Alpinumisoflavone Activates Disruption of Calcium Homeostasis, Mitochondria and Autophagosome to Suppress Development of Endometriosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Cell Culture
2.3. Detection of Cell Proliferative Capacity
2.4. Cell Cycle Assay
2.5. Cell Migration
2.6. Detection of Apoptotic Cells
2.7. Disruption of Mitochondria Membrane Polarity
2.8. Intracellular Calcium Ion Measurement
2.9. Seahorse XFe24 Mito Stress Assay
2.10. Intramitochondrial Calcium Ion Measurement
2.11. Immunoblotting
2.12. Statistical Analysis
3. Results
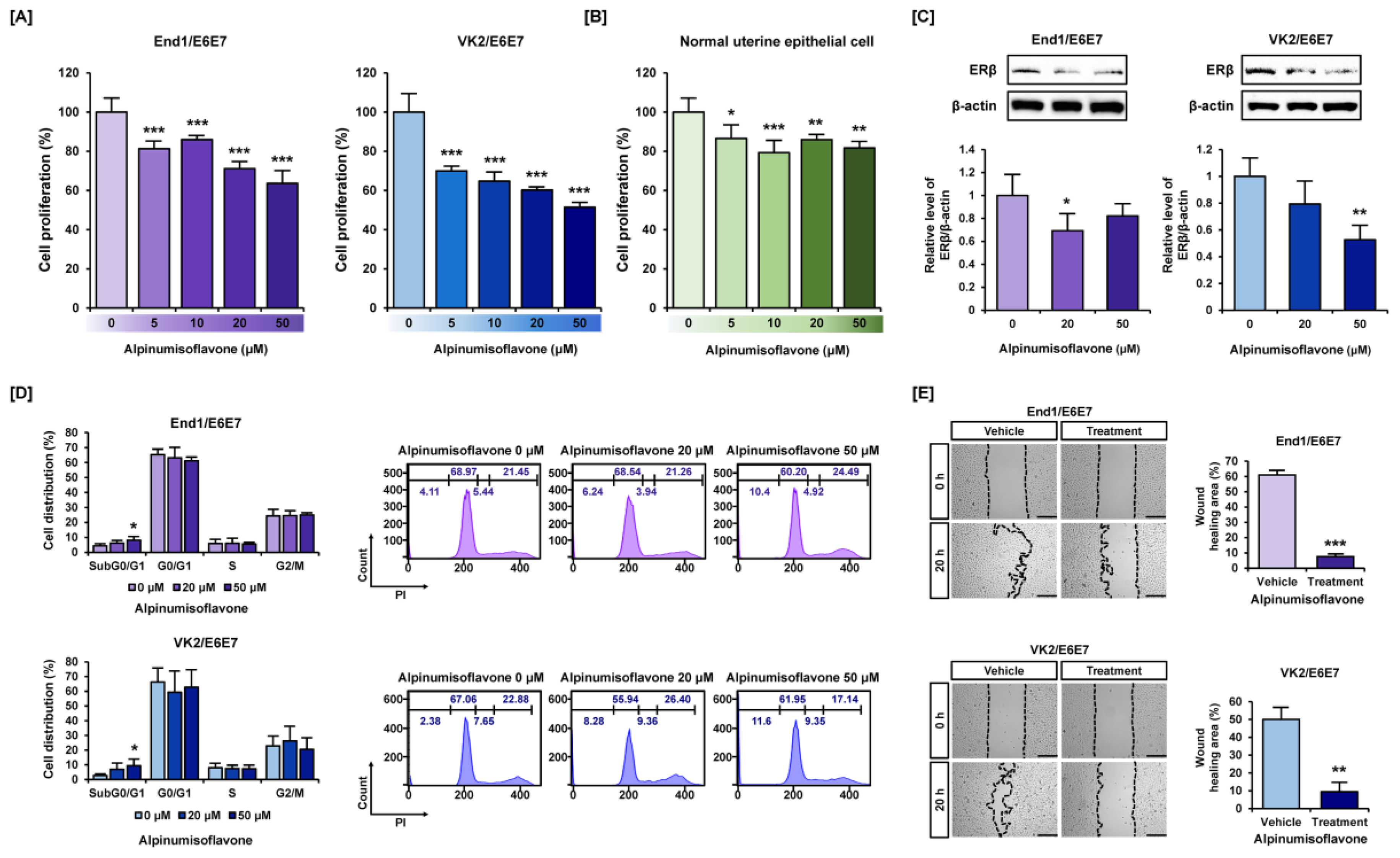
3.1. Alpinumisoflavone Suppressed Cell Proliferation and Migration in Human Endometriosis-like Cell Lines
