Topical Absorption of Glutathione–Cyclodextrin Nanoparticle Complex in Healthy Human Subjects Improves Immune Response against Mycobacterium avium Infection
Abstract
:1. Introduction
2. Materials and Methods
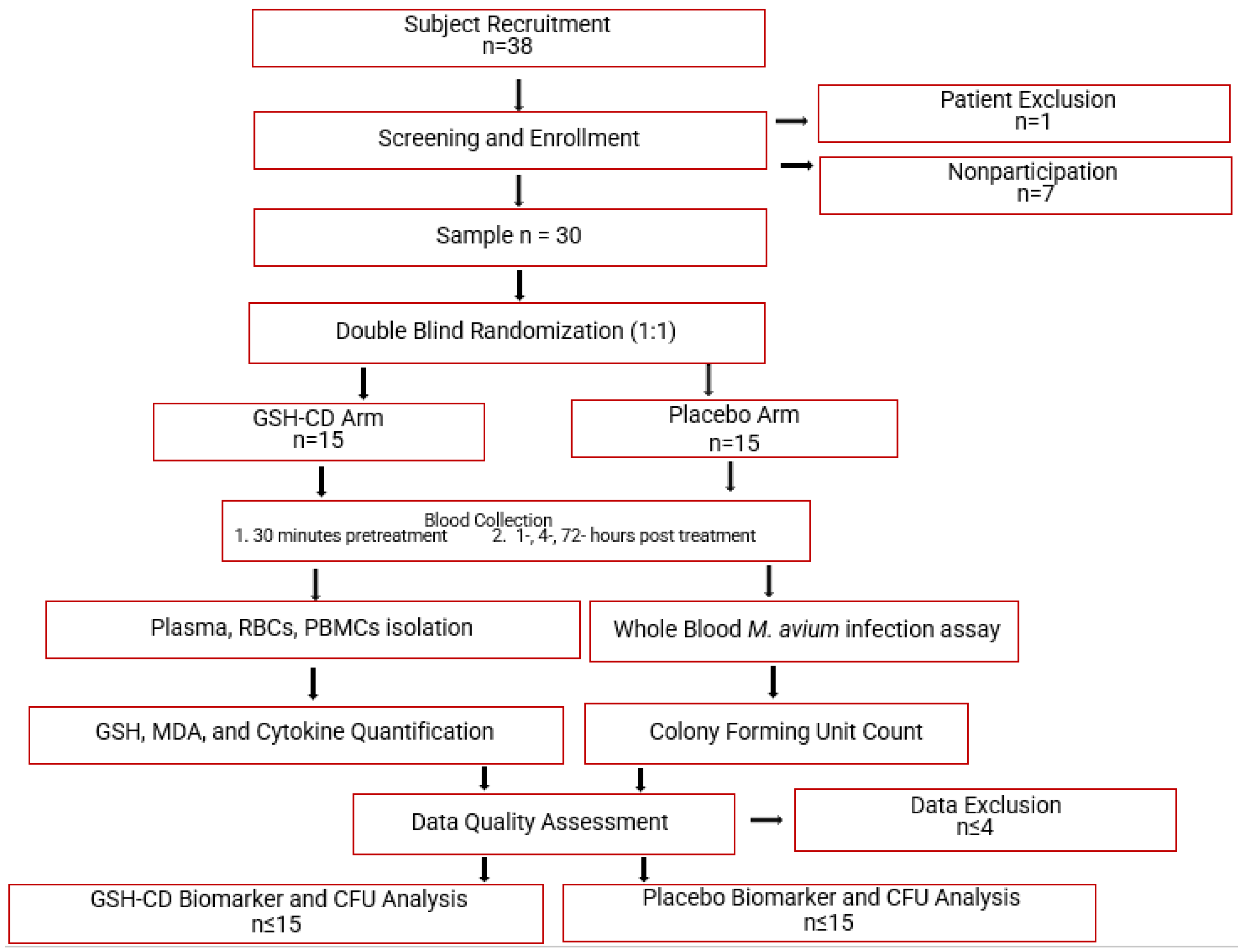
2.1. Study Design
2.2. Subject Selection, Clinical Encounters, Treatment Application
