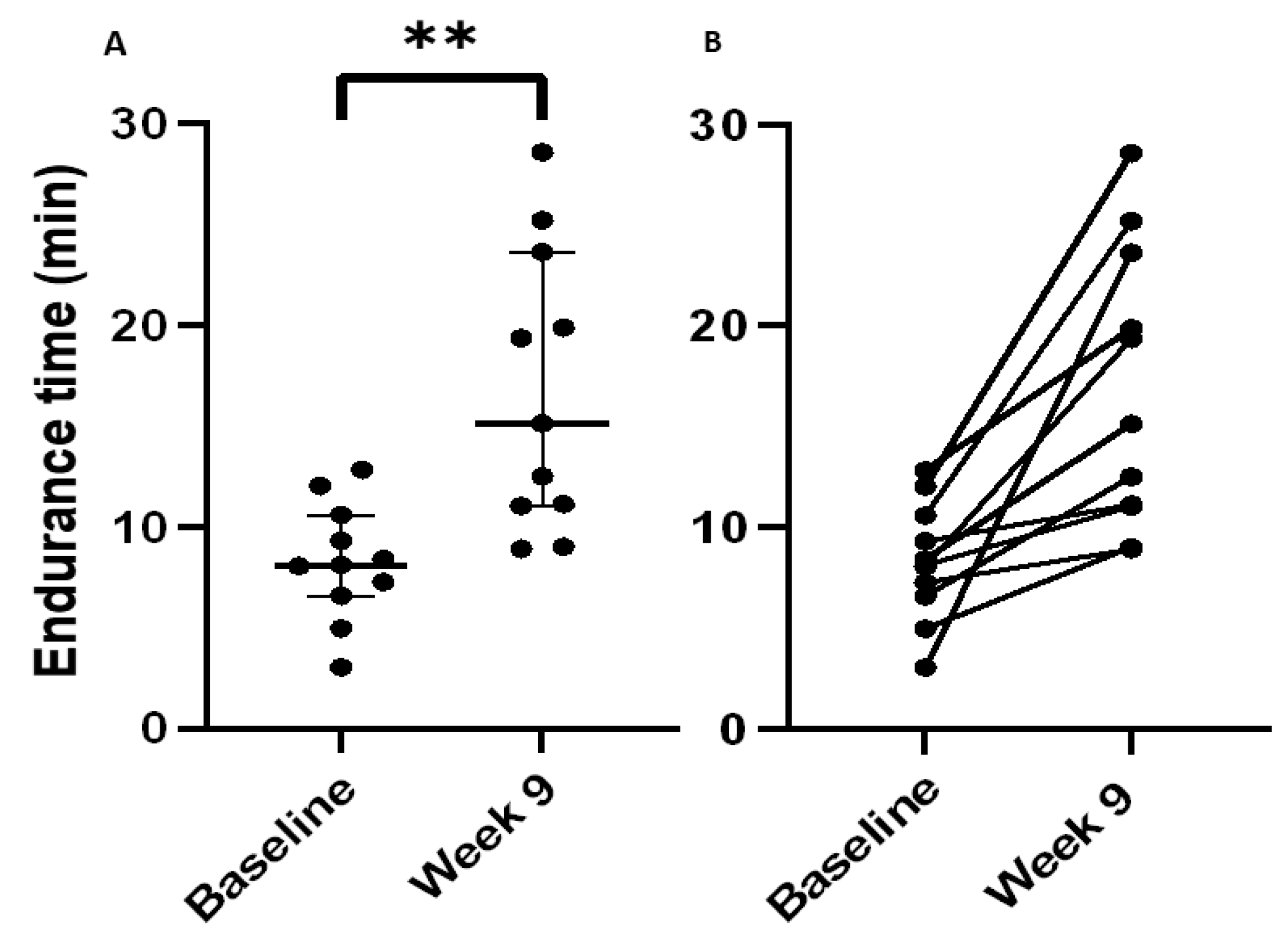
3.4. Physiological Outcomes following the SRETP in Patients with IPF
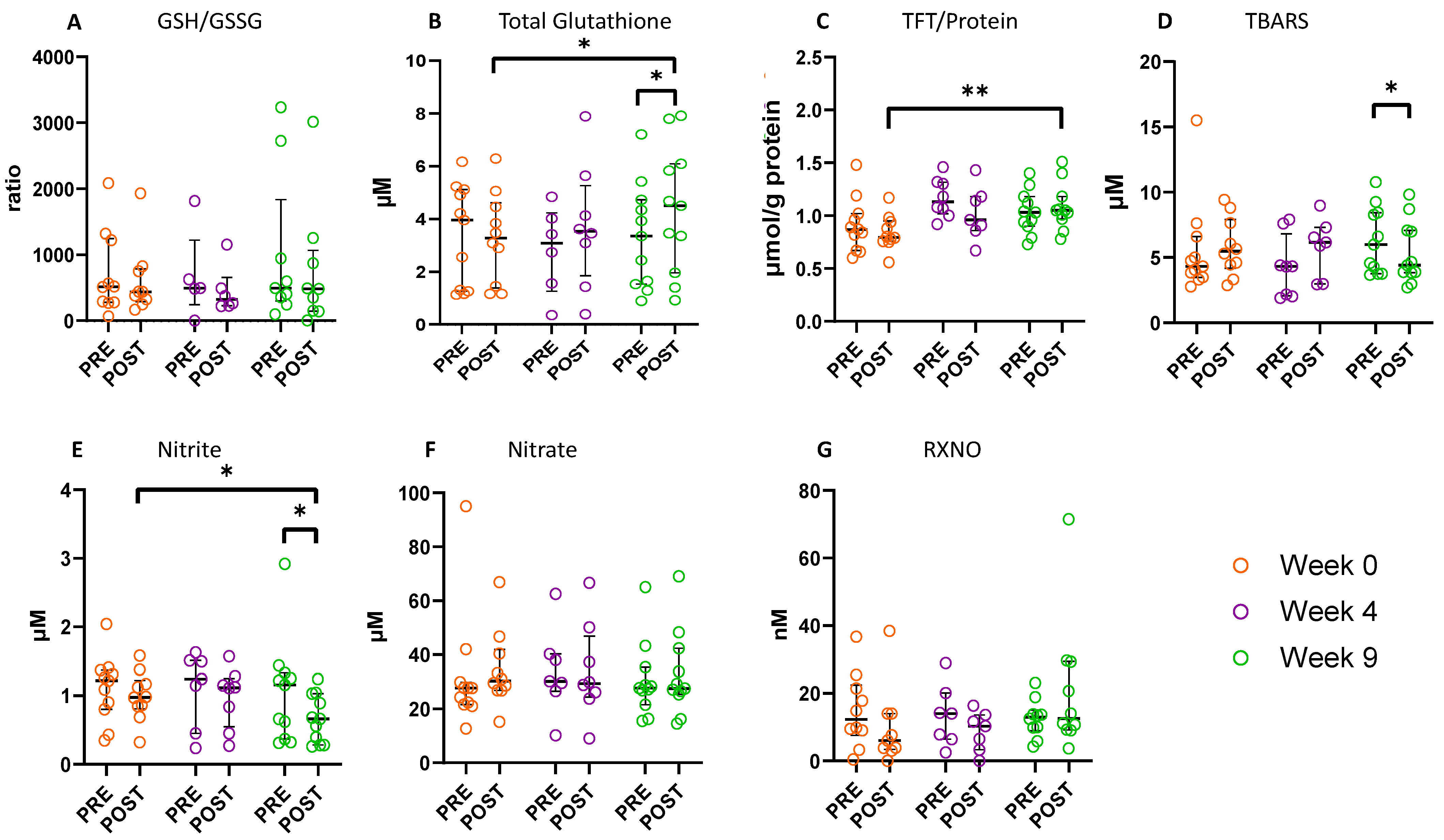
3.5. Effect of the SRETP on Systemic Redox Status
4. Discussion
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Jo, H.E.; Glaspole, I.; Grainge, C.; Goh, N.; Hopkins, P.M.; Moodley, Y.; Reynolds, P.N.; Chapman, S.; Walters, E.H.; Zappala, C.; et al. Baseline characteristics of idiopathic pulmonary fibrosis: Analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur. Respir. J. 2017, 49, 1601592. [Google Scholar] [CrossRef]
- Fernández-Fabrellas, E.; Molina-Molina, M.; Soriano, J.B.; Portal, J.A.R.; Ancochea, J.; Valenzuela, C.; Xaubet, A.; Aburto Barrenetxea, M.; Alfageme Michavila, I.; Bollo de Miguel, E.; et al. Demographic and clinical profile of idiopathic pulmonary fibrosis patients in Spain: The SEPAR National Registry. Respir. Res. 2019, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Swigris, J.J.; Kuschner, W.G.; Jacobs, S.S.; Wilson, S.R.; Gould, M.K. Health-related quality of life in patients with idiopathic pulmonary fibrosis: A systematic review. Thorax 2005, 60, 588–594. [Google Scholar] [CrossRef]
- Nathan, S.D.; Shlobin, O.A.; Weir, N.; Ahmad, S.; Kaldjob, J.M.; Battle, E.; Sheridan, M.J.; du Bois, R.M. Long-term Course and Prognosis of Idiopathic Pulmonary Fibrosis in the New Millennium. Chest 2011, 140, 221–229. [Google Scholar] [CrossRef]
- Banach, M.; Lewek, J.; Surma, S.; Penson, P.E.; Sahebkar, A.; Martin, S.S.; Bajraktari, G.; Henein, M.Y.; Reiner, Ž.; Bielecka-Dąbrowa, A.; et al. The association between daily step count and all-cause and cardiovascular mortality: A meta-analysis. Eur. J. Prev. Cardiol. 2023, zwad229, Epub ahead of print. [Google Scholar] [CrossRef]
- Olson, A.L.; Swigris, J.J.; Belkin, A.; Hannen, L.; Yagihashi, K.; Schenkman, M.; Brown, K.K. Physical functional capacity in idiopathic pulmonary fibrosis: Performance characteristics of the continuous-scale physical function performance test. Expert Rev. Respir. Med. 2015, 9, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wallaert, B.; Monge, E.; Le Rouzic, O.; Wémeau-Stervinou, L.; Salleron, J.; Grosbois, J.-M. Physical Activity in Daily Life of Patients With Fibrotic Idiopathic Interstitial Pneumonia. Chest 2013, 144, 1652–1658. [Google Scholar] [CrossRef]
