Abstract
Ischemic cardiomyopathy (ICM) is associated with abnormal microRNA expression levels that involve an altered gene expression profile. However, little is known about the underlying causes of microRNA disruption in ICM and whether microRNA maturation is compromised. Therefore, we focused on microRNA maturation defects analysis and the implication of the microRNA biogenesis pathway and redox-sensitive microRNAs (redoximiRs). Transcriptomic changes were investigated via ncRNA-seq (ICM, n = 22; controls, n = 8) and mRNA-seq (ICM, n = 13; control, n = 10). The effect of hypoxia on the biogenesis of microRNAs was evaluated in the AC16 cell line. ICM patients showed a reduction in microRNA maturation compared to control (4.30 ± 0.94 au vs. 5.34 ± 1.07 au, p ˂ 0.05), accompanied by a deregulation of the microRNA biogenesis pathway: a decrease in pre-microRNA export (XPO5, FC = −1.38, p ˂ 0.05) and cytoplasmic processing (DICER, FC = −1.32, p ˂ 0.01). Both processes were regulated by hypoxia in AC16 cells (XPO5, FC = −1.65; DICER1, FC = −1.55; p ˂ 0.01; Exportin-5, FC = −1.81; Dicer, FC = −1.15; p ˂ 0.05). Patients displayed deregulation of several redoximiRs, highlighting miR-122-5p (FC = −2.41, p ˂ 0.001), which maintained a good correlation with the ejection fraction (r = 0.681, p ˂ 0.01). We evidenced a decrease in microRNA maturation mainly linked to a decrease in XPO5-mediated pre-microRNA export and DICER1-mediated processing, together with a general effect of hypoxia through deregulation of biogenesis pathway and the redoximiRs.
1. Introduction
Heart failure was defined as a global pandemic. It constitutes a significant economic and public health burden with high morbidity and mortality rates worldwide [1], mainly due to ischemic heart diseases, and is a leading global cause of death [2]. The pathological process of the heart is associated with an altered expression profile of molecules that are important for cardiac function [3,4,5,6]; however, the molecular mechanisms of gene regulation remain incompletely understood. Most human protein-coding genes are likely under microRNA control through microRNA-binding sites located in their untranslated and amino acid coding regions [7,8] and, therefore, microRNA are probably involved in nearly all developmental and pathological processes. Thus, it was described that alteration in the expression of microRNA contributes to a range of human pathologies, including heart failure [9,10]. However, little is known about the underlying causes of microRNA disruption and whether microRNA maturation is compromised. In cancer, it was proposed that the global dysregulation of microRNA mainly involves transcriptional control and/or impaired biogenesis [11].
microRNA biogenesis is tightly regulated at multiple levels and involves both nuclear and cytoplasmic processing [12]. Altered expression and mutations of members of the microRNA processing machinery can lead to changes ranging the transcription of microRNA, processing, export and maturation [11], leading to a marked reduction in the production of mature microRNAs [13]. Emerging evidence suggests that microRNA biogenesis can be affected by oxidative stress, an important pathophysiological pathway in the development and progression of heart failure, especially in ischemic heart disease [14]; in a complex interplay between microRNA and ROS in which microRNA also can regulate cellular redox status [15]. The number of identified redox-sensitive microRNA is increasing [16,17], but this relationship may be broader [18,19]. This complex network between microRNAs and oxidative stress acts by modulating cell homeostasis, but despite the fact that several genome-wide microRNA studies identified microRNA differentially expressed in heart failure [9,10], suggesting their possible involvement in the pathogenesis, the state of microRNA maturity in heart failure and how the processing machinery and redox state control it is unknown.
In this study, we analyzed the state of microRNA maturity and its biogenesis process in ischemic cardiomyopathy (ICM). We focused especially on redox-sensitive microRNAs alterations that occur in these patients. We further delved into how oxidative stress affects the molecular machinery that controls microRNA biogenesis. This study demonstrates that global microRNA maturation is dysregulated in human ICM and sheds new light on the causes and potential implications of redox-sensitive microRNAs (redoximiRs) dysregulation. Here, we provide evidence that both changes in the microRNA biogenesis pathway and redoximiRs, particularly regulation by hypoxia, could participate in microRNA maturation defects.
2. Materials and Methods
2.1. Tissue Samples and Patients
Myocardial tissue samples were obtained from near the apex of the left ventricle of patients diagnosed with ICM undergoing heart transplantation and control donors. Specifically, 30 samples were used for non-coding RNA sequencing (ncRNA-seq; ICM, n = 22; control, n = 8), and 23 samples were used for mRNA sequencing (mRNA-seq; ICM, n = 13; control, n = 10). Tissue samples were preserved in 0.9% NaCl at 4 °C for 4.4 ± 3 h after coronary circulation loss and were storage at −80 °C until use. A reduced time between sample receipt and stored produced higher-quality samples, as evidenced by the RNA integrity numbers of ≥9.
Patients were diagnosed based on clinical history and results from hemodynamic, electrocardiographic, Doppler echocardiography and coronary angiography studies. The ICM diagnoses were based on the following inclusion criteria: (i) there were prior documented episodes of acute myocardial infarction, (ii) the echocardiography showed normal contractility segments coexisting with other dyskinetic or akinetic segments and iii) the electrocardiography showed signs of ischemia or myocardial necrosis.
All patients were classified according to the New York Heart Association (NYHA) functional criteria and were receiving medical treatment according to the guidelines of the European Society of Cardiology [20]. They were previously diagnosed with significant comorbidities, including hypertension and type 2 diabetes. Table 1 summarizes their clinical characteristics.

Table 1.
Clinical characteristics of ischemic cardiomyopathy patients.
All controls had normal left ventricular function (ejection fraction ≥ 50%) as determined by Doppler echocardiography, and they had no history of cardiac disease. The control samples were obtained from non-diseased hearts that could not be transplanted owing to surgical reasons or blood type incompatibility. The cause of death of these donors was a cerebrovascular event or a motor vehicle accident.
The investigation conforms to the principles outlined in the Declaration of Helsinki [21] and was approved by the Ethics Committee (Biomedical Investigation Ethics Committee of La Fe University Hospital of Valencia, Spain; protocol code 2016/0320, 15 November 2016). All participants signed their written informed consent to participate in the study.
2.2. RNA Extraction and Integrity
Heart samples were homogenized in a TissueLysser LT (Qiagen). RNA extractions were performed using a Quik-RNATM miniprep plus kit (Zymo Research) for non-coding RNA sequencing (ncRNA-seq; ICM, n = 22; control, n = 8) or a PureLink™ Kit (Ambion Life Technologies) for mRNA sequencing (mRNA-seq; ICM, n = 13; control, n = 10), according to the manufacturer’s instructions. RNA was quantified using a NanoDrop1000 spectrophotometer and at Qubit 3.0 fluorimeter (Thermo Fisher Scientific). The purity and integrity of RNA samples was measured using an Agilent 2100 Bioanalyzer with the RNA 6000 Nano LabChip kit (Agilent Technologies). All samples displayed a 260/280 absorbance ratio > 2.0 and RNA integrity numbers ≥ 9.
2.3. ncRNA-Seq Analysis
The cDNA libraries were obtained following Illumina’s recommendations. Briefly, 3′ and 5′adaptors were sequentially ligated to the RNA prior to reverse transcription and cDNA generation. The cDNA was enriched using PCR to create an indexed double-stranded cDNA library, and size selection was performed using a 6% polyacrylamide gel. The quality and quantity of the libraries were analyzed using a 4200 TapeStation D1000 High-Sensitivity assay. The cDNA libraries were pooled and the pools were sequenced using paired-end sequencing (100 × 2) in the Illumina HiSeq 2500 sequencer.
The quality control of the raw data was performed using the FastQC tool. For the adapter and quality filler of raw data, trim_galore was applied [http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/] (accessed on 15 May 2020). Then, the insufficient quality reads (phred score < 20) were eliminated using the Picard Tools software [22]. RNAs predictions were estimated using HT Seq software (version 0.6.0) [23].
2.4. Mature/Immature microRNA Ratio
The raw data for each sample were mapped against the human mature and hairpin sequences contained in mirBase [24] using the bowtie algorithm [22]. The low-quality reads were filtered using a Q20 threshold. Finally, only the unique mapped reads were considered for the next analyses. The fraction of mature reads mapped versus hairpin precursors was calculated. The statistical difference of fraction between conditions was evaluated using Wilcoxon test [25].
2.5. mRNA-Seq Analysis
PolyA-RNA was isolated form 25 micrograms of total RNA using the MicroPoly(A) Purist kit (Ambion). Total Poly(A) RNA samples were used to generate whole transcriptome libraries for sequencing on the SOLiD 5500XL platform, following the manufacturer’s recommendation (Life Technologies). No RNA spike-in controls were used. Amplified cDNA quality was analyzed by the Bioanalyzer 2100 DNA 1000 kit (Agilent Technologies) and quantified using the Qubit 2.0 Fluorimeter (Invitrogen). The whole transcriptome libraries were used for making SOLiD templated beads following the SOLiD Templated Bead Preparation guide. Bead quality was estimated based on WFA (workflow analysis) parameters. The samples were sequenced using the 50,625 paired-end protocol, generating 75 nt + 35 nt (Paired-End) + 5 nt (Barcode) sequences. Quality data were measured using software SETS parameters (SOLiD Experimental Tracking System).
The initial whole transcriptome paired-end reads obtained from sequencing were mapped against the latest version of the human genome (version GRchr37/hg19) using the Life Technologies mapping algorithm (http://www.lifetechnologies.com/, accessed on 15 May 2020), version 1.3. We used the standard Bioscope parameters of version 1.3 in paired-ends and whole transcriptome analysis. For both reads, forwards and reverse, the seed was the first 25 nucleotides with a maximum of 2 mismatches allowed. The aligned records were reported in BAM/SAM format [26]. Bad quality reads (Phred score < 10) were eliminated using Picard Tools software, version 1.83 (http://broadinstitute.github.io/picard/, accessed on 15 May 2020).
