Targeting Homocysteine and Hydrogen Sulfide Balance as Future Therapeutics in Cancer Treatment
Abstract
1. Introduction
2. Homocysteine Production and Hyperhomocysteinemia
3. Homocysteine Metabolism in Cancer
4. Association of Hyperhomocysteinemia and Cancer
4.1. High Plasma Hcy Levels and Cancer
4.2. Alteration in Hcy Metabolism Gene and Risk of Cancer
4.3. Homocysteine-Mediated Epigenetic Alterations and Risk of Cancer
4.3.1. Hcy-Mediated DNA Methylation and Cancer
4.3.2. Hcy-Mediated Histone Modification and Cancer
4.3.3. Hcy-Mediated RNA Interference and Cancer
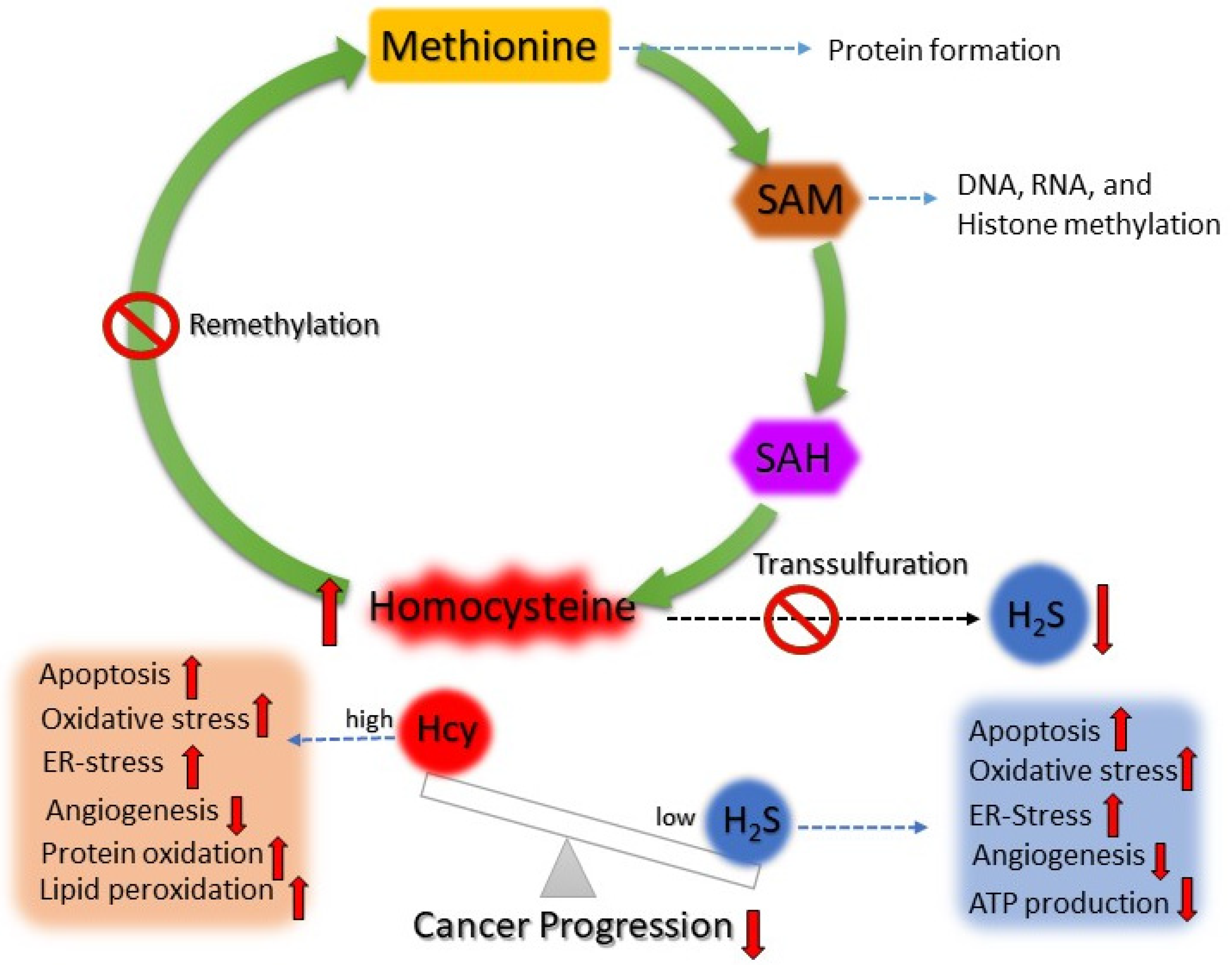
4.4. Hcy-Mediated H2S Production and Risk of Cancer
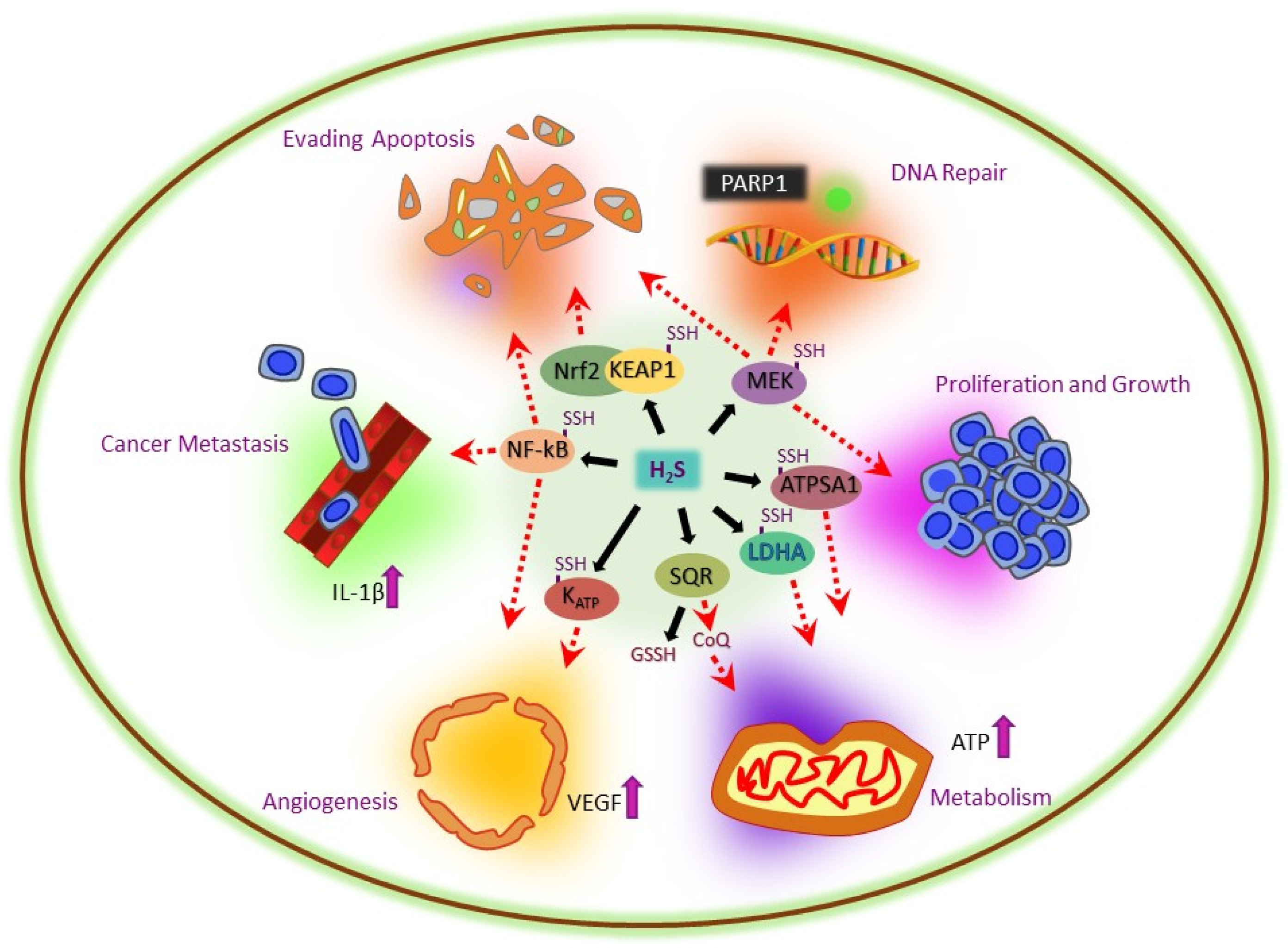
5. Multifactorial Role of H2S in Cancer
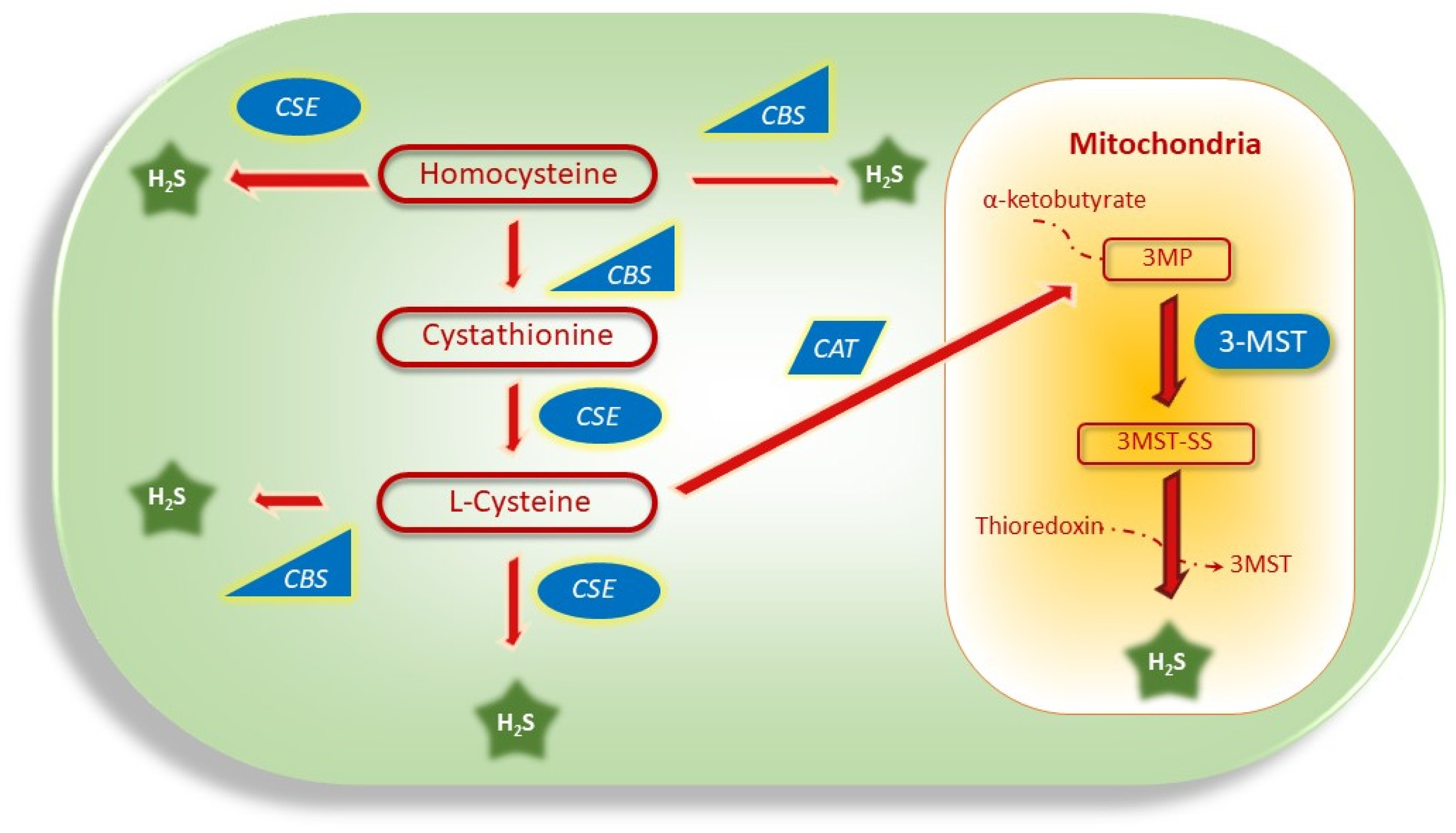
5.1. H2S Production via Dysregulation of CBS, CSE, and 3MST Genes in Cancer
5.1.1. Dysregulation of CBS in Cancer
5.1.2. Dysregulation of CSE in Cancer
5.1.3. Dysregulation of 3MST in Cancer
5.2. H2S-Mediated Redox Balance in Cancer
5.3. H2S-Mediated Recovery of Hypoxia in Cancer
5.4. H2S-Mediated Recovery of Apoptosis in Cancer
5.5. H2S-Mediated DNA Repair in Cancer
5.6. H2S-Mediated Tumor Growth and Metastasis in Cancer
5.7. H2S-Mediated Metabolism in Cancer
5.8. H2S-Mediated Angiogenesis in Cancer
5.9. H2S-Mediated Reduction in ER Stress in Cancer
6. Current Cancer Therapeutics Targeting the Hcy and H2S Signaling and Their Limitations
A Hypothesis of Targeting the Hcy and H2S Balance for Cancer Treatment and Its Application
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| SAH | S-Adenosyl Homocysteine |
| Hcy | Homocysteine |
| HHcy | Hyperhomocysteinemia |
| SAM | S-Adenosyl Methionine |
| H2S | Hydrogen Sulfide |
| GSH | Glutathione |
| ROS | Reactive Oxygen Species |
| ER | Endoplasmic Reticulum |
| MAT | Methionine Adenosyl Transferase |
| CBS | Cystathionine Β-Synthase |
| CSE | Cystathionine Γ-Lyase |
| MTHFR | Methylenetetrahydrofolate Reductase |
| MTRR | Methionine Synthase Reductase |
| MTR | Methionine Synthase |
| MTHFD | Methylenetetrahydrofolate Dehydrogenase |
| BHMT | Betaine Homocysteine Methyltransferase |
| TYMS | Thymidylate Synthase |
| TCN2 | Transcobalamin 2 |
| MTHFD1L | Methylenetetrahydrofolate dehydrogenase |
| DNMTS | DNA Methyltransferases |
| VSMCS | Vascular Smooth Muscle Cells |
| DDAH2 | Dimethylarginine Dimethylaminohydrolase 2 |
| HMT | Histone Methyltransferase |
| lncRNA | Long Non-Coding RNA |
| miRNA | MicroRNA |
| circRNA | CircularRNA |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MEK1 | Mitogen-activated protein kinase kinase1 |
| XIAP | X-linked inhibitor of apoptosis protein |
| cIAPs | Cellular Inhibitors of Apoptosis Proteins |
| Bcl-2 | B-cell lymphoma 2 gene |
| AREs | Antioxidant Response Elements |
References
- Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Altea-Manzano, P.; Cuadros, A.M.; Broadfield, L.A.; Fendt, S.M. Nutrient metabolism and cancer in the in vivo context: A metabolic game of give and take. EMBO Rep. 2020, 21, e50635. [Google Scholar] [CrossRef]
- Cellarier, E.; Durando, X.; Vasson, M.P.; Farges, M.C.; Demiden, A.; Maurizis, J.C.; Madelmont, J.C.; Chollet, P. Methionine dependency and cancer treatment. Cancer Treat. Rev. 2003, 29, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.; McCoy, J.; Simsek, M.; Majumdar, A.; Chang, S.H.; Rajbhandary, U.L.; Khorana, H.G. The bacteriorhodopsin gene. Proc. Natl. Acad. Sci. USA 1981, 78, 6744–6748. [Google Scholar] [CrossRef] [PubMed]
- Navik, U.; Sheth, V.G.; Khurana, A.; Jawalekar, S.S.; Allawadhi, P.; Gaddam, R.R.; Bhatti, J.S.; Tikoo, K. Methionine as a double-edged sword in health and disease: Current perspective and future challenges. Ageing Res. Rev. 2021, 72, 101500. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, M.; Behera, J.; Theilen, N.T.; George, A.K.; Tyagi, N.; Metreveli, N.; Tyagi, S.C. Hydrogen sulfide alleviates hyperhomocysteinemia-mediated skeletal muscle atrophy via mitigation of oxidative and endoplasmic reticulum stress injury. Am. J. Physiol. Cell Physiol. 2018, 315, C609–C622. [Google Scholar] [CrossRef]
- Majumder, A.; Behera, J.; Jeremic, N.; Tyagi, S.C. Hypermethylation: Causes and Consequences in Skeletal Muscle Myopathy. J. Cell. Biochem. 2017, 118, 2108–2117. [Google Scholar] [CrossRef]
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef]
- Wanders, D.; Hobson, K.; Ji, X. Methionine Restriction and Cancer Biology. Nutrients 2020, 12, 684. [Google Scholar] [CrossRef]
- Peng, H.; Yan, Y.; He, M.; Li, J.; Wang, L.; Jia, W.; Yang, L.; Jiang, J.; Chen, Y.; Li, F.; et al. SLC43A2 and NFκB signaling pathway regulate methionine/cystine restriction-induced ferroptosis in esophageal squamous cell carcinoma via a feedback loop. Cell Death Dis. 2023, 14, 347. [Google Scholar] [CrossRef] [PubMed]
- Khairan, P.; Sobue, T.; Eshak, E.S.; Zha, L.; Kitamura, T.; Sawada, N.; Iwasaki, M.; Inoue, M.; Yamaji, T.; Shimazu, T.; et al. Association of dietary intakes of vitamin B12, vitamin B6, folate, and methionine with the risk of esophageal cancer: The Japan Public Health Center-based (JPHC) prospective study. BMC Cancer 2021, 21, 982. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Lishko, V.K.; Herrera, H.; Groce, A.; Kubota, T.; Hoffman, R.M. Therapeutic tumor-specific cell cycle block induced by methionine starvation in vivo. Cancer Res. 1993, 53, 5676–5679. [Google Scholar]
- Epner, D.E.; Morrow, S.; Wilcox, M.; Houghton, J.L. Nutrient intake and nutritional indexes in adults with metastatic cancer on a phase I clinical trial of dietary methionine restriction. Nutr. Cancer 2002, 42, 158–166. [Google Scholar] [CrossRef]
- George, A.K.; Singh, M.; Homme, R.P.; Majumder, A.; Sandhu, H.S.; Tyagi, S.C. A hypothesis for treating inflammation and oxidative stress with hydrogen sulfide during age-related macular degeneration. Int. J. Ophthalmol. 2018, 11, 881–887. [Google Scholar] [CrossRef]
- Kaiser, P. Methionine Dependence of Cancer. Biomolecules 2020, 10, 568. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, M.; George, A.K.; Tyagi, S.C. Restoration of skeletal muscle homeostasis by hydrogen sulfide during hyperhomocysteinemia-mediated oxidative/ER stress condition. Can. J. Physiol. Pharmacol. 2019, 97, 441–456. [Google Scholar] [CrossRef]
- Hellmich, M.R.; Szabo, C. Hydrogen Sulfide and Cancer. Handb. Exp. Pharmacol. 2015, 230, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.C.; Maddocks, O.D.K. One-carbon metabolism in cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Arora, R.; Bansal, A.K.; Bhattacharya, R.; Sharma, G.S.; Singh, L.R. Disturbed homocysteine metabolism is associated with cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Skibola, C.F.; Smith, M.T.; Kane, E.; Roman, E.; Rollinson, S.; Cartwright, R.A.; Morgan, G. Polymorphisms in the methylenetetrahydrofolate reductase gene are associated with susceptibility to acute leukemia in adults. Proc. Natl. Acad. Sci. USA 1999, 96, 12810–12815. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Shen, Y. MTHFR C677T polymorphism and breast, ovarian cancer risk: A meta-analysis of 19,260 patients and 26,364 controls. OncoTargets Ther. 2017, 10, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Xu, J.H. MTHFR polymorphism and the risk of prostate cancer: A meta-analysis of case-control studies. Prostate Cancer Prostatic Dis. 2012, 15, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Mbemi, A.; Khanna, S.; Njiki, S.; Yedjou, C.G.; Tchounwou, P.B. Impact of Gene-Environment Interactions on Cancer Development. Int. J. Environ. Res. Public Health 2020, 17, 8089. [Google Scholar] [CrossRef]
- Chen, J.; Giovannucci, E.; Kelsey, K.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Spiegelman, D.; Willett, W.C.; Hunter, D.J. A methylenetetrahydrofolate reductase polymorphism and the risk of colorectal cancer. Cancer Res. 1996, 56, 4862–4864. [Google Scholar]
- Ma, E.; Iwasaki, M.; Junko, I.; Hamada, G.S.; Nishimoto, I.N.; Carvalho, S.M.; Motola, J., Jr.; Laginha, F.M.; Tsugane, S. Dietary intake of folate, vitamin B6, and vitamin B12, genetic polymorphism of related enzymes, and risk of breast cancer: A case-control study in Brazilian women. BMC Cancer 2009, 9, 122. [Google Scholar] [CrossRef]
- Suzuki, T.; Matsuo, K.; Sawaki, A.; Mizuno, N.; Hiraki, A.; Kawase, T.; Watanabe, M.; Nakamura, T.; Yamao, K.; Tajima, K.; et al. Alcohol drinking and one-carbon metabolism-related gene polymorphisms on pancreatic cancer risk. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2742–2747. [Google Scholar] [CrossRef]
- Chen, K.; Song, L.; Jin, M.J.; Fan, C.H.; Jiang, Q.T.; Yu, W.P. Association between genetic polymorphisms in folate metabolic enzyme genes and colorectal cancer: A nested case-control study. Zhonghua Zhong Liu Za Zhi 2006, 28, 429–432. [Google Scholar]
- Chen, J.; Giovannucci, E.; Hankinson, S.E.; Ma, J.; Willett, W.C.; Spiegelman, D.; Kelsey, K.T.; Hunter, D.J. A prospective study of methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms, and risk of colorectal adenoma. Carcinogenesis 1998, 19, 2129–2132. [Google Scholar] [CrossRef]
