Optimization of DIC-Tripolium Ecofriendly Extraction Process: Recovery of Hesperidin from Orange Byproducts, Antioxidant and α-Amylase Inhibition of Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. DIC Pretreatment and DIC–Tripolium Extraction
2.3. Scanning Electron Microscopy (SEM) Analysis
2.4. Analysis of Orange Byproduct Extracts
2.4.1. Analysis of Hesperidin by Ultra-High Performance Liquid Chromatography (UHPLC)
2.4.2. Determination of Radical Scavenging Activities Using DPPH Assay
2.4.3. Determination of Radical Scavenging Activity Using ABTS Assay
2.4.4. Iron Chelating Activity (ICA)
2.4.5. α-Amylase Inhibition Assay
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Effect of DIC Treatment on Tripolium Extraction Efficiency
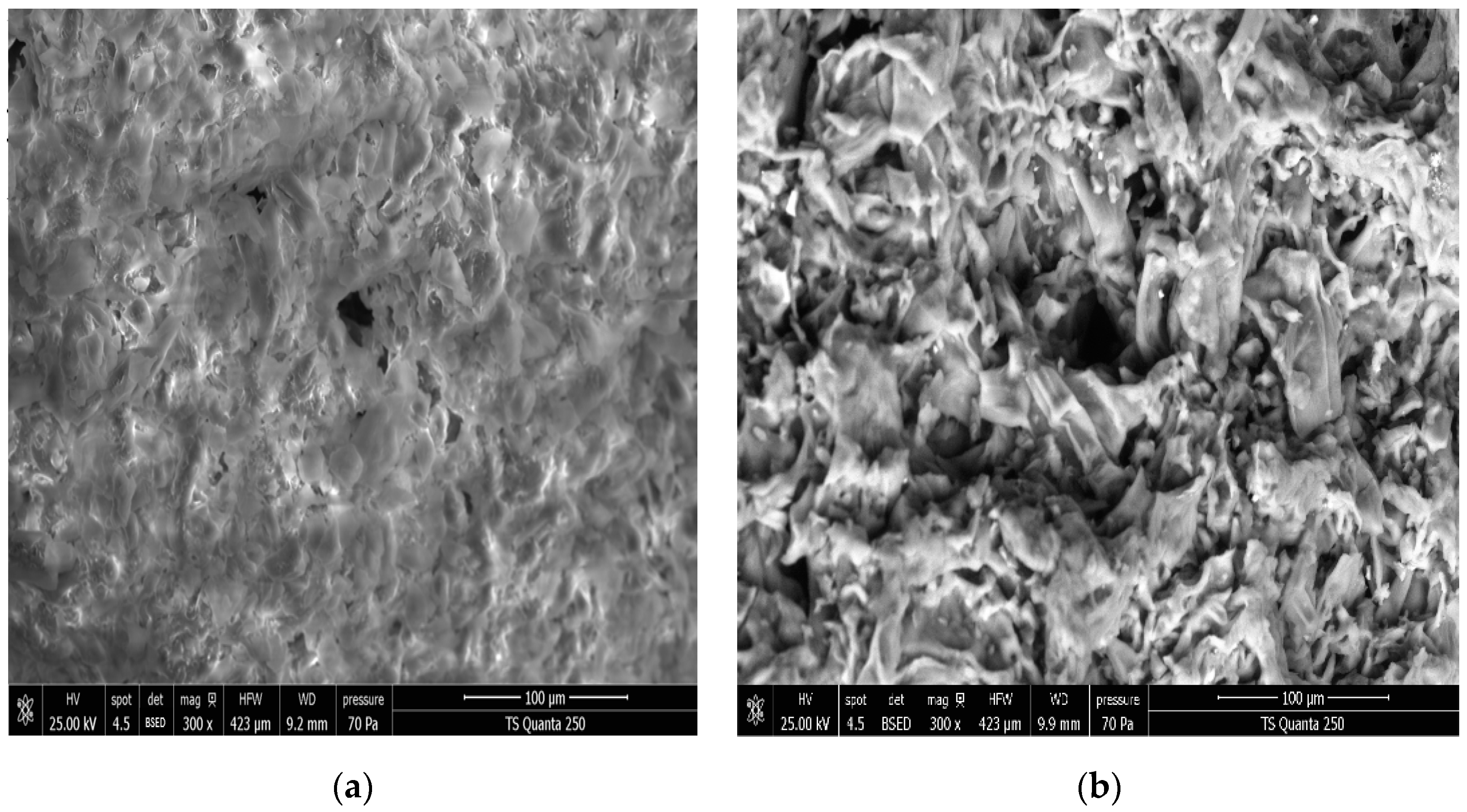