- Chang, J.A.; Curtis, J.R.; Patrick, D.L.; Raghu, G. Assessment of health-related quality of life in patients with interstitial lung disease. Chest 1999, 116, 1175–1182. [Google Scholar] [CrossRef]
- du Bois, R.M.; Albera, C.; Bradford, W.Z.; Costabel, U.; Leff, J.A.; Noble, P.W.; Sahn, S.A.; Valeyre, D.; Weycker, D.; King, T.E., Jr. 6-Minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2014, 43, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Bolton, C.E.; Blakey, J.D.; Morgan, M.D. The British Thoracic Society guideline on pulmonary rehabilitation in adults: Your opinion is noted. Thorax 2014, 69, 388–389. [Google Scholar] [CrossRef][Green Version]
- Dowman, L.; Hill, C.J.; May, A.; Holland, A.E. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst. Rev. 2021, 2, Cd006322. [Google Scholar] [CrossRef]
- Dowman, L.M.; McDonald, C.F.; Hill, C.J.; Lee, A.L.; Barker, K.; Boote, C.; Glaspole, I.; Goh, N.S.; Southcott, A.M.; Burge, A.T.; et al. The evidence of benefits of exercise training in interstitial lung disease: A randomised controlled trial. Thorax 2017, 72, 610–619. [Google Scholar] [CrossRef]
- Dowman, L.; McDonald, C.; Hill, C.; Lee, A.; Burge, A.; Holland, A.E. Achieving the minimal important difference following exercise in ILD. TSANZ Oral Present Respirol. 2018, 23, 21–103. [Google Scholar]
- Rahman, I.; Skwarska, E.; Henry, M.; Davis, M.; O’Connor, C.M.; FitzGerald, M.X.; Greening, A.; MacNee, W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic. Biol. Med. 1999, 27, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, Y.; Sugino, K.; Ishida, F.; Tatebe, J.; Morita, T.; Homma, S. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir. Investig. 2016, 54, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Daniil, Z.D.; Papageorgiou, E.; Koutsokera, A.; Kostikas, K.; Kiropoulos, T.; Papaioannou, A.I.; Gourgoulianis, K.I. Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2008, 21, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Kawashima, T.; Kuwabara, R.; Hayakawa, S.; Irie, T.; Yoshida, T.; Rikitake, H.; Wakabayashi, T.; Okada, N.; Kawashima, K.; et al. Change in serum marker of oxidative stress in the progression of idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2015, 32, 1–6. [Google Scholar] [CrossRef]