- Ascenção, K.; Szabo, C. Emerging roles of cystathionine β-synthase in various forms of cancer. Redox Biol. 2022, 53, 102331. [Google Scholar] [CrossRef]
- Ibrahim, H.; Serag, A.; Farag, M.A. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J. Adv. Res. 2021, 27, 137–153. [Google Scholar] [CrossRef]
- Wang, D.; Yang, H.; Zhang, Y.; Hu, R.; Hu, D.; Wang, Q.; Liu, Y.; Liu, M.; Meng, Z.; Zhou, W.; et al. Inhibition of cystathionine β-synthase promotes apoptosis and reduces cell proliferation in chronic myeloid leukemia. Signal Transduct. Target. Ther. 2021, 6, 52. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Ueki, I. Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. J. Inherit. Metab. Dis. 2011, 34, 17–32. [Google Scholar] [CrossRef]
- Veeranki, S.; Tyagi, S.C. Defective homocysteine metabolism: Potential implications for skeletal muscle malfunction. Int. J. Mol. Sci. 2013, 14, 15074–15091. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Arcamone, M.; Morelli, E.; Viscardi, D.; Russo, V.; De Franciscis, S.; Belli, A.; Accardo, R.; Caliendo, D.; De Luca, E.; et al. Erratum to: Multidisciplinary approach and anesthetic management of a surgical cancer patient with methylene tetrahydrofolate reductase deficiency: A case report and review of the literature. J. Med. Case Rep. 2015, 9, 218. [Google Scholar] [CrossRef] [PubMed]
- Hankey, G.J.; Eikelboom, J.W. Homocysteine and vascular disease. Lancet 1999, 354, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Singh, M.; George, A.K.; Homme, R.P.; Laha, A.; Tyagi, S.C. Remote ischemic conditioning as a cytoprotective strategy in vasculopathies during hyperhomocysteinemia: An emerging research perspective. J. Cell. Biochem. 2019, 120, 77–92. [Google Scholar] [CrossRef]
- Lahiri, K.D.; Datta, H.; Das, H.N. Reference interval determination of total plasma homocysteine in an Indian population. Indian J. Clin. Biochem. 2014, 29, 74–78. [Google Scholar] [CrossRef][Green Version]
- Tiahou, G.; Dupuy, A.M.; Jaussent, I.; Sees, D.; Cristol, J.P.; Badiou, S. Determinants of homocysteine levels in Ivorian rural population. Int. J. Vitam. Nutr. Res. 2009, 79, 319–327. [Google Scholar] [CrossRef]
- Maddocks, O.D.; Labuschagne, C.F.; Adams, P.D.; Vousden, K.H. Serine Metabolism Supports the Methionine Cycle and DNA/RNA Methylation through De Novo ATP Synthesis in Cancer Cells. Mol. Cell 2016, 61, 210–221. [Google Scholar] [CrossRef]
- Lehotsky, J.; Tothova, B.; Kovalska, M.; Dobrota, D.; Benova, A.; Kalenska, D.; Kaplan, P. Role of Homocysteine in the Ischemic Stroke and Development of Ischemic Tolerance. Front. Neurosci. 2016, 10, 538. [Google Scholar] [CrossRef]
- Banerjee, I.; Gupta, V.; Ganesh, S. Association of gene polymorphism with genetic susceptibility to stroke in Asian populations: A meta-analysis. J. Hum. Genet. 2007, 52, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, H.; Xiao, H.; Yao, G.; Shi, Y.; Wang, Y.; Zhou, X.; Yu, H. Association of MTHFR 677T variant allele with risk of intracerebral haemorrhage: A meta-analysis. J. Neurol. Sci. 2012, 323, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Zhao, X.; Liu, L.; Wu, W.; Zhang, D. Association of the C677T polymorphism in the MTHFR gene with hemorrhagic stroke: A meta-analysis. Genet. Test. Mol. Biomark. 2013, 17, 412–417. [Google Scholar] [CrossRef]
- Yu, H.H.; Zhang, W.L.; Shi, J.P. Relationship between methylenetetrahydrofolate reductase gene C677T polymorphism and susceptibility of ischemic stroke: A meta-analysis. Zhonghua Yi Xue Za Zhi 2011, 91, 2060–2064. [Google Scholar]
- Sen, U.; Mishra, P.K.; Tyagi, N.; Tyagi, S.C. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem. Biophys. 2010, 57, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Diakoumopoulou, E.; Tentolouris, N.; Kirlaki, E.; Perrea, D.; Kitsou, E.; Psallas, M.; Doulgerakis, D.; Katsilambros, N. Plasma homocysteine levels in patients with type 2 diabetes in a Mediterranean population: Relation with nutritional and other factors. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Singh, M.; George, A.K.; Behera, J.; Tyagi, N.; Tyagi, S.C. Hydrogen sulfide improves postischemic neoangiogenesis in the hind limb of cystathionine-β-synthase mutant mice via PPAR-γ/VEGF axis. Physiol. Rep. 2018, 6, e13858. [Google Scholar] [CrossRef]
- Laha, A.; Majumdar, A.; Singh, M.; Tyagi, S.C. Connecting homocysteine and obesity through pyroptosis, gut microbiome, epigenetics, peroxisome proliferator-activated receptor gamma, and zinc finger protein 407. Can. J. Physiol. Pharmacol. 2018, 96, 971–976. [Google Scholar] [CrossRef]
- Orendac, M.; Muskova, B.; Richterova, E.; Zvarova, J.; Stefek, M.; Zaykova, E.; Kraus, J.P.; Stribrny, J.; Hyanek, J.; Kozich, V. Is the common 844ins68 polymorphism in the cystathionine beta-synthase gene associated with atherosclerosis? J. Inherit. Metab. Dis. 1999, 22, 674–675. [Google Scholar] [CrossRef]
- Ding, R.; Lin, S.; Chen, D. The association of cystathionine beta synthase (CBS) T833C polymorphism and the risk of stroke: A meta-analysis. J. Neurol. Sci. 2012, 312, 26–30. [Google Scholar] [CrossRef]
- de Franchis, R.; Fermo, I.; Mazzola, G.; Sebastio, G.; Di Minno, G.; Coppola, A.; Andria, G.; D’Angelo, A. Contribution of the cystathionine beta-synthase gene (844ins68) polymorphism to the risk of early-onset venous and arterial occlusive disease and of fasting hyperhomocysteinemia. Thromb. Haemost. 2000, 84, 576–582. [Google Scholar] [CrossRef] [PubMed]
- McGimpsey, S.J.; Woodside, J.V.; Cardwell, C.; Cahill, M.; Chakravarthy, U. Homocysteine, methylenetetrahydrofolate reductase C677T polymorphism, and risk of retinal vein occlusion: A meta-analysis. Ophthalmology 2009, 116, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.G.; Shmorgun, D.; Chan, W.S. Common C677T polymorphism of the methylenetetrahydrofolate reductase gene and the risk of venous thromboembolism: Meta-analysis of 31 studies. Pathophysiol. Haemost. Thromb. 2002, 32, 51–58. [Google Scholar] [CrossRef]
- Cai, W.; Yin, L.; Yang, F.; Zhang, L.; Cheng, J. Association between Hcy levels and the CBS844ins68 and MTHFR C677T polymorphisms with essential hypertension. Biomed. Rep. 2014, 2, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Heifetz, E.M.; Birk, R.Z. MTHFR C677T polymorphism affects normotensive diastolic blood pressure independently of blood lipids. Am. J. Hypertens. 2015, 28, 387–392. [Google Scholar] [CrossRef]
- Yang, K.M.; Jia, J.; Mao, L.N.; Men, C.; Tang, K.T.; Li, Y.Y.; Ding, H.X.; Zhan, Y.Y. Methylenetetrahydrofolate reductase C677T gene polymorphism and essential hypertension: A meta-analysis of 10,415 subjects. Biomed. Rep. 2014, 2, 699–708. [Google Scholar] [CrossRef]
- Hua, Y.; Zhao, H.; Kong, Y.; Ye, M. Association between the MTHFR gene and Alzheimer’s disease: A meta-analysis. Int. J. Neurosci. 2011, 121, 462–471. [Google Scholar] [CrossRef]
- Ibrahim, S.; Maqbool, S.; Azam, M.; Iqbal, M.P.; Qamar, R. CBS mutations and MTFHR SNPs causative of hyperhomocysteinemia in Pakistani children. Mol. Biol. Rep. 2018, 45, 353–360. [Google Scholar] [CrossRef]
- Castro, R.; Rivera, I.; Blom, H.J.; Jakobs, C.; Tavares de Almeida, I. Homocysteine metabolism, hyperhomocysteinaemia and vascular disease: An overview. J. Inherit. Metab. Dis. 2006, 29, 3–20. [Google Scholar] [CrossRef]
- White, R.H. The epidemiology of venous thromboembolism. Circulation 2003, 107, I4–I8. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhou, Y.; Xie, J.; Lv, W.; Kang, B.; Liang, Y.; Chen, Y.; Li, Y. Association of C677T gene polymorphisms of methylenetetrahydrofolate reductase and plasma homocysteine level with hyperlipidemia. J. South. Med. Univ. 2014, 34, 1195–1198. [Google Scholar]
- Al-Rubeaan, K.; Siddiqui, K.; Saeb, A.T.; Nazir, N.; Al-Naqeb, D.; Al-Qasim, S. ACE I/D and MTHFR C677T polymorphisms are significantly associated with type 2 diabetes in Arab ethnicity: A meta-analysis. Gene 2013, 520, 166–177. [Google Scholar] [CrossRef]
- Chang, W.W.; Zhang, L.; Yao, Y.S.; Su, H.; Jin, Y.L.; Chen, Y. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism and susceptibility to diabetic nephropathy in Chinese type 2 diabetic patients: A meta-analysis. Ren. Fail. 2013, 35, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, J.; Feng, C.; Huang, G. MTHFR 677T variant contributes to diabetic nephropathy risk in Caucasian individuals with type 2 diabetes: A meta-analysis. Metab. Clin. Exp. 2013, 62, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, Y.; Han, L.; Ji, H.; Li, J. The effect of MTHFR C677T polymorphism on type 2 diabetes mellitus with vascular complications in Chinese Han population: A meta-analysis. Endocr. J. 2014, 61, 717–726. [Google Scholar] [CrossRef]
- Gouveia, L.O.; Canhao, P. MTHFR and the risk for cerebral venous thrombosis—A meta-analysis. Thromb. Res. 2010, 125, e153–e158. [Google Scholar] [CrossRef]