The mRNA-seq data discussed in this publication were deposited in NCBI’s Gene Expression Omnibus [27] and are accessible through GEO Series accession number GSE55296 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE55296, accessed on 15 May 2020).
2.6. Identification of microRNA-mRNA and Protein–Protein Interactions
We investigated experimentally validated targets of redoximiRs, among the microRNA biogenesis-related genes differentially expressed by accessing data from the miRTarBase [28] and TaRbase [29] databases. These databases contain experimentally verified microRNA targets on coding and non-coding RNAs. Protein–protein interactions were also accessed through the STRING v11.5 software (available at https://string-db.org/, accessed on 2 February 2023). The parameters that were evaluated included experiments, databases and co-expression. Furthermore, we investigated the relationships in the expression of these molecules and we overlapped this information with the predicted target microRNAs and protein–protein interactions to construct a microRNA–target gene regulatory network.
2.7. Cell Culture
AC16 Human Cardiomyocyte Cell Line (SCC109, Merck, St. Louis, MO, USA) was cultured in DMEM/F12 medium (Gibco™, Thermo Fisher Scientific, Horsham, UK), supplemented with 2 mM L-Glutamine (Gibco™, Thermo Fisher Scientific), 12.5% fetal bovine serum (FBS, LINUS), 50 U/mL penicillin and 50 µg/mL streptomycin (Gibco™, Thermo Fisher Scientific, Horsham, UK), at 37 °C in a 5% CO2 incubator. The culture medium was replaced every two to three days until 90% cell confluence was reached and was used in the following experimental assays.
In total, 8 × 104 human cardiomyocytes per well were seeded in 6-well plates. The cells were cultured at 37 °C in hypoxic conditions (5% CO2 and 2% O2), simulating the myocardial hypoxia of ICM, or normoxic conditions as control. At 48 h, the cells were harvested and the expression of the target molecules was analyzed.
2.8. Determination of XPO5 and DICER1 Expression Levels
Total cell RNA was extracted using a Qiagen miRNeasy Mini kit (normoxia, n = 6; hypoxia, n = 6) following the manufacturer’s instructions (Qiagen) and was quantified using a NanoDrop 2000c spectrophotometer. For determination of XPO5 and DICER expression levels, complementary DNA synthesis was carried out using M-MLV reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. qPCR was performed using Taqman® Gene Expression Assays (Applied Biosystems, Thermo Fisher Scientific, Horsham, UK) in a ViiA7 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Horsham, UK) according to the manufacturer’s instructions. The following TaqMan probes were obtained from Thermo Fisher Scientific: XPO5 (Hs00382453_m1), DICER1 (Hs00229023_m1), and GAPDH (Hs99999905_m1). The average Ct of GAPDH was used to normalize XPO5 and DICER1 in each sample. The Fold Change 2−∆∆Ct method was used to compare relative expression between samples of both conditions [30].
2.9. Determination of Exportin-5 and Endoribonuclease Dicer Protein Levels
Total cellular protein (normoxia, n = 6; hypoxia, n = 5) was extracted using total protein extraction buffer (2% SDS, 10 mM EDTA, 6 mM Tris–HCl, pH 7.4) supplemented with protease inhibitors (25 µg/mL aprotinin and 10 µg/mL leupeptin). Samples were sonicated and total protein was quantified by Peterson’s modification of the micro Lowry method (Sigma-Aldrich) with bovine serum albumin (BSA) as the standard.
The protein samples for detecting Exportin-5 and Endoribonuclease Dicer were subsequently separated by Bis-Tris 4–12% polyacrylamide gels under reducing conditions. After electrophoresis, proteins were transferred to a polyvinylidene difluoride membrane (PVDF) using the iBlot Dry Blotting System (Invitrogen Ltd., Waltham, MA, USA), blocked at 4 °C overnight with 1% BSA in Tris buffer solution containing 0.05% Tween 20 and were incubated for 2 hours with the primary antibody in the same buffer. The primary detection antibodies used were anti-Exportin-5 rabbit monoclonal antibody (1:1000; ab131281), anti-Dicer1 rabbit polyclonal antibody (1:1000; ab227518) and anti-GAPDH mouse monoclonal antibody (1:2000; ab8245) as a loading control, all obtained from Abcam.
The bands were visualized using an acid phosphatase-conjugated secondary antibody and nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP, Sigma-Aldrich, St. Louis, MO, USA) substrate system. Finally, the bands were digitalized using an image analyzer (DNR Bio-Imagining Systems, Jerusalem, Israel) and quantified with the GelQuant Pro (v. 12.2) program.
2.10. Statistical Methods
Data were expressed as the mean ± standard deviation for continuous variables and as percentage values for discrete variables. The Kolmogorov–Smirnov test was applied to analyze the data distribution. Clinical characteristics of patients were compared by using Student’s t-test for continuous variables and Fisher’s exact test for discrete variables. Differential ncRNA expression analysis between conditions was assessed using the DESeq2 method [31] (version 3.4). We considered those ncRNAs with a p value (P adj) corrected by FDR ≤ 0.05 as differently expressed to avoid identification of false positives across the differential expression data [32]. Gene predictions were estimated using the cufflinks method [33] and the expression levels were calculated using the HTSeq software, version 0.5.4p323. This method eliminated the multimapped reads, and only the unique reads were considered for gene expression estimation. The edgeR method, version 3.2.4, was applied for differential expression analysis between conditions [34]. This method relies on different normalizing processes based on in-depth global samples, CG composition and length of genes. In the differential expression process, this method relies on a Poisson model to estimate the variance of the RNA-seq data for differential expressions. Significant mean differences in protein levels were analyzed by using Student’s t-test for variables with a normal distribution and Mann–Whitney U test for variables with a non-normal distribution. Pearson’s correlation coefficient was calculated to analyze the association between variables with normal distribution and Spearman’s correlation coefficient for variables with non-normal distribution. p < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software (version 20.0) for Windows (IBM SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Patient Characteristics
The study populations for each assay (ncRNA-seq and mRNA-seq) were homogeneous based on the clinical characteristics of the patients. Table 1 shows the clinical characteristics of ICM patients of each assay. All of them were men; were classified as III–IV New York Heart Association functional classes; had several comorbidities, such as hypertension (33–40%) and diabetes (42–45%); and were mainly previous current smokers (81–92%). The control group was mainly men (62%) with a mean age of 50 ± 15 years. Comorbidities and other echocardiographic data were not available for the control group, in accordance with the Spanish Organic Law on Data Protection 15/1999.
3.2. Mature microRNA Expression Changes in ICM Patients: Implication of microRNA Biogenesis
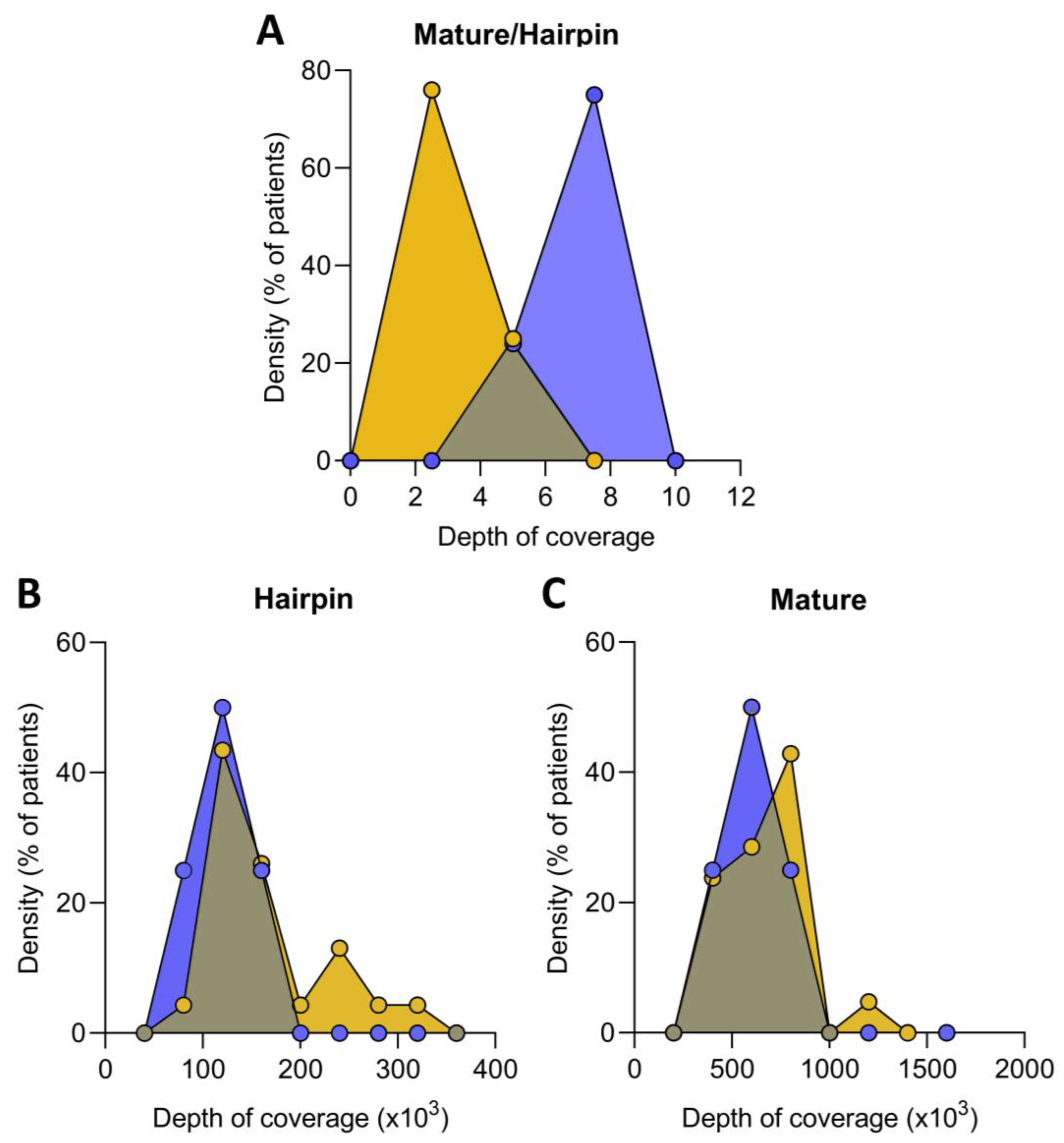
Non-coding RNA sequencing was performed to calculate the fraction of reads mapped against mature versus hairpin microRNAs to evaluate the ratio of mature and immature microRNA precursors present in the ICM patients compared to healthy controls. The mature/immature microRNA ratio in ICM patients was lower than in the control group (4.30 ± 0.94 au vs. 5.34 ± 1.07 au, p ˂ 0.05), indicating that these patients had a higher presence of immature microRNAs (Figure 1A). Indeed, we found an increase in hairpin microRNAs in ICM versus control (137,091 ± 60,204 vs. 93,887 ± 21,455 counts, p ˂ 0.05) (Figure 1B), while mature microRNAs were similar in both groups (607,612 ± 325,107 vs. 518,684 ± 202,203 counts, p ˃ 0.05) (Figure 1C).
