Abstract
Exercise produces oxidants from a variety of intracellular sources, including NADPH oxidases (NOX) and mitochondria. Exercise-derived reactive oxygen species (ROS) are beneficial, and the amount and location of these ROS is important to avoid muscle damage associated with oxidative stress. We discuss here some of the evidence that involves ROS production associated with skeletal muscle contraction and the potential oxidative stress associated with muscle contraction. We also discuss the potential role of H2O2 produced after NOX activation in the regulation of glucose transport in skeletal muscle. Finally, we propose a model based on evidence for the role of different populations of mitochondria in skeletal muscle in the regulation of ATP production upon exercise. The subsarcolemmal population of mitochondria has the enzymatic and metabolic components to establish a high mitochondrial membrane potential when fissioned at rest but lacks the capacity to produce ATP. Calcium entry into the mitochondria will further increase the metabolic input. Upon exercise, subsarcolemmal mitochondria will fuse to intermyofibrillar mitochondria and will transfer the mitochondria membrane potential to them. These mitochondria are rich in ATP synthase and will subsequentially produce the ATP needed for muscle contraction in long-term exercise. These events will optimize energy use and minimize mitochondria ROS production.
1. Introduction
Since the first report that muscular physiology is redox-dependent, several research lines in redox biology have grown significantly. Skeletal muscle is the human buffer against aging, some metabolism pathologies, and neuropathologies, so the understanding of how skeletal physiology processes are modulated by reactive oxygen species (ROS) is a source of potential intervention based on diet supplementation or exercise protocols. Evidence suggests that exercise produces oxidants from a variety of intracellular sources, including NADPH oxidases (NOX) and mitochondria as the main sources [1,2]. Exercise-derived ROS are beneficial, and the amount and location of these ROS is important to avoid muscle damage associated with oxidative stress. In this review, we propose a model in which the role of the fusion of different subpopulations of mitochondria after exercise is relevant, increasing energy saving and decreasing H2O2 production.
2. An Energy-Saving Mechanism in Skeletal Muscle
2.1. Mitochondria Distribution and Characteristics in Skeletal Muscle
Skeletal muscle fibers can change from a preferentially glycolytic metabolism to oxidative metabolism upon certain types of physiological exercise, a process for which the cellular mechanism needs to be better understood. In turn, the excitation–contraction (EC) mechanism is connected to a network of mitochondria in skeletal muscle fibers, showing a physiological communication between contractile machinery and mitochondria, in which Ca2+ has a relevant role. Moreover, mitochondria are mobile and plastic organelles, constantly changing shape, fusing, or fissioning with each other, and changing their role in parallel in cellular bioenergetics [3].
Adult skeletal muscle presents two populations of mitochondria: one group comprises both perinuclear (PN) and perivascular (PV) mitochondria, both considered peripherally located mitochondria (PLM) or subsarcolemmal mitochondria (SSM); the other group is called intermyofibrillar (IMF) mitochondria (see Figure 1) [4]. The SSM population comprises the mitochondria located beneath the plasma membrane of the muscle fiber (and, as nuclei have a similar location in adult skeletal muscle, mitochondria closely surrounding the myonuclei) and the IMF population of mitochondria, located regularly close to the sarcoplasmic reticulum terminal cisternae and the triad (the grouping of two terminal cisternae an one transverse tubule), at regular intervals along every myofibril [5,6].
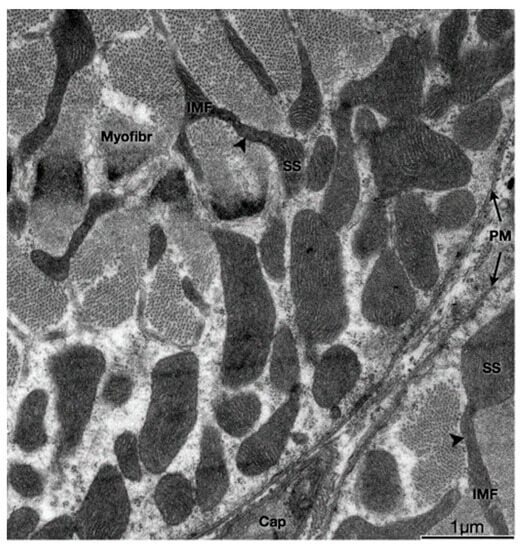
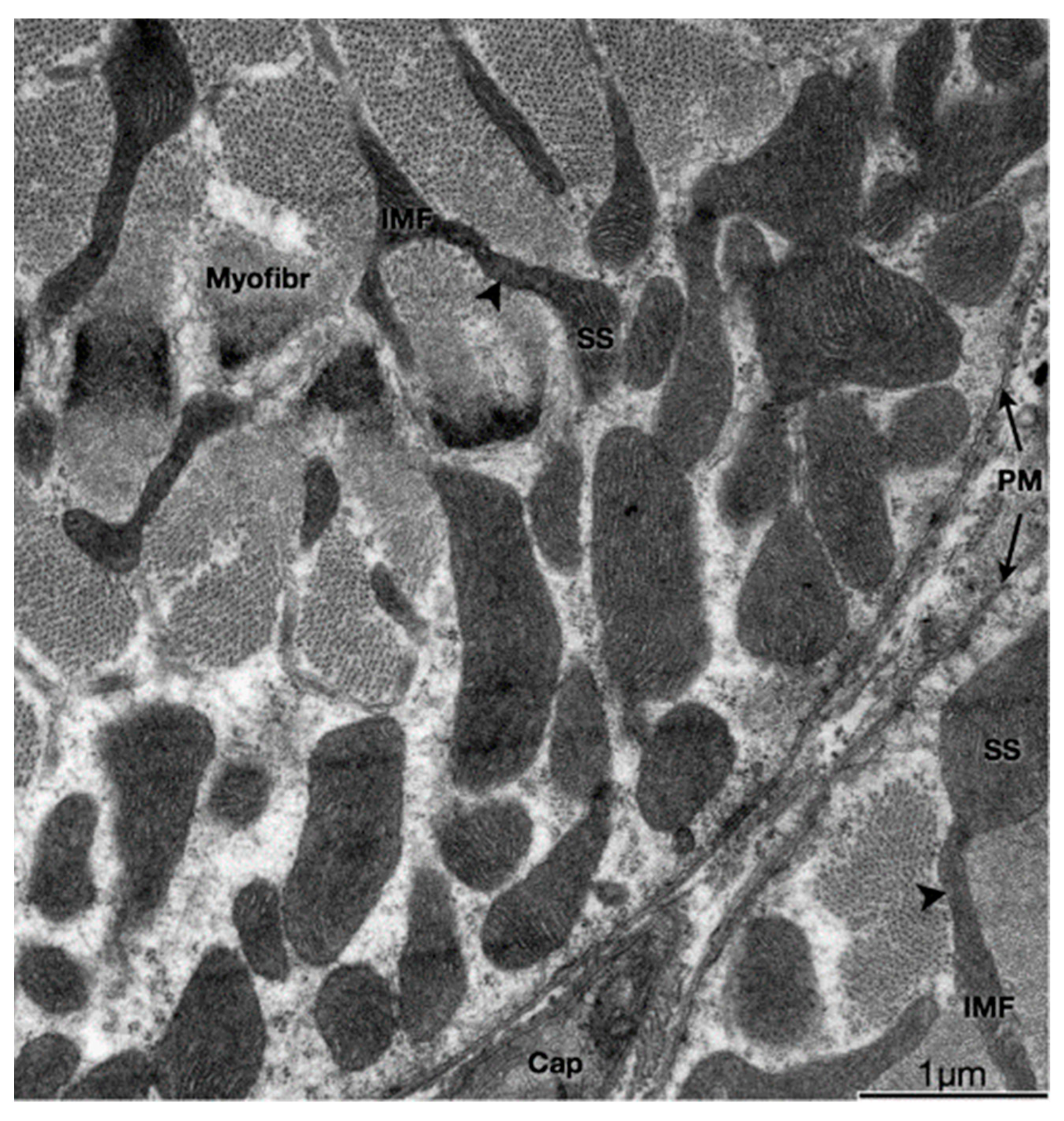
Figure 1.
Physical interactions between SS and IMF mitochondria. Transmission electron micrograph of myofibers in the transverse plane. SS and IMF mitochondria are distinct organelles. Some SS and IMF mitochondria form continuous organelles (arrowheads) that coexist in both subcellular compartments. SS, Subsarcolemmal; IMF, intermyofibrillar; PM, plasma membrane (sarcolemma); Myofibr, myofibrils; Cap, capillary (Reproduced with permission from M. Picard (J. Appl. Physiol., published by American Physiological Society, 2013) [7]).
IMF mitochondria form a structural arrangement characterized by the interaction of transverse mitochondrial tubules in the sarcomere, called “mitochondrial reticulum”, which has been proposed as an energetic conductive pathway from mitochondria to the contractile apparatus [4]. Specialized proteins play roles such as intermembrane linkers, which are formed by a single protein with two membrane-interacting domains in the sarcoplasmic reticulum [8]. This physical proximity, such as voltage-dependent anion channel (VDAC) and RyR1 or IP3R, allows the passage of calcium from the reticulum to the mitochondria to be more effective. It has also been described that H2O2 would diffuse from the mitochondrial space towards these contact domains to modulate Ca2+ release locally [8,9]. It is very interesting to note that these two populations of mitochondria with different membrane potential not only contribute both to muscle fiber oxidative capacity and bioenergetics but also do so each in a specialized way, allowing in the end, to optimize ATP production precisely where is needed for contraction, i.e., near the myofibrils, by producing mitochondria membrane potential propagation. In agreement with this, it has been proposed that SSM supports the IMF energy, based on the presence of higher oxidative enzyme activity [10].
