Extract of Isatidis Radix Inhibits Lipid Accumulation in In Vitro and In Vivo by Regulating Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of IR
2.3. Differentiation of 3T3-L1 Cells and hAMSCs
2.4. Cell Viability
2.5. Oil Red O Staining
2.6. Measurement of Cellular ROS Production
2.7. ROS Assay
2.8. RNA Extraction and Real-Time RT PCR
2.9. Western Blot Analysis
2.10. HFD-Induced Obese Mice
2.11. Serum Analysis
2.12. Hematoxylin and Eosin (H&E) Staining
2.13. Chromatography Analysis of WIR and EtIR
2.14. Statistical Analysis
3. Results
3.1. WIR and EtIR Reduced Lipid Accumulation of 3T3-L1 Cells
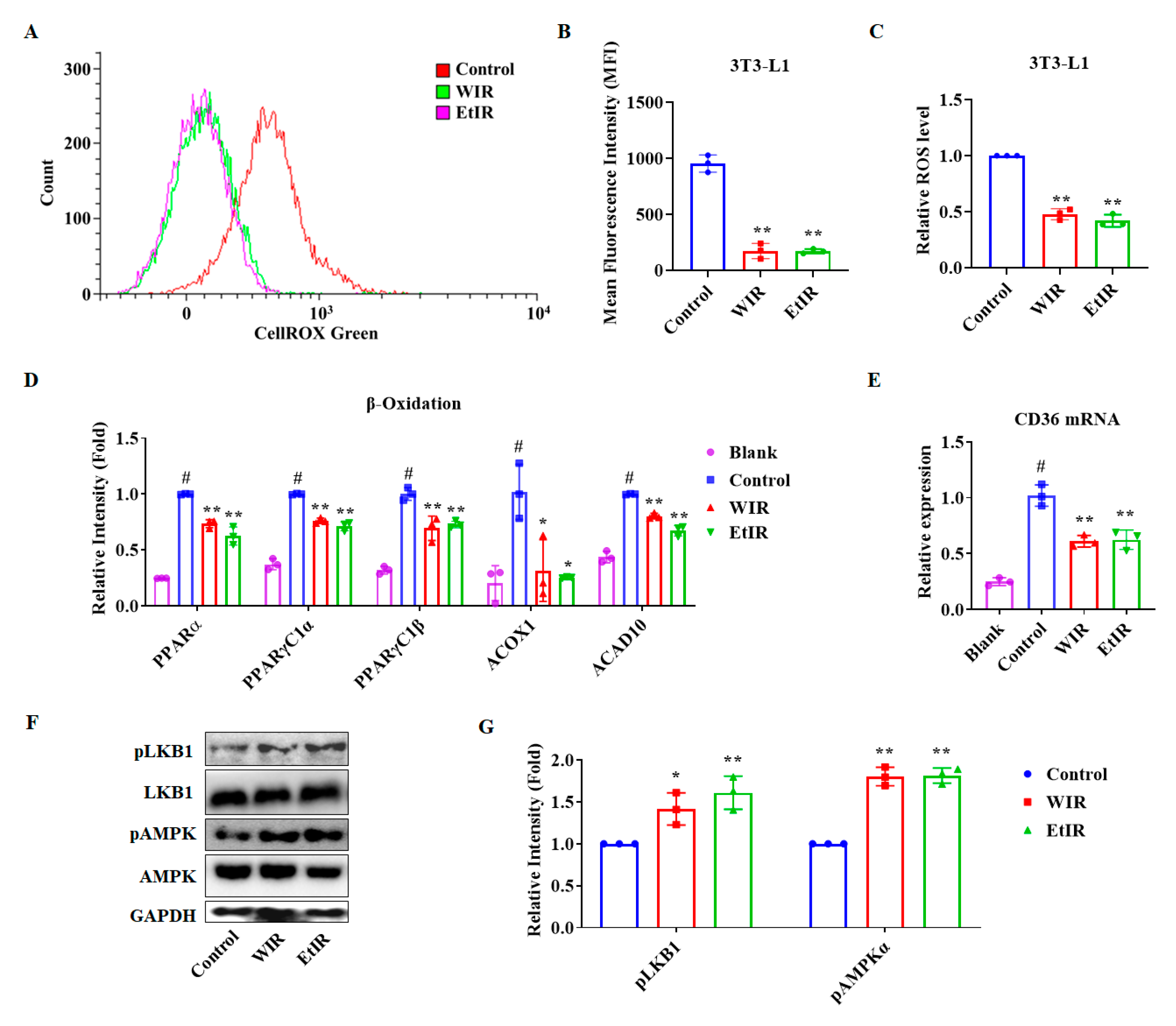
3.2. WIR and EtIR Inhibited the Production of ROS through the CD36-AMPK Pathway during the Adipogenesis
3.3. WIR and EtIR Inhibited the Adipogenesis of 3T3-L1 Adipocytes
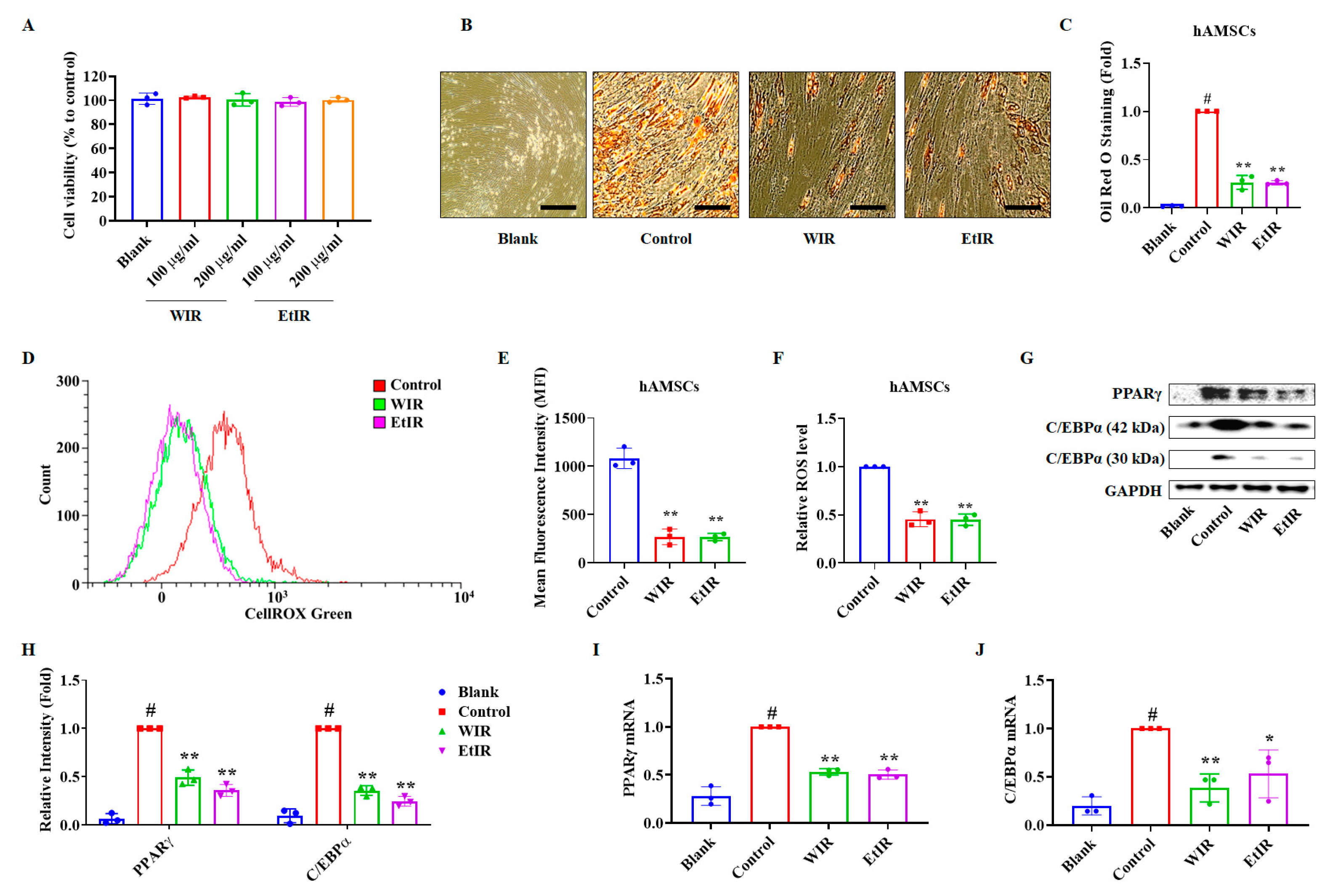
3.4. WIR and EtIR Inhibited hAMSCs Differentiation into Adipocytes by Suppression of ROS
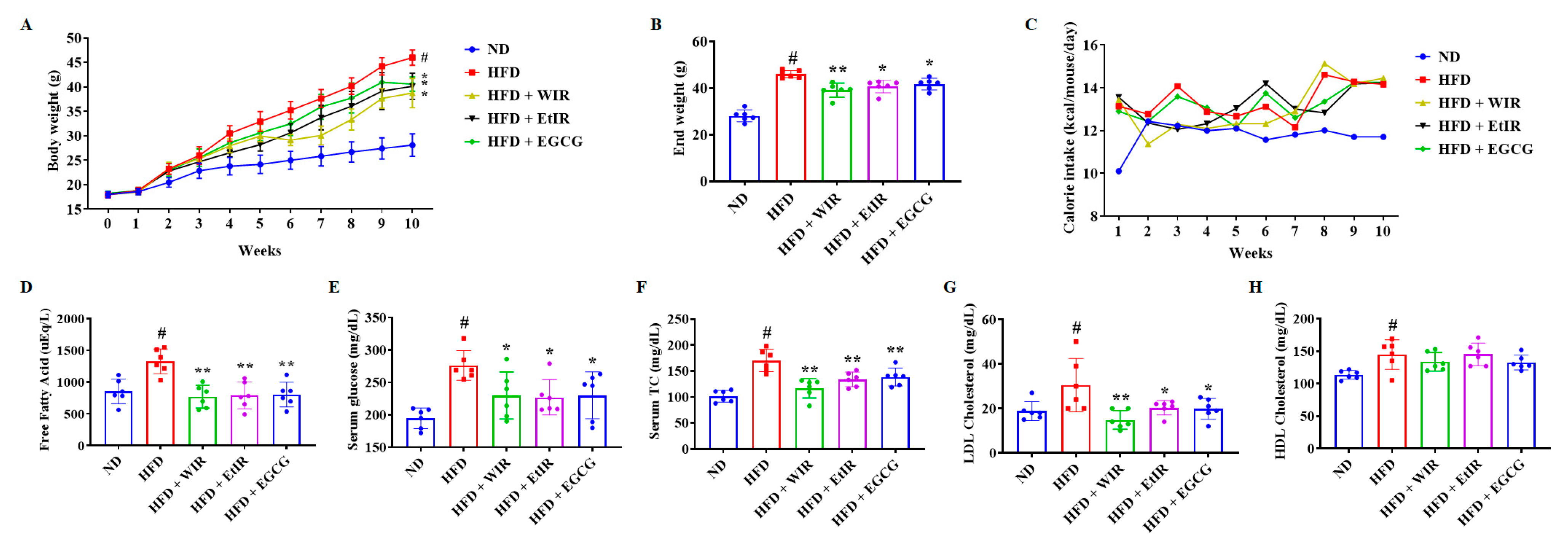
3.5. WIR and EtIR Reduced Body Weight and Regulated Serum Lipid Levels in Obese Mice
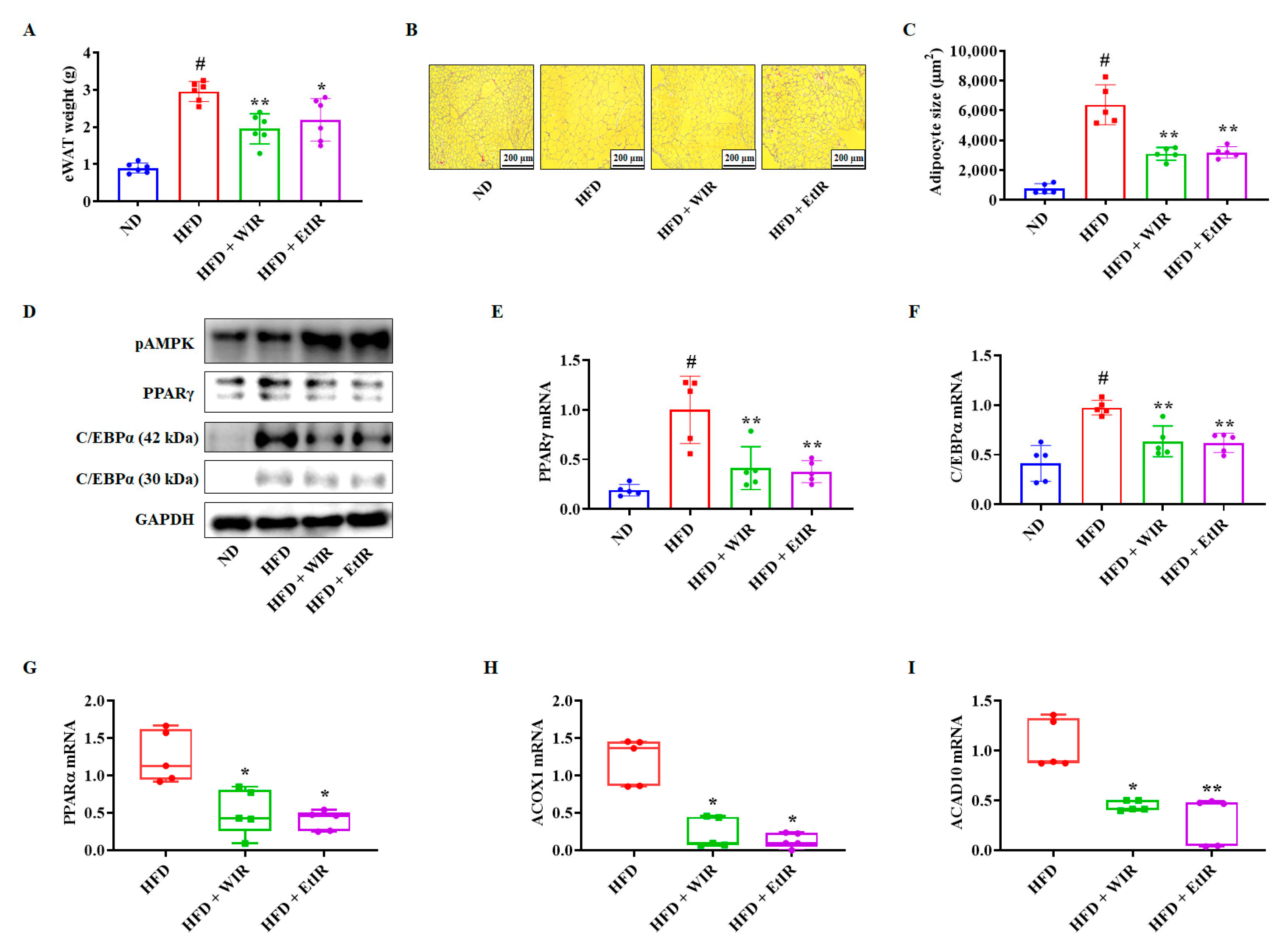
3.6. WIR and EtIR Decreased Lipids in eWAT by Inhibiting Adipogenesis and β-Oxidation
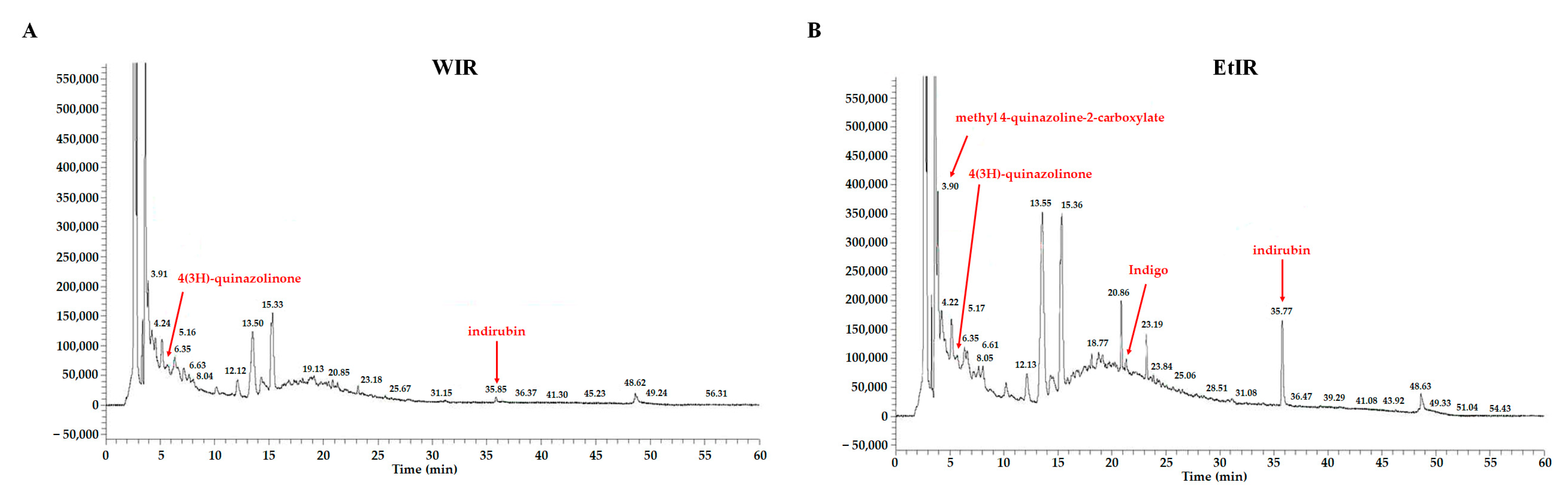
3.7. Chromatography Analysis of WIR and EtIR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Shah, A.S.; Nakamura, T. Extracellular Vesicles: A Potential Novel Regulator of Obesity and Its Associated Complications. Children 2018, 5, 152. [Google Scholar] [CrossRef]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.M.; Postolache, T.T.; Snitker, S. Childhood Obesity and Depression: Connection between these Growing Problems in Growing Children. Int. J. Child. Health Hum. Dev. 2008, 1, 103–114. [Google Scholar] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- de Villiers, D.; Potgieter, M.; Ambele, M.A.; Adam, L.; Durandt, C.; Pepper, M.S. The Role of Reactive Oxygen Species in Adipogenic Differentiation. Adv. Exp. Med. Biol. 2018, 1083, 125–144. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Christiaens, V.; Van Hul, M.; Lijnen, H.R.; Scroyen, I. CD36 promotes adipocyte differentiation and adipogenesis. Biochim. Biophys. Acta 2012, 1820, 949–956. [Google Scholar] [CrossRef]