- Jackson, R.; Ramos, C.; Gupta, C.; Gomez-Marin, O. Exercise decreases plasma antioxidant capacity and increases urinary isoprostanes of IPF patients. Respir. Med. 2010, 104, 1919–1928. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, R.M.; Gomez-Marin, O.W.; Ramos, C.F.; Sol, C.M.; Cohen, M.I.; Gaunaurd, I.A.; Cahalin, L.P.; Cardenas, D.D. Exercise limitation in IPF patients: A randomized trial of pulmonary rehabilitation. Lung 2014, 192, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Dowman, L.M.; McDonald, C.F.; Bozinovski, S.; Vlahos, R.; Gillies, R.; Pouniotis, D.; Hill, C.J.; Goh, N.S.L.; Holland, A.E. Greater endurance capacity and improved dyspnoea with acute oxygen supplementation in idiopathic pulmonary fibrosis patients without resting hypoxaemia. Respirology 2017, 22, 957–964. [Google Scholar] [CrossRef]
- de Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Wasfy, M.M.; Baggish, A.L. Exercise Dose in Clinical Practice. Circulation 2016, 133, 2297–2313. [Google Scholar] [CrossRef]
- Loughney, L.; West, M.A.; Moyses, H.; Bates, A.; Kemp, G.J.; Hawkins, L.; Varkonyi-Sepp, J.; Burke, S.; Barben, C.P.; Calverley, P.M.; et al. The effects of neoadjuvant chemoradiotherapy and an in-hospital exercise training programme on physical fitness and quality of life in locally advanced rectal cancer patients: A randomised controlled trial (The EMPOWER Trial). Perioper. Med. 2021, 10, 23. [Google Scholar] [CrossRef]
- West, M.A.; Loughney, L.; Lythgoe, D.; Barben, C.P.; Sripadam, R.; Kemp, G.J.; Grocott, M.P.; Jack, S. Effect of prehabilitation on objectively measured physical fitness after neoadjuvant treatment in preoperative rectal cancer patients: A blinded interventional pilot study. Br. J. Anaesth. 2015, 114, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.; Cellura, D.; Minnion, M.; Fernandez, B.O.; Spalluto, C.M.; Levett, D.; Bates, A.; Wallis, T.; Watson, A.; Jack, S.; et al. Exercise Training Induces a Shift in Extracellular Redox Status with Alterations in the Pulmonary and Systemic Redox Landscape in Asthma. Antioxidants 2021, 10, 1926. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br. J. Sports Med. 2016, 50, 1428–1437. [Google Scholar] [CrossRef]