- Wu, C.Y.; Yang, M.; Lin, M.; Li, L.P.; Wen, X.Z. MTHFR C677T polymorphism was an ethnicity-dependent risk factor for cervical cancer development: Evidence based on a meta-analysis. Arch. Gynecol. Obstet. 2013, 288, 595–605. [Google Scholar] [CrossRef]
- Wu, Y.L.; Ding, X.X.; Sun, Y.H.; Yang, H.Y.; Sun, L. Methylenetetrahydrofolate reductase (MTHFR) C677T/A1298C polymorphisms and susceptibility to Parkinson’s disease: A meta-analysis. J. Neurol. Sci. 2013, 335, 14–21. [Google Scholar] [CrossRef]
- Laster, L.; Mudd, S.H.; Finkelstein, J.D.; Irreverre, F. Homocystinuria due to cystathionine synthase deficiency: The metabolism of L-methionine. J. Clin. Investig. 1965, 44, 1708–1719. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J. Biol. Chem. 1984, 259, 9508–9513. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D.; Kyle, W.E.; Martin, J.L.; Pick, A.M. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun. 1975, 66, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Steegborn, C.; Wahl, M.C.; Oliveriusova, J.; Kraus, J.P.; Allen, R.H.; Wagner, C.; Mudd, S.H. Elevated plasma total homocysteine in severe methionine adenosyltransferase I/III deficiency. Metab. Clin. Exp. 2002, 51, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg-Solomon, R.Z.; Miller, E.R., 3rd; Maguire, M.G.; Selhub, J.; Appel, L.J. Association of dietary protein intake and coffee consumption with serum homocysteine concentrations in an older population. Am. J. Clin. Nutr. 1999, 69, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J. Homocysteine metabolism. Annu. Rev. Nutr. 1999, 19, 217–246. [Google Scholar] [CrossRef]
- Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; Rogers, G.; Bowman, B.A.; Gunter, E.W.; Wright, J.D.; Johnson, C.L. Serum total homocysteine concentrations in the third National Health and Nutrition Examination Survey (1991–1994): Population reference ranges and contribution of vitamin status to high serum concentrations. Ann. Intern. Med. 1999, 131, 331–339. [Google Scholar] [CrossRef]
- Eloranta, T.O.; Martikainen, V.; Smith, T.K. Adaptation of adenosylmethionine metabolism and methionine recycling to variations in dietary methionine in the rat. Proc. Soc. Exp. Biol. Med. 1990, 194, 364–371. [Google Scholar] [CrossRef]
- Veeranki, S.; Winchester, L.J.; Tyagi, S.C. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochim. Biophys. Acta 2015, 1852, 732–741. [Google Scholar] [CrossRef]
- Veeranki, S.; Tyagi, S.C. Mechanisms of hyperhomocysteinemia induced skeletal muscle myopathy after ischemia in the CBS-/+ mouse model. Int. J. Mol. Sci. 2015, 16, 1252–1265. [Google Scholar] [CrossRef]
- Winchester, L.; Veeranki, S.; Givvimani, S.; Tyagi, S.C. Exercise mitigates the adverse effects of hyperhomocysteinemia on macrophages, MMP-9, skeletal muscle, and white adipocytes. Can. J. Physiol. Pharmacol. 2014, 92, 575–582. [Google Scholar] [CrossRef]
- Ishii, I.; Akahoshi, N.; Yamada, H.; Nakano, S.; Izumi, T.; Suematsu, M. Cystathionine gamma-Lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J. Biol. Chem. 2010, 285, 26358–26368. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Ascherio, A.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Alcohol, low-methionine--low-folate diets, and risk of colon cancer in men. J. Natl. Cancer Inst. 1995, 87, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stampfer, M.J.; Christensen, B.; Giovannucci, E.; Hunter, D.J.; Chen, J.; Willett, W.C.; Selhub, J.; Hennekens, C.H.; Gravel, R.; et al. A polymorphism of the methionine synthase gene: Association with plasma folate, vitamin B12, homocyst(e)ine, and colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 1999, 8, 825–829. [Google Scholar]
- Bravatà, V. Controversial roles of methylenetetrahydrofolate reductase polymorphisms and folate in breast cancer disease. Int. J. Food Sci. Nutr. 2015, 66, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Dnistrian, A.M.; Schwartz, M.; Toniolo, P.; Koenig, K.; Shore, R.E.; Akhmedkhanov, A.; Zeleniuch-Jacquotte, A.; Riboli, E. Serum folate, homocysteine and colorectal cancer risk in women: A nested case-control study. Br. J. Cancer 1999, 79, 1917–1922. [Google Scholar] [CrossRef]
- de Jong, M.M.; Nolte, I.M.; te Meerman, G.J.; van der Graaf, W.T.; de Vries, E.G.; Sijmons, R.H.; Hofstra, R.M.; Kleibeuker, J.H. Low-penetrance genes and their involvement in colorectal cancer susceptibility. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1332–1352. [Google Scholar]
- Matsuo, K.; Hamajima, N.; Hirai, T.; Kato, T.; Inoue, M.; Takezaki, T.; Tajima, K. Methionine Synthase Reductase Gene A66G Polymorphism is Associated with Risk of Colorectal Cancer. Asian Pac. J. Cancer Prev. 2002, 3, 353–359. [Google Scholar]
- Robien, K.; Ulrich, C.M. 5,10-Methylenetetrahydrofolate reductase polymorphisms and leukemia risk: A HuGE minireview. Am. J. Epidemiol. 2003, 157, 571–582. [Google Scholar] [CrossRef][Green Version]
- Krajinovic, M.; Lamothe, S.; Labuda, D.; Lemieux-Blanchard, E.; Theoret, Y.; Moghrabi, A.; Sinnett, D. Role of MTHFR genetic polymorphisms in the susceptibility to childhood acute lymphoblastic leukemia. Blood 2004, 103, 252–257. [Google Scholar] [CrossRef]
- Singal, R.; Ferdinand, L.; Das, P.M.; Reis, I.M.; Schlesselman, J.J. Polymorphisms in the methylenetetrahydrofolate reductase gene and prostate cancer risk. Int. J. Oncol. 2004, 25, 1465–1471. [Google Scholar] [CrossRef]
- Matsuo, K.; Ito, H.; Wakai, K.; Hirose, K.; Saito, T.; Suzuki, T.; Kato, T.; Hirai, T.; Kanemitsu, Y.; Hamajima, H.; et al. One-carbon metabolism related gene polymorphisms interact with alcohol drinking to influence the risk of colorectal cancer in Japan. Carcinogenesis 2005, 26, 2164–2171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, L.L.; Wu, J.T. Hyperhomocysteinemia is a risk factor for cancer and a new potential tumor marker. Clin. Chim. Acta 2002, 322, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Refsum, H. Plasma homocysteine, a risk factor for vascular disease: Plasma levels in health, disease, and drug therapy. J. Lab. Clin. Med. 1989, 114, 473–501. [Google Scholar]
- Corona, G.; Toffoli, G.; Fabris, M.; Viel, A.; Zarrelli, A.; Donada, C.; Boiocchi, M. Homocysteine accumulation in human ovarian carcinoma ascitic/cystic fluids possibly caused by metabolic alteration of the methionine cycle in ovarian carcinoma cells. Eur. J. Cancer 1997, 33, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Santotoribio, J.D.; Cañavate-Solano, C.; Garcia-de la Torre, A.; Del Valle-Vazquez, L.; Arce-Matute, F.; Cuadros-Muñoz, J.F.; Sanchez del Pino, M.J.; Bandez-Ruiz, M.J.; Piñuela-Rojas, C.; Perez-Ramos, S. Homocysteine: New tumor marker in pleural fluid. Tumor Biol. 2015, 36, 7941–7945. [Google Scholar] [CrossRef]
- Shujuan, Y.; Jianxing, Z.; Xin-Yue, C. Methylenetetrahydrofolate reductase genetic polymorphisms and esophageal squamous cell carcinoma susceptibility: A meta-analysis of case-control studies. Pak. J. Med. Sci. 2013, 29, 693–698. [Google Scholar] [PubMed]
- Marugame, T.; Tsuji, E.; Inoue, H.; Shinomiya, S.; Kiyohara, C.; Onuma, K.; Hamada, H.; Koga, H.; Handa, K.; Hayabuchi, H.; et al. Methylenetetrahydrofolate reductase polymorphism and risk of colorectal adenomas. Cancer Lett. 2000, 151, 181–186. [Google Scholar] [CrossRef]
- Paynter, R.A.; Hankinson, S.E.; Hunter, D.J.; De Vivo, I. No association between MTHFR 677 C->T or 1298 A->C polymorphisms and endometrial cancer risk. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1088–1089. [Google Scholar] [CrossRef]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S. Relationship between three polymorphisms of methylenetetrahydrofolate reductase (MTHFR C677T, A1298C, and G1793A) gene and risk of prostate cancer: A case-control study. Prostate 2010, 70, 1645–1657. [Google Scholar] [CrossRef]
- Fang, D.H.; Ji, Q.; Fan, C.H.; An, Q.; Li, J. Methionine synthase reductase A66G polymorphism and leukemia risk: Evidence from published studies. Leuk. Lymphoma 2014, 55, 1910–1914. [Google Scholar] [CrossRef]
- Wang, P.; Li, S.; Wang, M.; He, J.; Xi, S. Association of MTRR A66G polymorphism with cancer susceptibility: Evidence from 85 studies. J. Cancer 2017, 8, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.P.; Tang, R.N.; An, L. A meta-analysis of MTRR A66G polymorphism and colorectal cancer susceptibility. J. BUON 2015, 20, 918–922. [Google Scholar] [PubMed]
- Yoo, J.Y.; Kim, S.Y.; Hwang, J.A.; Hong, S.H.; Shin, A.; Choi, I.J.; Lee, Y.S. Association Study between Folate Pathway Gene Single Nucleotide Polymorphisms and Gastric Cancer in Koreans. Genom. Inform. 2012, 10, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zou, T.; Cao, N.; Ni, J.; Xu, W.; Zhou, T.; Wang, X. Plasma homocysteine levels and genetic polymorphisms in folate metablism are associated with breast cancer risk in chinese women. Hered. Cancer Clin. Pract. 2014, 12, 2. [Google Scholar] [CrossRef]