- Ly, L.D.; Xu, S.; Choi, S.K.; Ha, C.M.; Thoudam, T.; Cha, S.K.; Wiederkehr, A.; Wollheim, C.B.; Lee, I.K.; Park, K.S. Oxidative stress and calcium dysregulation by palmitate in type 2 diabetes. Exp. Mol. Med. 2017, 49, e291. [Google Scholar] [CrossRef]
- Marechal, L.; Laviolette, M.; Rodrigue-Way, A.; Sow, B.; Brochu, M.; Caron, V.; Tremblay, A. The CD36-PPARgamma Pathway in Metabolic Disorders. Int. J. Mol. Sci. 2018, 19, 1529. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, D.; Yang, P.; Zhao, L.; Wei, L.; Chen, Y.; Ruan, X.Z. Suppression of CD36 attenuates adipogenesis with a reduction of P2X7 expression in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2017, 491, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Kee, J.Y.; Hong, S.H. Gomisin A Alleviates Obesity by Regulating the Phenotypic Switch between White and Brown Adipocytes. Am. J. Chin. Med. 2021, 49, 1929–1948. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Kee, J.Y.; Kim, D.S.; Park, J.; Jeong, M.Y.; Mun, J.G.; Park, S.J.; Lee, J.H.; Um, J.Y.; Hong, S.H. Anti-obesity effects of Arctii Fructus (Arctium lappa) in white/brown adipocytes and high-fat diet-induced obese mice. Food Funct. 2016, 7, 5025–5033. [Google Scholar] [CrossRef]
- Sinha, K.; Kumar, S.; Rawat, B.; Singh, R.; Purohit, R.; Kumar, D.; Padwad, Y. Kutkin, iridoid glycosides enriched fraction of Picrorrhiza kurroa promotes insulin sensitivity and enhances glucose uptake by activating PI3K/Akt signaling in 3T3-L1 adipocytes. Phytomedicine 2022, 103, 154204. [Google Scholar] [CrossRef]
- Han, Y.H.; Kee, J.Y.; Park, S.H.; Mun, J.G.; Jeon, H.D.; Park, J.; Zou, Q.P.; Liu, X.Q.; Hong, S.H. Rubrofusarin-6-beta-gentiobioside inhibits lipid accumulation and weight gain by regulating AMPK/mTOR signaling. Phytomedicine 2019, 62, 152952. [Google Scholar] [CrossRef]