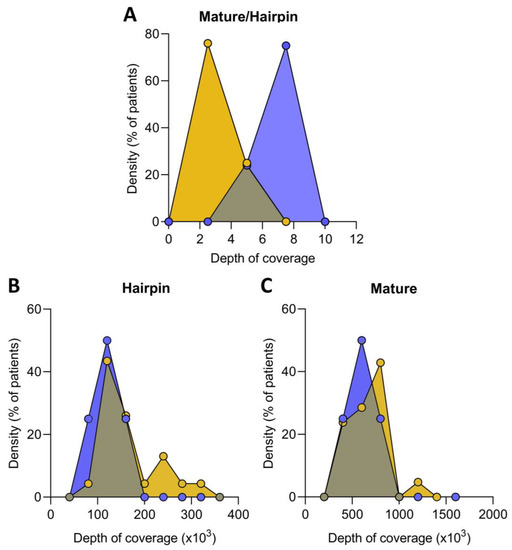
Figure 1.
Relative expression of mature and immature microRNA in ischemic cardiomyopathy (ICM, n = 22) and controls (n = 8) expressed as the ratio of mature/immature microRNA (A). Distribution of hairpin (B) and mature (C) microRNA coverage depth in ICM patients and control. Data are expressed as the fraction of reads mapped against mature microRNA versus hairpin microRNA precursors. ICM patients (orange), control subjects (blue).
Subsequently, an mRNA-seq study was performed to analyze differences in transcriptome between microRNA biogenesis-related genes from ICM and control. We focused on the differential gene expression analysis of the main microRNA mechanisms of transcription, processing, export and maturation. All genes analyzed are described in Table 2. This analysis showed that the lower amount of mature microRNA described is accompanied by a deregulation of the microRNA biogenesis process.

Table 2.
Genes involved in the microRNA biogenesis pathway.
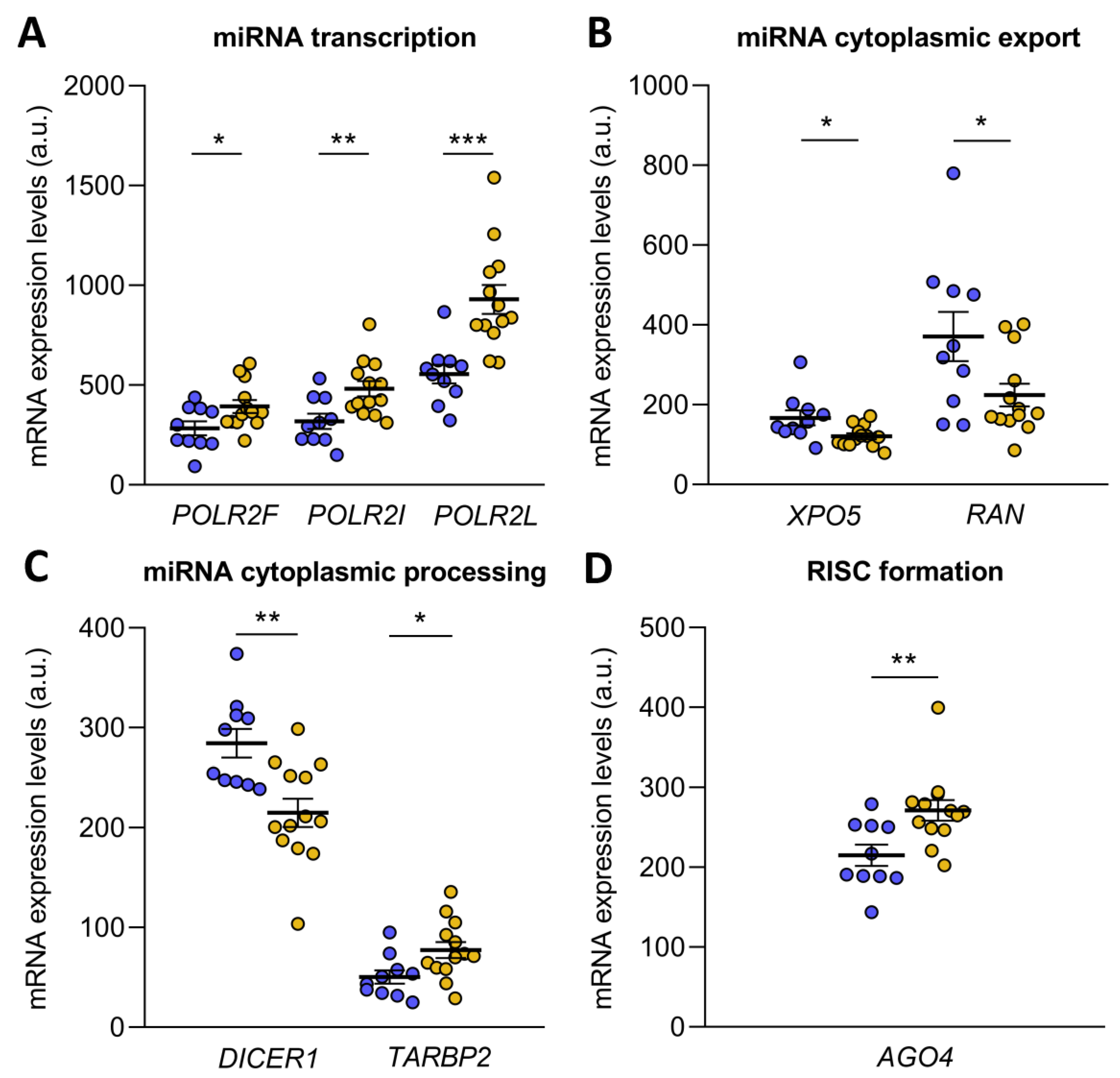
microRNA biogenesis starts with the transcription of pri-microRNA by RNA polymerase II, an enzyme that has 12 subunits, of which POLR2F (FC = 1.38, p ˂ 0.05), POLR2I (FC = 1.51, p ˂ 0.01) and POLR2L (FC = 1.67, p ˂ 0.001) were overexpressed in ICM patients (Figure 2A). Then, the pri-microRNA was cleaved to form the pre-microRNA, which was exported to the cytoplasm in an Exportin-5/RanGTP-dependent manner. Both XPO5 (FC = −1.38) and RAN (FC = −1.66) were underexpressed in ICM patients (p ˂ 0.05) (Figure 2B). Upon export to the cytoplasm, pre-microRNA was cleaved by the Endoribonuclease Dicer and TRBP, liberating a small RNA duplex. Both molecules involved in cytoplasmic pre-microRNA processing, DICER1 (FC = −1.32, p ˂ 0.01) and TARBP2 (a gene that encodes TRBP; FC = 1.54, p ˂ 0.05), were found to be deregulated in ischemic origin heart failure observed in the patients (Figure 2C). The small RNA duplex generated by the Endoribonuclease Dicer was subsequently loaded onto an AGO protein to form the RNA-induced silencing complex (RISC), the effector complex of microRNA-induced gene silencing. Of all the Argonaute proteins, only AGO4 (FC = 1.26, p ˂ 0.01) showed an overexpression in ICM patients (Figure 2D).
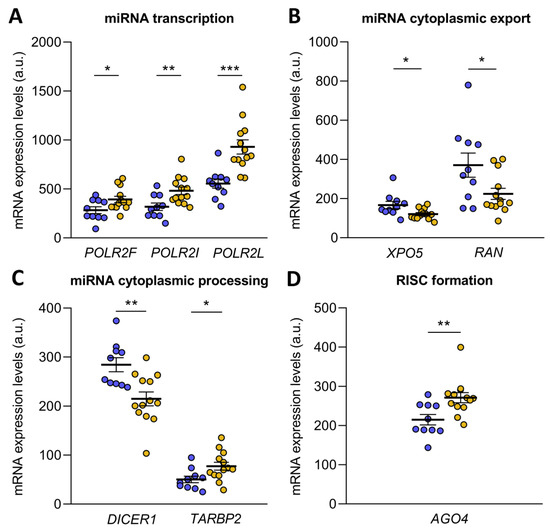
Figure 2.
Transcriptome differences in microRNA biogenesis-related genes from ischemic cardiomyopathy patients (ICM, n = 13) and control subjects (n = 10). Differential gene expression analysis of microRNA transcription (A), cytoplasmic export (B), cytoplasmic processing (C) and RNA-induced silencing complex (RISC) formation (D). Data are presented as the mean ± SEM. au, arbitrary units. ICM patients (orange), control subjects (blue). * p < 0.05, ** p < 0.01, *** p < 0.001.
3.3. Cellular Redox Homeostasis Modulation in ICM Patients: Interplay between Oxidative Stress and microRNA
Emerging evidence suggests that microRNA biogenesis can be affected by oxidative stress, and so, an exhaustive literature review was carried out in order to identify the set of microRNAs described as redoximiRs, microRNAs that participate in redox responses both as direct regulatory molecules of the post-transcriptional expression of several pathways and as indirect modulators of the redox homeostatic response, and those microRNAs whose biogenesis is directly modulated by reactive oxygen species (Table S1). The microRNA expression data obtained in the ncRNA-seq were used to compare the microRNA profile of ICM patients to the control group.