- Koning, A.M.; Meijers, W.C.; Pasch, A.; Leuvenink, H.G.D.; Frenay, A.S.; Dekker, M.M.; Feelisch, M.; de Boer, R.A.; van Goor, H. Serum free thiols in chronic heart failure. Pharmacol. Res. 2016, 111, 452–458. [Google Scholar] [CrossRef]
- Sutton, T.R.; Minnion, M.; Barbarino, F.; Koster, G.; Fernandez, B.O.; Cumpstey, A.F.; Wischmann, P.; Madhani, M.; Frenneaux, M.P.; Postle, A.D.; et al. A robust and versatile mass spectrometry platform for comprehensive assessment of the thiol redox metabolome. Redox Biol. 2018, 16, 359–380. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159, e61122. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Denninger, J.W.; Marletta, M.A. Guanylate cyclase and the ⋅NO/cGMP signaling pathway. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1411, 334–350. [Google Scholar] [CrossRef]
- Puente-Maestu, L.; Villar, F.; de Miguel, J.; Stringer, W.W.; Sanz, P.; Sanz, M.L.; García de Pedro, J.; Martínez-Abad, Y. Clinical relevance of constant power exercise duration changes in COPD. Eur. Respir. J. 2009, 34, 340–345. [Google Scholar] [CrossRef]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44 (Suppl. S1), S35–S45. [Google Scholar] [CrossRef]
- Santolini, J.; Wootton, S.A.; Jackson, A.A.; Feelisch, M. The Redox architecture of physiological function. Curr. Opin. Physiol. 2019, 9, 34–47. [Google Scholar] [CrossRef]
- Feelisch, M.; Cortese-Krott, M.M.; Santolini, J.; Wootton, S.A.; Jackson, A.A. Systems redox biology in health and disease. EXCLI J. 2022, 21, 623–646. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.G.C.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef]
- Jones, D.P. [11] Redox potential of GSH/GSSG couple: Assay and biological significance. In Methods in Enzymology; Sies, H., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2002; Volume 348, pp. 93–112. [Google Scholar]
- Done, A.J.; Traustadóttir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Estornut, C.; Milara, J.; Bayarri, M.A.; Belhadj, N.; Cortijo, J. Targeting Oxidative Stress as a Therapeutic Approach for Idiopathic Pulmonary Fibrosis. Front. Pharmacol. 2022, 12, 794997. [Google Scholar] [CrossRef] [PubMed]