- Rana, S.; Blowers, E.C.; Natarajan, A. Small molecule adenosine 5’-monophosphate activated protein kinase (AMPK) modulators and human diseases. J. Med. Chem. 2015, 58, 2–29. [Google Scholar] [CrossRef]
- Daval, M.; Foufelle, F.; Ferre, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, T.; Xiao, H. The Implication of Oxidative Stress and AMPK-Nrf2 Antioxidative Signaling in Pneumonia Pathogenesis. Front. Endocrinol. 2020, 11, 400. [Google Scholar] [CrossRef]
- Jeon, H.D.; Han, Y.H.; Mun, J.G.; Yoon, D.H.; Lee, Y.G.; Kee, J.Y.; Hong, S.H. Dehydroevodiamine inhibits lung metastasis by suppressing survival and metastatic abilities of colorectal cancer cells. Phytomedicine 2022, 96, 153809. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.; Xu, A.; Wu, D. Effects of an AMP-activated protein kinase inhibitor, compound C, on adipogenic differentiation of 3T3-L1 cells. Biol. Pharm. Bull. 2008, 31, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Chen, Z.; Liu, F.; Qiao, Y.; Chen, T.; Wang, X. Antiviral activities of Radix isatidis polysaccharide against pseudorabies virus in swine testicle cells. BMC Complement. Med. Ther. 2020, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wan, J.; Tang, J.; Wang, W.; Liu, Y. Radix isatidis and infectious diseases caused by viruses. Zhongguo Zhong Yao Za Zhi 2009, 34, 3169–3172. [Google Scholar]
- Xiao, P.; Huang, H.; Chen, J.; Li, X. In vitro antioxidant and anti-inflammatory activities of Radix Isatidis extract and bioaccessibility of six bioactive compounds after simulated gastro-intestinal digestion. J. Ethnopharmacol. 2014, 157, 55–61. [Google Scholar] [CrossRef]
- Li, J.P.; Yuan, Y.; Zhang, W.Y.; Jiang, Z.; Hu, T.J.; Feng, Y.T.; Liu, M.X. Effect of Radix isatidis polysaccharide on alleviating insulin resistance in type 2 diabetes mellitus cells and rats. J. Pharm. Pharmacol. 2019, 71, 220–229. [Google Scholar] [CrossRef]
- Han, Y.H.; Kee, J.Y.; Park, J.; Kim, D.S.; Shin, S.; Youn, D.H.; Kang, J.; Jung, Y.; Lee, Y.M.; Park, J.H.; et al. Lipin1-Mediated Repression of Adipogenesis by Rutin. Am. J. Chin. Med. 2016, 44, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O. An established preadipose cell line and its differentiation in culture. Cell 1975, 5, 19–27. [Google Scholar] [CrossRef]