- Cui, L.H.; Song, Y.; Si, H.; Shen, F.; Shin, M.H.; Kim, H.N.; Choi, J.S. Folate metabolism-related gene polymorphisms and susceptibility to primary liver cancer in North China. Med. Oncol. 2012, 29, 1837–1842. [Google Scholar] [CrossRef]
- Tao, M.H.; Shields, P.G.; Nie, J.; Marian, C.; Ambrosone, C.B.; McCann, S.E.; Platek, M.; Krishnan, S.S.; Xie, B.; Edge, S.B.; et al. DNA promoter methylation in breast tumors: No association with genetic polymorphisms in MTHFR and MTR. Cancer Epidemiol. Biomark. Prev. 2009, 18, 998–1002. [Google Scholar] [CrossRef]
- Semmler, A.; Simon, M.; Moskau, S.; Linnebank, M. The methionine synthase polymorphism c.2756A>G alters susceptibility to glioblastoma multiforme. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2314–2316. [Google Scholar] [CrossRef]
- Ott, N.; Geddert, H.; Sarbia, M. Polymorphisms in methionine synthase (A2756G) and cystathionine beta-synthase (844ins68) and susceptibility to carcinomas of the upper gastrointestinal tract. J. Cancer Res. Clin. Oncol. 2008, 134, 405–410. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Z.; Ma, Y.; Xia, Q.; Zhang, F.; Fu, D.; Wang, X.F. Lack of association between methionine synthase A2756G polymorphism and digestive system cancer risk: Evidence from 39327 subjects. PLoS ONE 2013, 8, e61511. [Google Scholar] [CrossRef]
- Wang, L.; Ke, Q.; Chen, W.; Wang, J.; Tan, Y.; Zhou, Y.; Hua, Z.; Ding, W.; Niu, J.; Shen, J.; et al. Polymorphisms of MTHFD, plasma homocysteine levels, and risk of gastric cancer in a high-risk Chinese population. Clin. Cancer Res. 2007, 13, 2526–2532. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, H.; Li, L.; Zhang, Z.; Xu, Y. Association of methylenetetrahydrofolate dehydrogenase 1 polymorphisms with cancer: A meta-analysis. PLoS ONE 2013, 8, e69366. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.M.; Galbiatti, A.L.; Ruiz, M.T.; Raposo, L.S.; Maniglia, J.V.; Pavarino, E.C.; Goloni-Bertollo, E.M. MTHFD1 G1958A, BHMT G742A, TC2 C776G and TC2 A67G polymorphisms and head and neck squamous cell carcinoma risk. Mol. Biol. Rep. 2012, 39, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gammon, M.D.; Zeisel, S.H.; Lee, Y.L.; Wetmur, J.G.; Teitelbaum, S.L.; Bradshaw, P.T.; Neugut, A.I.; Santella, R.M.; Chen, J. Choline metabolism and risk of breast cancer in a population-based study. FASEB J. 2008, 22, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Mostowska, A.; Myka, M.; Lianeri, M.; Roszak, A.; Jagodziński, P.P. Folate and choline metabolism gene variants and development of uterine cervical carcinoma. Clin. Biochem. 2011, 44, 596–600. [Google Scholar] [CrossRef]
- Pawlik, P.; Mostowska, A.; Lianeri, M.; Sajdak, S.; Kędzia, H.; Jagodzinski, P.P. Folate and choline metabolism gene variants in relation to ovarian cancer risk in the Polish population. Mol. Biol. Rep. 2012, 39, 5553–5560. [Google Scholar] [CrossRef]
- Hazra, A.; Wu, K.; Kraft, P.; Fuchs, C.S.; Giovannucci, E.L.; Hunter, D.J. Twenty-four non-synonymous polymorphisms in the one-carbon metabolic pathway and risk of colorectal adenoma in the Nurses’ Health Study. Carcinogenesis 2007, 28, 1510–1519. [Google Scholar] [CrossRef]
- Kurzwelly, D.; Knop, S.; Guenther, M.; Loeffler, J.; Korfel, A.; Thiel, E.; Hebart, H.; Simon, M.; Weller, M.; Linnebank, M.; et al. Genetic variants of folate and methionine metabolism and PCNSL incidence in a German patient population. J. Neurooncol. 2010, 100, 187–192. [Google Scholar] [CrossRef]
- Gao, C.M.; Takezaki, T.; Wu, J.Z.; Liu, Y.T.; Ding, J.H.; Li, S.P.; Su, P.; Hu, X.; Kai, H.T.; Li, Z.Y.; et al. Polymorphisms in thymidylate synthase and methylenetetrahydrofolate reductase genes and the susceptibility to esophageal and stomach cancer with smoking. Asian Pac. J. Cancer Prev. 2004, 5, 133–138. [Google Scholar]
- Stathopoulou, A.; Vlachonikolis, I.; Mavroudis, D.; Perraki, M.; Kouroussis, C.; Apostolaki, S.; Malamos, N.; Kakolyris, S.; Kotsakis, A.; Xenidis, N.; et al. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: Evaluation of their prognostic significance. J. Clin. Oncol. 2002, 20, 3404–3412. [Google Scholar] [CrossRef]
- Refsum, H.; Nurk, E.; Smith, A.D.; Ueland, P.M.; Gjesdal, C.G.; Bjelland, I.; Tverdal, A.; Tell, G.S.; Nygård, O.; Vollset, S.E. The Hordaland Homocysteine Study: A community-based study of homocysteine, its determinants, and associations with disease. J. Nutr. 2006, 136, 1731s–1740s. [Google Scholar] [CrossRef]
- Gatt, A.; Makris, A.; Cladd, H.; Burcombe, R.J.; Smith, J.M.; Cooper, P.; Thompson, D.; Makris, M. Hyperhomocysteinemia in women with advanced breast cancer. Int. J. Lab. Hematol. 2007, 29, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Goyette, P.; Sumner, J.S.; Milos, R.; Duncan, A.M.; Rosenblatt, D.S.; Matthews, R.G.; Rozen, R. Human methylenetetrahydrofolate reductase: Isolation of cDNA, mapping and mutation identification. Nat. Genet. 1994, 7, 195–200. [Google Scholar] [CrossRef]
- Goyette, P.; Frosst, P.; Rosenblatt, D.S.; Rozen, R. Seven novel mutations in the methylenetetrahydrofolate reductase gene and genotype/phenotype correlations in severe methylenetetrahydrofolate reductase deficiency. Am. J. Hum. Genet. 1995, 56, 1052–1059. [Google Scholar] [PubMed]
- Kluijtmans, L.A.; Wendel, U.; Stevens, E.M.; van den Heuvel, L.P.; Trijbels, F.J.; Blom, H.J. Identification of four novel mutations in severe methylenetetrahydrofolate reductase deficiency. Eur. J. Hum. Genet. 1998, 6, 257–265. [Google Scholar] [CrossRef]
- Weisberg, I.; Tran, P.; Christensen, B.; Sibani, S.; Rozen, R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol. Genet. Metab. 1998, 64, 169–172. [Google Scholar] [CrossRef]
- Sibani, S.; Christensen, B.; O’Ferrall, E.; Saadi, I.; Hiou-Tim, F.; Rosenblatt, D.S.; Rozen, R. Characterization of six novel mutations in the methylenetetrahydrofolate reductase (MTHFR) gene in patients with homocystinuria. Hum. Mutat. 2000, 15, 280–287. [Google Scholar] [CrossRef]
- Tonetti, C.; Amiel, J.; Munnich, A.; Zittoun, J. Impact of new mutations in the methylenetetrahydrofolate reductase gene assessed on biochemical phenotypes: A familial study. J. Inherit. Metab. Dis. 2001, 24, 833–842. [Google Scholar] [CrossRef]
- Yano, H.; Nakaso, K.; Yasui, K.; Wakutani, Y.; Nakayasu, H.; Kowa, H.; Adachi, Y.; Nakashima, K. Mutations of the MTHFR gene (428C>T and [458G>T+459C>T]) markedly decrease MTHFR enzyme activity. Neurogenetics 2004, 5, 135–140. [Google Scholar] [CrossRef]
- Ma, J.; Stampfer, M.J.; Giovannucci, E.; Artigas, C.; Hunter, D.J.; Fuchs, C.; Willett, W.C.; Selhub, J.; Hennekens, C.H.; Rozen, R. Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res. 1997, 57, 1098–1102. [Google Scholar]
- Esteller, M.; Garcia, A.; Martinez-Palones, J.M.; Xercavins, J.; Reventos, J. Germ line polymorphisms in cytochrome-P450 1A1 (C4887 CYP1A1) and methylenetetrahydrofolate reductase (MTHFR) genes and endometrial cancer susceptibility. Carcinogenesis 1997, 18, 2307–2311. [Google Scholar] [CrossRef]
- Song, C.; Xing, D.; Tan, W.; Wei, Q.; Lin, D. Methylenetetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res. 2001, 61, 3272–3275. [Google Scholar] [PubMed]
- Krajinovic, M.; Lemieux-Blanchard, E.; Chiasson, S.; Primeau, M.; Costea, I.; Moghrabi, A. Role of polymorphisms in MTHFR and MTHFD1 genes in the outcome of childhood acute lymphoblastic leukemia. Pharmacogenom. J. 2004, 4, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Li, Y.T.; Yang, S.Y.; Li, W. Meta-analysis on MTHFR polymorphism and lung cancer susceptibility in East Asian populations. Biomed. Rep. 2013, 1, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Dick, D.M. Gene-environment interaction in psychological traits and disorders. Annu. Rev. Clin. Psychol. 2011, 7, 383–409. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA hypomethylation in cancer cells. Epigenomics 2009, 1, 239–259. [Google Scholar] [CrossRef]
- Martin, C.; Zhang, Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, J.; Deng, C.; Xia, Y.; Li, B.; Meng, S.; Jin, X.; Cheng, L.; Li, H.; Zhang, C.; et al. Crosstalk of Histone and RNA Modifications Identified a Stromal-Activated Subtype with Poor Survival and Resistance to Immunotherapy in Gastric Cancer. Front. Pharmacol. 2022, 13, 868830. [Google Scholar] [CrossRef]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef]
- Santos-Rebouças, C.B.; Pimentel, M.M. Implication of abnormal epigenetic patterns for human diseases. Eur. J. Hum. Genet. 2007, 15, 10–17. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Xu, G. Notable epigenetic role of hyperhomocysteinemia in atherogenesis. Lipids Health Dis. 2014, 13, 134. [Google Scholar] [CrossRef]
- Jiang, L.; Gonda, T.A.; Gamble, M.V.; Salas, M.; Seshan, V.; Tu, S.; Twaddell, W.S.; Hegyi, P.; Lazar, G.; Steele, I.; et al. Global hypomethylation of genomic DNA in cancer-associated myofibroblasts. Cancer Res. 2008, 68, 9900–9908. [Google Scholar] [CrossRef] [PubMed]