Together with the different localization and oxidative capacity, both populations express different types of proteins and different membrane potentials. For example, SSM expresses mitochondrial calcium uniporter regulator 1 (MICU1) [11]. Mitochondrial calcium uniporter (MCU) is a highly selective and highly regulated calcium channel inserted in the inner mitochondrial membrane that allows calcium uptake from the cytosol to mitochondria after contraction [10,12]. This complex (when regulated by MICU1) is basally closed and is activated upon cytoplasmic Ca2+ increases, allowing then the ion to enter the mitochondria matrix. ROS production is normally high when the mitochondrial potential (ΔΨ) is elevated. Therefore, MICU1 expression, by regulating Ca2+ entry, would protect against mitochondrial H2O2-generated damage [13].
This idea is consistent with the evidence showing that, in neurons, MCU promoted the activity of the electron transport chain and the chemical reduction of NAD+ to NADH, which would imply that electrons are not available for ROS formation [14]. Moreover, it is important to highlight that high MCU activity also induces mitochondrial membrane potential dissipation, facilitating the activity of the ATP synthase [15]. It has been observed that the lower expression of MCU generates muscle atrophy, intimately relating the regulation of mitochondrial Ca2+ to the size of the muscle fiber [16]. The action of MCU is crucial for ATP synthesis in aerobic conditions because Ca2+ increase is an essential cofactor for the tricarboxylic acid (TCA) cycle’s enzymes such as glycerol phosphate dehydrogenase (GPDH), pyruvate dehydrogenase (PDH), isocitrate dehydrogenase (ICDH), and α-ketoglutarate dehydrogenase (α-KGDH) [17]. Thus, Ca2+ increase can elevate the efficiency of complexes I, III, and IV of OXPHOS. The Ca2+-induced activation of mitochondrial sodium–calcium exchanger (NCLX) results in a Na+ influx into the matrix, and Na+ interacts with phospholipids in the inner leaflet of the inner membrane, decreases its fluidity, and slows down ubiquinol (UQH2) diffusion, increasing electron flux due to major NADH availability. In simple words, calcium potentiates the establishment of the proton gradient needed for ATP synthesis, which is used for sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) to pump back calcium from the cytosol to the SR [18].
When we talk about muscle activity, it is important to remember that there are different types of muscle fibers (as different types of motor units). Slow, fatigue-resistant fibers possess a larger number of mitochondria, having a preferential oxidative metabolism that allows them to contract for long periods of time. Fast, fatigable fibers have lower mitochondria content, relying on an anaerobic glycolytic metabolism, which is responsible for their poor fatigue resistance. These different types of muscle fibers can change from one phenotype to another depending on external demands. One of the main actors in maintaining or changing the muscle phenotype is the pattern of stimulation coming from motor neurons. In this way, it has been shown that low-frequency electrical stimulation induces the expression of genes belonging to the slow phenotype, while high frequencies lead to the expression of genes typical for fast phenotypes. Among the slow phenotype transcriptional profile, we can find slow isoforms of proteins from the contractile apparatus and of factors that lead to increased mitochondrial biogenesis as well as oxidative enzymes [19,20]. Interestingly, Quezada et al. have also shown that low-frequency electrical stimulation (which allows fibers to convert to slow-phenotype fibers) can decrease the expression of the MCU complex in isolated adult fibers [21].
Higher protein levels of MCU and MICU1 per mitochondrion have been observed in fast-phenotypes muscles (like flexor digitorium longus) than in slow phenotype muscles, such as the soleus. From these data, we can propose a hypothesis in which the decrease in mRNA of the MCU complex after the low-frequency electrical stimulation of isolated fibers from a fast muscle would favor a lower protein level of MCU and MICU1 per mitochondrion, being an early metabolic response to the phenotypic shift from fast to slow phenotype muscle fiber [22]. Also, a gradual increase in the number of mitochondria, together with a decrease in levels of the MCU complex in response to a low-frequency electrical stimulus, could allow adapting mitochondrial Ca2+ homeostasis to finally reach that of a slow muscle. On the other hand, Mcu gen deletion produces a decrease in Ca2+-stimulated ATP synthesis, impairment in TCA cycle substrate flux, and a turn toward fatty acid metabolism [12].
These data suggest that both mitochondria calcium transients and the total volume of mitochondria are somehow conjointly modulating metabolism to provide either a fast-fatigable or a slow-fatigue resistant response.
2.2. Function of the Heterogeneity of Mitochondria within the Muscle Fiber
The SS mitochondria are quite different to the IMF mitochondria [7,11,23]. It has been shown that electron transport chain elements needed to establish the mitochondria membrane potential are differentially distributed among SSM and IMF; in particular, complex IV and cytochrome C are located mostly in the SSM [11,23]. There are also differences in the distribution of complex V (ATP synthase), which is located mostly in the IMF mitochondria [23]. In contrast, the importin translocase of the outer membrane, TOM20, has homogeneous distribution in all the cell’s mitochondria, suggesting a specific compartmentalization of different mitochondrial proteins between mitochondria subpopulations [11].
This evidence, together with the fact that there is a shift in mitochondria membrane potential (higher in SSM in resting conditions) towards the center of the muscle fiber upon electrical stimulation [11], prompted us to propose that, in the SSM pool, the main proton motive force is generated by the activation of complexes I-IV. In contrast, ATP is generated in complex V, which is in the IMF mitochondria, and this ATP production will occur only after the fusion of both mitochondria populations with the consequent spreading of mitochondria membrane potential. As mitochondria membrane potential is established across the inner mitochondria membrane, it can only propagate via the fusion of mitochondria membranes. Mitochondrial ROS production will be minimal since the proton motive force is used for ATP synthesis when the electron transport chain works at a maximum level induced by exercise.
In fact, Mcu deletion produces a decrease in Ca2+-stimulated ATP synthesis, impairment in TCA cycle substrate flux, and a turn toward fatty acid metabolism [12]. Stimuli, such as extracellular ATP or electrical stimulation, can increase the expression of MCU in isolated adult fibers [21]. In turn, it has been shown that the MCU-dependent increase in mitochondrial ROS is necessary for optimal skeletal muscle repair after an injury via the induction of actin polymerization dependent on RhoA [24]. It is interesting to note that, using the direct measurement of superoxide via electron paramagnetic resonance, Crochemore et al. demonstrated that SSM produce more superoxide than IMF mitochondria [25].