- Samovski, D.; Sun, J.; Pietka, T.; Gross, R.W.; Eckel, R.H.; Su, X.; Stahl, P.D.; Abumrad, N.A. Regulation of AMPK activation by CD36 links fatty acid uptake to beta-oxidation. Diabetes 2015, 64, 353–359. [Google Scholar] [CrossRef]
- Han, L.; Yang, Q.; Li, J.; Cheng, F.; Zhang, Y.; Li, Y.; Wang, M. Protocatechuic Acid-Ameliorated Endothelial Oxidative Stress through Regulating Acetylation Level via CD36/AMPK Pathway. J. Agric. Food Chem. 2019, 67, 7060–7072. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Tzeng, T.F.; Chang, C.J.; Liu, I.M. 6-gingerol inhibits rosiglitazone-induced adipogenesis in 3T3-L1 adipocytes. Phytother. Res. 2014, 28, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Ruperez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.H.; Kee, J.Y.; Park, J.; Kim, H.L.; Jeong, M.Y.; Kim, D.S.; Jeon, Y.D.; Jung, Y.; Youn, D.H.; Kang, J.; et al. Arctigenin Inhibits Adipogenesis by Inducing AMPK Activation and Reduces Weight Gain in High-Fat Diet-Induced Obese Mice. J. Cell Biochem. 2016, 117, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, P.; Wojtczak, L. Fatty acids decrease mitochondrial generation of reactive oxygen species at the reverse electron transport but increase it at the forward transport. Biochim. Biophys. Acta 2007, 1767, 1032–1040. [Google Scholar] [CrossRef]
- Liu, T.W.; Heden, T.D.; Matthew Morris, E.; Fritsche, K.L.; Vieira-Potter, V.J.; Thyfault, J.P. High-Fat Diet Alters Serum Fatty Acid Profiles in Obesity Prone Rats: Implications for In Vitro Studies. Lipids 2015, 50, 997–1008. [Google Scholar] [CrossRef]
- Deng, J.; Ma, Y.; He, Y.; Yang, H.; Chen, Y.; Wang, L.; Huang, D.; Qiu, S.; Tao, X.; Chen, W. A Network Pharmacology-Based Investigation to the Pharmacodynamic Material Basis and Mechanisms of the Anti-Inflammatory and Anti-Viral Effect of Isatis indigotica. Drug Des. Devel Ther. 2021, 15, 3193–3206. [Google Scholar] [CrossRef]