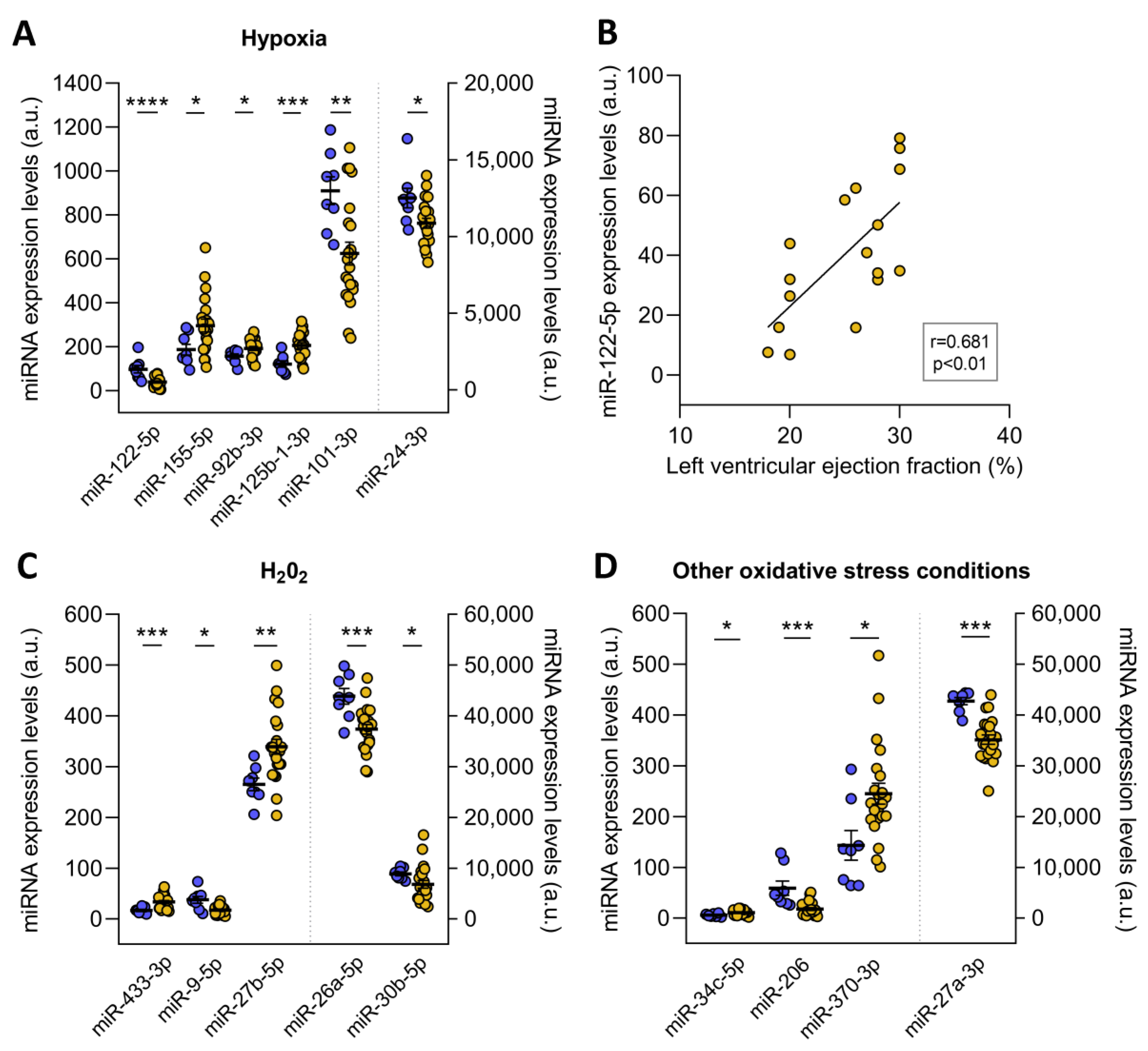
The redoximiRs that we identified as differentially expressed are involved in the regulation of ROS production, apoptosis, or DNA repair and are regulated by stress conditions such as hypoxia and H2O2 (Figure 3). On the one hand, we found altered microRNA in ICM patients that are regulated under conditions of hypoxia: miR-122-5p (FC = −2.41, p ˂ 0.001), miR-155-5p (FC = 1.59, p ˂ 0.05), miR-92b-3p (FC = 1.23, p ˂ 0.05), miR-125b-1-3p (FC = 1.69, p ˂ 0.001), miR-101-3p (FC = −1.46, p ˂ 0.01), and miR-24-3p (FC = −1.15, p ˂ 0.05) (Figure 3A). Interestingly, we observed significant correlations between miR-122-5p and left ventricular ejection fraction (r = 0.681, p ˂ 0.01) (Figure 3B). On the other hand, these patients also showed deregulated expression of the miR-433-3p (FC = 1.99, p ˂ 0.001), miR-9-5p (FC = −2.14, p ˂ 0.05), miR-27b-5p (FC = 1.28, p ˂ 0.01), miR-26a-5p (FC = −1.17, p ˂ 0.001), and miR-30b-5p (FC = −1.30, p ˂ 0.05), which are microRNAs that are H2O2-sensitive (Figure 3C). Finally, we found changes in miR-34c-5p (FC = 1.83, p ˂ 0.05), miR-206 (FC = −3.27, p ˂ 0.001), miR-370-3p (FC = 1.71, p ˂ 0.05), and miR-27a-3p (FC = −1.22, p ˂ 0.001) in ICM patients, which are redoximiRs regulated by other oxidative conditions (Figure 3D).
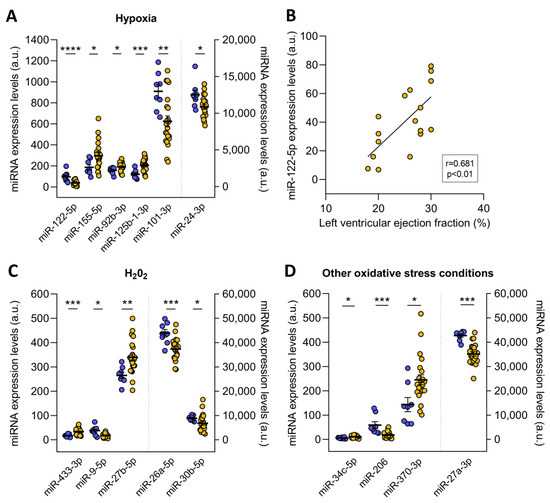
Figure 3.
RedoximiRs regulated by stress conditions and differentially expressed in patients with ischemic cardiomyopathy (ICM, n = 22) compared to control subjects (n = 8). RedoximiRs regulated by hypoxia (A). Relationship of miR-122-5p with left ventricular ejection fraction (B). RedoximiRs regulated by the presence of H2O2 (C) and redoximiRs regulated by other oxidative stress conditions (D). Data are presented as the mean ± SEM. au, arbitrary units. ICM patients (orange), control (blue). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.4. Construction of the microRNA−mRNA Regulatory Network
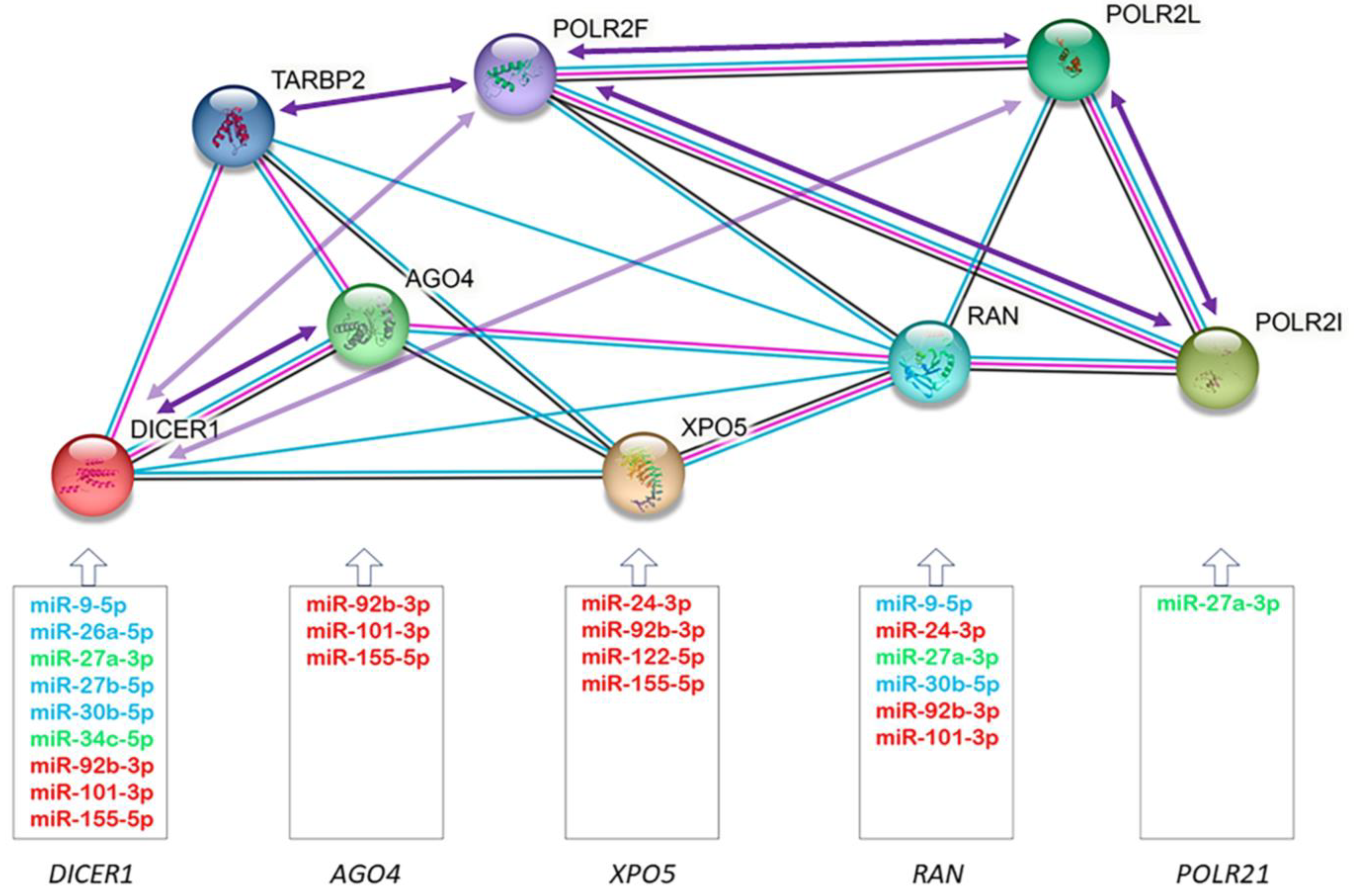
Through the miRTarBase and TaRbase databases, we established a microRNA-mRNA regulatory network, where we observed that 73% of redoximiRs altered in the ICM patients regulated one or more of the differentially expressed genes of the microRNA biogenesis pathway (Figure 4). This figure also shows a protein–protein interaction network’s functional enrichment analysis using the String tool, the correlations in the expression of the redoximiRs (Table 3) and the genes of the microRNA biogenesis pathway (Table 4). It should be noted that the export of microRNAs to the cytoplasm (XPO5 and RAN) and their cytoplasmic processing (DICER1) were regulated by more than 60% of redoximiRs in both cases, while only 20% of redoximiRs regulated the formation of RISC (AGO4) and 1% regulated the transcription of pri-microRNA by RNA polymerase II (POLR2I).