- Arizono, S.; Taniguchi, H.; Sakamoto, K.; Kondoh, Y.; Kimura, T.; Kataoka, K.; Ogawa, T.; Watanabe, F.; Nishiyama, O.; Nishimura, K.; et al. Endurance time is the most responsive exercise measurement in idiopathic pulmonary fibrosis. Respir. Care 2014, 59, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.C.; Burnley, M.; Vanhatalo, A.; Rossiter, H.B.; Jones, A.M. Critical Power: An Important Fatigue Threshold in Exercise Physiology. Med. Sci. Sports Exerc. 2016, 48, 2320–2334. [Google Scholar] [CrossRef]
- Casaburi, R.; Merrill, D.D.; Harding, G.; Leidy, N.K.; Rossiter, H.B.; Tal-Singer, R.; Hamilton, A. A Conceptual Framework for Use of Increased Endurance Time During Constant Work Rate Cycle Ergometry as a Patient-Focused Meaningful Outcome in COPD Clinical Trials. Chronic Obstr. Pulm. Dis. 2022, 9, 252–265. [Google Scholar] [CrossRef]
- Rassaf, T.; Lauer, T.; Heiss, C.; Balzer, J.; Mangold, S.; Leyendecker, T.; Rottler, J.; Drexhage, C.; Meyer, C.; Kelm, M. Nitric oxide synthase-derived plasma nitrite predicts exercise capacity. Br. J. Sports Med. 2007, 41, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Miller, E.M.; Schwark, E.; Robbins, J.L.; Duscha, B.D.; Annex, B.H. Plasma nitrite response and arterial reactivity differentiate vascular health and performance. Nitric Oxide 2009, 20, 231–237. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Umbrello, M.; Dyson, A.; Feelisch, M.; Singer, M. The key role of nitric oxide in hypoxia: Hypoxic vasodilation and energy supply-demand matching. Antioxid. Redox Signal. 2013, 19, 1690–1710. [Google Scholar] [CrossRef]
- Elshazly, M.; Hosny, H.; Abdel-Hafiz, H.; Zakaria, A.; Elkaffas, K.; Okasha, N. Assessment of endothelial dysfunction in idiopathic pulmonary fibrosis. Egypt. J. Chest Dis. Tuberc. 2013, 62, 589–592. [Google Scholar] [CrossRef]
- Avdeev, S.; Makarova, M.; Chuchalin, A. Arterial stiffness and endothelial dysfunction in idiopathic pulmonary fibrosis (IPF). Eur. Respir. J. 2011, 38 (Suppl. S55), 647. [Google Scholar]
- Bhayadia, R.; Schmidt, B.M.W.; Melk, A.; Hömme, M. Senescence-Induced Oxidative Stress Causes Endothelial Dysfunction. J. Gerontol. Ser. A 2016, 71, 161–169. [Google Scholar] [CrossRef]
- Tiso, M.; Schechter, A.N. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS ONE 2015, 10, e0119712. [Google Scholar] [CrossRef]