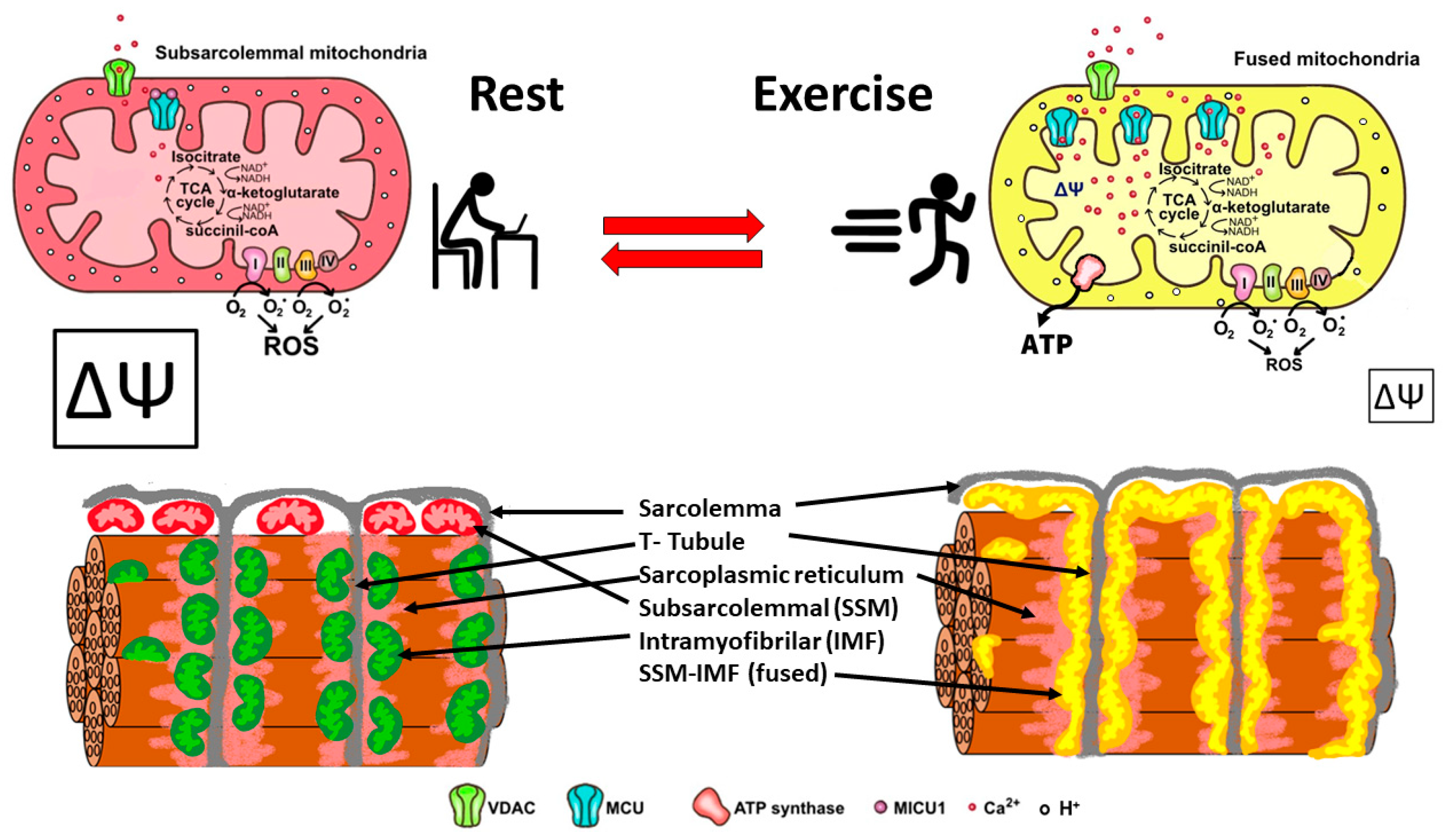
In summary, skeletal muscle mitochondria achieve this performance by separating two important functions: the first is mitochondria membrane potential generation via calcium-sensitive oxidative phosphorylation (which occurs mainly in the subsarcolemmal population of mitochondria), and the second function is ATP production mediated by ATP synthase, which occurs in the intermyofibrillar population of mitochondria. To achieve this amazing performance, the two populations of mitochondria are not connected at rest and have a different resting protein composition. The intermyofibrillar mitochondria are enriched in ATP synthase and have a high content of mitochondrial calcium uptake 1 (MICU1), MICU1, as a key regulator of mitochondrial Ca2+ uptake, which negatively regulates calcium entry into the mitochondrial matrix through the MCU calcium channel [26,27]. This protein is a Ca2+ sensor, and its functioning does not impact ΔΨ or oxygen consumption [26,28]. On the other hand, the subsarcolemmal mitochondria are enriched in the electron transport chain complex proteins and, having no MICU1, can reach a high calcium content upon muscle activation (Figure 2). The model in Figure 2 shows that exercise induces the mitochondrial fusion of SSM and IMF, transferring electrical properties and proteins from SSM to the mitochondrial network, decreasing the ROS generation, and improving energy-saving design. This process is reversible, and, consequently, in resting conditions after exercise, the fission of mitochondria will occur, and the high mitochondria membrane potential in SSM can be recovered.
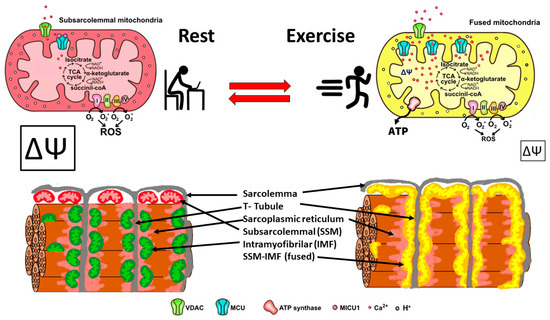
Figure 2.
Energy efficient distribution of mitochondria that saves energy and minimizes mitochondria ROS production. Under resting conditions, skeletal muscle has different pools of mitochondria, SSM have a high mitochondrial potential (ΔΨ) and a basal rate of ROS generation from superoxide anions generated by the electron transport chain. Thus, the respiratory chain may allow the “leakage of electrons” and generate ROS. IMF mitochondria have a high expression of MICU1, which prevents calcium from massively entering the mitochondrial space. A condition of exercise implies that the SSM and the IMF mitochondria fuse, generating a network characterized by a lower density of MICU1, a high density of MCU, and an extended proton gradient (enough ΔΨ) needed to generate ATP and to minimize ROS generation. Colors of mitochondria represent diversity (red and green), and yellow represents fused mitochondria.
2.3. Mitochondria Dynamics Are Altered in Skeletal Muscle of Aging Subjects and in Pathological Conditions
Mitochondrial fusion events in skeletal muscle fibers are hard to evidence due to the highly restricted space in which IMF mitochondria are located; fusion events nevertheless take place, and they were shown for the first time by Eisner et al. in 2014 [29].
When we consider the above-described model, it is reasonable to assume that mitochondria dynamics (fission and fusion) play an essential role in skeletal muscle function and wellbeing. Maintaining optimal skeletal muscle health requires dynamic mitochondrial function, which becomes disrupted in aging and various pathological conditions. Age-related mitochondrial dysfunction is characterized by impaired fusion/fission processes and mitophagy, leading to sarcopenia and reduced exercise capacity. In addition, diseases such as muscular dystrophies, mitochondrial myopathies, and type 2 diabetes exhibit mitochondrial fusion and fission imbalances, contributing to impaired excitation–transcription coupling (ETC, see Figure 3).
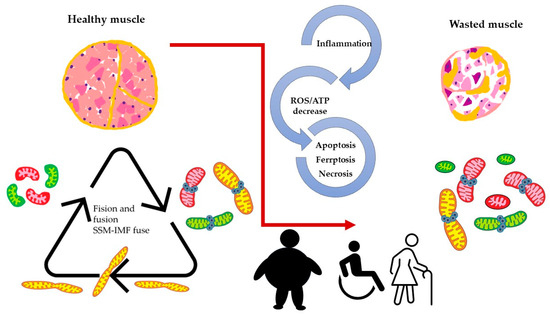
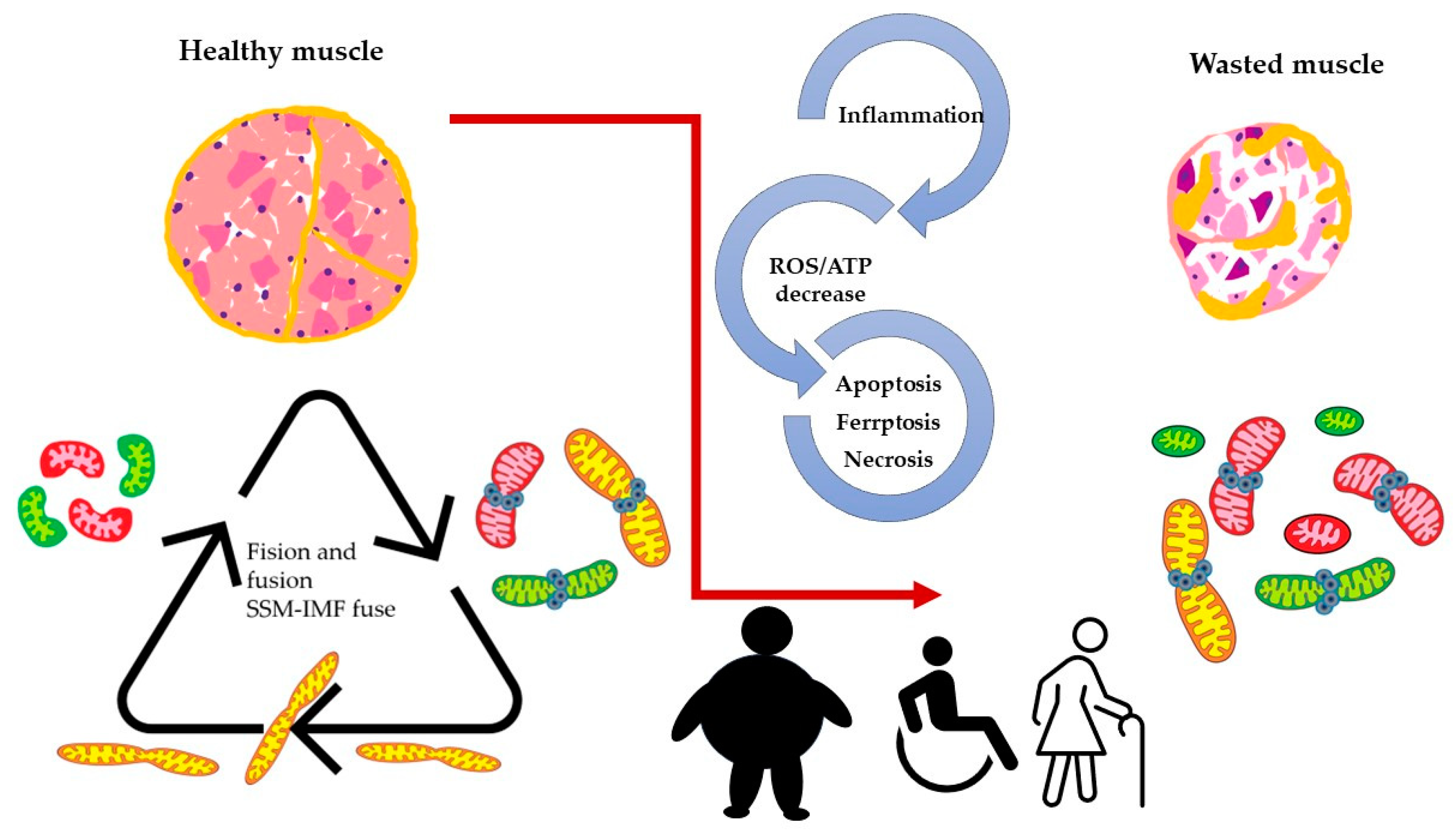
Figure 3.