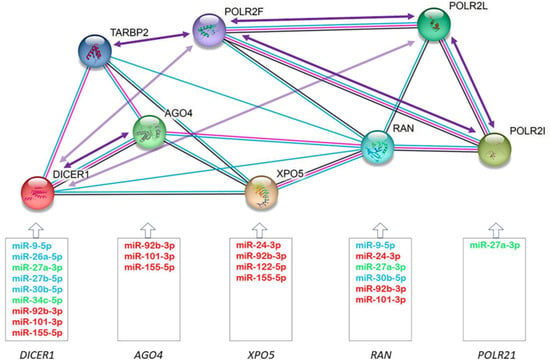
Figure 4.
Schematic of the regulatory network showing the validated targets of redoximiRs (miRTarBAse and TaRbase) among differentially expressed genes, protein–protein interactions (STRING), and correlations in the expression of the genes of the microRNA biogenesis pathway. Experimentally validated redoximiRs targets obtained from the miRTarBase and TaRbase databases are shown: redoximiRs regulated under conditions of hypoxia (red), H2O2 (light blue) and other conditions (light green). Edges represent protein–protein associations: known interactions from curated databases (light blue) and experimentally determined (pink) and co-expression (black). Arrows represent positive (dark purple) or negative (light purple) correlations between mRNA expressions.

Table 3.
Relationships between altered redoximiRs in patients with ischemic cardiomyopathy.

Table 4.
Relationships between differentially expressed genes of the microRNA biogenesis pathway in ischemic cardiomyopathy.
3.5. Effect of Hypoxia on microRNA Biogenesis: Export to the Cytoplasm and Cytoplasmic Processing
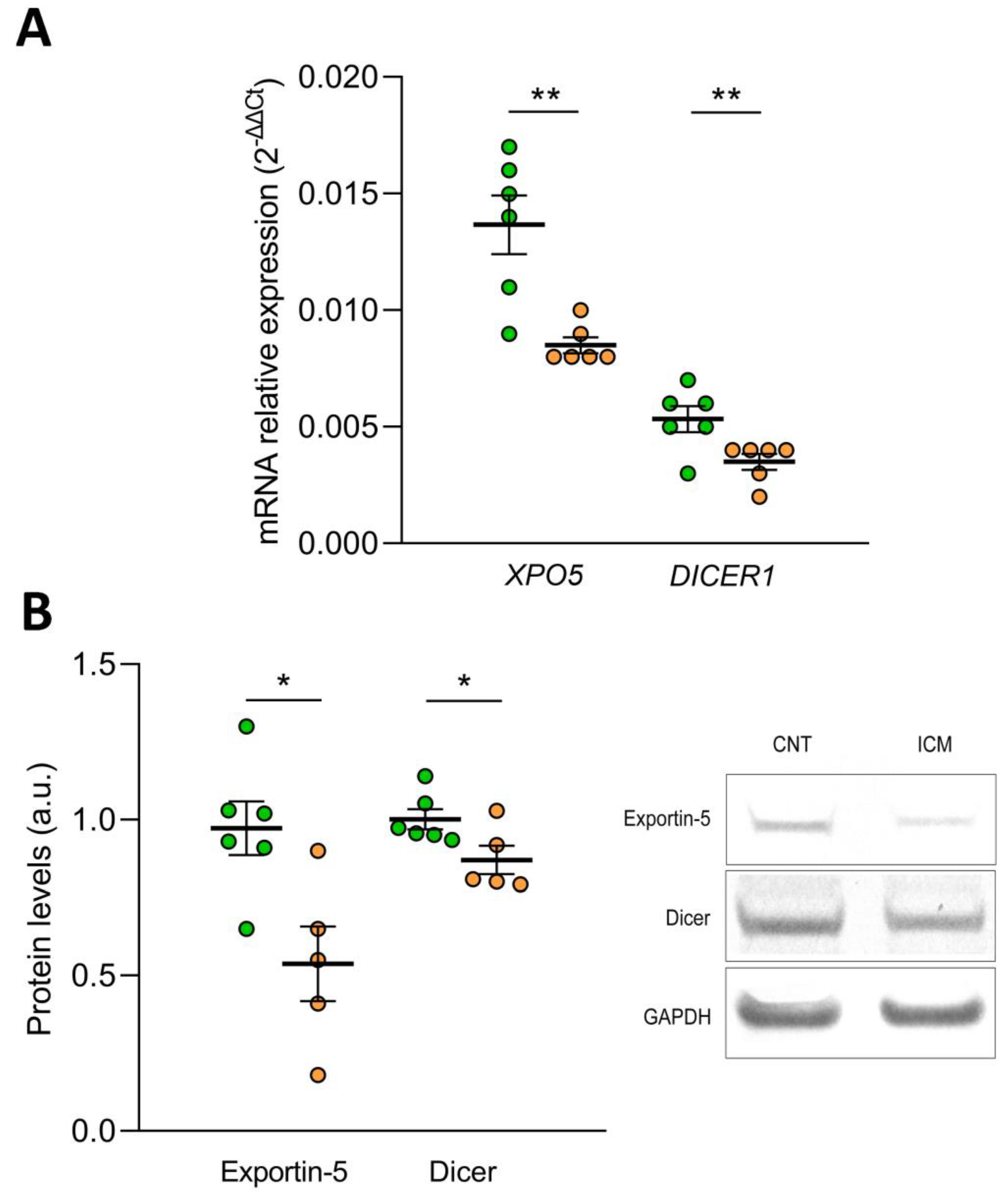
To elucidate the effect of oxidative stress on the biogenesis of microRNAs, we determined the mRNA and protein expression levels of Exportin-5 and the Endoribonuclease Dicer in the AC16 cell line under hypoxic conditions. We found that after 48 h of hypoxia, the mRNA levels of XPO5 (FC= −1.65) and DICER1 (FC = −1.55) were reduced in the human cardiomyocyte cell line AC16 (p ˂ 0.01) (Figure 5A). In addition, the protein levels of both molecules were also affected by hypoxia, both Exportin-5 (FC = −1.81) and Endoribonuclease Dicer (FC = −1.15) were downregulated (p ˂ 0.05) (Figure 5B).
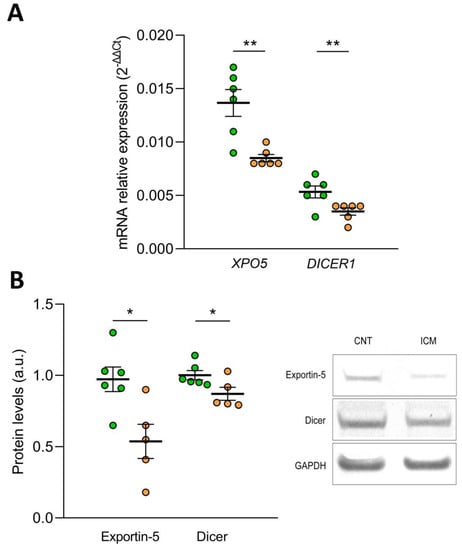
Figure 5.
Effect of hypoxia on the expression of Exportin-5/XPO5 and Endoribonuclease Dicer/DICER1 in human cardiomyocyte cell line AC16 at mRNA by RT-qPCR (A) and protein level by Western Blot (B). Data are presented as the mean ± SEM. au, arbitrary units. AC16 under hypoxic conditions (n = 6, orange). AC16 under normoxic conditions (n = 6, green). * p < 0.05, ** p < 0.01.
4. Discussion
Numerous studies described abnormal expression levels of microRNA in ICM that involved differential expression of their target genes, implicating them in the pathophysiology of this syndrome [9,10,35]. However, little is known about the underlying causes of microRNA disruption in ICM and whether microRNA maturation is compromised. For this purpose, we investigated the state of microRNA maturity and the expression of the major microRNA biogenesis pathway components. This study demonstrated that global microRNA maturation is dysregulated in human ICM and shed new light on the causes and potential implications of redoximiRs dysregulation. We provided evidence that both changes in the microRNA biogenesis pathway and in redoximiRs, particularly the regulation by hypoxia, could participate in microRNA maturation defects.