- Srihirun, S.; Park, J.W.; Teng, R.; Sawaengdee, W.; Piknova, B.; Schechter, A.N. Nitrate uptake and metabolism in human skeletal muscle cell cultures. Nitric Oxide 2020, 94, 1–8. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.; Sue, D.Y.; Stringer, W.; Sietsema, K.; Sun, X.-G.; Whipp, B.J. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications, 5th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Levett, D.Z.H.; Jack, S.; Swart, M.; Carlisle, J.; Wilson, J.; Snowden, C.; Riley, M.; Danjoux, G.; Ward, S.A.; Older, P.; et al. Perioperative cardiopulmonary exercise testing (CPET): Consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br. J. Anaesth. 2018, 120, 484–500. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; American College of Chest Physicans. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir. J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [PubMed]
- Stenton, C. The MRC breathlessness scale. Occup. Med. 2008, 58, 226–227. [Google Scholar] [CrossRef]
- Yorke, J.; Jones, P.W.; Swigris, J.J. Development and validity testing of an IPF-specific version of the St George′s Respiratory Questionnaire. Thorax 2010, 65, 921–926. [Google Scholar] [CrossRef]
- Ellis, R. The use of N-ethylmaleimide in stabilizing and measuring inorganic sulphur compounds. Biochem. J. 1968, 110, 43P. [Google Scholar] [CrossRef]
- Rassaf, T.; Bryan, N.S.; Kelm, M.; Feelisch, M. Concomitant presence of N-nitroso and S-nitroso proteins in human plasma. Free Radic. Biol. Med. 2002, 33, 1590–1596. [Google Scholar] [CrossRef]
- Feelisch, M.; Rassaf, T.; Mnaimneh, S.; Singh, N.; Bryan, N.S.; Jourd′Heuil, D.; Kelm, M. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: Implications for the fate of NO in vivo. Faseb J. 2002, 16, 1775–1785. [Google Scholar] [CrossRef]
- Hansen, J.E.; Sue, D.Y.; Oren, A.; Wasserman, K. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am. J. Cardiol. 1987, 59, 669–674. [Google Scholar] [CrossRef]
- Mitchell, S.H.; Steele, N.P.; Leclerc, K.M.; Sullivan, M.; Levy, W.C. Oxygen Cost of Exercise Is Increased in Heart Failure After Accounting for Recovery Costs. Chest 2003, 124, 572–579. [Google Scholar] [CrossRef] [PubMed]



| Demographics (n = 15) | |
|---|---|
| Age (years) | 72.5 (69–80) |
| Male | 87% (13) |
| BMI (kg·m−2) | 28.0 (26–32) |
| FVC% predicted | 78 (73–88) |
| FEV1/FVC | 0.82 (0.79–0.84) |
| TLCO% predicted | 52.0 (41.0–62.0) |
| MRC Breathlessness Score | 1–7% (n = 1) 2–40% (n = 6) 3–53% (n = 8) |
| Antifibrotics | 33% (5) |