2.3. Blood Processing and Storage
2.4. GSH Quantification
2.5. Malondialdehyde (MDA) Quantification
2.6. Cytokine Assessment
2.7. Total Protein Quantification
2.8. Bacterial Preparation
2.9. Whole Blood Infection Assay
2.10. Statistical Analysis
3. Results
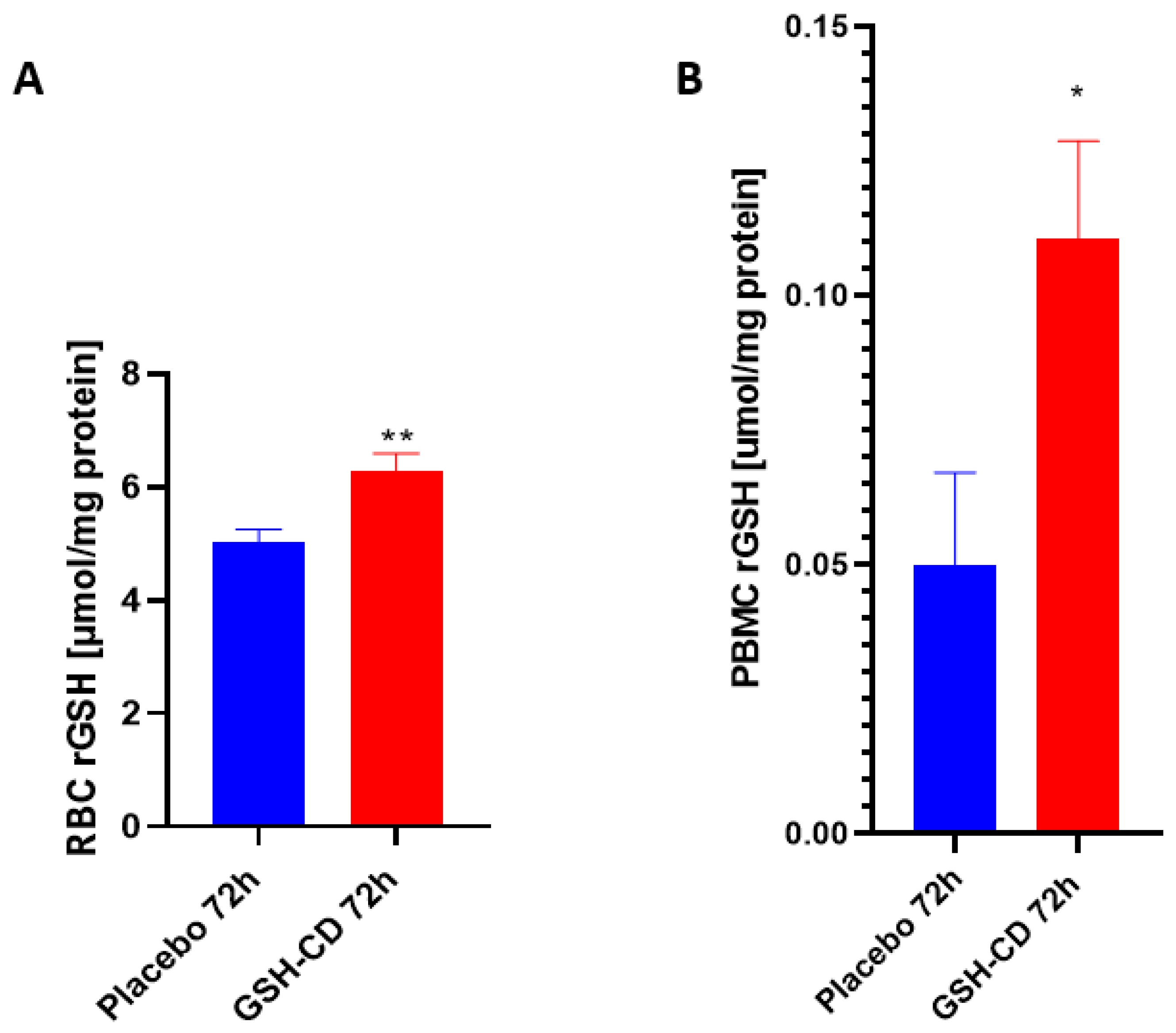