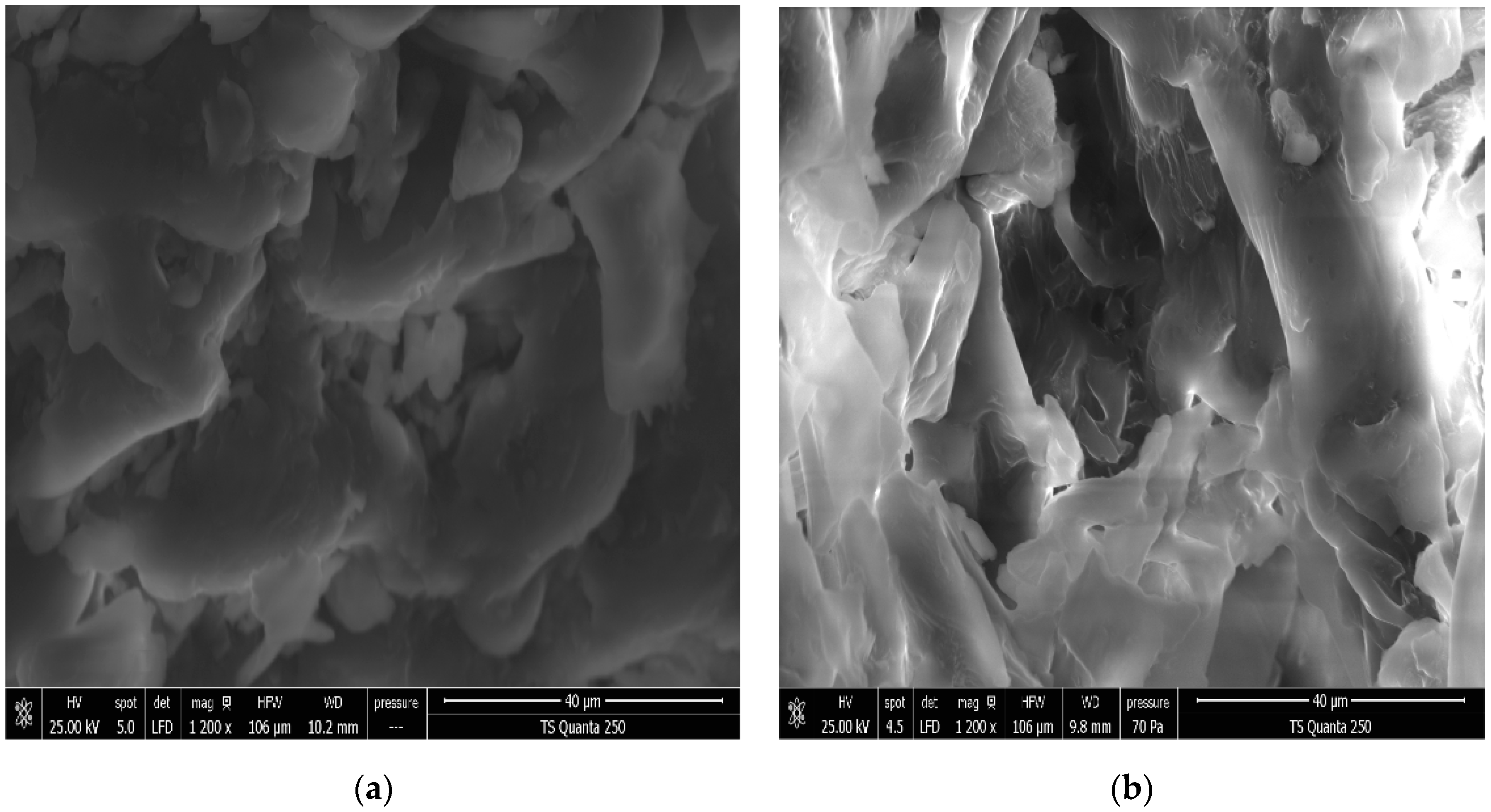
3.2. Effect of DIC Treatment on Microstructure
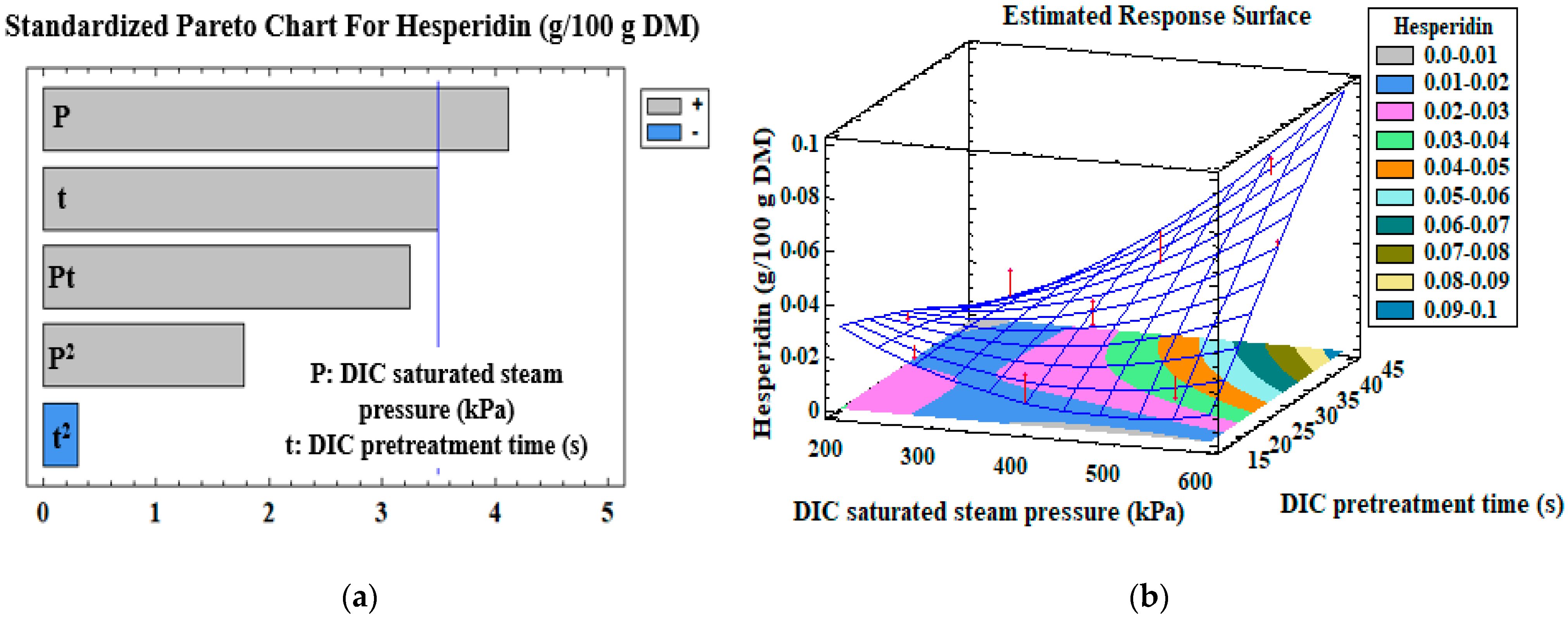
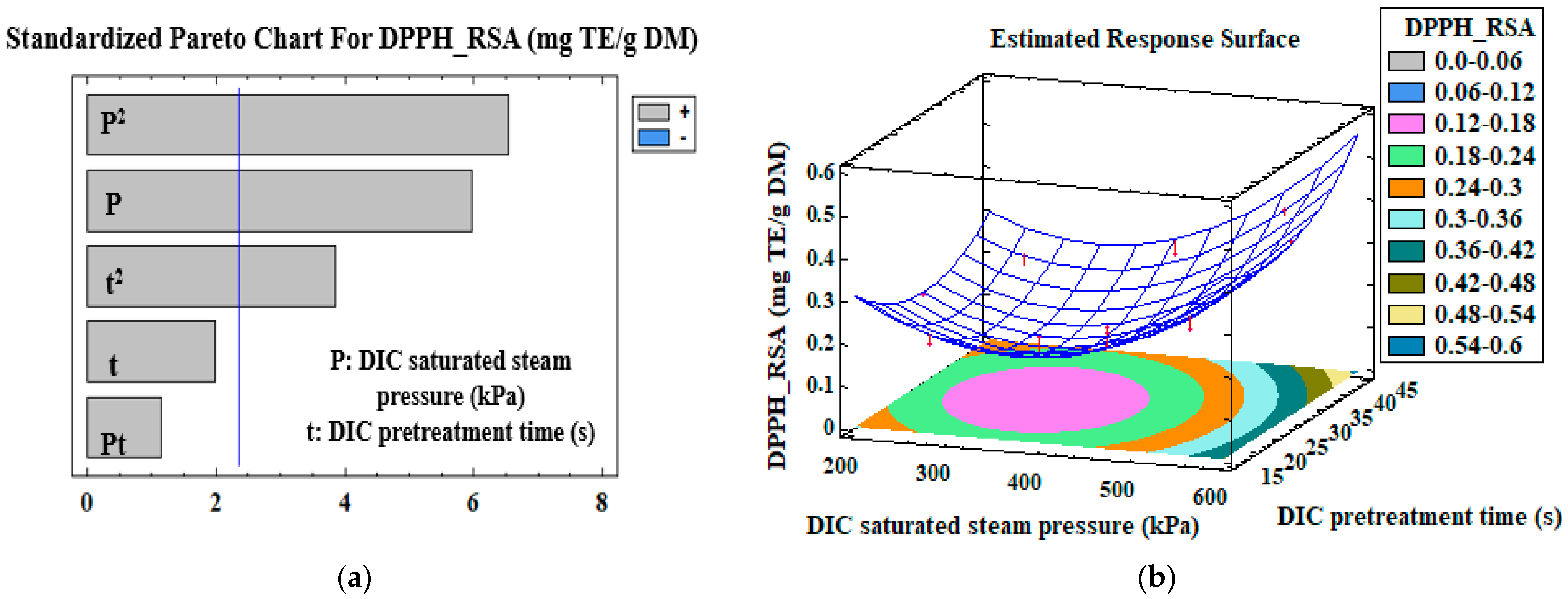
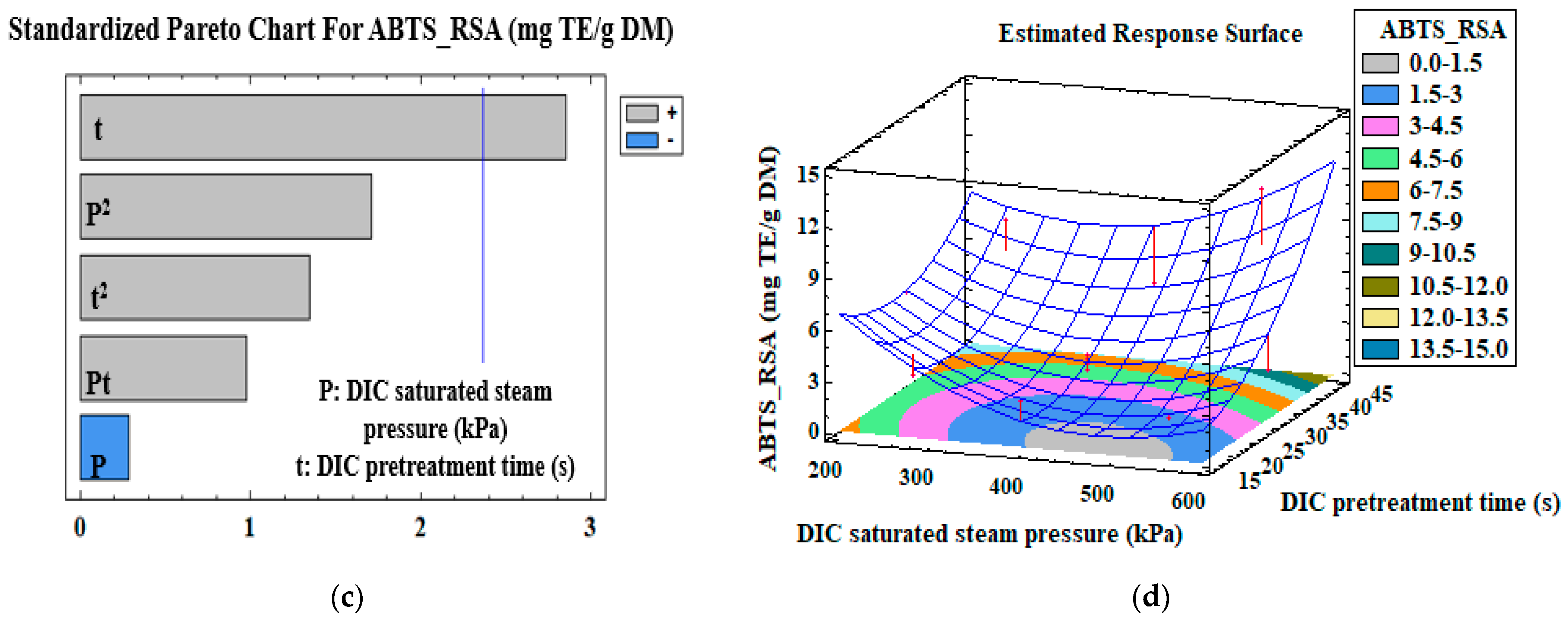
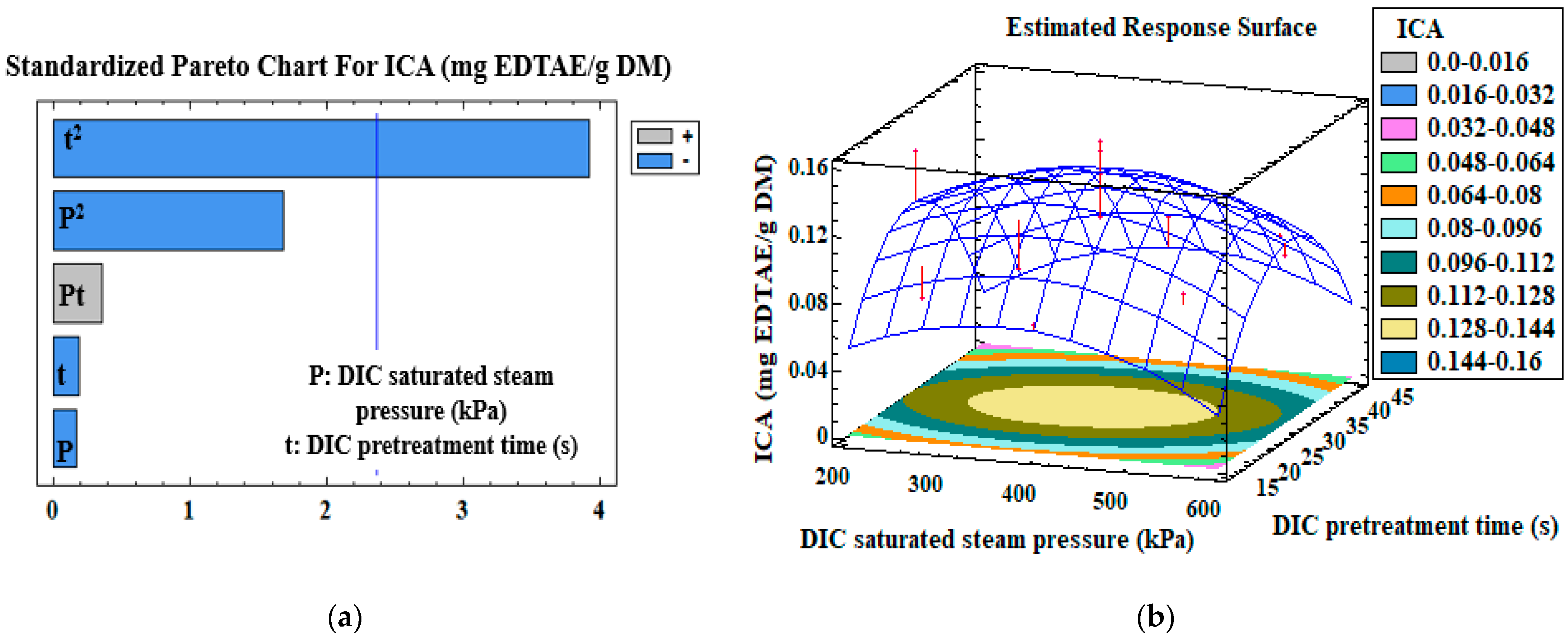