- Berchuck, J.E.; Baca, S.C.; McClure, H.M.; Korthauer, K.; Tsai, H.K.; Nuzzo, P.V.; Kelleher, K.M.; He, M.; Steinharter, J.A.; Zacharia, S.; et al. Detecting Neuroendocrine Prostate Cancer Through Tissue-Informed Cell-Free DNA Methylation Analysis. Clin. Cancer Res. 2022, 28, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Cahill, N.; Rosenquist, R. Uncovering the DNA methylome in chronic lymphocytic leukemia. Epigenetics 2013, 8, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Malik, S.; Xing, S. Epigenetic Mechanisms Involved in HCV-Induced Hepatocellular Carcinoma (HCC). Front. Oncol. 2021, 11, 677926. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA hypomethylation, cancer, the immunodeficiency, centromeric region instability, facial anomalies syndrome and chromosomal rearrangements. J. Nutr. 2002, 132, 2424s–2429s. [Google Scholar] [CrossRef] [PubMed]
- Pappalardo, X.G.; Barra, V. Losing DNA methylation at repetitive elements and breaking bad. Epigenetics Chromatin 2021, 14, 25. [Google Scholar] [CrossRef]
- Burns, K.H. Transposable elements in cancer. Nat. Rev. Cancer 2017, 17, 415–424. [Google Scholar] [CrossRef]
- Zhang, N. Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Anim. Nutr. 2018, 4, 11–16. [Google Scholar] [CrossRef]
- Lund, G.; Andersson, L.; Lauria, M.; Lindholm, M.; Fraga, M.F.; Villar-Garea, A.; Ballestar, E.; Esteller, M.; Zaina, S. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J. Biol. Chem. 2004, 279, 29147–29154. [Google Scholar] [CrossRef]
- Devlin, A.M.; Arning, E.; Bottiglieri, T.; Faraci, F.M.; Rozen, R.; Lentz, S.R. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood 2004, 103, 2624–2629. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Kalani, A.; Givvimani, S.; Kamat, P.K.; Familtseva, A.; Tyagi, S.C. Differential regulation of DNA methylation versus histone acetylation in cardiomyocytes during HHcy in vitro and in vivo: An epigenetic mechanism. Physiol. Genom. 2014, 46, 245–255. [Google Scholar] [CrossRef]
- Izadi, P.; Noruzinia, M.; Karimipoor, M.; Karbassian, M.H.; Akbari, M.T. Promoter hypermethylation of estrogen receptor alpha gene is correlated to estrogen receptor negativity in Iranian patients with sporadic breast cancer. Cell J. 2012, 14, 102–109. [Google Scholar]
- Zhang, J.G.; Liu, J.X.; Li, Z.H.; Wang, L.Z.; Jiang, Y.D.; Wang, S.R. Dysfunction of endothelial NO system originated from homocysteine-induced aberrant methylation pattern in promoter region of DDAH2 gene. Chin. Med. J. 2007, 120, 2132–2137. [Google Scholar] [CrossRef]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.M.; et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef]
- Mariño-Ramírez, L.; Kann, M.G.; Shoemaker, B.A.; Landsman, D. Histone structure and nucleosome stability. Expert Rev. Proteom. 2005, 2, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications—Cause and consequence of genome function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Wu, Q.; Li, J.; Sun, S.; Sun, S. S-adenosylmethionine: A metabolite critical to the regulation of autophagy. Cell Prolif. 2020, 53, e12891. [Google Scholar] [CrossRef] [PubMed]
- Helin, K.; Dhanak, D. Chromatin proteins and modifications as drug targets. Nature 2013, 502, 480–488. [Google Scholar] [CrossRef]
- Garraway, L.A.; Lander, E.S. Lessons from the cancer genome. Cell 2013, 153, 17–37. [Google Scholar] [CrossRef]
- Halsall, J.A.; Andrews, S.; Krueger, F.; Rutledge, C.E.; Ficz, G.; Reik, W.; Turner, B.M. Histone modifications form a cell-type-specific chromosomal bar code that persists through the cell cycle. Sci. Rep. 2021, 11, 3009. [Google Scholar] [CrossRef]
- Yang, J.X.; Rastetter, R.H.; Wilhelm, D. Non-coding RNAs: An Introduction. Adv. Exp. Med. Biol. 2016, 886, 13–32. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Volovat, S.R.; Volovat, C.; Hordila, I.; Hordila, D.A.; Mirestean, C.C.; Miron, O.T.; Lungulescu, C.; Scripcariu, D.V.; Stolniceanu, C.R.; Konsoulova-Kirova, A.A.; et al. MiRNA and LncRNA as Potential Biomarkers in Triple-Negative Breast Cancer: A Review. Front. Oncol. 2020, 10, 526850. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Kamat, P.K.; Tyagi, S.C.; Tyagi, N. Synergy of homocysteine, microRNA, and epigenetics: A novel therapeutic approach for stroke. Mol. Neurobiol. 2013, 48, 157–168. [Google Scholar] [CrossRef] [PubMed]
- George, A.K.; Master, K.; Majumder, A.; Homme, R.P.; Laha, A.; Sandhu, H.S.; Tyagi, S.C.; Singh, M. Circular RNAs constitute an inherent gene regulatory axis in the mammalian eye and brain. Can. J. Physiol. Pharmacol. 2019, 97, 463–472. [Google Scholar] [CrossRef]
- Singh, M.; George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Sandhu, H.S.; Tyagi, S.C. Circular RNAs profiling in the cystathionine-β-synthase mutant mouse reveals novel gene targets for hyperhomocysteinemia induced ocular disorders. Exp. Eye Res. 2018, 174, 80–92. [Google Scholar] [CrossRef] [PubMed]
- George, A.K.; Homme, R.P.; Majumder, A.; Tyagi, S.C.; Singh, M. Effect of MMP-9 gene knockout on retinal vascular form and function. Physiol. Genom. 2019, 51, 613–622. [Google Scholar] [CrossRef]
- Homme, R.P.; Singh, M.; Majumder, A.; George, A.K.; Nair, K.; Sandhu, H.S.; Tyagi, N.; Lominadze, D.; Tyagi, S.C. Remodeling of Retinal Architecture in Diabetic Retinopathy: Disruption of Ocular Physiology and Visual Functions by Inflammatory Gene Products and Pyroptosis. Front. Physiol. 2018, 9, 1268. [Google Scholar] [CrossRef]
- Liu, X.; Tong, Y.; Xia, D.; Peng, E.; Yang, X.; Liu, H.; Ye, T.; Wang, X.; He, Y.; Ye, Z.; et al. Circular RNAs in prostate cancer: Biogenesis, biological functions, and clinical significance. Mol. Ther.-Nucleic Acids 2021, 26, 1130–1147. [Google Scholar] [CrossRef]
- Singh, M.; George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Sandhu, H.S.; Tyagi, S.C. Expression Analysis of the Circular RNA Molecules in the Human Retinal Cells Treated with Homocysteine. Curr. Eye Res. 2019, 44, 287–293. [Google Scholar] [CrossRef] [PubMed]
- George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Metreveli, N.; Sandhu, H.S.; Tyagi, S.C.; Singh, M. Hydrogen sulfide intervention in cystathionine-β-synthase mutant mouse helps restore ocular homeostasis. Int. J. Ophthalmol. 2019, 12, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, R.E.; Mohammad, I.Z.; Meram, A.T.; Kim, D.; Alotaibi, F.; Patel, S.; Ghali, G.E.; Kevil, C.G. Molecular Functions of Hydrogen Sulfide in Cancer. Pathophysiology 2021, 28, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002, 26, 13–19. [Google Scholar] [CrossRef]
- Guo, H.; Gai, J.W.; Wang, Y.; Jin, H.F.; Du, J.B.; Jin, J. Characterization of hydrogen sulfide and its synthases, cystathionine β-synthase and cystathionine γ-lyase, in human prostatic tissue and cells. Urology 2012, 79, 483.e1–483.e5. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Saha, S.; Giri, K.; Lanza, I.R.; Nair, K.S.; Jennings, N.B.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Basal, E.; Weaver, A.L.; et al. Cystathionine beta-synthase (CBS) contributes to advanced ovarian cancer progression and drug resistance. PLoS ONE 2013, 8, e79167. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, H.X.; Wu, C.T.; Wang, W.Q.; Jin, W.; Gao, H.L.; Li, H.; Zhang, S.R.; Xu, J.Z.; Qi, Z.H.; et al. Angiogenesis in pancreatic cancer: Current research status and clinical implications. Angiogenesis 2019, 22, 15–36. [Google Scholar] [CrossRef]
- Cai, W.J.; Wang, M.J.; Ju, L.H.; Wang, C.; Zhu, Y.C. Hydrogen sulfide induces human colon cancer cell proliferation: Role of Akt, ERK and p21. Cell Biol. Int. 2010, 34, 565–572. [Google Scholar] [CrossRef]
- Untereiner, A.A.; Pavlidou, A.; Druzhyna, N.; Papapetropoulos, A.; Hellmich, M.R.; Szabo, C. Drug resistance induces the upregulation of H2S-producing enzymes in HCT116 colon cancer cells. Biochem. Pharmacol. 2018, 149, 174–185. [Google Scholar] [CrossRef]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Szabo, C.; Coletta, C.; Chao, C.; Módis, K.; Szczesny, B.; Papapetropoulos, A.; Hellmich, M.R. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 12474–12479. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kawahara, B.; Gupta, D.; Tsai, R.; Khachatryan, M.; Roy-Chowdhuri, S.; Bose, S.; Yoon, A.; Faull, K.; Farias-Eisner, R.; et al. Role of cystathionine β-synthase in human breast Cancer. Free Radic. Biol. Med. 2015, 86, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Turbat-Herrera, E.A.; Kilpatrick, M.J.; Chen, J.; Meram, A.T.; Cotelingam, J.; Ghali, G.; Kevil, C.G.; Coppola, D.; Shackelford, R.E. Cystathione β-Synthase Is Increased in Thyroid Malignancies. Anticancer Res. 2018, 38, 6085–6090. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, Z.; Liu, Z.; Zou, Q.; Yuan, Y. Clinical Significance of CBS and CCL21 in Gallbladder Adenocarcinomas and Squamous Cell/Adenosquamous Carcinomas. Appl. Immunohistochem. Mol. Morphol. 2020, 28, 103–110. [Google Scholar] [CrossRef]