3.1. Elevated Levles of Reduced GSH after GSH-CD Topical Treatment
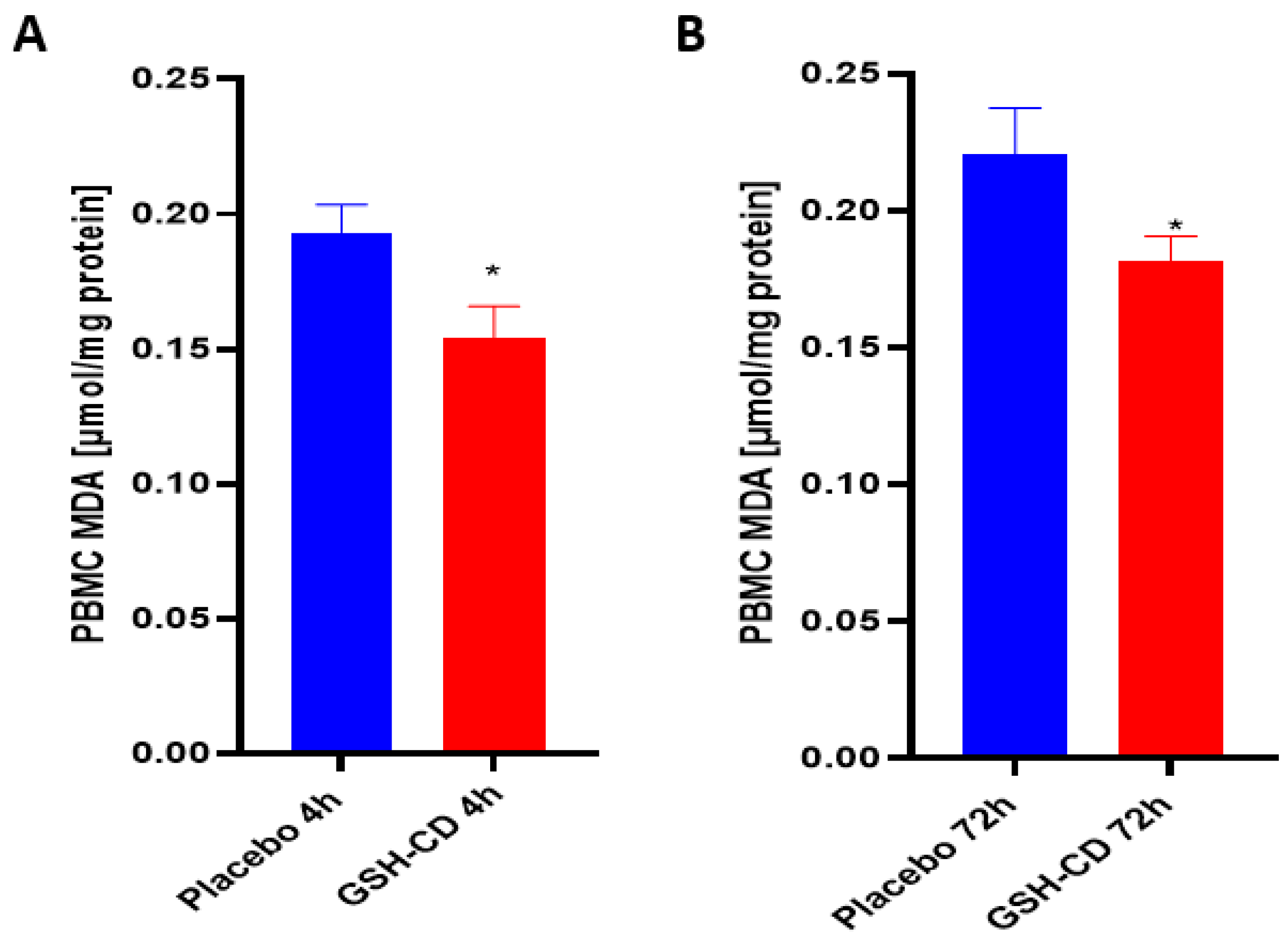
3.2. MDA Levels Are Decreased after Topical GSH-CD Treatment
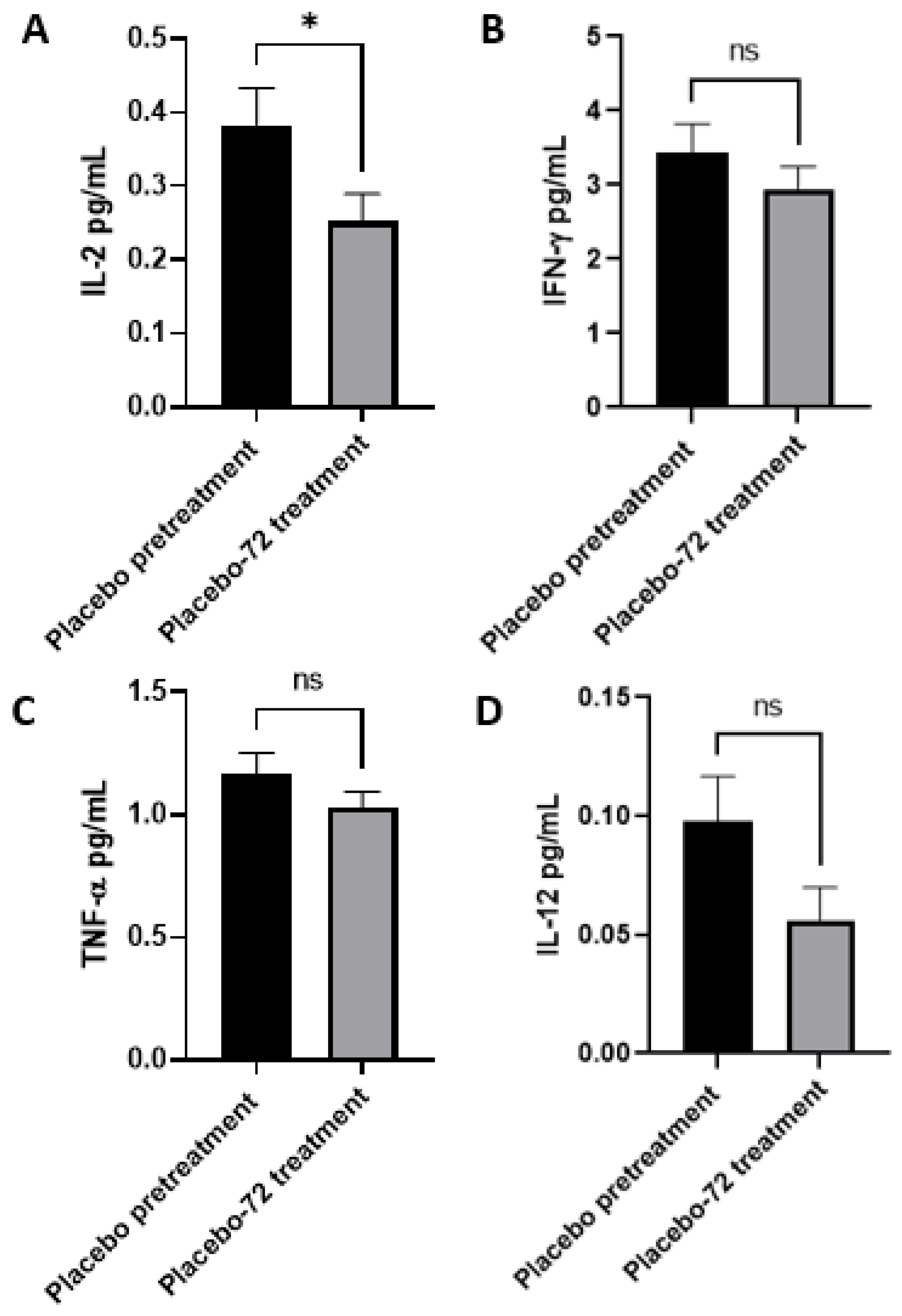
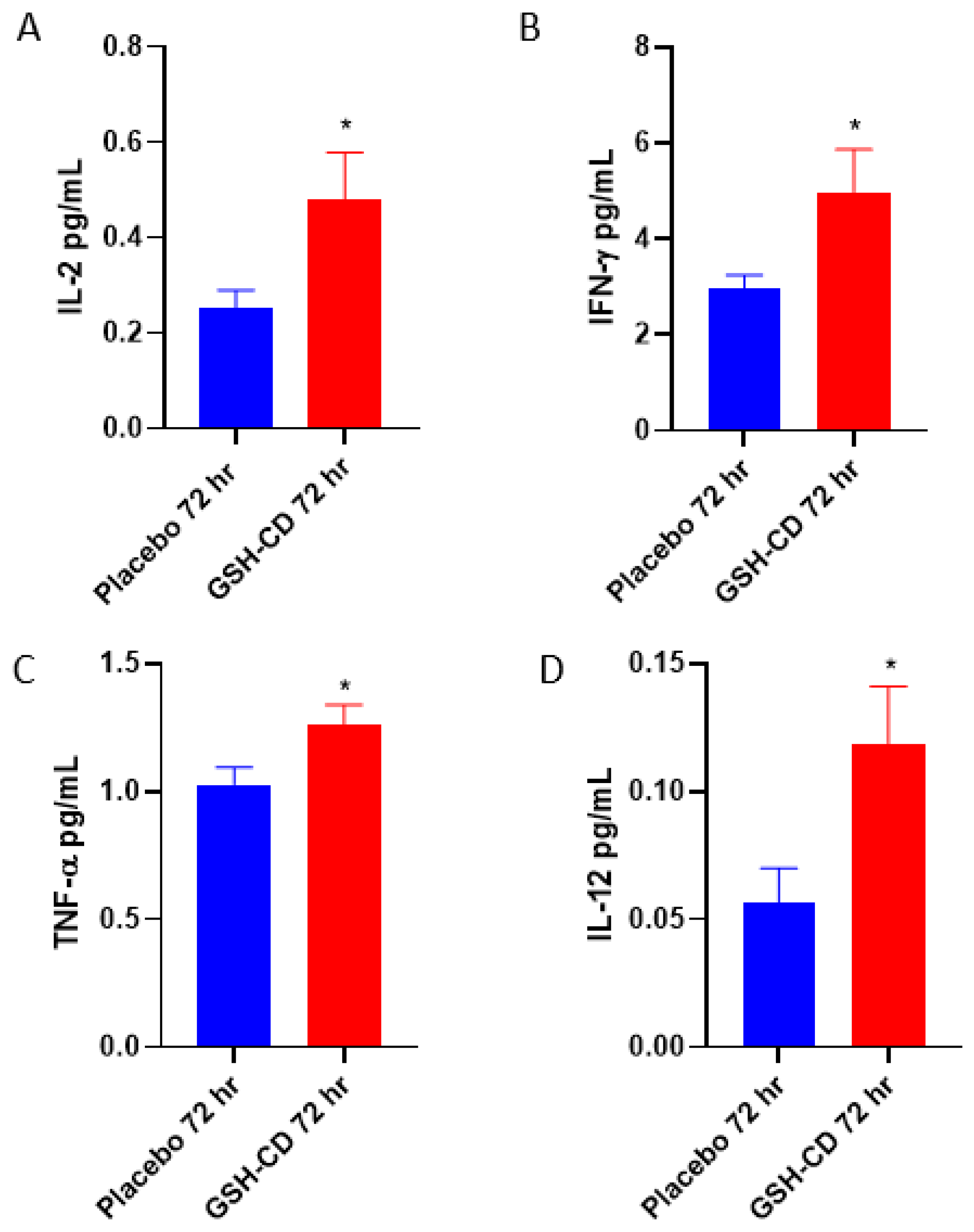
3.3. Increased Levels of IL-12, IL-2, IFN-γ and TNF-α in Plasma Post-Initial GSH-CD Treatment
3.4. Topical GSH-CD Treatment Is Associated with the Reduction in the Burden of M. avium (In Vitro)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P., Jr. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2018, 72, 105–111. [Google Scholar] [CrossRef]
- Cao, R.; Kolloli, A.; Kumar, R.; Owens, J.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Kachour, N.; Beever, A.; et al. Effects of Glutathione Diminishment on the Immune Responses against Mycobacterium tuberculosis Infection. Appl. Sci. 2021, 11, 8274. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/publications/i/item/9789240061729 (accessed on 5 April 2023).