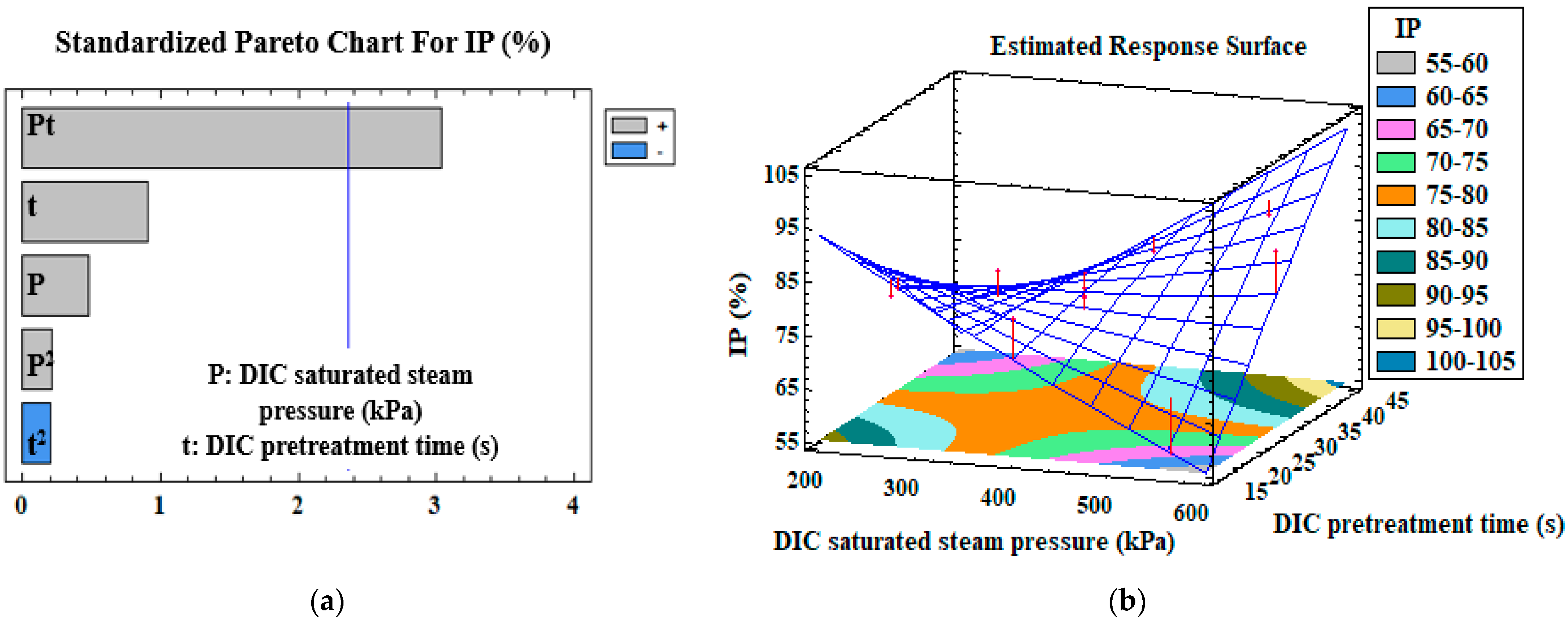
3.3. Mono-Criterion Optimization
3.4. Multi-Criteria Optimization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Triopicsafe: Scientific Innovation Factsheet. 2021. Available online: http://www.tropicsafe.eu (accessed on 10 May 2023).