Altered dynamics and fusion capacity of mitochondria in disease. A common factor in many pathological conditions affecting skeletal muscle is an imbalance in mitochondrial dynamics. This imbalance is both a cause and consequence of inflammation, increased ROS production, and cell death, leading to muscle deterioration characterized by dysfunctional mitochondria. Different colors of mitochondria refer to SSM, IFM and fused mitochondria as pictures in Figure 2.
Alterations in mitochondria dynamics have been shown to occur in middle age and more advanced age in mice [30], and they parallel dramatic decreases in muscle function. This was evidenced by changes in mitochondria orientation as seen by confocal microscopy, changes in both mitochondria size and shape as seen by electron microscopy, and changes in the expression of proteins involved in fission and fusion processes. Furthermore, mitochondria dynamics appeared to be altered in the skeletal muscle of a mouse model of alcohol consumption [29]. Oxidative stress and reduced antioxidants reinforce mitochondrial fragmentation, suppress fusion/fission, and impair the electron transport chain, decrease ATP production, and cause DNA damage. Mitochondrial dynamics depend on proteins such as mitofusin (MFN) 1 and 2 and optic atrophy protein 1(OPA1) for the fusion of the outer and inner mitochondrial membranes. Mitochondrial fission involves the recruitment of cytoplasmic Drp1 to the mitochondrial outer membrane, forming a ring-like structure with adaptors Mff, MiD49, and MiD51. As schematized in Figure 3, these protein interactions ensure a balance between fusion and fission, which is crucial for maintaining mitochondrial dynamics and cellular functions [31]. The deletion of OPA1 leads to mitochondrial dysfunction, reduced myogenic stem cells, decreased protein synthesis, and the activation of protein breakdown. Opa1 deletion in skeletal muscle affects the entire body, causing a premature aging phenotype that ultimately leads to animal death [32]. Opa1 deficiency in myopathy engages TLR9, activating NF-κB and triggering muscle inflammation. This localized inflammation can become systemic, impacting the entire body. An altered growth hormone/IGF1 axis and enhanced FGF21 expression are observed, contributing to impaired growth. Opa1 deficiency disrupts growth-related processes and promotes inflammation via inflammatory pathway activation. These processes collectively contribute to the development and progression of myopathy [33]. Aging is associated with sarcopenia, and when obesity is associated, the term sarcopenic obesity (SO) is applied. Dysfunctional adipose tissue, fatty acid excess inside the bloodstream, and low-grade systemic inflammation are combined, resulting in lipotoxicity, oxidative stress, insulin resistance, and inflammation in the skeletal muscle [34]. It has been reported that a high-fat diet suppresses mitochondrial biogenesis in the skeletal muscle of zebrafish and decreases the number of SSM, Mfn2, and OPA1. On the contrary, Fis1 and Drp1, both fission proteins, were found to be increased in an obese mouse model, as opposed to in non-obese conditions in mice [31]. On the other hand, mitochondrial uncoupling attenuates SO by enhancing skeletal muscle mitophagy, reducing muscle inflammation, promoting mitochondrial turnover via STAT3 signaling, and mitigating muscle degradation [35].
Exercise enhances the healthy mitochondrial network, promoting fusion/fission markers and biogenesis. Sarcopenia diminishes mitochondrial dynamics, mitophagy markers, and network efficiency, while exercise stimulates mitochondrial biogenesis via PGC1-α activation. Also, PGC-1α overexpression mitigates age-related increases in mitophagy markers, including Fis1 and Drp1 proteins, improves mitochondrial function, and reduces oxidative damage in mouse muscle [36]. Moderate-intensity exercise can be a non-invasive treatment, activating pathways that regulate the mitochondrial network in skeletal muscle [37]. Reduced MICU3 expression during aging leads to decreased mitochondrial Ca2+ uptake, and studies on aged mice and senescent C2C12 cells revealed that MICU3 downregulation is associated with decreased myogenesis, increased ROS, and apoptosis. Restoring MICU3 levels increases antioxidant defenses and promotes myogenesis. These findings highlight that MICU3 is a contributing factor to ROS production and apoptotic processes during aging [38]. In the same line, mitochondrial dynamics, and ROS production play crucial roles in the pathogenesis of Duchenne muscular dystrophy (DMD), a genetic skeletal muscle disorder characterized by mutations in the gene that encodes dystrophin. Studies have shown that DMD patients present disruptions in mitochondrial fusion and fission processes, leading to mitochondrial dysfunction and generating fragmented and dysfunctional mitochondria in muscle fibers. Intracellular Ca2+ disruption also has been reported to be derived from both increased Ca2+ influx and altered calcium release, leading to abnormally elevated resting cytosolic Ca2+ concentration [39]. Consequently, these effects contribute to the apoptosis of muscle cells. These abnormalities increase ROS production and oxidative stress, compromise energy production, and impair cellular signaling, exacerbating muscle weakness and degeneration in DMD. Recent studies have highlighted the involvement of ferroptosis, a form of regulated cell death involving iron-dependent lipid peroxidation, in DMD pathology [40,41]. The dysregulation of NRF2 in DMD may promote ferroptosis in muscle cells by contributing to increased susceptibility to cell death. In turn, this can exacerbate muscle degeneration and inflammation observed in DMD.
Some studies have reported cases of statin-induced, muscle-related side effects, including myopathy or rhabdomyolysis. Atorvastatin dose-dependently inhibits C2C12 cell viability, resulting in increased intracellular iron ions, ROS, and lipid peroxidation. These effects primarily occur in mitochondria, leading to mitochondrial dysfunction. Biomarkers of myocardial injury are elevated during atorvastatin treatment, but ferroptosis inhibitors can counteract these effects [42]. Mechanistically, GSH depletion, along with the decrease in Nrf2, GPx4, and xCT cystine-glutamate antiporter, contributes to atorvastatin-induced muscular cell ferroptosis and damage [42]. Moreover, an increase in iron overload, senescence, and muscle atrophy markers was found in old senescence-accelerated mouse-prone 8 (SAMP8), a sarcopenia-like phenotype, suggesting that iron-overload-induced ferroptosis plays an essential role in sarcopenia [43].
3. H2O2-Mediated Physiological Signals in Skeletal Muscle
3.1. Role of H2O2 in the Excitation–Contraction (EC) Coupling Mechanism
In the EC coupling mechanism, there is direct evidence pointing to hydrogen peroxide as the oxidizing agent that modulates the interaction between the Cav.1.1 and the ryanodine-1 receptor (RyR1) and, on the other hand, the oxidation of the contractile apparatus. In EC coupling, Cav1.1 is an L-type Ca2+ channel located in the T tubule of skeletal muscle cells, composed of 5 subunits (α1S, α2, β, γ and δ), of which the α1S subunit is the one carrying the transmembrane barrels responsible for its voltage sensor capacity during a change in membrane potential, as occurs during an action potential [44,45]. Cav 1.1 plays a fundamental role in changing its spatial conformation after membrane depolarization, which induces RyR1 channel opening, and the following massive release of Ca2+ from the sarcoplasmic reticulum to the cytoplasm, which triggers the contraction of the muscle fiber [46,47,48]. A modulatory role of H2O2 in skeletal muscle contraction has been suggested for decades, based on the idea that skeletal muscle has many redox-sensitive proteins involved in the contractile apparatus and mitochondria [49]. However, the intracellular H2O2 concentration in skeletal muscle fibers is relatively low, estimated to be less than 10−7 M (100 nM). Contractions induced a smaller increase in intracellular H2O2 compared to exposure to 10–6 M (1 μM) H2O2 [50]. H2O2 induces a delay in action potential associated with excitation processes in mouse myoblasts, which was reverted using the antioxidant N-acetylcysteine (NAC) [51]. Indeed, a decrease in the action potential amplitude was observed in H2O2-treated fibers after high-frequency stimulation with respect to non-treated fibers [52]. The addition of H2O2 is known to affect EC coupling, but contradictory information is found in the literature. Some reports show that µM doses apparently do not affect EC coupling, but mM doses have an inhibitory effect [53]. On the other hand, 10 mM H2O2 could increase the Ca2+ sensitivity of contractile machinery. Moreover, the effect of 1mM H2O2 on contractile response also depends on the fiber type: depolarization-induced contraction of slow but not fast twitch fibers [54]. One of the central stimuli that increase H2O2 production is the electrical stimulation of isolated fibers or cultured muscle cells, an in vitro strategy mimicking physical activity. For example, contraction by the electrical stimulation of myotubes or nude fibers can generate intracellular increase in both H2O2 and NO [55,56,57].
A particularly interesting protein is SERCA. This calcium pump is responsible for decreasing Ca2+ concentrations to allow muscle relaxation. It has been reported that SERCA expression decreases upon oxidative conditions [58].
Exercise could be considered an acute stressor, which at low doses induces H2O2 production as a “hormetic” signal, inducing a gene expression program of defense against oxidative stress [59,60]. In this line, acute exercise significantly increased mitochondrial H2O2 and FOXO3a, a key transcription factor involved in the activation of transcription of mitochondrial superoxide dismutase and catalase, both key antioxidants enzymes [28]. The same exercise pattern upregulates redox effector factor-1 (Ref1) and nuclear factor erythroid 2-related factor 2 (Nrf2) and increases reduced glutathione (GSH) content and manganese superoxide dismutase activity, suggesting that a low level of ROS production is a stimulus for antioxidant gene expression. Along the same lines, it has been observed that cultured myotubes incubated with low doses of H2O2 increase the expression of hemoxygenase 1, an enzyme related to antioxidant protection [34].