To our knowledge, this is the first study that globally analyzed the state of microRNA maturity in ICM or in any pathology in general, while studies generally focused on the analysis of a handful of microRNAs [13]. Our analysis showed a global lower amount of mature microRNA in ICM patients, consistent with previous, mainly cancer, publications based on microRNA clusters [13]. Several authors proposed that in cancer mutation or aberrant expression of any component of the microRNA biogenesis machinery could lead to abnormal microRNA expression [36]. However, although some specific maturation molecules were independently studied, the whole biogenesis pathway was not previously studied. Our cardiac analysis showed that the overall lower amount of mature microRNA we described in ICM was accompanied by a deregulation of the microRNA biogenesis process at four levels: transcription of pri-microRNA by RNA polymerase II, export of pre-microRNA to the cytoplasm, processing of cytoplasmic pre-microRNA and formation of the RISC complex. Additionally, the expression levels of several microRNAs can also be influenced by oxidative stress, an important contributing factor to many diseases including ICM. In turn, microRNAs may control the expression of redox sensors, change important components of the cellular antioxidants and influence DNA repair mechanism [15]. In patients with ICM, we noticed that many of these microRNAs, known as redoximiRs, presented an alteration in their expression levels, mainly regulated by hypoxia, and that most of them targeted one or more of the differentially expressed genes of the microRNA biogenesis pathway.
The transcription of microRNA genes was carried out by RNA polymerase II, a 12-subunit enzyme essential in the transcription process. Increased expression of its subunits may reflect a higher transcriptional activity, and although there are few studies associating its altered expression with disease pathophysiology, beyond elucidating the understanding of the transcription process, it appeared to be disease-specific [37,38]. As far as we know, the relationship between polymerase alteration and ICM was not previously reported. The overexpression of POLR2F, POLR2I and POLR2I that we observed in ICM also showed a good relationship between the three subunits, which could be a response to a lower overall maturation of microRNAs. This premise was also supported by the relationship shown by the expression of these subunits with the expression of the cytoplasmic pre-microRNA processing molecules DICER1 and TARBP2 in ICM patients. DICER1 plays an important role in the maintenance of the cellular response to hypoxia, significantly reducing its protein levels under hypoxic conditions [17], as we observed in the AC16 cell line. Interestingly, DICER1 knockdown, which was previously associated with the development of heart failure [39], is critical for canonical microRNA biogenesis [13]. Therefore, we observed an underexpression in ICM patients with marked reduction in microRNA maturation. Nevertheless, although we observed a dysregulation of TARBP2, it appeared to have no significant effect on overall microRNA abundance but affected Dicer processing in a subset of pre-microRNAs [40].
Regardless of whether there may be an increase or decrease in microRNA transcription, its transport from the nucleus to the cytoplasm is necessary to continue cytoplasmic processing to produce the mature microRNAs, considering it a rate-limiting step during microRNA biogenesis. Any alteration of XPO5 can impair its nucleus-to-cytoplasmic transport ability, leading to downregulation of mature microRNAs [13,41]. We showed a reduction in both XPO5 and RAN in ICM patients, and confirmed that oxidative stress also regulates microRNA export, observing in AC16 a decrease in both XPO5 and Exportin-5 levels under hypoxic conditions. AC16 are stable proliferating cell lines, which exhibit ultrastructural, molecular genetics and immunocytochemical characteristics of human cardiomyocytes. They are widely used as an in vitro model to study the function and expression of human cardiac genes, during normal and pathological conditions at the cellular, organellar and molecular levels [42].
The redoximiRs that we found to be differentially expressed are controlled by stress conditions, namely hypoxia, and are involved in the regulation of ROS generation, apoptosis, or DNA repair. Furthermore, several validated targets of these differentially expressed microRNAs correspond to genes from the microRNA biogenesis pathway. Thus, the links between ICM hypoxia and microRNA expression raise the possibility of a general effect of hypoxia on microRNA biogenesis and function. In this sense, altered microRNA biogenesis could modify the availability and functionality of microRNA becoming a causal factor of the adverse effects of hypoxia in ICM patients. Accordingly, we observed a strong relationship between miR-122-5p expression, a microRNA regulated by hypoxia and ventricular dysfunction in ICM patients.
The wide variation in subjects and their treatments, some of which may affect the results, is a major limitation of research that focuses on cardiac tissues from end-stage human heart failure. Additionally, the samples analyzed were mainly from men, due to the higher prevalence of ICM. Consequently, our research population had a homogeneous etiology, and all of the patients examined in this study were receiving medical treatment in accordance with the recommendations of the European Society of Cardiology [20]. In addition, it is essential to underline the significance of conducting this investigation on a sizable number of ICM samples from explanted human hearts undergoing cardiac transplantation and control donors. AC16 Human Cardiomyocyte Cell Line is a proliferating cell line that was derived from the fusion of primary cells from adult human ventricular heart tissues with SV40 transformed, uridine auxotroph human fibroblasts, devoid of mitochondrial DNA. Although in vitro data need to be interpreted with caution, these analyses provide valuable information as a first step to provide concrete answers before further in vivo studies can be performed.
5. Conclusions
We showed that microRNA biogenesis is strongly compromised in the human ICM. We evidenced a decrease in microRNA maturation mainly linked to a decrease in XPO5 mediated pre-microRNA export and a lower DICER1-mediated processing, together with a general effect of hypoxia through deregulation of the biogenesis pathway and redoximiRs. Consequently, the availability and functionality of microRNA could become a causal factor of the adverse effects of hypoxia in ICM patients, highlighting the role of miR-122-5p. Since most human protein-coding genes are likely under microRNA control, manipulation of microRNA activity may influence the course of a disease, improving health-related outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12071337/s1, Table S1: microRNAs regulating antioxidant responses and/or being regulated by ROS (redoximiRs) analyzed. References [16,19,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90] are cited in the supplementary materials.
Author Contributions
Conceptualization, E.T. and E.R.-L.; methodology, L.P.-C., I.G.-E. and M.G.-M.; validation, E.T.; formal analysis, L.P.-C., E.T. and J.C.T.; investigation, E.T.; resources, L.M.-D.; writing—original draft preparation, L.P.-C., M.G.-M., M.P. and E.T.; writing—review and editing, L.P.-C., I.G.-E., M.G.-M., J.C.T., S.F.-B., A.A.-H., F.L., L.M.-D., M.P., E.T. and E.R.-L.; supervision. E.T.; funding acquisition, L.M.-D., E.T. and E.R.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institute of Health “Fondo de Investigaciones Sanitarias del Instituto de Salud Carlos III” [Projects: PI20/01469 and PI20/00071 co-funded by European Union; Miguel Servet contracts: CP18/00145 co-funded by European Union, European Social Fund (ESF) “The ESF invests in your future” and CP21/00041 co-funded by European Union; contract FI21/00186 and FI21/00034]. “Consorcio Centro de Investigación Biomédica en Red, M.P.” [CIBERCV, under Grant CB16/11/00261].
Institutional Review Board Statement
The investigation was approved by the Ethics Committee (Biomedical Investigation Ethics Committee of La Fe University Hospital of Valencia, Spain; protocol code 2016/0320, 15 November 2016) and all participants signed a written informed consent to participate in the study.
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
The mRNA-seq data discussed in this publication were deposited in NCBI’s Gene Expression Omnibus [27] and are accessible through GEO Series Accession Number GSE55296 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE55296, accessed on 28 April 2014).