- Zhang, J.; Bo, S.; Zhang, Y.; Xu, H. Extraction technology of radix Isatidis. Zhongguo Zhong Yao Za Zhi 1990, 15, 287–289, 318. [Google Scholar]
- Zhou, W.; Zhang, X.Y.; Lv, Y.P.; Liu, X.D.; Xu, C.; Duan, G.L. RSM-Optimized IRAE Sample Pretreatment and HPLC Simultaneous Determination of Tryptanthrin, Indigo, and Indirubin from Chinese Herbal Medicine Radix Isatidis. Acta Chromatogr. 2013, 25, 297–315. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Flier, J.S. The adipocyte: Storage depot or node on the energy information superhighway? Cell 1995, 80, 15–18. [Google Scholar] [CrossRef]
- Serrero, G.; Lepak, N. Endocrine and paracrine negative regulators of adipose differentiation. Int. J. Obes. Relat. Metab. Disord. 1996, 20 (Suppl. S3), S58–S64. [Google Scholar] [PubMed]
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-Approved Anti-Obesity Drugs in the United States. Am. J. Med. 2016, 129, 879.e871–e876. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. EClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, J.; Xue, J.; Qin, Z.; Shen, F.; Liu, J.; Chen, X.; Li, X.; Wu, Z.; Xiao, W.; et al. In silico-based screen synergistic drug combinations from herb medicines: A case using Cistanche tubulosa. Sci. Rep. 2017, 7, 16364. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Gomez, C.I.; Rocha-Guzman, N.E.; Gallegos-Infante, J.A.; Moreno-Jimenez, M.R.; Vazquez-Cabral, B.D.; Gonzalez-Laredo, R.F. Plants with potential use on obesity and its complications. EXCLI J. 2015, 14, 809–831. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B. Origins of metabolic complications in obesity: Adipose tissue and free fatty acid trafficking. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 535–541. [Google Scholar] [CrossRef]
- Kim, S.M.; Lun, M.; Wang, M.; Senyo, S.E.; Guillermier, C.; Patwari, P.; Steinhauser, M.L. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab. 2014, 20, 1049–1058. [Google Scholar] [CrossRef]