The controlled production of ROS exerted by exercise is a factor that allows the muscles to adapt to training, triggering the activation of transcription factors and the expression of genes such as peroxisome proliferator-activated receptor gamma co-activator 1 α (PGC1 α) and peroxisome proliferator-activated receptor γ (PPAR γ), the first of which is related to the induction of mitochondrial myogenesis, while the second is related to the increase in uncoupling protein-3 (UCP3), a mitochondrial uncoupling protein, which is considered the first line of defense against mitochondrial ROS production [13,56]. In addition, the upregulation of UCP3 may help reduce ROS production by increasing the proton gradient and protecting muscle mitochondria from oxidative stress during exercise. However, it also compromises energy coupling efficiency, as indicated by an increased respiration rate [35].
Another mechanism involved in ROS production with skeletal muscle physiology is extracellular ATP release. This mechanism has been described in macrophages where ROS increase triggers the activation of pathways needed for cytokine release [61]. When skeletal muscle fibers are electrically stimulated at low frequencies (20 Hz), ATP is released from the muscle fibers and binds to their P2Y purinergic receptors, thereby activating a signal transduction pathway, which culminates in the regulation of the transcription of some genes by Ca2+ release via inositol 1,4,5-trisphosphate receptors (IP3R) from the sarcoplasmic reticulum. Indeed, Cav1.1 is also responsible for triggering an excitation–transcription coupling (ETC) process, which is mediated by the release of ATP [62,63,64]. H2O2 may also have a role in the ETC process, considering that, after contraction, ATP binds to P2Y1 receptors and then activates NOX2 via a protein kinase C (PKC)-dependent mechanism [62]. This evidence shows that exercise results in skeletal muscle’s exposure to H2O2, which is involved in adaptive physiological responses.
3.2. Role of H2O2 in Glucose Uptake in Skeletal Muscle
Among the physiological functions of H2O2 in skeletal muscle, in addition to its role as a modulator of EC and ET coupling, metabolic participation also has been described. Muscle contraction is a process with high energy demand, so the EC coupling must have machinery associated with the constant generation of ATP. In addition, it has been shown that the production of ROS by various sources activates the entry of glucose into the cell. Myotubes treated with NAC for 24 h decreased the mRNA and protein contents of glucose transporter type 4 (GLUT4), the mRNA content and activity of phosphofructokinase (PFK), and lactate production and glucose uptake [65]. As for muscle contraction, exogenous stimulation with H2O2 increased 2-Deoxy-D-glucose uptake, adenosine monophosphate-activated protein kinase (AMPK) [66], and glycolytic activity [67]. These findings suggest that ROS produced after muscle contraction play an important role in increasing glycolytic activity and glucose uptake after exercise. On the other hand, H2O2 stimulation in high doses is capable of activating peroxisome proliferator-activated receptor γ co-activator 1 α (PGC-1 α) transcription via AMPK activation [68]. In turn, H2O2 induces glucose uptake, but this is completel independently of AMPK activation [69]—apparently by activating a phosphatidylinositol 3-kinase (PI3K)-dependent mechanism [67,68].
NOX4 has been related to the physiological adaptation to acute exercise. Particularly, NOX4 deletion results in impaired glucose and fatty acid oxidization and decreased mitochondrial uncoupling protein 3 (UCP3) protein expression in response to acute exercise [70]. During muscle fiber electrical stimulation or insulin stimulation, NOX2 activation also increases H2O2 production, and the inhibition of this isoform decreases glucose uptake in myotubes [71]. Moreover, a specific source of ROS has been linked to exercise-stimulated glucose uptake, where H2O2 from NOX2 could be primarily responsible for GLUT-4 translocation during moderate-intensity exercise [72]. Moreover, H2O2 enhances GLUT4 translocation in skeletal muscle cells, independently of insulin, and is reduced by antioxidants and NOX2 inhibition. RyR-mediated Ca2+ release and IP3R-mediated mitochondrial Ca2+ uptake, alongside the canonical pathway, jointly promote glucose uptake in response to insulin [73]. Indeed, piperine administration induces intracellular Ca2+ and ROS generation via TRPV1, leading to calcium/calmodulin-dependent protein kinase kinase β-dependent AMPK phosphorylation and, consecutively, GLUT4 translocation and AMPK activation in L6 myotubes [74]. On the other hand, our research group has found an important connection between the electrical stimulation of skeletal muscle and the release of ATP into the extracellular medium. This ATP release appears to be also mediated by NOX2 [62]. Also, extracellular ATP is capable of increasing glucose uptake in skeletal muscle in response to exercise by PI3K activation [70]. All these findings point to a role of extracellular ATP in activating NOX2, which could modulate glucose uptake. Therefore, NOX2-dependent ROS production is a crucial mechanism for increasing muscle glucose uptake during exercise.
3.3. Mitochondrial ROS Associated to Physical Activity
Another hormetic response to physical activity is the induction of mitochondrial biogenesis, also called mitohormesis, which is mainly induced to enhance respiratory capacity and endurance [60]. Thus, exercise-induced mitochondrial ROS production might have an adaptive role at different levels, including the spread of energy between different pools of mitochondria, the fusion/fission state, and the status and location of mitochondrial Ca2+.
ROS production depends on exercise characteristics regarding the previous training level, intensity, and duration. Exercise can be moderate or intense, and it could be aerobic/endurance or resistance. Mitochondria and NADPH oxidases are the main sources of ROS production in aerobic exercise. Considering that complexes I and III of the electron transport chain are the main sites of mitochondrial superoxide production [75], the logical thinking is that increased ROS generation by contractile activity is associated with increased mitochondrial activity, increasing the superoxide generation dramatically by skeletal muscle during aerobic contractions [76]. However, during aerobic exercise, there is a reduction in mitochondria-derived superoxide anion formation compared to at rest, which is attributed to changes in the redox status of muscles shifting towards a more oxidative status to contraction. Indeed, when isolated mitochondria were incubated in a medium mimicking the cytosol of rat skeletal muscle during mild or intense aerobic exercise, the total rate of H2O2 production decreased to about 25% or 15%, respectively, of the rate in a medium mimicking rest [77]. On the other hand, a cytosolic source of ROS in aerobic exercise is NADPH oxidase, and interplay between the NADPH oxidases and mitochondrial ROS sources has been proposed, probably as an adaptive mechanism involved in slow and fast twitch-plasticity [2].
In aerobic exercise, the mitochondrial ratio of reduced NADH to oxidized NAD+ (NADH/NAD+) decreases. Low NADH/NAD+ ratio is associated with a decrease in the release of free radical oxygen species via complex I of the electron transport chain [78]. However, high-intensity aerobic exercise adversely affects mitochondrial function together with a decline in glucose tolerance [79]. Moreover, endurance exercise impacts the mitochondrial life cycle because Drp1 deficiency impairs muscle endurance and running performance and alters exercise-induced muscle adaptations [80]. On the other hand, resistance training enhances muscle strength and hypertrophy but may decrease mitochondrial volume due to hypertrophy outpacing mitochondrial biogenesis. Despite this reduction, the evidence does not support a net loss of mitochondria, and specific aspects of mitochondrial function may be improved with resistance training [81]. In aging rats, quadriceps femoris muscle mitochondria exhibit Ca2+ accumulation, ROS increase, reduced mitochondrial membrane potential, and decreased fusion protein levels. However, resistance training effectively mitigates these alterations [82]. Alternatively, in humans, resistance training for moderate periods, such as 12 weeks, does not significantly affect mitochondrial ROS production [83]. But resistance training induces mtDNA shifting; mitochondrial fusion via Mfn1, Mfn2, and Opa1; and mitochondrial biogenesis markers, contributing to improved mitochondrial function [32,84,85].
The transient increase in ROS production resulting from fission events during exercise may act as a signaling mechanism, triggering adaptive responses in the body, and orchestrating the beneficial adaptations that arise from physical activity.
4. Conclusions
Many questions remain when studying both oxidative metabolism and ROS production in skeletal muscle. The aim of this review is to bring attention, on one side, to the beneficial role of ROS in skeletal muscle physiology as opposed to their deleterious effects. On the other side, the particular distribution of skeletal muscle mitochondria and their different composition regarding key metabolic enzymes, together with the evidence pointing to both fusion events and fast sequential changes in mitochondria membrane potential, allow us to propose an energy-saving model consisting of distinct compartments where mitochondria membrane potential is generated in one compartment and, when needed, is transferred to a second compartment where ATP synthesis can take place. This distribution of functions saves energy and minimizes ROS production in muscle cells. It is important to note that mitochondria dynamics, i.e., the balance between mitochondria fusion and fission, is essential to this process and that normal muscle functioning depends on the capacity of mitochondria to fuse and fission at the right moment. This may explain why alterations in the mitochondria dynamics machinery have been reported in conditions (obesity and aging sarcopenia) in which muscle function is altered.