- Kim, J.; Hong, S.J.; Park, J.H.; Park, S.Y.; Kim, S.W.; Cho, E.Y.; Do, I.G.; Joh, J.W.; Kim, D.S. Expression of cystathionine beta-synthase is downregulated in hepatocellular carcinoma and associated with poor prognosis. Oncol. Rep. 2009, 21, 1449–1454. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Wang, J.; Su, X.; Ng, K.M.; Qiu, T.; Shan, L.; Ling, Y.; Wang, L.; Cai, J.; et al. Frequent epigenetic silencing of the folate-metabolising gene cystathionine-beta-synthase in gastrointestinal cancer. PLoS ONE 2012, 7, e49683. [Google Scholar] [CrossRef]
- You, J.; Shi, X.; Liang, H.; Ye, J.; Wang, L.; Han, H.; Fang, H.; Kang, W.; Wang, T. Cystathionine-γ-lyase promotes process of breast cancer in association with STAT3 signaling pathway. Oncotarget 2017, 8, 65677–65686. [Google Scholar] [CrossRef]
- Pei, Y.; Wu, B.; Cao, Q.; Wu, L.; Yang, G. Hydrogen sulfide mediates the anti-survival effect of sulforaphane on human prostate cancer cells. Toxicol. Appl. Pharmacol. 2011, 257, 420–428. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Q.; Yang, J.; Sun, D.; Li, C.; Xue, Y.; Jiang, Q.; Tian, Y.; Xu, C.; Wang, R. An Anticancer Role of Hydrogen Sulfide in Human Gastric Cancer Cells. Oxid. Med. Cell. Longev. 2015, 2015, 636410. [Google Scholar] [CrossRef]
- Gai, J.W.; Qin, W.; Liu, M.; Wang, H.F.; Zhang, M.; Li, M.; Zhou, W.H.; Ma, Q.T.; Liu, G.M.; Song, W.H.; et al. Expression profile of hydrogen sulfide and its synthases correlates with tumor stage and grade in urothelial cell carcinoma of bladder. Urol. Oncol. 2016, 34, 166.e15. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ye, S.; Yuan, D.; Zhang, J.; Bai, Y.; Shao, C. Hydrogen sulfide (H2S)/cystathionine γ-lyase (CSE) pathway contributes to the proliferation of hepatoma cells. Mutat. Res. 2014, 763–764, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Li, N.; Qi, J.; Yin, P.; Zhao, C.; Wang, L.; Li, Z.; Zha, X. Wnt/β-catenin signaling induces the transcription of cystathionine-γ-lyase, a stimulator of tumor in colon cancer. Cell. Signal. 2014, 26, 2801–2808. [Google Scholar] [CrossRef]
- Breza, J., Jr.; Soltysova, A.; Hudecova, S.; Penesova, A.; Szadvari, I.; Babula, P.; Chovancova, B.; Lencesova, L.; Pos, O.; Breza, J.; et al. Endogenous H2S producing enzymes are involved in apoptosis induction in clear cell renal cell carcinoma. BMC Cancer 2018, 18, 591. [Google Scholar] [CrossRef]
- Wróbel, M.; Czubak, J.; Bronowicka-Adamska, P.; Jurkowska, H.; Adamek, D.; Papla, B. Is development of high-grade gliomas sulfur-dependent? Molecules 2014, 19, 21350–21362. [Google Scholar] [CrossRef]
- Oláh, G.; Módis, K.; Törö, G.; Hellmich, M.R.; Szczesny, B.; Szabo, C. Role of endogenous and exogenous nitric oxide, carbon monoxide and hydrogen sulfide in HCT116 colon cancer cell proliferation. Biochem. Pharmacol. 2018, 149, 186–204. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ding, L.; Xie, Z.Z.; Yang, Y.; Whiteman, M.; Moore, P.K.; Bian, J.S. A Review of Hydrogen Sulfide Synthesis, Metabolism, and Measurement: Is Modulation of Hydrogen Sulfide a Novel Therapeutic for Cancer? Antioxid. Redox Signal. 2019, 31, 1–38. [Google Scholar] [CrossRef]
- Tu, X.H.; Huang, S.X.; Li, W.S.; Song, J.X. Correlation of methylation of CpG island in cystathionine beta synthase promoter and clinicopathological features in colorectal cancer. Zhonghua Zhong Liu Za Zhi 2013, 35, 351–355. [Google Scholar] [CrossRef]
- Módis, K.; Coletta, C.; Asimakopoulou, A.; Szczesny, B.; Chao, C.; Papapetropoulos, A.; Hellmich, M.R.; Szabo, C. Effect of S-adenosyl-L-methionine (SAM), an allosteric activator of cystathionine-β-synthase (CBS) on colorectal cancer cell proliferation and bioenergetics in vitro. Nitric Oxide 2014, 41, 146–156. [Google Scholar] [CrossRef]
- Niu, W.; Wang, J.; Qian, J.; Wang, M.; Wu, P.; Chen, F.; Yan, S. Allosteric control of human cystathionine β-synthase activity by a redox active disulfide bond. J. Biol. Chem. 2018, 293, 2523–2533. [Google Scholar] [CrossRef]
- Takano, N.; Sarfraz, Y.; Gilkes, D.M.; Chaturvedi, P.; Xiang, L.; Suematsu, M.; Zagzag, D.; Semenza, G.L. Decreased expression of cystathionine β-synthase promotes glioma tumorigenesis. Mol. Cancer Res. 2014, 12, 1398–1406. [Google Scholar] [CrossRef]
- Kimura, Y.; Toyofuku, Y.; Koike, S.; Shibuya, N.; Nagahara, N.; Lefer, D.; Ogasawara, Y.; Kimura, H. Identification of H2S3 and H2S produced by 3-mercaptopyruvate sulfurtransferase in the brain. Sci. Rep. 2015, 5, 14774. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Gadalla, M.M.; Sen, N.; Kim, S.; Mu, W.; Gazi, S.K.; Barrow, R.K.; Yang, G.; Wang, R.; Snyder, S.H. H2S signals through protein S-sulfhydration. Sci. Signal. 2009, 2, ra72. [Google Scholar] [CrossRef] [PubMed]
- d’Emmanuele di Villa Bianca, R.; Mitidieri, E.; Esposito, D.; Donnarumma, E.; Russo, A.; Fusco, F.; Ianaro, A.; Mirone, V.; Cirino, G.; Russo, G.; et al. Human Cystathionine-β-Synthase Phosphorylation on Serine227 Modulates Hydrogen Sulfide Production in Human Urothelium. PLoS ONE 2015, 10, e0136859. [Google Scholar] [CrossRef]
- Sbodio, J.I.; Snyder, S.H.; Paul, B.D. Regulators of the transsulfuration pathway. Br. J. Pharmacol. 2019, 176, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The gasotransmitter hydrogen sulfide induces nrf2-target genes by inactivating the keap1 ubiquitin ligase substrate adaptor through formation of a disulfide bond between cys-226 and cys-613. Antioxid. Redox Signal. 2013, 19, 465–481. [Google Scholar] [CrossRef]
- Yang, G.; Pei, Y.; Teng, H.; Cao, Q.; Wang, R. Specificity protein-1 as a critical regulator of human cystathionine gamma-lyase in smooth muscle cells. J. Biol. Chem. 2011, 286, 26450–26460. [Google Scholar] [CrossRef]
- Sen, N.; Paul, B.D.; Gadalla, M.M.; Mustafa, A.K.; Sen, T.; Xu, R.; Kim, S.; Snyder, S.H. Hydrogen sulfide-linked sulfhydration of NF-κB mediates its antiapoptotic actions. Mol. Cell 2012, 45, 13–24. [Google Scholar] [CrossRef]
- Wang, Y.H.; Huang, J.T.; Chen, W.L.; Wang, R.H.; Kao, M.C.; Pan, Y.R.; Chan, S.H.; Tsai, K.W.; Kung, H.J.; Lin, K.T.; et al. Dysregulation of cystathionine γ-lyase promotes prostate cancer progression and metastasis. EMBO Rep. 2019, 20, e45986. [Google Scholar] [CrossRef]
- Nagahara, N.; Katayama, A. Post-translational regulation of mercaptopyruvate sulfurtransferase via a low redox potential cysteine-sulfenate in the maintenance of redox homeostasis. J. Biol. Chem. 2005, 280, 34569–34576. [Google Scholar] [CrossRef] [PubMed]
- Augsburger, F.; Randi, E.B.; Jendly, M.; Ascencao, K.; Dilek, N.; Szabo, C. Role of 3-Mercaptopyruvate Sulfurtransferase in the Regulation of Proliferation, Migration, and Bioenergetics in Murine Colon Cancer Cells. Biomolecules 2020, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Whiteman, M.; Armstrong, J.S.; Chu, S.H.; Jia-Ling, S.; Wong, B.S.; Cheung, N.S.; Halliwell, B.; Moore, P.K. The novel neuromodulator hydrogen sulfide: An endogenous peroxynitrite ‘scavenger’? J. Neurochem. 2004, 90, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Cheung, N.S.; Zhu, Y.Z.; Chu, S.H.; Siau, J.L.; Wong, B.S.; Armstrong, J.S.; Moore, P.K. Hydrogen sulphide: A novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem. Biophys. Res. Commun. 2005, 326, 794–798. [Google Scholar] [CrossRef]
- Yan, S.K.; Chang, T.; Wang, H.; Wu, L.; Wang, R.; Meng, Q.H. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2006, 351, 485–491. [Google Scholar] [CrossRef]
- Greiner, R.; Palinkas, Z.; Basell, K.; Becher, D.; Antelmann, H.; Nagy, P.; Dick, T.P. Polysulfides link H2S to protein thiol oxidation. Antioxid. Redox Signal. 2013, 19, 1749–1765. [Google Scholar] [CrossRef]
- Szabo, C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007, 6, 917–935. [Google Scholar] [CrossRef]
- Al-Magableh, M.R.; Kemp-Harper, B.K.; Ng, H.H.; Miller, A.A.; Hart, J.L. Hydrogen sulfide protects endothelial nitric oxide function under conditions of acute oxidative stress in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 67–74. [Google Scholar] [CrossRef]
- Predmore, B.L.; Lefer, D.J.; Gojon, G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012, 17, 119–140. [Google Scholar] [CrossRef]
- Whiteman, M.; Li, L.; Kostetski, I.; Chu, S.H.; Siau, J.L.; Bhatia, M.; Moore, P.K. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem. Biophys. Res. Commun. 2006, 343, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.; Chang, L.; Pan, C.; Qi, Y.; Zhao, J.; Pang, Y.; Du, J.; Tang, C. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem. Biophys. Res. Commun. 2004, 318, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Kimura, H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004, 18, 1165–1167. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Hu, L.F.; Hu, G.; Bian, J.S. Hydrogen sulfide protects astrocytes against H2O2-induced neural injury via enhancing glutamate uptake. Free Radic. Biol. Med. 2008, 45, 1705–1713. [Google Scholar] [CrossRef]
- Tyagi, N.; Moshal, K.S.; Sen, U.; Vacek, T.P.; Kumar, M.; Hughes, W.M., Jr.; Kundu, S.; Tyagi, S.C. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid. Redox Signal. 2009, 11, 25–33. [Google Scholar] [CrossRef]