- Guerra, C.; Morris, D.; Sipin, A.; Kung, S.; Franklin, M.; Gray, D.; Tanzil, M.; Guilford, F.; Khasawneh, F.T.; Venketaraman, V. Glutathione and adaptive immune responses against Mycobacterium tuberculosis infection in healthy and HIV infected individuals. PLoS ONE 2011, 6, e28378. [Google Scholar] [CrossRef]
- Morris, D.; Guerra, C.; Donohue, C.; Oh, H.; Khurasany, M.; Venketaraman, V. Unveiling the mechanisms for decreased glutathione in individuals with HIV infection. Clin. Dev. Immunol. 2012, 2012, 734125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagman, M.; Ly, J.; Saing, T.; Kaur Singh, M.; Vera Tudela, E.; Morris, D.; Chi, P.T.; Ochoa, C.; Sathananthan, A.; Venketaraman, V. Investigating the causes for decreased levels of glutathione in individuals with type II diabetes. PLoS ONE 2015, 10, e0118436. [Google Scholar] [CrossRef] [Green Version]
- Buonocore, D.; Grosini, M.; Giardina, S.; Michelotti, A.; Carrabetta, M.; Seneci, A.; Verri, M.; Dossena, M.; Marzatico, F. Bioavailability Study of an Innovative Orobuccal Formulation of Glutathione. Oxidative Med. Cell. Longev. 2016, 2016, 3286365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: A comparative crossover study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Ly, J.; Lagman, M.; Saing, T.; Singh, M.K.; Tudela, E.V.; Morris, D.; Anderson, J.; Daliva, J.; Ochoa, C.; Patel, N.; et al. Liposomal Glutathione Supplementation Restores TH1 Cytokine Response to Mycobacterium tuberculosis Infection in HIV-Infected Individuals. J. Interferon Cytokine Res. 2015, 35, 875–887. [Google Scholar] [CrossRef] [Green Version]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Sathananthan, A.; Venketaraman, V. The Effects of Oral Liposomal Glutathione and In Vitro Everolimus in Altering the Immune Responses against Mycobacterium bovis BCG Strain in Individuals with Type 2 Diabetes. Biomol. Concepts 2021, 12, 16–26. [Google Scholar] [CrossRef]
- Wahab, S.; Anwar, A.I.; Zainuddin, A.N.; Hutabarat, E.N.; Anwar, A.A.; Kurniadi, I. Combination of topical and oral glutathione as a skin-whitening agent: A double-blind randomized controlled clinical trial. Int. J. Dermatol. 2021, 60, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Tewari-Singh, N.; Agarwal, C.; Huang, J.; Day, B.J.; White, C.W.; Agarwal, R. Efficacy of glutathione in ameliorating sulfur mustard analog-induced toxicity in cultured skin epidermal cells and in SKH-1 mouse skin in vivo. J. Pharmacol. Exp. Ther. 2011, 336, 450–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopal, C.; Deveci, M.; Oztürk, S.; Sengezer, M. Effects of topical glutathione treatment in rat ischemic wound model. Ann. Plast. Surg. 2007, 58, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione synthesis. Biochim. Biophys. Acta. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [Green Version]
- Oppenheimer, L.; Wellner, V.P.; Griffith, O.W.; Meister, A. Glutathione synthetase. Purification from rat kidney and mapping of the substrate binding sites. J. Biol. Chem. 1979, 254, 5184–5190. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Checa, J.C.; Kaplowitz, N.; García-Ruiz, C.; Colell, A.; Miranda, M.; Marí, M.; Ardite, E.; Morales, A. GSH transport in mitochondria: Defense against TNF-induced oxidative stress and alcohol-induced defect. Am. J. Physiol. 1997, 273, G7–G17. [Google Scholar] [CrossRef]
- Panday, S.; Talreja, R.; Kavdia, M. The role of glutathione and glutathione peroxidase in regulating cellular level of reactive oxygen and nitrogen species. Microvasc. Res. 2020, 131, 104010. [Google Scholar] [CrossRef]
- Kwon, D.H.; Lee, H.; Park, C.; Hong, S.H.; Hong, S.H.; Kim, G.Y.; Cha, H.J.; Kim, S.; Kim, H.S.; Hwang, H.J.; et al. Glutathione Induced Immune-Stimulatory Activity by Promoting M1-Like Macrophages Polarization via Potential ROS Scavenging Capacity. Antioxidants 2019, 8, 413. [Google Scholar] [CrossRef] [Green Version]
- Kachour, N.; Beever, A.; Owens, J.; Cao, R.; Kolloli, A.; Kumar, R.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; et al. Liposomal Glutathione Helps to Mitigate Mycobacterium tuberculosis Infection in the Lungs. Antioxidants 2022, 11, 673. [Google Scholar] [CrossRef] [PubMed]