Author Contributions
E.J. conceived the review, conceptualized, and wrote several sections, A.E. wrote several sections, conceived and performed the drawings, M.C. wrote a section, and all authors critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad de Chile ICBM Puente 2022.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
AMPK, Adenosine monophosphate-activated protein kinase; xCT, cystine-glutamate antiporter; DMD, Duchenne muscular dystrophy; EC, excitation–contraction; ETC, excitation–transcription coupling; FGF21, fibroblast growth factor 21; GLUT4, glucose transporter type 4; GPDH, glycerol phosphate dehydrogenase; GSH, reduced glutathione, GPx4, glutathione peroxidase 4; ICDH, isocitrate dehydrogenase; IGF1, insulin-like growth factor 1; IMF, intermyofibrillar, IP3R, inositol 1,4,5-trisphosphate receptor; MCU, mitochondrial calcium uniporter; α-KGDH, α-ketoglutarate dehydrogenase; MFN, mitofusin; MICU1, mitochondrial calcium uniporter regulator 1; NAC, N-acetylcysteine; NCLX, sodium–calcium exchanger; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells, NOX, NADPH oxidases; Nrf2, nuclear factor erythroid 2-related factor 2; OPA1, optic atrophy protein 1; OXPHOS, oxidative phosphorylation; PDH, pyruvate dehydrogenase; PFK, phosphofructokinase; PGC1α, peroxisome proliferator-activated receptor gamma co-activator 1 α; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; PLM, peripherally located mitochondria, PN, perinuclear; PV, perivascular; PPAR γ, peroxisome proliferator-activated receptor γ; Ref1, redox effector factor-1; ROS, reactive oxygen species; RyR1, ryanodine receptor type 1; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; SAMP8, senescence-accelerated mouse-prone 8; SR, sarcoplasmic reticulum; SS, subsarcolemmal; SSM, subsarcolemmal mitochondria; STAT3, signal transducer and activator of transcription 3; TCA, tricarboxylic acid; TLR9, Toll-like receptor 9; TRPV1, transient receptor potential channel V1; UCP3, mitochondria uncoupling protein-3; UQH2, ubiquinol; VDAC, voltage-dependent anion channel.
References
- Wang, P.; Li, C.G.; Qi, Z.; Cui, D.; Ding, S. Acute Exercise Induced Mitochondrial H₂O₂ Production in Mouse Skeletal Muscle: Association with p(66Shc) and FOXO3a Signaling and Antioxidant Enzymes. Oxid. Med. Cell. Longev. 2015, 2015, 536456. [Google Scholar] [CrossRef] [PubMed]
- Osório Alves, J.; Matta Pereira, L.; Cabral Coutinho do Rêgo Monteiro, I.; Pontes Dos Santos, L.H.; Soares Marreiros Ferraz, A.; Carneiro Loureiro, A.C.; Calado Lima, C.; Leal-Cardoso, J.H.; Pires Carvalho, D.; Soares Fortunato, R.; et al. Strenuous Acute Exercise Induces Slow and Fast Twitch-Dependent NADPH Oxidase Expression in Rat Skeletal Muscle. Antioxidants 2020, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Favaro, G.; Romanello, V.; Varanita, T.; Andrea Desbats, M.; Morbidoni, V.; Tezze, C.; Albiero, M.; Canato, M.; Gherardi, G.; De Stefani, D.; et al. DRP1-Mediated Mitochondrial Shape Controls Calcium Homeostasis and Muscle Mass. Nat. Commun. 2019, 10, 2576. [Google Scholar] [CrossRef]
- Willingham, T.B.; Ajayi, P.T.; Glancy, B. Subcellular Specialization of Mitochondrial Form and Function in Skeletal Muscle Cells. Front. Cell Dev. Biol. 2021, 9, 757305. [Google Scholar] [CrossRef]
- Takahashi, M.; Hood, D.A. Protein Import into Subsarcolemmal and Intermyofibrillar Skeletal Muscle Mitochondria. Differential Import Regulation in Distinct Subcellular Regions. J. Biol. Chem. 1996, 271, 27285–27291. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.; Vitorino, R.; Alves, R.M.P.; Appell, H.J.; Powers, S.K.; Duarte, J.A.; Amado, F. Subsarcolemmal and Intermyofibrillar Mitochondria Proteome Differences Disclose Functional Specializations in Skeletal Muscle. Proteomics 2010, 10, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; White, K.; Turnbull, D.M. Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: A quantitative three-dimensional electron microscopy study. J. Appl. Physiol. 2013, 114, 161–171. [Google Scholar] [CrossRef]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic Reticulum-Mitochondrial Contactology: Structure and Signaling Functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef]
- Booth, D.M.; Enyedi, B.; Geiszt, M.; Várnai, P.; Hajnóczky, G. Redox Nanodomains Are Induced by and Control Calcium Signaling at the ER-Mitochondrial Interface. Mol. Cell 2016, 63, 240–248. [Google Scholar] [CrossRef]
- Elander, A.; Sjöström, M.; Lundgren, F.; Scherstén, T.; Bylund-Fellenius, A.C. Biochemical and Morphometric Properties of Mitochondrial Populations in Human Muscle Fibres. Clin. Sci. 1985, 69, 153–164. [Google Scholar] [CrossRef]
- Díaz-Vegas, A.R.; Cordova, A.; Valladares, D.; Llanos, P.; Hidalgo, C.; Gherardi, G.; De Stefani, D.; Mammucari, C.; Rizzuto, R.; Contreras-Ferrat, A.; et al. Mitochondrial Calcium Increase Induced by RyR1 and IP3R Channel Activation After Membrane Depolarization Regulates Skeletal Muscle Metabolism. Front. Physiol. 2018, 9, 791. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.Q.; Huo, J.; Bround, M.J.; Boyer, J.G.; Schwanekamp, J.A.; Ghazal, N.; Maxwell, J.T.; Jang, Y.C.; Khuchua, Z.; Shi, K.; et al. The Mitochondrial Calcium Uniporter Underlies Metabolic Fuel Preference in Skeletal Muscle. JCI Insight 2018, 3, e121689. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Harper, M.-E. Uncoupling Proteins and the Control of Mitochondrial Reactive Oxygen Species Production. Free Radic. Biol. Med. 2011, 51, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Groten, C.J.; MacVicar, B.A. Mitochondrial Ca2+ Uptake by the MCU Facilitates Pyramidal Neuron Excitability and Metabolism during Action Potential Firing. Commun. Biol. 2022, 5, 900. [Google Scholar] [CrossRef] [PubMed]
- Patron, M.; Raffaello, A.; Granatiero, V.; Tosatto, A.; Merli, G.; De Stefani, D.; Wright, L.; Pallafacchina, G.; Terrin, A.; Mammucari, C.; et al. The Mitochondrial Calcium Uniporter (MCU): Molecular Identity and Physiological Roles. J. Biol. Chem. 2013, 288, 10750–10758. [Google Scholar] [CrossRef]
- Mammucari, C.; Gherardi, G.; Zamparo, I.; Raffaello, A.; Boncompagni, S.; Chemello, F.; Cagnin, S.; Braga, A.; Zanin, S.; Pallafacchina, G.; et al. The Mitochondrial Calcium Uniporter Controls Skeletal Muscle Trophism in Vivo. Cell Rep. 2015, 10, 1269–1279. [Google Scholar] [CrossRef]
- Li, A.; Yi, J.; Li, X.; Zhou, J. Physiological Ca2+ Transients Versus Pathological Steady-State Ca2+ Elevation, Who Flips the ROS Coin in Skeletal Muscle Mitochondria. Front. Physiol. 2020, 11, 595800. [Google Scholar] [CrossRef]
- Alevriadou, B.R.; Patel, A.; Noble, M.; Ghosh, S.; Gohil, V.M.; Stathopulos, P.B.; Madesh, M. Molecular Nature and Physiological Role of the Mitochondrial Calcium Uniporter Channel. Am. J. Physiol. Cell Physiol. 2021, 320, C465–C482. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber Types in Mammalian Skeletal Muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Khodabukus, A.; Baar, K. Contractile and Metabolic Properties of Engineered Skeletal Muscle Derived from Slow and Fast Phenotype Mouse Muscle. J. Cell. Physiol. 2015, 230, 1750–1757. [Google Scholar] [CrossRef]
- Quezada, E.R.; Díaz-Vegas, A.; Jaimovich, E.; Casas, M. Changes in Gene Expression of the MCU Complex Are Induced by Electrical Stimulation in Adult Skeletal Muscle. Front. Physiol. 2020, 11, 601313. [Google Scholar] [CrossRef] [PubMed]