- Mir-Cerdà, A.; Nuñez, O.; Granados, M.; Sentellas, S.; Saurina, J. An overview of the extraction and characterization of bioactive phenolic compounds from agri-food waste within the framework of circular bioeconomy. Trends Anal. Chem. 2023, 161, 1–12. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, A.D.; Melgar, B.; Conidi, C.; Barros, L.; Ferreira, I.C.F.R.; Cassano, A.; Garcia-Castello, E.M. Food industry by-products valorization and new ingredients. In Sustainability of the Food System; Academic Press: Cambridge, MA, USA, 2020; pp. 71–99. [Google Scholar] [CrossRef]
- Chaudhari, S.Y.; Ruknuddin, G.; Prajapati, P. Ethno medicinal values of Citrus genus: A review. Med. J. DY Patil Univ. 2016, 9, 560–565. [Google Scholar] [CrossRef]
- Kaura, S.S.; Panesara, P.S.; Choprab, H.K. Citrus processing by-products: An overlooked repository of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 67–86. [Google Scholar] [CrossRef]
- M’hiri, N.; Ioannou, I.; Mihoubi Boudhrioua, N.; Ghoul, M. Antioxidants of Maltease orange peel: Comparative investigation of the efficiency of four extraction methods. J. Appl. Pharm. Sci. 2017, 11, 126–135. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef]
- Mir Khan, U.; Sameen, A.; Aadil, R.M.; Shahid, M.; Sezen, S.; Zarrabi, A.; Ozdemir, B.; Sevindik, M.; Nur Kaplan, D.; Selamoglu, Z.; et al. Citrus Genus and Its Waste Utilization: A Review on Health-Promoting Activities and Industrial Application. Evid. Based Complement. Altern. Med. 2021, 2488804. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. [Google Scholar] [CrossRef]
- Singh, B.; Pal Singh, J.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavonone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Asabi, O.A.; Oisemuzeimen, J.O.; Abiodun, O.O.; Blessing, K. Antioxidant and In-vitro Antidiabetic Activities of Fermented Peels of Citrus × Sinensis (L.) Osbeck (Rutaceae). Prog. Chem. Biochem. Res. 2021, 4, 414–425. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of Evidence Available on Hesperidin-Rich Products as Potential Tools against COVID-19 and Hydrodynamic Cavitation-Based Extraction as a Method of Increasing Their Production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Teshome, E.; Teka, T.A.; Nandasiri, R.; Rout, J.R.; Harouna, D.V.; Astatkie, T.; Urugo, M.M. Fruit By-Products and Their Industrial Applications for Nutritional Benefits and Health Promotion: A Comprehensive Review. Sustainability 2023, 15, 7840. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of Antioxidant Compounds Extraction from Fruit Byproducts: Apple Pomace, Orange and Banana Peel. J. Food Process. Preserv. 2015, 40, 103–115. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abd El Aziz, N.M.; Youssef, M.M.; El-Sohaimy, S.A. Optimization conditions of ultrasound-assisted extraction of phenolic compounds from orange peels using response surface methodology. J. Food Process. Preserv. 2021, 45, e15870. [Google Scholar] [CrossRef]
- .Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds de-rived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2021, 63, 719–752. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Li, Q.; Putra, N.R.; Rizkiyah, D.N.; Abdul Aziz, A.H.; Irianto, I.; Qomariyah, L. Orange Pomace and Peel Extraction Processes towards Sustainable Utilization: A Short Review. Molecules 2023, 28, 3550. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Bioref. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Pech-Almeida, J.L.; Téllez-Pérez, C.; Alonzo-Macías, M.; Teresa-Martínez, G.D.; Allaf, K.; Allaf, T.; Carda-dor-Martínez, A. An Overview on Food Applications of the Instant Controlled Pressure-Drop Technology, an Innovative High Pressure-Short Time Process. Molecules 2021, 26, 6519. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Alonzo-Macías, M.; Mounir, S.; Besombes, C.; Allaf, T.; Amami, E.; Allaf, K. Instant Controlled Pressure-Drop DIC as a Strategic Technology for Different Types of Natural. In Functional Foods; IntechOpen: London, UK, 2019; pp. 1–25. [Google Scholar]