Acknowledgments
The authors are grateful to Manuel Vázquez-Carrera from School of Pharmacy and Food Sciences of University of Barcelona for their technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Moran, A.E.; Forouzanfar, M.H.; Roth, G.A.; Mensah, G.A.; Ezzati, M.; Murray, C.J.; Naghavi, M. Temporal Trends in Ischemic Heart Disease Mortality in 21 World Regions, 1980 to 2010: The Global Burden of Disease 2010 study. Circulation 2014, 129, 1483–1492. [Google Scholar] [CrossRef]
- Tarazón, E.; Pérez-Carrillo, L.; Giménez-Escamilla, I.; Ramos-Castellanos, P.; Martínez-Dolz, L.; Portolés, M.; Roselló-Lletí, E. Relationships of Telomere Homeostasis with Oxidative Stress and Cardiac Dysfunction in Human Ischaemic Hearts. Antioxidants 2021, 10, 1750. [Google Scholar] [CrossRef]
- Gil-Cayuela, C.; Rivera, M.; Ortega, A.; Tarazón, E.; Triviño, J.C.; Lago, F.; González-Juanatey, J.R.; Almenar, L.; Martínez-Dolz, L.; Portolés, M. RNA Sequencing Analysis Identifies New Human Collagen Genes Involved in Cardiac Remodeling. J. Am. Coll. Cardiol. 2015, 65, 1265–1267. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Ye, S.Q.; Irizarry, R.A.; Minhas, K.M.; Edness, G.; Conte, J.V.; Parmigiani, G.; Miller, L.W.; Chen, Y.; Hall, J.L.; et al. Identification of a Gene Expression Profile That Differentiates between Ischemic and Nonischemic Cardiomyopathy. Circulation 2004, 110, 3444–3451. [Google Scholar] [CrossRef]
- Cortés, R.; Roselló-Lletí, E.; Rivera, M.; Martínez-Dolz, L.; Salvador, A.; Azorín, I.; Portolés, M. Influence of heart failure on nucleocytoplasmic transport in human cardiomyocytes. Cardiovasc. Res. 2009, 85, 464–472. [Google Scholar] [CrossRef]
- Miranda, K.C.; Huynh, T.; Tay, Y.; Ang, Y.-S.; Tam, W.-L.; Thomson, A.M.; Lim, B.; Rigoutsos, I. A Pattern-Based Method for the Identification of MicroRNA Binding Sites and Their Corresponding Heteroduplexes. Cell 2006, 126, 1203–1217. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Wong, L.L.; Wang, J.; Liew, O.W.; Richards, A.M.; Chen, Y.-T. MicroRNA and Heart Failure. Int. J. Mol. Sci. 2016, 17, 502. [Google Scholar] [CrossRef]
- Shen, N.-N.; Wang, J.-L.; Fu, Y.-P. The microRNA Expression Profiling in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9. [Google Scholar] [CrossRef]
- Lujambio, A.; Lowe, S.W. The microcosmos of cancer. Nature 2012, 482, 347–355. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, B.; Kim, V.N. Re-evaluation of the roles of DROSHA, Export in 5, and DICER in microRNA biogenesis. Proc. Natl. Acad. Sci. USA 2016, 113, E1881–E1889. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the regulation of cellular redox status and its implications in myocardial ischemia-reperfusion injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Wang, X.; Tang, Y.; Zhang, W.; Cui, B.; Liu, Q.; Xing, L. Dicer mediating the expression of miR-143 and miR-155 regulates hexokinase II associated cellular response to hypoxia. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2014, 307, L829–L837. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.J.D.; Metcalf, J.L.; Yan, M.S.; Turgeon, P.J.; Wang, J.J.; Chalsev, M.; Petruzziello-Pellegrini, T.N.; Tsui, A.K.Y.; He, J.Z.; Dhamko, H.; et al. Functional Importance of Dicer Protein in the Adaptive Cellular Response to Hypoxia. J. Biol. Chem. 2012, 287, 29003–29020. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I.; Yamagata, K.; Sugimoto, K.; Iwamoto, T.; Kato, S.; Miyazono, K. Modulation of microRNA processing by p53. Nature 2009, 460, 529–533. [Google Scholar] [CrossRef]
- Berardino, B.G.; Fesser, E.A.; Cánepa, E.T. Perinatal protein malnutrition alters expression of miRNA biogenesis genes Xpo5 and Ago2 in mice brain. Neurosci. Lett. 2017, 647, 38–44. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar]
- Macrae, D.J. The Council for International Organizations and Medical Sciences (CIOMS) Guidelines on Ethics of Clinical Trials. Proc. Am. Thorac. Soc. 2007, 4, 176–179; discussion 178–179. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Bürkner, P.-C.; Doebler, P.; Holling, H. Optimal design of the Wilcoxon-Mann-Whitney-test. Biom. J. 2016, 59, 25–40. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Chou, C.H.; Shrestha, S.; Yang, C.D.; Chang, N.W.; Lin, Y.L.; Liao, K.W.; Huang, W.C.; Sun, T.H.; Tu, S.J.; Lee, W.H.; et al. miRTarBase update 2018: A resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018, 46, D296–D302. [Google Scholar] [CrossRef]
- Karagkouni, D.; Paraskevopoulou, M.D.; Chatzopoulos, S.; Vlachos, I.S.; Tastsoglou, S.; Kanellos, I.; Papadimitriou, D.; Kavakiotis, I.; Maniou, S.; Skoufos, G.; et al. DIANA-TarBase v8: A decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res. 2018, 46, D239–D245. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Kong, A.S.-Y.; Lai, K.-S.; Lim, S.-H.E.; Sivalingam, S.; Loh, J.-Y.; Maran, S. miRNA in Ischemic Heart Disease and Its Potential as Biomarkers: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 9001. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Xu, J.; Wu, B.; Wu, S.; Zhang, Y.; Sun, Y.; Zhang, N. Comprehensive Bioinformatics Analysis Identifies POLR2I as a Key Gene in the Pathogenesis of Hypertensive Nephropathy. Front. Genet. 2021, 12, 698570. [Google Scholar] [CrossRef] [PubMed]
- Antonacopoulou, A.G.; Grivas, P.D.; Skarlas, L.; Kalofonos, M.; Scopa, C.D.; Kalofonos, H.P. POLR2F, ATP6V0A1 and PRNP expression in colorectal cancer: New molecules with prognostic significance? Anticancer Res. 2008, 28, 1221–1227. [Google Scholar]
- Chen, J.-F.; Murchison, E.P.; Tang, R.; Callis, T.E.; Tatsuguchi, M.; Deng, Z.; Rojas, M.; Hammond, S.M.; Schneider, M.D.; Selzman, C.H.; et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. USA 2008, 105, 2111–2116. [Google Scholar] [CrossRef]
- Kim, Y.; Yeo, J.; Lee, J.H.; Cho, J.; Seo, D.; Kim, J.-S.; Kim, V.N. Deletion of Human tarbp2 Reveals Cellular MicroRNA Targets and Cell-Cycle Function of TRBP. Cell Rep. 2014, 9, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; He, J.; Pu, W.; Peng, Y. The Role of Exportin-5 in MicroRNA Biogenesis and Cancer. Genom. Proteom. Bioinform. 2018, 16, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Dong, X.; Dong, C.; Hou, G.; Liu, W.; Jiang, X.; Xin, Y. LncRNA MHRT Prevents Angiotensin II-Induced Myocardial Oxidative Stress and NLRP3 Inflammasome via Nrf2 Activation. Antioxidants 2023, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, K.S.; Lee, D.K.; Kim, J.; Kwak, S.N.; Ha, K.S.; Choe, J.; Won, M.H.; Cho, B.R.; Jeoung, D.; et al. Hypoxia-responsive microRNA-101 promotes angiogenesis via heme oxygenase-1/vascular endothelial growth factor axis by targeting cullin 3. Antioxid. Redox Signal. 2014, 21, 2469–2482. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, L.; Wang, F.; Wang, Y.; Huo, X.S.; Yin, Y.X.; Wang, Y.Q.; Zhang, L.; Sun, S.H. Modulation of the unfolded protein response is the core of microRNA-122-involved sensitivity to chemotherapy in hepatocellular carcinoma. Neoplasia 2011, 13, 590–600. [Google Scholar] [CrossRef]
- Zhi, F.; Shao, N.; Xue, L.; Xu, Y.; Kang, X.; Yang, Y.; Xia, Y. Characteristic MicroRNA Expression Induced by delta-Opioid Receptor Activation in the Rat Liver Under Prolonged Hypoxia. Cell Physiol. Biochem. 2017, 44, 2296–2309. [Google Scholar] [CrossRef]
- Varga, Z.V.; Zvara, A.; Farago, N.; Kocsis, G.F.; Pipicz, M.; Gaspar, R.; Bencsik, P.; Gorbe, A.; Csonka, C.; Puskas, L.G.; et al. MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: ProtectomiRs. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H216–H227. [Google Scholar] [CrossRef]
- Szabo, M.R.; Gaspar, R.; Pipicz, M.; Zsindely, N.; Dioszegi, P.; Sarkozy, M.; Bodai, L.; Csont, T. Hypercholesterolemia Interferes with Induction of miR-125b-1-3p in Preconditioned Hearts. Int. J. Mol. Sci. 2020, 21, 3744. [Google Scholar] [CrossRef]
- Caggiano, R.; Cattaneo, F.; Moltedo, O.; Esposito, G.; Perrino, C.; Trimarco, B.; Ammendola, R.; Faraonio, R. miR-128 Is Implicated in Stress Responses by Targeting MAFG in Skeletal Muscle Cells. Oxid. Med. Cell Longev. 2017, 2017, 9308310. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Li, W.; Zheng, H. miR-130a can predict response to temozolomide in patients with glioblastoma multiforme, independently of O6-methylguanine-DNA methyltransferase. J. Transl. Med. 2015, 13, 69. [Google Scholar] [CrossRef]
- Liu, X.; Tong, Z.; Chen, K.; Hu, X.; Jin, H.; Hou, M. The Role of miRNA-132 against Apoptosis and Oxidative Stress in Heart Failure. BioMed Res. Int. 2018, 2018, 3452748. [Google Scholar] [CrossRef]
- Brabletz, S.; Brabletz, T. The ZEB/miR-200 feedback loop--a motor of cellular plasticity in development and cancer? EMBO Rep. 2010, 11, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Sangokoya, C.; Telen, M.J.; Chi, J.T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood 2010, 116, 4338–4348. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Meng, F.; Xue, N.; Pang, G.; Wang, Q.; Ma, H. Inducible miR-145 expression by HIF-1a protects cardiomyocytes against apoptosis via regulating SGK1 in simulated myocardial infarction hypoxic microenvironment. Cardiol. J. 2018, 25, 268–278. [Google Scholar] [PubMed]