- Loucif, H.; Dagenais-Lussier, X.; Beji, C.; Telittchenko, R.; Routy, J.-P.; van Grevenynghe, J. Plasticity in T-Cell Mitochondrial Metabolism: A Necessary Peacekeeper during the Troubled Times of Persistent HIV-1 Infection. Cytokine Growth Factor. Rev. 2020, 55, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Hartnell, L.M.; Malide, D.; Yu, Z.-X.; Combs, C.A.; Connelly, P.S.; Subramaniam, S.; Balaban, R.S. Mitochondrial Reticulum for Cellular Energy Distribution in Muscle. Nature 2015, 523, 617–620. [Google Scholar] [CrossRef]
- Horn, A.; Van der Meulen, J.H.; Defour, A.; Hogarth, M.; Sreetama, S.C.; Reed, A.; Scheffer, L.; Chandel, N.S.; Jaiswal, J.K. Mitochondrial Redox Signaling Enables Repair of Injured Skeletal Muscle Cells. Sci. Signal. 2017, 10, eaaj1978. [Google Scholar] [CrossRef] [PubMed]
- Crochemore, C.; Mekki, M.; Corbière, C.; Karoui, A.; Noël, R.; Vendeville, C.; Vaugeois, J.-M.; Monteil, C. Subsarcolemmal and Interfibrillar Mitochondria Display Distinct Superoxide Production Profiles. Free Radic. Res. 2015, 49, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Debattisti, V.; Horn, A.; Singh, R.; Seifert, E.L.; Hogarth, M.W.; Mazala, D.A.; Huang, K.T.; Horvath, R.; Jaiswal, J.K.; Hajnóczky, G. Dysregulation of Mitochondrial Ca2+ Uptake and Sarcolemma Repair Underlie Muscle Weakness and Wasting in Patients and Mice Lacking MICU1. Cell Rep. 2019, 29, 1274–1286.e6. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative Genomics Identifies MCU as an Essential Component of the Mitochondrial Calcium Uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.G.; Qi, Z.; Cui, D.; Ding, S. Acute Exercise Stress Promotes Ref1/Nrf2 Signalling and Increases Mitochondrial Antioxidant Activity in Skeletal Muscle. Exp. Physiol. 2016, 101, 410–420. [Google Scholar] [CrossRef]
- Eisner, V.; Lenaers, G.; Hajnóczky, G. Mitochondrial Fusion Is Frequent in Skeletal Muscle and Supports Excitation-Contraction Coupling. J. Cell Biol. 2014, 205, 179–195. [Google Scholar] [CrossRef]
- Del Campo, A.; Contreras-Hernández, I.; Castro-Sepúlveda, M.; Campos, C.A.; Figueroa, R.; Tevy, M.F.; Eisner, V.; Casas, M.; Jaimovich, E. Muscle Function Decline and Mitochondria Changes in Middle Age Precede Sarcopenia in Mice. Aging 2018, 10, 34–55. [Google Scholar] [CrossRef]
- Fealy, C.E.; Grevendonk, L.; Hoeks, J.; Hesselink, M.K.C. Skeletal Muscle Mitochondrial Network Dynamics in Metabolic Disorders and Aging. Trends Mol. Med. 2021, 27, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389.e6. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nuevo, A.; Díaz-Ramos, A.; Noguera, E.; Díaz-Sáez, F.; Duran, X.; Muñoz, J.P.; Romero, M.; Plana, N.; Sebastián, D.; Tezze, C.; et al. Mitochondrial DNA and TLR9 Drive Muscle Inflammation upon Opa1 Deficiency. EMBO J. 2018, 37, e96553. [Google Scholar] [CrossRef] [PubMed]
- McArdle, F.; Spiers, S.; Aldemir, H.; Vasilaki, A.; Beaver, A.; Iwanejko, L.; McArdle, A.; Jackson, M.J. Preconditioning of Skeletal Muscle against Contraction-Induced Damage: The Role of Adaptations to Oxidants in Mice. J. Physiol. 2004, 561, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, G.; Bo, H.; Qu, J.; Ma, G.; Cao, D.; Wen, L.; Liu, S.; Ji, L.L.; Zhang, Y. Upregulation of Uncoupling Protein-3 in Skeletal Muscle during Exercise: A Potential Antioxidant Function. Free Radic. Biol. Med. 2009, 46, 138–145. [Google Scholar] [CrossRef]
- Yeo, D.; Kang, C.; Gomez-Cabrera, M.C.; Vina, J.; Ji, L.L. Intensified Mitophagy in Skeletal Muscle with Aging Is Downregulated by PGC-1alpha Overexpression in Vivo. Free Radic. Biol. Med. 2019, 130, 361–368. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H.; Laher, I.; Knechtle, B.; Zouhal, H. Exercise and Mitochondrial Mechanisms in Patients with Sarcopenia. Front. Physiol. 2022, 13, 1040381. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Yang, W.; Liao, Z.-Y.; Wu, Y.-X.; Fan, Z.; Guo, A.; Yu, J.; Chen, Q.-N.; Wu, J.-H.; Zhou, J.; et al. MICU3 Regulates Mitochondrial Ca2+-Dependent Antioxidant Response in Skeletal Muscle Aging. Cell Death Dis. 2021, 12, 1115. [Google Scholar] [CrossRef]
- Altamirano, F.; López, J.R.; Henríquez, C.; Molinski, T.; Allen, P.D.; Jaimovich, E. Increased Resting Intracellular Calcium Modulates NF-ΚB-Dependent Inducible Nitric-Oxide Synthase Gene Expression in Dystrophic Mdx Skeletal Myotubes. J. Biol. Chem. 2012, 287, 20876–20887. [Google Scholar] [CrossRef]
- Mechler, F.; Imre, S.; Dioszeghy, P. Lipid Peroxidation and Superoxide Dismutase Activity in Muscle and Erythrocytes in Duchenne Muscular Dystrophy. J. Neurol. Sci. 1984, 63, 279–283. [Google Scholar] [CrossRef]
- Łoboda, A.; Dulak, J. Nuclear Factor Erythroid 2-Related Factor 2 and Its Targets in Skeletal Muscle Repair and Regeneration. Antioxid. Redox Signal. 2023, 38, 619–642. [Google Scholar] [CrossRef]
- Zhang, Q.; Qu, H.; Chen, Y.; Luo, X.; Chen, C.; Xiao, B.; Ding, X.; Zhao, P.; Lu, Y.; Chen, A.F.; et al. Atorvastatin Induces Mitochondria-Dependent Ferroptosis via the Modulation of Nrf2-XCT/GPx4 Axis. Front. Cell Dev. Biol. 2022, 10, 806081. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, B.; Shen, D.; Chen, J.; Yu, Z.; Chen, C. Ferroptosis in a Sarcopenia Model of Senescence Accelerated Mouse Prone 8 (SAMP8). Int. J. Biol. Sci. 2021, 17, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Bannister, R.A.; Beam, K.G. Ca(V)1.1: The Atypical Prototypical Voltage-Gated Ca2+ Channel. Biochim. Biophys. Acta 2013, 1828, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Ríos, E.; Gillespie, D.; Franzini-Armstrong, C. The Binding Interactions That Maintain Excitation-Contraction Coupling Junctions in Skeletal Muscle. J. Gen. Physiol. 2019, 151, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, R.T.; Karunasekara, Y.; Board, P.G.; Beard, N.A.; Casarotto, M.G.; Dulhunty, A.F. Skeletal Muscle Excitation-Contraction Coupling: Who Are the Dancing Partners? Int. J. Biochem. Cell Biol. 2014, 48, 28–38. [Google Scholar] [CrossRef]
- Huang, C.L.-H.; Pedersen, T.H.; Fraser, J.A. Reciprocal Dihydropyridine and Ryanodine Receptor Interactions in Skeletal Muscle Activation. J. Muscle Res. Cell Motil. 2011, 32, 171–202. [Google Scholar] [CrossRef]
- Lu, X.; Xu, L.; Meissner, G. Phosphorylation of Dihydropyridine Receptor II-III Loop Peptide Regulates Skeletal Muscle Calcium Release Channel Function. Evidence for an Essential Role of the Beta-OH Group of Ser687. J. Biol. Chem. 1995, 270, 18459–18464. [Google Scholar] [CrossRef]
- Zuo, L.; Pannell, B.K. Redox Characterization of Functioning Skeletal Muscle. Front. Physiol. 2015, 6, 338. [Google Scholar] [CrossRef]
- Jackson, M.J.; Stretton, C.; McArdle, A. Hydrogen Peroxide as a Signal for Skeletal Muscle Adaptations to Exercise: What Do Concentrations Tell Us about Potential Mechanisms? Redox Biol. 2020, 35, 101484. [Google Scholar] [CrossRef]
- Luin, E.; Giniatullin, R.; Sciancalepore, M. Effects of H2O2 on Electrical Membrane Properties of Skeletal Myotubes. Free Radic. Biol. Med. 2011, 50, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Ishikawa, T.; Takaishi, T.; Aoki, T.; Yamaguchi, M. Hydrogen Peroxide Decelerates Recovery of Action Potential after High-Frequency Fatigue in Skeletal Muscle. Muscle Nerve 2000, 23, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Posterino, G.S.; Lamb, G.D. Effects of Reducing Agents and Oxidants on Excitation-Contraction Coupling in Skeletal Muscle Fibres of Rat and Toad. J. Physiol. 1996, 496 Pt 3, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Plant, D.R.; Lynch, G.S.; Williams, D.A. Hydrogen Peroxide Increases Depolarization-Induced Contraction of Mechanically Skinned Slow Twitch Fibres from Rat Skeletal Muscles. J. Physiol. 2002, 539, 883–891. [Google Scholar] [CrossRef]