- Louati, I.; Bahloul, N.; Besombes, C.; Allaf, K.; Kechaou, N. Instant Controlled Pressure-Drop as Texturing Pre-treatment for Intensifying both Final Drying Stage and Extraction of Phenolic Compounds to valorize Orange Industry Byproducts (Citrus sinensis L.). Food Bioprod. Process. 2018, 114, 85–94. [Google Scholar] [CrossRef]
- Allaf, T.; Mounir, S.; Tomao, V.; Chemat, F. Instant Controlled Pressure-drop Combined to Ultrasounds as Innova-tive Ex-traction Process Combination: Fundamental Aspects. Procedia Eng. 2012, 42, 1061–1078. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Chadni, M.; M’hiri, N.; Brunissen, F.; Rokbeni, N.; Allaf, K.; Besombes, C.; Ioannou, I.; Boudhrioua, N. Intensifying Effect of Instant Controlled Pressure-drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts. Molecules 2023, 28, 1858. [Google Scholar] [CrossRef]
- Xynos, N.; Papaefstathiou, G.; Gikas, E.; Argyropoulou, A.; Aligiannis, N.; Skaltsounis, A.L. Design optimization study of the extraction of olive leaves performed with pressurized liquid extraction using response surface methodology. Sep. Purif. Technol. 2014, 122, 323–330. [Google Scholar] [CrossRef]
- Özcan, M.M.; Ghafoor, K.; Al Juhaimi, F.; Uslu, N.; Babiker, E.E.; Mohamed Ahmed, I.A.; Almusallam, I.A. Influence of drying techniques on bioactive properties, phenolic compounds and fatty acid compositions of dried lemon and orange peel powders. J. Food Sci. Technol. 2020, 58, 147–158. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L.) Peel, a Valuable Byproduct from Northeastern Morocco. Biomolecules 2021, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.N.; Kausar, T.; Jabbar, S.; Mumtaz, A.; Ahad, K.; Saddozai, A.A. Extraction and quantification of poly-phenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J. Food Drug Anal. 2017, 25, 488–500. [Google Scholar] [CrossRef]
- Allaf, T.; Tomao, V.; Ruiz, K.; Chemat, F. Instant controlled Pressure-drop technology and ultrasound assisted extraction for sequential extraction of essential oil and antioxidants. Ultrason. Sonochem. 2013, 20, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Louka, N.; Allaf, K. Expansion ratio and color improvement of dried vegetables texturized by a new process “Con-trolled Sudden Decompression to the vacuum”. J. Food Eng. 2004, 65, 233–243. [Google Scholar] [CrossRef]
- Lefebvre, T.; Destandau, E.; Lesellier, E. Selective extraction of bioactive compounds from plants using recent ex-traction techniques: A Review. J. Chromatogr. A. 2021, 1635, 461770. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some methodical problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Senol, F.S.; Ankli, A.; Reich, E.; Orhan, I.E. HPTLC Finger-Printing and Cholinesterase Inhibitory and Metal-Chelating Capacity of Various Citrus Cultivars and Olea europaea. Food Technol. Biotechnol. 2016, 54, 275–281. [Google Scholar] [CrossRef] [PubMed]
| DIC Saturated Steam Pressure (kPa) | DIC Pretreatment Time (s) | |
|---|---|---|
| Point min (−α) | 200 | 15 |
| Point (−1): | 259 | 19 |
| Central point | 400 | 30 |
| Point (+1): | 541 | 41 |
| Point max (+α) | 600 | 45 |
| Time (min) | Flow (mL/min) | Acetonitrile (%) | Formic Acid 0.1% (v/v) in Water (%) |
|---|---|---|---|
| 0 | 0.8 | 2 | 98 |
| 3 | 0.8 | 14 | 86 |
| 5.5 | 0.8 | 20 | 80 |
| 9 | 0.8 | 50 | 50 |
| 9.5 | 0.8 | 50 | 50 |
| 10 | 0.8 | 95 | 5 |
| 11.5 | 0.8 | 95 | 5 |
| 12 | 0.8 | 2 | 98 |
| 14 | 0.8 | 2 | 98 |
| Trials | P (kPa) | T (°C) | t (s) | DPPH—RSA (mg TE/g DM) | ABTS—RSA (mg TE/g DM) | ICA (mg EDTAE/g DM) | IP (%) | Hesperidin (g/100 g DM) | HY (%) |
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 3) | C | C | C | 0.138 ± 0.004 | 2.594 ± 0.05 | 0.104 ± 0.01 | 82 ± 0.1 | 0.016 ± 0.002 | 0 |