| Ex-smoker | 67% (10) |
| Never-smoker | 33% (5) |
| COPD | 20% (3) |
| T2DM | 20% (3) |
| IHD | 13% (2) |
| Baseline exercise capacity (n = 15) | |
| Endurance time (min) | 8.05 (5.8–10.6) |
| O2peak % predicted | 66.7 (57.1–75.9) |
| Peak WR (Watts) | 90 (66–112) |
| 75% O2peak WR (Watts) | 65 (45–85) |
| Variable (Unit) | Median Difference (IQR) | p Value |
|---|---|---|
| Endurance time (minutes) | +6.7 (2.9–14.6) | 0.003 ** |
| FVC% predicted (%) | +5 (1–5) | 0.023 * |
| TLCO% predicted (%) | −2 (−5–+2) | 0.121 |
| BMI | −0.26 (−1.25–+1.00) | 0.213 |
| AT (mL·kg−1·min−1) | −0.28 (−1.1–+0.46) | 0.484 |
| O2peak (mL·kg−1·min−1) | +0.60 (−0.5–+1.2) | 0.328 |
| Peak WR (Watts) | +8 (6–16) | 0.010 * |
| E/CO2 slope | +2.9 (−6.0–+7.8) | 0.286 |
| peak E (l·min−1) | +9 (2–19) | 0.033 * |
| SpO2 at O2peak (%) | −2.0 (−5 to 4) | 0.789 |
| SGRQ-I Total Score | −3.9 (−11–+10) | 0.790 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallis, T.J.M.; Minnion, M.; Freeman, A.; Bates, A.; Otto, J.M.; Wootton, S.A.; Fletcher, S.V.; Grocott, M.P.W.; Feelisch, M.; Jones, M.G.; et al. Individualised Exercise Training Enhances Antioxidant Buffering Capacity in Idiopathic Pulmonary Fibrosis. Antioxidants 2023, 12, 1645. https://doi.org/10.3390/antiox12081645
Wallis TJM, Minnion M, Freeman A, Bates A, Otto JM, Wootton SA, Fletcher SV, Grocott MPW, Feelisch M, Jones MG, et al. Individualised Exercise Training Enhances Antioxidant Buffering Capacity in Idiopathic Pulmonary Fibrosis. Antioxidants. 2023; 12(8):1645. https://doi.org/10.3390/antiox12081645
Chicago/Turabian StyleWallis, Tim J. M., Magdalena Minnion, Anna Freeman, Andrew Bates, James M. Otto, Stephen A. Wootton, Sophie V. Fletcher, Michael P. W. Grocott, Martin Feelisch, Mark G. Jones, and et al. 2023. "Individualised Exercise Training Enhances Antioxidant Buffering Capacity in Idiopathic Pulmonary Fibrosis" Antioxidants 12, no. 8: 1645. https://doi.org/10.3390/antiox12081645
APA StyleWallis, T. J. M., Minnion, M., Freeman, A., Bates, A., Otto, J. M., Wootton, S. A., Fletcher, S. V., Grocott, M. P. W., Feelisch, M., Jones, M. G., & Jack, S. (2023). Individualised Exercise Training Enhances Antioxidant Buffering Capacity in Idiopathic Pulmonary Fibrosis. Antioxidants, 12(8), 1645. https://doi.org/10.3390/antiox12081645