- Kesherwani, V.; Nelson, K.S.; Agrawal, S.K. Effect of sodium hydrosulphide after acute compression injury of spinal cord. Brain Res. 2013, 1527, 222–229. [Google Scholar] [CrossRef]
- Huang, C.; Kan, J.; Liu, X.; Ma, F.; Tran, B.H.; Zou, Y.; Wang, S.; Zhu, Y.Z. Cardioprotective effects of a novel hydrogen sulfide agent-controlled release formulation of S-propargyl-cysteine on heart failure rats and molecular mechanisms. PLoS ONE 2013, 8, e69205. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.Y.; Liu, Z.W.; Fu, Y.; Zhao, B. Effect of endogenous hydrogen sulfide on oxidative stress in oleic acid-induced acute lung injury in rats. Chin. Med. J. 2011, 124, 3476–3480. [Google Scholar]
- Sen, U.; Basu, P.; Abe, O.A.; Givvimani, S.; Tyagi, N.; Metreveli, N.; Shah, K.S.; Passmore, J.C.; Tyagi, S.C. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am. J. Physiol. Ren. Physiol. 2009, 297, F410–F419. [Google Scholar] [CrossRef]
- Guo, C.; Liang, F.; Shah Masood, W.; Yan, X. Hydrogen sulfide protected gastric epithelial cell from ischemia/reperfusion injury by Keap1 s-sulfhydration, MAPK dependent anti-apoptosis and NF-kappaB dependent anti-inflammation pathway. Eur. J. Pharmacol. 2014, 725, 70–78. [Google Scholar] [CrossRef]
- Cui, J.; Liu, L.; Zou, J.; Qiao, W.; Liu, H.; Qi, Y.; Yan, C. Protective effect of endogenous hydrogen sulfide against oxidative stress in gastric ischemia-reperfusion injury. Exp. Ther. Med. 2013, 5, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tang, Z.; Cong, B.; Du, J.; Wang, C.; Wang, L.; Ni, X.; Lu, J. Estrogens increase cystathionine-gamma-lyase expression and decrease inflammation and oxidative stress in the myocardium of ovariectomized rats. Menopause 2013, 20, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Liu, X.; Geng, B.; Fang, L.; Tang, C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci. 2008, 82, 1196–1202. [Google Scholar] [CrossRef]
- Wen, Y.D.; Wang, H.; Kho, S.H.; Rinkiko, S.; Sheng, X.; Shen, H.M.; Zhu, Y.Z. Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PLoS ONE 2013, 8, e53147. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Nagpure, B.V.; Wong, P.T.; Bian, J.S. Hydrogen sulfide protects SH-SY5Y neuronal cells against d-galactose induced cell injury by suppression of advanced glycation end products formation and oxidative stress. Neurochem. Int. 2013, 62, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Benetti, L.R.; Campos, D.; Gurgueira, S.A.; Vercesi, A.E.; Guedes, C.E.; Santos, K.L.; Wallace, J.L.; Teixeira, S.A.; Florenzano, J.; Costa, S.K.; et al. Hydrogen sulfide inhibits oxidative stress in lungs from allergic mice in vivo. Eur. J. Pharmacol. 2013, 698, 463–469. [Google Scholar] [CrossRef]
- Su, Y.W.; Liang, C.; Jin, H.F.; Tang, X.Y.; Han, W.; Chai, L.J.; Zhang, C.Y.; Geng, B.; Tang, C.S.; Du, J.B. Hydrogen sulfide regulates cardiac function and structure in adriamycin-induced cardiomyopathy. Circ. J. 2009, 73, 741–749. [Google Scholar] [CrossRef]
- Kai, S.; Tanaka, T.; Daijo, H.; Harada, H.; Kishimoto, S.; Suzuki, K.; Takabuchi, S.; Takenaga, K.; Fukuda, K.; Hirota, K. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid. Redox Signal. 2012, 16, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Guo, Z.; Wang, S. Regulation of cystathionine γ-lyase in mammalian cells by hypoxia. Biochem. Genet. 2014, 52, 29–37. [Google Scholar] [CrossRef]
- Giuffrè, A.; Tomé, C.S.; Fernandes, D.G.F.; Zuhra, K.; Vicente, J.B. Hydrogen Sulfide Metabolism and Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1219, 335–353. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.H.; Zhang, C.C.; Wang, M.J.; Xue, W.L.; Wu, D.D.; Ma, F.F.; Li, W.W.; Tao, B.B.; Zhu, Y.C. Hydrogen sulfide promotes angiogenesis by downregulating miR-640 via the VEGFR2/mTOR pathway. Am. J. Physiol. Cell Physiol. 2016, 310, C305–C317. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yan, J.; Cao, X.; Hua, P.; Li, Z. Hydrogen sulfide modulates epithelial-mesenchymal transition and angiogenesis in non-small cell lung cancer via HIF-1α activation. Biochem. Pharmacol. 2020, 172, 113775. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C.; Papapetropoulos, A. Hydrogen sulphide and angiogenesis: Mechanisms and applications. Br. J. Pharmacol. 2011, 164, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Rose, P.; Moore, P.K.; Ming, S.H.; Nam, O.C.; Armstrong, J.S.; Whiteman, M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J. Gastroenterol. 2005, 11, 3990–3997. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, Z.; Chen, J.; Zhuang, X.; Feng, J.; Li, J. Exogenous hydrogen sulfide exerts proliferation, anti-apoptosis, migration effects and accelerates cell cycle progression in multiple myeloma cells via activating the Akt pathway. Oncol. Rep. 2016, 36, 1909–1916, Corrigendum in Oncol. Rep. 2021, 45, 1315. [Google Scholar] [CrossRef]
- Zhen, Y.; Pan, W.; Hu, F.; Wu, H.; Feng, J.; Zhang, Y.; Chen, J. Exogenous hydrogen sulfide exerts proliferation/anti-apoptosis/angiogenesis/migration effects via amplifying the activation of NF-κB pathway in PLC/PRF/5 hepatoma cells. Int. J. Oncol. 2015, 46, 2194–2204. [Google Scholar] [CrossRef]
- Tiong, C.X.; Lu, M.; Bian, J.S. Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/Akt pathway. Br. J. Pharmacol. 2010, 161, 467–480. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef]
- Zhao, K.; Ju, Y.; Li, S.; Altaany, Z.; Wang, R.; Yang, G. S-sulfhydration of MEK1 leads to PARP-1 activation and DNA damage repair. EMBO Rep. 2014, 15, 792–800. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Szczesny, B.; Marcatti, M.; Zatarain, J.R.; Druzhyna, N.; Wiktorowicz, J.E.; Nagy, P.; Hellmich, M.R.; Szabo, C. Inhibition of hydrogen sulfide biosynthesis sensitizes lung adenocarcinoma to chemotherapeutic drugs by inhibiting mitochondrial DNA repair and suppressing cellular bioenergetics. Sci. Rep. 2016, 6, 36125. [Google Scholar] [CrossRef] [PubMed]
- Ascenção, K.; Dilek, N.; Augsburger, F.; Panagaki, T.; Zuhra, K.; Szabo, C. Pharmacological induction of mesenchymal-epithelial transition via inhibition of H2S biosynthesis and consequent suppression of ACLY activity in colon cancer cells. Pharmacol. Res. 2021, 165, 105393. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Zatarain, J.R.; Nicholls, M.E.; Porter, C.; Widen, S.G.; Thanki, K.; Johnson, P.; Jawad, M.U.; Moyer, M.P.; Randall, J.W.; et al. Upregulation of Cystathionine-β-Synthase in Colonic Epithelia Reprograms Metabolism and Promotes Carcinogenesis. Cancer Res. 2017, 77, 5741–5754. [Google Scholar] [CrossRef]
- Módis, K.; Ju, Y.; Ahmad, A.; Untereiner, A.A.; Altaany, Z.; Wu, L.; Szabo, C.; Wang, R. S-Sulfhydration of ATP synthase by hydrogen sulfide stimulates mitochondrial bioenergetics. Pharmacol. Res. 2016, 113, 116–124. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis: An organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007, 6, 273–286. [Google Scholar] [CrossRef]
- Dicks, N.; Gutierrez, K.; Michalak, M.; Bordignon, V.; Agellon, L.B. Endoplasmic reticulum stress, genome damage, and cancer. Front. Oncol. 2015, 5, 11. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, R.; Jin, H.; Liu, D.; Tang, X.; Tang, C.; Du, J. Hydrogen sulfide attenuates hyperhomocysteinemia-induced cardiomyocytic endoplasmic reticulum stress in rats. Antioxid. Redox Signal. 2010, 12, 1079–1091. [Google Scholar] [CrossRef]
- Li, C.; Hu, M.; Wang, Y.; Lu, H.; Deng, J.; Yan, X. Hydrogen sulfide preconditioning protects against myocardial ischemia/reperfusion injury in rats through inhibition of endo/sarcoplasmic reticulum stress. Int. J. Clin. Exp. Pathol. 2015, 8, 7740–7751. [Google Scholar] [PubMed]
- Li, X.; Zhang, K.Y.; Zhang, P.; Chen, L.X.; Wang, L.; Xie, M.; Wang, C.Y.; Tang, X.Q. Hydrogen sulfide inhibits formaldehyde-induced endoplasmic reticulum stress in PC12 cells by upregulation of SIRT-1. PLoS ONE 2014, 9, e89856. [Google Scholar] [CrossRef]
- Bełtowski, J. Protein homocysteinylation: A new mechanism of atherogenesis? Adv. Hyg. Exp. Med. 2005, 59, 392–404. [Google Scholar]
- Chen, S.M.; Tang, X.Q. Homocysteinylation and Sulfhydration in Diseases. Curr. Neuropharmacol. 2022, 20, 1726–1735. [Google Scholar] [CrossRef]
- Majumder, A.; Singh, M.; Tyagi, S.C. Post-menopausal breast cancer: From estrogen to androgen receptor. Oncotarget 2017, 8, 102739–102758. [Google Scholar] [CrossRef] [PubMed]
- Majumder, A.; Sandhu, M.; Banerji, D.; Steri, V.; Olshen, A.; Moasser, M.M. The role of HER2 and HER3 in HER2-amplified cancers beyond breast cancers. Sci. Rep. 2021, 11, 9091. [Google Scholar] [CrossRef]
- Gao, X.; Sanderson, S.M.; Dai, Z.; Reid, M.A.; Cooper, D.E.; Lu, M.; Richie, J.P., Jr.; Ciccarella, A.; Calcagnotto, A.; Mikhael, P.G.; et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 2019, 572, 397–401. [Google Scholar] [CrossRef]
- Mitsuboshi, S.; Niimura, T.; Kanda, M.; Ishida, S.; Zamami, Y.; Ishizawa, K. Risk of Hematologic Events With Coadministration of Methotrexate and the Breast Cancer Resistance Protein Inhibitor Febuxostat. Ann. Pharmacother. 2022, 56, 910–915. [Google Scholar] [CrossRef]