- Guillermier, C.; Fazeli, P.K.; Kim, S.; Lun, M.; Zuflacht, J.P.; Milian, J.; Lee, H.; Francois-Saint-Cyr, H.; Horreard, F.; Larson, D.; et al. Imaging mass spectrometry demonstrates age-related decline in human adipose plasticity. JCI Insight 2017, 2, e90349. [Google Scholar] [CrossRef]
- Jakab, J.; Miskic, B.; Miksic, S.; Juranic, B.; Cosic, V.; Schwarz, D.; Vcev, A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPalpha induces adipogenesis through PPARgamma: A unified pathway. Genes. Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Ebbert, J.O.; Jensen, M.D. Fat depots, free fatty acids, and dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Elshourbagy, N.A.; Meyers, H.V.; Abdel-Meguid, S.S. Cholesterol: The good, the bad, and the ugly—Therapeutic targets for the treatment of dyslipidemia. Med. Princ. Pract. 2014, 23, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent. Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Han, Y.; Zhu, Y.; Almuntashiri, S.; Wang, X.; Somanath, P.R.; Owen, C.A.; Zhang, D. Extracellular vesicle-encapsulated CC16 as novel nanotherapeutics for treatment of acute lung injury. Mol. Ther. 2023, 31, 1346–1364. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Catani, M.V.; Evangelista, D.; Gasperi, V.; Avigliano, L. Obesity-associated oxidative stress: Strategies finalized to improve redox state. Int. J. Mol. Sci. 2013, 14, 10497–10538. [Google Scholar] [CrossRef]
- Lee, D.K.; Jang, H.D. Carnosic Acid Attenuates an Early Increase in ROS Levels during Adipocyte Differentiation by Suppressing Translation of Nox4 and Inducing Translation of Antioxidant Enzymes. Int. J. Mol. Sci. 2021, 22, 6096. [Google Scholar] [CrossRef]
- Rosca, M.G.; Vazquez, E.J.; Chen, Q.; Kerner, J.; Kern, T.S.; Hoppel, C.L. Oxidation of fatty acids is the source of increased mitochondrial reactive oxygen species production in kidney cortical tubules in early diabetes. Diabetes 2012, 61, 2074–2083. [Google Scholar] [CrossRef] [PubMed]
- Gathercole, L.L.; Morgan, S.A.; Bujalska, I.J.; Hauton, D.; Stewart, P.M.; Tomlinson, J.W. Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PLoS ONE 2011, 6, e26223. [Google Scholar] [CrossRef]