- Wang, Q.; Chen, W.; Bai, L.; Chen, W.; Padilla, M.T.; Lin, A.S.; Shi, S.; Wang, X.; Lin, Y. Receptor-interacting protein 1 increases chemoresistance by maintaining inhibitor of apoptosis protein levels and reducing reactive oxygen species through a microRNA-146a-mediated catalase pathway. J. Biol. Chem. 2014, 289, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, M.; Riar, A.K.; Rathinam, M.L.; Vedpathak, D.; Henderson, G.; Mahimainathan, L. Hydrogen peroxide responsive miR153 targets Nrf2/ARE cytoprotection in paraquat induced dopaminergic neurotoxicity. Toxicol. Lett. 2014, 228, 179–191. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Gao, X.G. microRNA-181a is upregulated in human atherosclerosis plaques and involves in the oxidative stress-induced endothelial cell dysfunction through direct targeting Bcl-2. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3092–3100. [Google Scholar]
- Zhang, D.; Lee, H.; Cao, Y.; Dela Cruz, C.S.; Jin, Y. miR-185 mediates lung epithelial cell death after oxidative stress. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L700–L710. [Google Scholar] [CrossRef]
- Yu, S.; Lu, Y.; Zong, M.; Tan, Q.; Fan, L. Hypoxia-induced miR-191-C/EBPbeta signaling regulates cell proliferation and apoptosis of fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 78. [Google Scholar] [CrossRef]
- Hou, W.; Tian, Q.; Zheng, J.; Bonkovsky, H.L. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology 2010, 51, 1494–1504. [Google Scholar] [CrossRef]
- Rane, S.; He, M.; Sayed, D.; Vashistha, H.; Malhotra, A.; Sadoshima, J.; Vatner, D.E.; Vatner, S.F.; Abdellatif, M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ. Res. 2009, 104, 879–886. [Google Scholar] [CrossRef]
- Eades, G.; Yang, M.; Yao, Y.; Zhang, Y.; Zhou, Q. miR-200a regulates Nrf2 activation by targeting Keap1 mRNA in breast cancer cells. J. Biol. Chem. 2011, 286, 40725–40733. [Google Scholar] [CrossRef]
- Riehle, C.; Sieweke, J.T.; Bakshi, S.; Ha, C.M.; Junker Udesen, N.L.; Moller-Helgestad, O.K.; Froese, N.; Berg Ravn, H.; Bahre, H.; Geffers, R.; et al. miRNA-200b-A Potential Biomarker Identified in a Porcine Model of Cardiogenic Shock and Mechanical Unloading. Front. Cardiovasc. Med. 2022, 9, 881067. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, S.; Geng, Y.; Xue, J.; Wang, Z.; Xie, X.; Wang, J.; Zhang, S.; Hou, Y. MicroRNA profiling of atrial fibrillation in canines: miR-206 modulates intrinsic cardiac autonomic nerve remodeling by regulating SOD1. PLoS ONE 2015, 10, e0122674. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Li, L.; Kim, I.K.; Sun, P.; Gupta, S. NF-kappaB mediated miR-21 regulation in cardiomyocytes apoptosis under oxidative stress. Free Radic. Res. 2014, 48, 282–291. [Google Scholar] [CrossRef]
- Turunen, T.A.; Roberts, T.C.; Laitinen, P.; Vaananen, M.A.; Korhonen, P.; Malm, T.; Yla-Herttuala, S.; Turunen, M.P. Changes in nuclear and cytoplasmic microRNA distribution in response to hypoxic stress. Sci. Rep. 2019, 9, 10332. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wu, J.; Pan, C.; Wang, H.; Ying, X.; Zhou, Y.; Yu, H.; Zuo, Y.; Pan, Z.; Liu, R.Y.; et al. Genetic and epigenetic down-regulation of microRNA-212 promotes colorectal tumor metastasis via dysregulation of MnSOD. Gastroenterology 2013, 145, 426–436. [Google Scholar] [CrossRef]
- Liu, W.; Zabirnyk, O.; Wang, H.; Shiao, Y.H.; Nickerson, M.L.; Khalil, S.; Anderson, L.M.; Perantoni, A.O.; Phang, J.M. miR-23b targets proline oxidase, a novel tumor suppressor protein in renal cancer. Oncogene 2010, 29, 4914–4924. [Google Scholar] [CrossRef]
- Fiedler, J.; Stohr, A.; Gupta, S.K.; Hartmann, D.; Holzmann, A.; Just, A.; Hansen, A.; Hilfiker-Kleiner, D.; Eschenhagen, T.; Thum, T. Functional microRNA library screening identifies the hypoxamir miR-24 as a potent regulator of smooth muscle cell proliferation and vascularization. Antioxid. Redox Signal. 2014, 21, 1167–1176. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, W.; Guo, S.; Jian, Z.; Li, S.; Li, K.; Ge, R.; Dai, W.; Wang, G.; Gao, T.; et al. Oxidative stress-induced overexpression of miR-25: The mechanism underlying the degeneration of melanocytes in vitiligo. Cell Death Differ. 2016, 23, 496–508. [Google Scholar] [CrossRef]
- Peng, J.; He, X.; Zhang, L.; Liu, P. MicroRNA-26a protects vascular smooth muscle cells against H2O2-induced injury through activation of the PTEN/AKT/mTOR pathway. Int. J. Mol. Med. 2018, 42, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, T.W.; Zhou, Y.; Peng, H.; Liu, T.; Zandi, E.; Martinez-Chantar, M.L.; Mato, J.M.; Lu, S.C. Activation of a novel c-Myc-miR27-prohibitin 1 circuitry in cholestatic liver injury inhibits glutathione synthesis in mice. Antioxid. Redox Signal. 2015, 22, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Thulasingam, S.; Massilamany, C.; Gangaplara, A.; Dai, H.; Yarbaeva, S.; Subramaniam, S.; Riethoven, J.J.; Eudy, J.; Lou, M.; Reddy, J. miR-27b*, an oxidative stress-responsive microRNA modulates nuclear factor-kB pathway in RAW 264.7 cells. Mol. Cell Biochem. 2011, 352, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; Chun, E.; Howell, J.C.; Sengupta, T.; Chen, D.; Kim, H. MicroRNA-30b-mediated regulation of catalase expression in human ARPE-19 cells. PLoS ONE 2012, 7, e42542. [Google Scholar] [CrossRef]
- Kao, Y.Y.; Chou, C.H.; Yeh, L.Y.; Chen, Y.F.; Chang, K.W.; Liu, C.J.; Fan Chiang, C.Y.; Lin, S.C. MicroRNA miR-31 targets SIRT3 to disrupt mitochondrial activity and increase oxidative stress in oral carcinoma. Cancer Lett. 2019, 456, 40–48. [Google Scholar] [CrossRef]
- Li, N.; Muthusamy, S.; Liang, R.; Sarojini, H.; Wang, E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech. Ageing Dev. 2011, 132, 75–85. [Google Scholar] [CrossRef]
- Minones-Moyano, E.; Porta, S.; Escaramis, G.; Rabionet, R.; Iraola, S.; Kagerbauer, B.; Espinosa-Parrilla, Y.; Ferrer, I.; Estivill, X.; Marti, E. MicroRNA profiling of Parkinson's disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum. Mol. Genet 2011, 20, 3067–3078. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, X.; Sun, J.; Li, M.; Ma, J.; Ge, L. miR-361-3p mitigates hypoxia-induced cardiomyocyte injury via targeting apoptosis initiators caspase-2/-8/-9. Vitr. Cell Dev. Biol. Anim. 2022, 58, 116–123. [Google Scholar] [CrossRef]
- Gao, Y.T.; Chen, X.B.; Liu, H.L. Up-regulation of miR-370-3p restores glioblastoma multiforme sensitivity to temozolomide by influencing MGMT expression. Sci. Rep. 2016, 6, 32972. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, S.H.; Kim, M.J.; Lee, Y.M. MicroRNA-382 induced by HIF-1alpha is an angiogenic miR targeting the tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res. 2014, 42, 8062–8072. [Google Scholar] [CrossRef]
- Ghosh, G.; Subramanian, I.V.; Adhikari, N.; Zhang, X.; Joshi, H.P.; Basi, D.; Chandrashekhar, Y.S.; Hall, J.L.; Roy, S.; Zeng, Y.; et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J. Clin. Investig. 2010, 120, 4141–4154. [Google Scholar] [CrossRef] [PubMed]
- Bartoszewska, S.; Kochan, K.; Piotrowski, A.; Kamysz, W.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1alpha expression in human endothelial cells through a negative feedback loop. FASEB J. 2015, 29, 1467–1479. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Fierro-Fernandez, M.; Sanchez-Gomez, F.; Rodriguez-Pascual, F.; Alique, M.; Ruiz-Ortega, M.; Beraza, N.; Martinez-Chantar, M.L.; Fernandez-Hernando, C.; Lamas, S. Targeting of Gamma-Glutamyl-Cysteine Ligase by miR-433 Reduces Glutathione Biosynthesis and Promotes TGF-beta-Dependent Fibrogenesis. Antioxid. Redox Signal. 2015, 23, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Shi, Y.P.; He, H.J.; Wang, Y.H.; Chen, T.; Yang, L.W.; Yang, T.; Chen, J.; Cao, J.; Yao, W.M.; et al. MiR-4673 Modulates Paclitaxel-Induced Oxidative Stress and Loss of Mitochondrial Membrane Potential by Targeting 8-Oxoguanine-DNA Glycosylase-1. Cell Physiol. Biochem. 2017, 42, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Ren, X.; Zhang, X.; Luo, Y.; Wang, G.; Huang, K.; Feng, S.; Bao, X.; Huang, K.; He, X.; et al. Selective killing of lung cancer cells by miRNA-506 molecule through inhibiting NF-kappaB p65 to evoke reactive oxygen species generation and p53 activation. Oncogene 2015, 34, 691–703. [Google Scholar] [CrossRef]
- Song, Y.H.; Wang, J.; Nie, G.; Chen, Y.J.; Li, X.; Jiang, X.; Cao, W.H. MicroRNA-509-5p functions as an anti-oncogene in breast cancer via targeting SOD2. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3617–3625. [Google Scholar]
- Xu, X.; Wells, A.; Padilla, M.T.; Kato, K.; Kim, K.C.; Lin, Y. A signaling pathway consisting of miR-551b, catalase and MUC1 contributes to acquired apoptosis resistance and chemoresistance. Carcinogenesis 2014, 35, 2457–2466. [Google Scholar] [CrossRef]
- Salinas-Vera, Y.M.; Gallardo-Rincon, D.; Garcia-Vazquez, R.; Hernandez-de la Cruz, O.N.; Marchat, L.A.; Gonzalez-Barrios, J.A.; Ruiz-Garcia, E.; Vazquez-Calzada, C.; Contreras-Sanzon, E.; Resendiz-Hernandez, M.; et al. Corrigendum: HypoxamiRs Profiling Identify miR-765 as a Regulator of the Early Stages of Vasculogenic Mimicry in SKOV3 Ovarian Cancer Cells. Front. Oncol. 2020, 10, 889. [Google Scholar] [CrossRef]
- Fierro-Fernandez, M.; Busnadiego, O.; Sandoval, P.; Espinosa-Diez, C.; Blanco-Ruiz, E.; Rodriguez, M.; Pian, H.; Ramos, R.; Lopez-Cabrera, M.; Garcia-Bermejo, M.L.; et al. miR-9-5p suppresses pro-fibrogenic transformation of fibroblasts and prevents organ fibrosis by targeting NOX4 and TGFBR2. EMBO Rep. 2015, 16, 1358–1377. [Google Scholar] [CrossRef]
- Lee, J.; Heo, J.; Kang, H. miR-92b-3p-TSC1 axis is critical for mTOR signaling-mediated vascular smooth muscle cell proliferation induced by hypoxia. Cell Death Differ. 2019, 26, 1782–1795. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).