- Davids, L.M.; Van Wyk, J.C.; Khumalo, N.P. Intravenous glutathione for skin lightening: Inadequate safety data. S. Afr. Med. J. 2016, 106, 782–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beever, A.; Kachour, N.; Owens, J.; Sasaninia, K.; Kolloli, A.; Kumar, R.; Ramasamy, S.; Sisliyan, C.; Khamas, W.; Subbian, S.; et al. L-GSH Supplementation in Conjunction with Rifampicin Augments the Treatment Response to Mycobacterium tuberculosis in a Diabetic Mouse Model. Front. Pharmacol. 2022, 13, 879729. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Bradley, R.D. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J. Altern. Complement. Med. 2011, 17, 827–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, L.; Xia, T.; Li, S.; Yan, T.; Wu, S.; Qiu, G.; Liu, Z. Toward a biomarker of oxidative stress: A fluorescent probe for exogenous and endogenous malondialdehyde in living cells. Anal. Chem. 2015, 87, 8052–8056. [Google Scholar] [CrossRef]
- Gideon, H.P.; Phuah, J.; Myers, A.J.; Bryson, B.D.; Rodgers, M.A.; Coleman, M.T.; Maiello, P.; Rutledge, T.; Marino, S.; Fortune, S.M.; et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog. 2015, 11, e1004603. [Google Scholar] [CrossRef] [Green Version]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef]
- Murray, H.W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann. Intern. Med. 1988, 108, 595–608. [Google Scholar] [CrossRef]
- Malek, T.R. The biology of interleukin-2. Annu. Rev. Immunol. 2008, 26, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Trotta, E.; Simeonov, D.R.; Marson, A.; Bluestone, J.A. Revisiting IL-2: Biology and therapeutic prospects. Sci. Immunol. 2018, 3, eaat1482. [Google Scholar] [CrossRef] [Green Version]
- Kokolakis, G.; Sabat, R.; Krüger-Krasagakis, S.; Eberle, J. Ambivalent Effects of Tumor Necrosis Factor Alpha on Apoptosis of Malignant and Normal Human Keratinocytes. Skin Pharmacol. Physiol. 2021, 34, 94–102. [Google Scholar] [CrossRef]
- Maphasa, R.E.; Meyer, M.; Dube, A. The Macrophage Response to Mycobacterium tuberculosis and Opportunities for Autophagy Inducing Nanomedicines for Tuberculosis Therapy. Front. Cell. Infect. Microbiol. 2021, 10, 618414. [Google Scholar] [CrossRef]
- Johnson, M.M.; Odell, J.A. Nontuberculous mycobacterial pulmonary infections. J. Thorac. Dis. 2014, 6, 210–220. [Google Scholar] [CrossRef]
- Franzblau, S.G.; Takeda, T.; Nakamura, M. Mycobacterial plasmids: Screening and possible relationship to antibiotic resistance in Mycobacterium avium/Mycobacterium intracellulare. Microbiol. Immunol. 1986, 30, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.L. Mycobacterium avium Complex Disease. Microbiol. Spectr. 2017, 5, 18. [Google Scholar] [CrossRef]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Nguyen, T.; Sasaninia, K.; Vaughn, C.; Singh, M.; Truong, E.; Medina, A.; et al. Effects of Oral Liposomal Glutathione in Altering the Immune Responses against Mycobacterium tuberculosis and the Mycobacterium bovis BCG Strain in Individuals with Type 2 Diabetes. Front. Cell. Infect. Microbiol. 2021, 11, 657775. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaninia, K.; Kelley, M.; Abnousian, A.; Badaoui, A.; Alexander, L.; Sheren, N.; Owens, J.; Rajurkar, S.; Razo-Botello, B.; Chorbajian, A.; et al. Topical Absorption of Glutathione–Cyclodextrin Nanoparticle Complex in Healthy Human Subjects Improves Immune Response against Mycobacterium avium Infection. Antioxidants 2023, 12, 1375. https://doi.org/10.3390/antiox12071375
Sasaninia K, Kelley M, Abnousian A, Badaoui A, Alexander L, Sheren N, Owens J, Rajurkar S, Razo-Botello B, Chorbajian A, et al. Topical Absorption of Glutathione–Cyclodextrin Nanoparticle Complex in Healthy Human Subjects Improves Immune Response against Mycobacterium avium Infection. Antioxidants. 2023; 12(7):1375. https://doi.org/10.3390/antiox12071375
Chicago/Turabian StyleSasaninia, Kayvan, Melissa Kelley, Arbi Abnousian, Ali Badaoui, Logan Alexander, Nisar Sheren, James Owens, Shlok Rajurkar, Brianna Razo-Botello, Abraham Chorbajian, and et al. 2023. "Topical Absorption of Glutathione–Cyclodextrin Nanoparticle Complex in Healthy Human Subjects Improves Immune Response against Mycobacterium avium Infection" Antioxidants 12, no. 7: 1375. https://doi.org/10.3390/antiox12071375