- Li, W.; Febbraio, M.; Reddy, S.P.; Yu, D.Y.; Yamamoto, M.; Silverstein, R.L. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J. Clin. Investig. 2010, 120, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Farmer, S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Karunakaran, U.; Elumalai, S.; Lee, I.K.; Lee, H.W.; Kim, Y.W.; Won, K.C. Metformin prevents glucotoxicity by alleviating oxidative and ER stress-induced CD36 expression in pancreatic beta cells. J. Diabetes Complicat. 2017, 31, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Susa, S.T.; Hussain, A.; Preuss, C.V. Drug Metabolism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Sun, Q.; Leng, J.; Tang, L.; Wang, L.; Fu, C. A Comprehensive Review of the Chemistry, Pharmacokinetics, Pharmacology, Clinical Applications, Adverse Events, and Quality Control of Indigo Naturalis. Front. Pharmacol. 2021, 12, 664022. [Google Scholar] [CrossRef]


| Gene (Accession Number) | Sequences |
|---|---|
| mPPARα (XM_030248424) | 5′-AACTGGATGACAGTGACATTTCC-3′ (sense) |
| 5′-CCCTCCTGCAACTTCTCAAT-3′ (antisense) | |
| mPPARγC1α (XM_006503775) | 5′-CCCTGCCATTGTTAAGAC-3′ (sense) |
| 5′-GCTGCTGTTCCTGTTTTC-3′ (antisense) | |
| mPPARγC1β (XM_006525698) | 5′-GAGGGCTCCGGCACTTCC-3′ (sense) |
| 5′-GTACTTGCTTTTCCCAGATG-3′ (antisense) | |
| mACOX1 (NM_001377522) | 5′-GCATCGCAGACCCTGAAGAA-3′ (sense) |
| 5′-GGTGCATCCATTTCTCCTGC -3′ (antisense) | |
| mACAD10 (XM_030254818) | 5′-CTTGGAGAAGTACCTGAAGG-3′ (sense) |
| 5′-CAGCCTGATGTAGTACGTTG-3′ (antisense) | |
| mCD36 (XM_030254088) | 5′-ATGGGCTGTGATCGGAACTG-3′ (sense) |
| 5′-GTCTTCCCAATAAGCATGTCTCC-3′ (antisense) | |
| mPPARγ (XM_017321455) | 5′-TTTCAAGGGTGCCAGTTTC-3′ (sense) |
| 5′-TTATTCATCAGGGAGGCCAG-3′ (antisense) | |
| mC/EBPα (NM_001287514) | 5′-GCCGAGATAAAGCCAAACAA-3′ (sense) |
| 5′-CGTAAATGGGGATTTGGTCA-3′ (antisense) | |
| mFAS (XM_030245556) | 5′-TGGTGGGTTTGGTGAATTGTC-3′ (sense) |
| 5′-GCTTGTCCTGCTCTAACTGGAAGT-3′ (antisense) | |
| mGAPDH (NM_001411843) | 5′-AACTTTGGCATTGTGGAAGG-3′ (sense) |
| 5′-GGATGCAGGGATGATGTTCT-3′ (antisense) | |