- Diddens, H.; Niethammer, D.; Jackson, R.C. Patterns of cross-resistance to the antifolate drugs trimetrexate, metoprine, homofolate, and CB3717 in human lymphoma and osteosarcoma cells resistant to methotrexate. Cancer Res. 1983, 43, 5286–5292. [Google Scholar]
- Mullarky, E.; Lucki, N.C.; Beheshti Zavareh, R.; Anglin, J.L.; Gomes, A.P.; Nicolay, B.N.; Wong, J.C.; Christen, S.; Takahashi, H.; Singh, P.K.; et al. Identification of a small molecule inhibitor of 3-phosphoglycerate dehydrogenase to target serine biosynthesis in cancers. Proc. Natl. Acad. Sci. USA 2016, 113, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Pacold, M.E.; Brimacombe, K.R.; Chan, S.H.; Rohde, J.M.; Lewis, C.A.; Swier, L.J.; Possemato, R.; Chen, W.W.; Sullivan, L.B.; Fiske, B.P.; et al. A PHGDH inhibitor reveals coordination of serine synthesis and one-carbon unit fate. Nat. Chem. Biol. 2016, 12, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Chen, L.; Morscher, R.J.; Ghergurovich, J.M.; Esposito, M.; Teng, X.; Kang, Y.; Rabinowitz, J.D. Reversal of Cytosolic One-Carbon Flux Compensates for Loss of the Mitochondrial Folate Pathway. Cell Metab. 2016, 23, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Hellmich, M.R.; Coletta, C.; Chao, C.; Szabo, C. The therapeutic potential of cystathionine β-synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 2015, 22, 424–448. [Google Scholar] [CrossRef]
- Lee, Z.W.; Zhou, J.; Chen, C.S.; Zhao, Y.; Tan, C.H.; Li, L.; Moore, P.K.; Deng, L.W. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS ONE 2011, 6, e21077. [Google Scholar] [CrossRef]
- Lu, S.; Gao, Y.; Huang, X.; Wang, X. GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int. J. Oncol. 2014, 44, 1259–1267. [Google Scholar] [CrossRef]
- Sakuma, S.; Minamino, S.; Takase, M.; Ishiyama, Y.; Hosokura, H.; Kohda, T.; Ikeda, Y.; Fujimoto, Y. Hydrogen sulfide donor GYY4137 suppresses proliferation of human colorectal cancer Caco-2 cells by inducing both cell cycle arrest and cell death. Heliyon 2019, 5, e02244. [Google Scholar] [CrossRef]
- Tanase, S.; Morino, Y. Irreversible inactivation of aspartate aminotransferases during transamination with L-propargylglycine. Biochem. Biophys. Res. Commun. 1976, 68, 1301–1308. [Google Scholar] [CrossRef]
- Burnett, G.; Marcotte, P.; Walsh, C. Mechanism-based inactivation of pig heart L-alanine transaminase by L-propargylglycine. Half-site reactivity. J. Biol. Chem. 1980, 255, 3487–3491. [Google Scholar] [CrossRef]
- Mitra, J.; Bhattacharyya, D. Irreversible inactivation of snake venom l-amino acid oxidase by covalent modification during catalysis of l-propargylglycine. FEBS Open Bio 2013, 3, 135–143. [Google Scholar] [CrossRef]
- Yadav, P.K.; Yamada, K.; Chiku, T.; Koutmos, M.; Banerjee, R. Structure and kinetic analysis of H2S production by human mercaptopyruvate sulfurtransferase. J. Biol. Chem. 2013, 288, 20002–20013. [Google Scholar] [CrossRef]
- Hanaoka, K.; Sasakura, K.; Suwanai, Y.; Toma-Fukai, S.; Shimamoto, K.; Takano, Y.; Shibuya, N.; Terai, T.; Komatsu, T.; Ueno, T.; et al. Discovery and Mechanistic Characterization of Selective Inhibitors of H2S-producing Enzyme: 3-Mercaptopyruvate Sulfurtransferase (3MST) Targeting Active-site Cysteine Persulfide. Sci. Rep. 2017, 7, 40227. [Google Scholar] [CrossRef] [PubMed]
- Bantzi, M.; Augsburger, F.; Loup, J.; Berset, Y.; Vasilakaki, S.; Myrianthopoulos, V.; Mikros, E.; Szabo, C.; Bochet, C.G. Novel Aryl-Substituted Pyrimidones as Inhibitors of 3-Mercaptopyruvate Sulfurtransferase with Antiproliferative Efficacy in Colon Cancer. J. Med. Chem. 2021, 64, 6221–6240. [Google Scholar] [CrossRef]
- Zuhra, K.; Augsburger, F.; Majtan, T.; Szabo, C. Cystathionine-β-Synthase: Molecular Regulation and Pharmacological Inhibition. Biomolecules 2020, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulou, A.; Panopoulos, P.; Chasapis, C.T.; Coletta, C.; Zhou, Z.; Cirino, G.; Giannis, A.; Szabo, C.; Spyroulias, G.A.; Papapetropoulos, A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br. J. Pharmacol. 2013, 169, 922–932. [Google Scholar] [CrossRef] [PubMed]


| Genes | Polymorphisms | Condition | Associated Complications | References |
|---|---|---|---|---|
| CBS | 844INS68 | HHcy | Peripheral artery occlusive disease | [50] |
| T833C | HHcy | Stroke | [51] | |
| 844INS68 | HHcy | Thrombosis | [52] | |
| MTHFR | C677T | HHcy | Retinal vein occlusion | [53] |
| C677T | HHcy | Stroke | [42,43,44,45] | |
| C677T | HHcy | Venous thromboembolism | [54] | |
| C677T | HHcy | Hypertension | [55,56,57] | |
| C677T | HHcy | Alzheimer’s Disease | [58] | |
| A1298C | HHcy | Cerebral venous sinus thrombosis | [59,60,61] | |
| C677T | HHcy | Hyperlipidemia | [62] | |
| C677T | HHcy | Diabetic nephropathy | [63,64,65,66] | |
| C677T | HHcy | Cerebral venous thrombosis | [67] | |
| C677T | HHcy | Parkinson’s Disease | [68,69] |
| Genes | Polymorphisms | Cancer Types | Significant Association (OR) | References |
|---|---|---|---|---|
| MTHFR | 677C-> T | Breast Cancer | Positive Association (1.19) | [22] |
| Ovarian Cancer | No association (1.03) | [22] | ||
| Esophageal Squamous Cell Carcinoma | Positive Association (1.47) | [96] | ||
| Acute Lymphocytic Leukemia | Negative Association (0.99) | [21] | ||
| Prostate Cancer | Negative association (0.78) | [23] | ||
| Colorectal Adenomas | Negative association (0.76) | [97] | ||
| Late-stage colorectal tumorigenesis | Positive Association (1.32) | [29] | ||
| Endometrial Cancer | No association (1.10) | [98] | ||
| 1298A->C | Prostate Cancer | Negative Association (0.58) | [99] | |
| Acute Lymphocytic Leukemia | Negative Association (0.33) | [21] | ||
| Acute Myeloid Leukemia | No association (1.00) | [88] | ||
| Endometrial Cancer | No association (1.00) | [98] | ||
| MTRR | 66A->G | Acute Myeloid Leukemia | Positive association for Asian population (1.40) | [100] |
| Head and Neck Cancer | Positive Association (1.24) | [101] | ||
| Colorectal Cancer | Positive Association (2.77, 1.15) | [87,102] | ||
| Gastric Cancer | Positive Association (1.39) | [103] | ||
| Breast Cancer | Positive Association (4.45) | [104] | ||
| MTR | b2756A->G | Colorectal Cancer | Positive Association (2.04) | [28] |
| Primary Liver Cancer | No association (1.00) | [105] | ||
| Breast Cancer | No association (1.00) | [106] | ||
| Glioblastoma Multiforme | No association (1.00) | [107] | ||
| Upper Gastrointestinal Tract cancer | No association (1.00) | [108] | ||
| Digestive System Cancer | No association (1.00) | [109] | ||
| MTHFD1 | 1958G->A | Gastric Cancer | Positive Association (2.05) | [110] |
| G1958A | Colon Cancer | Negative Association (0.89) | [111] | |
| BHMT | 742G->A | Head and Neck Squamous Cell Carcinoma | Positive Association (1.34) | [112] |
| Breast Cancer | No association (0.98) | [113] | ||
| Cervical Cancer | Negative Association (0.433) | [114] | ||
| Ovarian Cancer | No association (1.00) | [115] | ||
| Colorectal Adenoma | Positive Association (1.09) | [116] | ||
| TCN 2 | 776G>C | Glioblastoma Multiforme | No association (1.00) | [107] |
| Primary Central Nervous System Lymphoma | No association (1.00) | [117] | ||
| TYMS | TS 3′-UTR | Esophageal and Stomach Cancer | No association (1.00) | [118] |
| Enzymes | Cancer Types | Upregulation/Downregulation | Reference |
|---|---|---|---|
| CBS | Colon Cancer | Upregulation | [182] |
| Ovarian Cancer | Upregulation | [176] | |
| Breast Cancer | Upregulation | [183] | |
| Thyroid Cancer | Upregulation | [184] | |
| Gallbladder Adenocarcinoma | Upregulation | [185] | |
| Hepatocellular Carcinoma | Downregulation | [186] | |
| Gastrointestinal Cancer | Downregulation | [187] | |
| CSE | Breast Cancer | Upregulation | [188] |
| Prostate Cancer | Upregulation | [189] | |
| Gastric Cancer | Upregulation | [190] | |
| Bladder Cancer | Upregulation | [191] | |
| Hepatoma | Upregulation | [192] | |
| Colon Cancer | Upregulation | [193] | |
| Renal Cell Carcinoma | Downregulation | [194] | |
| 3MST | Glioma Tumor | Upregulation | [195] |
| Colon Cancer | Upregulation | [196] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majumder, A. Targeting Homocysteine and Hydrogen Sulfide Balance as Future Therapeutics in Cancer Treatment. Antioxidants 2023, 12, 1520. https://doi.org/10.3390/antiox12081520
Majumder A. Targeting Homocysteine and Hydrogen Sulfide Balance as Future Therapeutics in Cancer Treatment. Antioxidants. 2023; 12(8):1520. https://doi.org/10.3390/antiox12081520
Chicago/Turabian StyleMajumder, Avisek. 2023. "Targeting Homocysteine and Hydrogen Sulfide Balance as Future Therapeutics in Cancer Treatment" Antioxidants 12, no. 8: 1520. https://doi.org/10.3390/antiox12081520
APA StyleMajumder, A. (2023). Targeting Homocysteine and Hydrogen Sulfide Balance as Future Therapeutics in Cancer Treatment. Antioxidants, 12(8), 1520. https://doi.org/10.3390/antiox12081520