- Espinosa, A.; Leiva, A.; Peña, M.; Müller, M.; Debandi, A.; Hidalgo, C.; Carrasco, M.A.; Jaimovich, E. Myotube Depolarization Generates Reactive Oxygen Species through NAD(P)H Oxidase; ROS-Elicited Ca2+ Stimulates ERK, CREB, Early Genes. J. Cell. Physiol. 2006, 209, 379–388. [Google Scholar] [CrossRef]
- Silveira, L.R.; Pereira-Da-Silva, L.; Juel, C.; Hellsten, Y. Formation of Hydrogen Peroxide and Nitric Oxide in Rat Skeletal Muscle Cells during Contractions. Free Radic. Biol. Med. 2003, 35, 455–464. [Google Scholar] [CrossRef]
- Palomero, J.; Pye, D.; Kabayo, T.; Spiller, D.G.; Jackson, M.J. In Situ Detection and Measurement of Intracellular Reactive Oxygen Species in Single Isolated Mature Skeletal Muscle Fibers by Real Time Fluorescence Microscopy. Antioxid. Redox Signal. 2008, 10, 1463–1474. [Google Scholar] [CrossRef]
- Qaisar, R.; Bhaskaran, S.; Ranjit, R.; Sataranatarajan, K.; Premkumar, P.; Huseman, K.; Van Remmen, H. Restoration of SERCA ATPase Prevents Oxidative Stress-Related Muscle Atrophy and Weakness. Redox Biol. 2019, 20, 68–74. [Google Scholar] [CrossRef]
- Goto, S.; Radák, Z. Hormetic Effects of Reactive Oxygen Species by Exercise: A View from Animal Studies for Successful Aging in Human. Dose Response 2009, 8, 68–72. [Google Scholar] [CrossRef]
- Musci, R.V.; Hamilton, K.L.; Linden, M.A. Exercise-Induced Mitohormesis for the Maintenance of Skeletal Muscle and Healthspan Extension. Sports 2019, 7, 170. [Google Scholar] [CrossRef]
- Cruz, C.M.; Rinna, A.; Forman, H.J.; Ventura, A.L.M.; Persechini, P.M.; Ojcius, D.M. ATP Activates a Reactive Oxygen Species-Dependent Oxidative Stress Response and Secretion of Proinflammatory Cytokines in Macrophages. J. Biol. Chem. 2007, 282, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Vegas, A.; Campos, C.A.; Contreras-Ferrat, A.; Casas, M.; Buvinic, S.; Jaimovich, E.; Espinosa, A. ROS Production via P2Y1-PKC-NOX2 Is Triggered by Extracellular ATP after Electrical Stimulation of Skeletal Muscle Cells. PLoS ONE 2015, 10, e0129882. [Google Scholar] [CrossRef]
- Jorquera, G.; Altamirano, F.; Contreras-Ferrat, A.; Almarza, G.; Buvinic, S.; Jacquemond, V.; Jaimovich, E.; Casas, M. Cav1.1 Controls Frequency-Dependent Events Regulating Adult Skeletal Muscle Plasticity. J. Cell Sci. 2013, 126, 1189–1198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Casas, M.; Buvinic, S.; Jaimovich, E. ATP Signaling in Skeletal Muscle: From Fiber Plasticity to Regulation of Metabolism. Exerc. Sport Sci. Rev. 2014, 42, 110–116. [Google Scholar] [CrossRef]
- Da Justa Pinheiro, C.H.; Silveira, L.R.; Nachbar, R.T.; Vitzel, K.F.; Curi, R. Regulation of Glycolysis and Expression of Glucose Metabolism-Related Genes by Reactive Oxygen Species in Contracting Skeletal Muscle Cells. Free Radic. Biol. Med. 2010, 48, 953–960. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Higaki, Y.; Mikami, T.; Fujii, N.; Hirshman, M.F.; Koyama, K.; Seino, T.; Tanaka, K.; Goodyear, L.J. Oxidative Stress Stimulates Skeletal Muscle Glucose Uptake through a Phosphatidylinositol 3-Kinase-Dependent Pathway. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E889–E897. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP Kinase Activity in the Regulation of PGC-1alpha Transcription in Skeletal Muscle Cells. Am. J. Physiol. Cell Physiol. 2009, 296, C116–C123. [Google Scholar] [CrossRef]
- Jensen, T.E.; Schjerling, P.; Viollet, B.; Wojtaszewski, J.F.P.; Richter, E.A. AMPK Alpha1 Activation Is Required for Stimulation of Glucose Uptake by Twitch Contraction, but Not by H2O2, in Mouse Skeletal Muscle. PLoS ONE 2008, 3, e2102. [Google Scholar] [CrossRef]
- Specht, K.S.; Kant, S.; Addington, A.K.; McMillan, R.P.; Hulver, M.W.; Learnard, H.; Campbell, M.; Donnelly, S.R.; Caliz, A.D.; Pei, Y.; et al. Nox4 Mediates Skeletal Muscle Metabolic Responses to Exercise. Mol. Metab. 2021, 45, 101160. [Google Scholar] [CrossRef]
- Espinosa, A.; Campos, C.; Díaz-Vegas, A.; Galgani, J.; Juretic, N.; Osorio-Fuentealba, C.; Bucarey, J.; Tapia, G.; Valenzuela, R.; Contreras-Ferrat, A.; et al. Insulin-Dependent H2O2 Production Is Higher in Muscle Fibers of Mice Fed with a High-Fat Diet. Int. J. Mol. Sci. 2013, 14, 15740–15754. [Google Scholar] [CrossRef]
- Henríquez-Olguin, C.; Knudsen, J.R.; Raun, S.H.; Li, Z.; Dalbram, E.; Treebak, J.T.; Sylow, L.; Holmdahl, R.; Richter, E.A.; Jaimovich, E.; et al. Cytosolic ROS Production by NADPH Oxidase 2 Regulates Muscle Glucose Uptake during Exercise. Nat. Commun. 2019, 10, 4623. [Google Scholar] [CrossRef]
- Contreras-Ferrat, A.; Llanos, P.; Vásquez, C.; Espinosa, A.; Osorio-Fuentealba, C.; Arias-Calderon, M.; Lavandero, S.; Klip, A.; Hidalgo, C.; Jaimovich, E. Insulin Elicits a ROS-Activated and an IP₃-Dependent Ca2+ Release, Which Both Impinge on GLUT4 Translocation. J. Cell Sci. 2014, 127, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Shirao, T.; Shirasaya, D.; Yoshioka, Y.; Yamashita, Y.; Akagawa, M.; Ashida, H. Piperine Promotes Glucose Uptake through ROS-Dependent Activation of the CAMKK/AMPK Signaling Pathway in Skeletal Muscle. Mol. Nutr. Food Res. 2018, 62, e1800086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of Mitochondrial and Nonmitochondrial Sources Implicate Nicotinamide Adenine Dinucleotide Phosphate Oxidase(s) in the Increased Skeletal Muscle Superoxide Generation That Occurs during Contractile Activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef]
- Goncalves, R.L.S.; Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Brand, M.D. Sites of Superoxide and Hydrogen Peroxide Production by Muscle Mitochondria Assessed Ex Vivo under Conditions Mimicking Rest and Exercise. J. Biol. Chem. 2015, 290, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, G.K.; Jackson, M.J.; Vasilaki, A. Redefining the Major Contributors to Superoxide Production in Contracting Skeletal Muscle. The Role of NAD(P)H Oxidases. Free Radic. Res. 2014, 48, 12–29. [Google Scholar] [CrossRef]
- Flockhart, M.; Nilsson, L.C.; Tais, S.; Ekblom, B.; Apró, W.; Larsen, F.J. Excessive Exercise Training Causes Mitochondrial Functional Impairment and Decreases Glucose Tolerance in Healthy Volunteers. Cell Metab. 2021, 33, 957–970.e6. [Google Scholar] [CrossRef]
- Moore, T.M.; Zhou, Z.; Cohn, W.; Norheim, F.; Lin, A.J.; Kalajian, N.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; et al. The Impact of Exercise on Mitochondrial Dynamics and the Role of Drp1 in Exercise Performance and Training Adaptations in Skeletal Muscle. Mol. Metab. 2019, 21, 51–67. [Google Scholar] [CrossRef]
- Parry, H.A.; Roberts, M.D.; Kavazis, A.N. Human Skeletal Muscle Mitochondrial Adaptations Following Resistance Exercise Training. Int. J. Sports Med. 2020, 41, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-H.; Yuan, Q.-K.; Xiao, R.; Chen, J.; Li, Q.; Zhang, S.-C. Effects of resistance training on mitochondrial function in skeletal muscle of aging rats. Chin. J. Appl. Physiol. 2020, 36, 165–170. [Google Scholar] [CrossRef]
- Flack, K.D.; Davy, B.M.; DeBerardinis, M.; Boutagy, N.E.; McMillan, R.P.; Hulver, M.W.; Frisard, M.I.; Anderson, A.S.; Savla, J.; Davy, K.P. Resistance Exercise Training and in Vitro Skeletal Muscle Oxidative Capacity in Older Adults. Physiol. Rep. 2016, 4, e12849. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A. Mitochondrial DNA Shifting in Older Adults Following Resistance Exercise. Appl. Physiol. Nutr. Metab. 2009, 34, 348–354. [Google Scholar] [CrossRef]
- Mesquita, P.H.C.; Lamb, D.A.; Parry, H.A.; Moore, J.H.; Smith, M.A.; Vann, C.G.; Osburn, S.C.; Fox, C.D.; Ruple, B.A.; Huggins, K.W.; et al. Acute and Chronic Effects of Resistance Training on Skeletal Muscle Markers of Mitochondrial Remodeling in Older Adults. Physiol. Rep. 2020, 8, e14526. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).