| hPPARγ (NM_001354666) | 5′-TGAATGTGAAGCCCATTGAA-3′ (sense) |
| 5′-CTGCAGTAGCTGCACGTGTT-3′ (antisense) | |
| hC/EBPα (NM_001287424) | 5′-TGTATACCCCTGGTGGGAGA-3′ (sense) |
| 5′-TCATAACTCCGGTCCCTCTG-3′ (antisense) | |
| h36b4 (NM_001002) | 5′-AAACTGCTGCCTCATATCCGG-3′ (sense) |
| 5′-TTGTAGATGCTGCCATTGTCGA-3′ (antisense) |
| Parameter | Condition | |
|---|---|---|
| Flow Rate | 1.0 mL/min | |
| Column | YMC Pack-Pro C18 | |
| Injection Vol. | 20 μL | |
| Column Temp. | 30 °C | |
| Heater Temp. | 250 °C | |
| Sheath Gas Flow Rate | 35 arb (N2) | |
| Spray Voltage | 5 kV | |
| Capillary Temp. | 275 °C | |
| Gradient condition | ||
| Time (min) | Water (0.1% formic acid) | Acetonitrile (0.1% formic acid) |
| 10 | 83 | 17 |
| 40 | 30 | 70 |
| 45 | 0 | 100 |
| 50 | 83 | 17 |
| 60 | 83 | 17 |
| ND | HFD | HFD + WIR | HFD + EtIR | |
|---|---|---|---|---|
| AST (IU/L) | 142.67 ± 24.07 | 185.17 ± 51.70 | 159.83 ± 62.23 | 171.50 ± 52.08 |
| ALT (IU/L) | 41.33 ± 10.89 | 135.167 ± 67.72 # | 60.83 ± 38.25 | 123.67 ± 58.00 |
| Creatinine (mg/dL) | 0.16 ± 0.03 | 0.16 ± 0.03 | 0.15 ± 0.06 | 0.18 ± 0.04 |
| BUN (mg/dL) | 17.00 ± 1.26 | 22.33 ± 2.42 # | 14.17 ± 3.19 * | 12.67 ± 2.16 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.-H.; Kee, J.-Y. Extract of Isatidis Radix Inhibits Lipid Accumulation in In Vitro and In Vivo by Regulating Oxidative Stress. Antioxidants 2023, 12, 1426. https://doi.org/10.3390/antiox12071426
Han Y-H, Kee J-Y. Extract of Isatidis Radix Inhibits Lipid Accumulation in In Vitro and In Vivo by Regulating Oxidative Stress. Antioxidants. 2023; 12(7):1426. https://doi.org/10.3390/antiox12071426
Chicago/Turabian StyleHan, Yo-Han, and Ji-Ye Kee. 2023. "Extract of Isatidis Radix Inhibits Lipid Accumulation in In Vitro and In Vivo by Regulating Oxidative Stress" Antioxidants 12, no. 7: 1426. https://doi.org/10.3390/antiox12071426
APA StyleHan, Y.-H., & Kee, J.-Y. (2023). Extract of Isatidis Radix Inhibits Lipid Accumulation in In Vitro and In Vivo by Regulating Oxidative Stress. Antioxidants, 12(7), 1426. https://doi.org/10.3390/antiox12071426