| Central point (n = 5) | 400 | 143.67 | 30 | 0.151 ± 0.01 | 2.471 ± 0.02 | 0.140 ± 0.01 | 77 ± 0.1 | 0.027 ± 0.003 | 68.75 |
| 1 | 600 | 158.90 | 30 | 0.411 ± 0.01 | 2.914 ± 0.03 | 0.099 ± 0.0006 | 88.2 ± 0.1 | 0.058 ± 0.003 | 262.5 |
| 2 | 400 | 143.67 | 45 | 0.240 ± 0.01 | 4.553 ± 0.03 | 0.0862 ± 0.0006 | 76.50 ± 0.01 | 0.028 ± 0.07 | 75 |
| 3 | 541 | 154.92 | 40.6 | 0.399 ± 0.07 | 11.460 ± 0.01 | 0.0891 ± 0.004 | 88.3 ± 0.01 | 0.076 ± 0.003 | 375 |
| 4 | 541 | 154.92 | 19.4 | 0.266 ± 0.01 | 1.699 ± 0.003 | 0.0941 ± 0.0005 | 55.6 ± 0.02 | 0.01 ± 0,05 | 6.25 |
| 5 | 259 | 128.55 | 19.4 | 0.179 ± 0.04 | 2.833 ± 0.06 | 0.0774 ± 0.0006 | 81.90 ± 0.05 | 0.016 ± 0.008 | 0 |
| 6 | 259 | 128.55 | 40.6 | 0.239 ± 0.002 | 8.299 ± 0.01 | 0.0559 ± 0.0007 | 73.3 ± 0.1 | 0.025 ± 0.001 | 56.25 |
| 7 | 200 | 120.22 | 30 | 0.210 ± 0.09 | 5.607 ± 0.04 | 0.1380 ± 0.02 | 73.60 ± 0.05 | 0.017 ± 0.004 | 6.25 |
| 8 | 400 | 143.67 | 15 | 0.253 ± 0.01 | 2.760 ± 0.006 | 0.0765 ± 0.0006 | 81.1 ± 0.1 | 0.016 ± 0.002 | 0 |
| Response Variable | Source | Significant Regression Coefficients | p-Value | |
|---|---|---|---|---|
| Hesperidin (g/100 g DM) | Model | β0 | 7.680 × 10−2 | |
| P (kPa) | β1 | −2 × 10−4 | 0.004 | |
| t (s) | β2 | −3 × 10−3 | 0.01 | |
| P × t | β12 | 1 × 10−5 | 0.01 | |
| R2 | 0.80 | |||
| DPPH—RSA (mg TE/g DM) | Model | β0 | 9.6 × 10−1 | |
| P (kPa) | β1 | −2.7 × 10−3 | 0.0006 | |
| P2 | β11 | 4 × 10−6 | 0.0003 | |
| t2 | β22 | 4.1 × 10−4 | 0.009 | |
| R2 | 0.88 | |||
| ABTS—RSA (mg TE/g DM) | Model | β0 | −2.25 | |
| t (s) | β2 | 0.21 | 0.02 | |
| R2 | 0.40 | |||
| ICA (mg EDTAE/g DM) | Model | β0 | −0.13 | |
| t2 | β22 | −3 × 10−4 | 0.002 | |
| R2 | 0.60 | |||
| IP (%) | Model | β0 | 160.1 | |
| P × t | β12 | 7 × 10−3 | 0.005 | |
| R2 | 0.53 | |||
| Optimal Condition | Hesperidin (g/100 g DM) | DPPH—RSA (mg TE/g DM) | ABTS—RSA (mg TE/g DM) | ICA (mg EDTAE/g DM) | IP (%) |
|---|---|---|---|---|---|
| DIC saturated steam pressure (kPa) | 599.4 | 599.4 | 400 | 400 | 200.6 |
| DIC pretreatment time (s) | 44.99 | 44.99 | 44.99 | 30 | 15.01 |
| Predicted values | 0.091 | 0.493 | 7.176 | 0.130 | 97.93 |
| Response | Optimal Value |
|---|---|
| Hesperidin (g/100 g DM) | 0.071 |
| DPPH-RSA (mg TE/g DM) | 0.427 |
| ICA (mg EDTAE/g DM) | 0.111 |
| IP (%) | 88.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Abdallah, M.; Chadni, M.; M’hiri, N.; Brunissen, F.; Rokbeni, N.; Ioannou, I.; Allaf, K.; Besombes, C.; Boudhrioua, N. Optimization of DIC-Tripolium Ecofriendly Extraction Process: Recovery of Hesperidin from Orange Byproducts, Antioxidant and α-Amylase Inhibition of Extracts. Antioxidants 2023, 12, 1346. https://doi.org/10.3390/antiox12071346
Ben Abdallah M, Chadni M, M’hiri N, Brunissen F, Rokbeni N, Ioannou I, Allaf K, Besombes C, Boudhrioua N. Optimization of DIC-Tripolium Ecofriendly Extraction Process: Recovery of Hesperidin from Orange Byproducts, Antioxidant and α-Amylase Inhibition of Extracts. Antioxidants. 2023; 12(7):1346. https://doi.org/10.3390/antiox12071346
Chicago/Turabian StyleBen Abdallah, Mariem, Morad Chadni, Nouha M’hiri, Fanny Brunissen, Nesrine Rokbeni, Irina Ioannou, Karim Allaf, Colette Besombes, and Nourhene Boudhrioua. 2023. "Optimization of DIC-Tripolium Ecofriendly Extraction Process: Recovery of Hesperidin from Orange Byproducts, Antioxidant and α-Amylase Inhibition of Extracts" Antioxidants 12, no. 7: 1346. https://doi.org/10.3390/antiox12071346
APA StyleBen Abdallah, M., Chadni, M., M’hiri, N., Brunissen, F., Rokbeni, N., Ioannou, I., Allaf, K., Besombes, C., & Boudhrioua, N. (2023). Optimization of DIC-Tripolium Ecofriendly Extraction Process: Recovery of Hesperidin from Orange Byproducts, Antioxidant and α-Amylase Inhibition of Extracts. Antioxidants, 12(7), 1346. https://doi.org/10.3390/antiox12071346