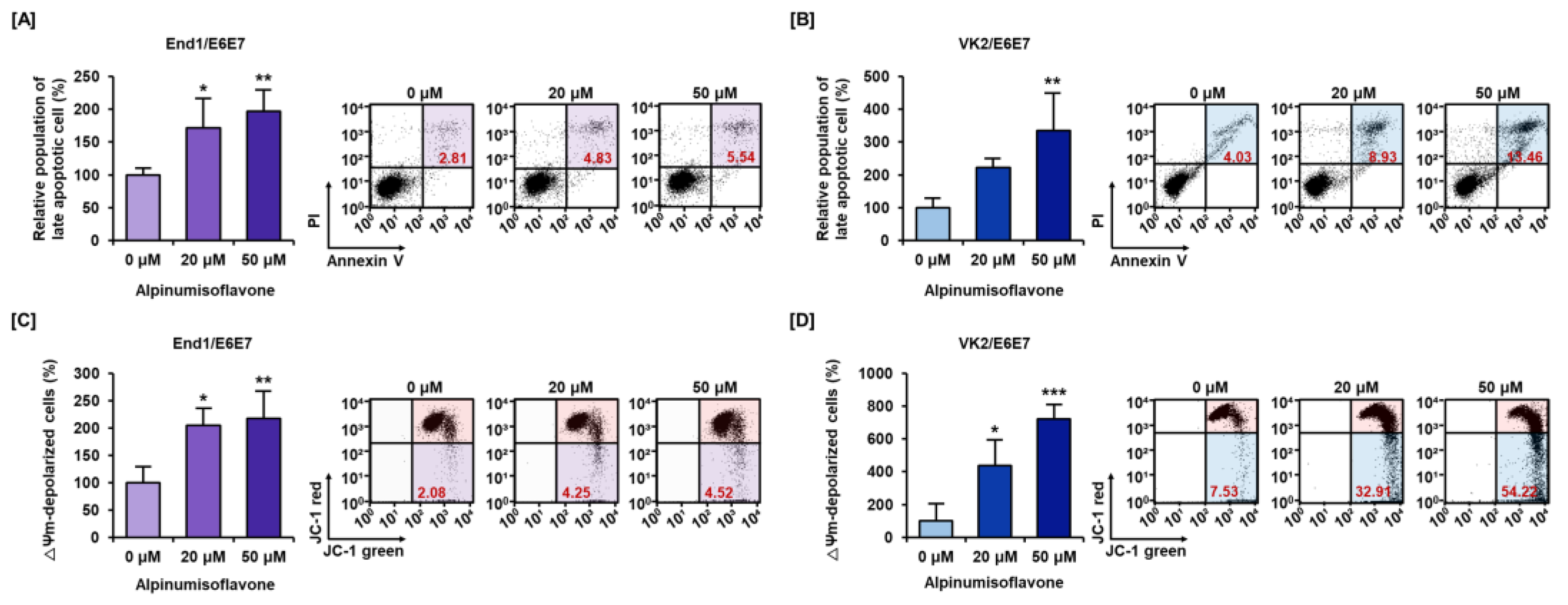
3.2. Alpinumisoflavone Caused Cell Death and Depolarized Mitochondria Membrane Potential (MMP) in Human Endometriosis Cells
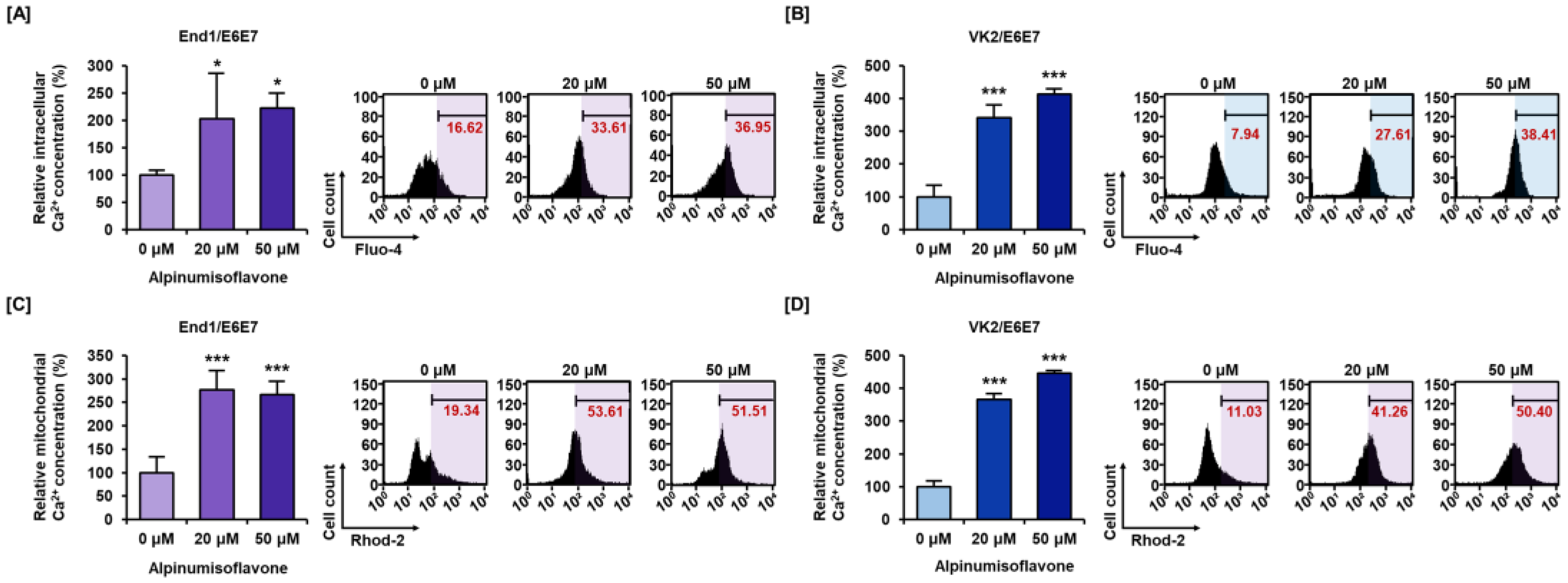
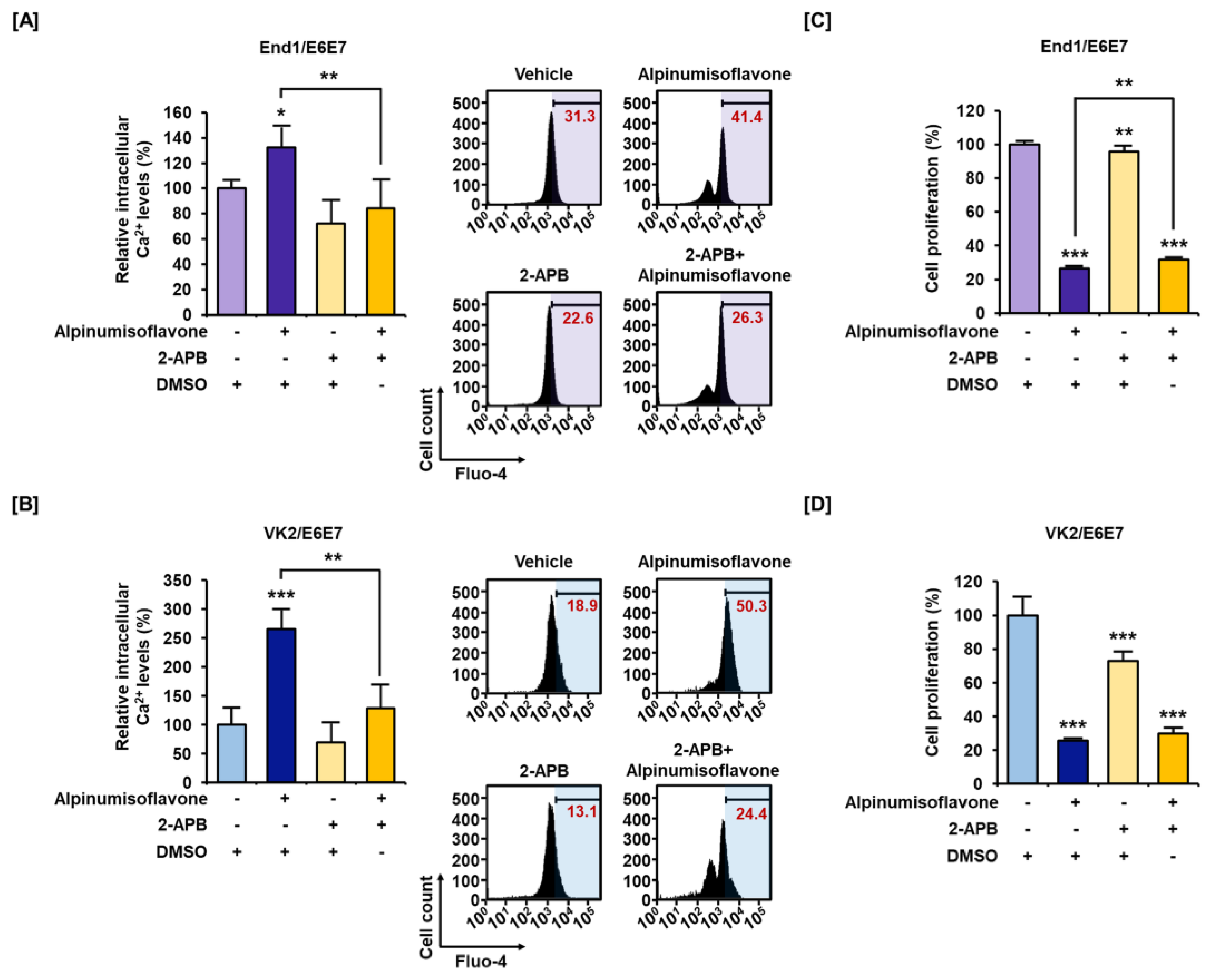
3.3. Alpinumisoflavone Disrupted Calcium Homeostasis in Cytosolic and Mitochondrial Matrix in End1/E6E7 and VK2/E6E7 Cells
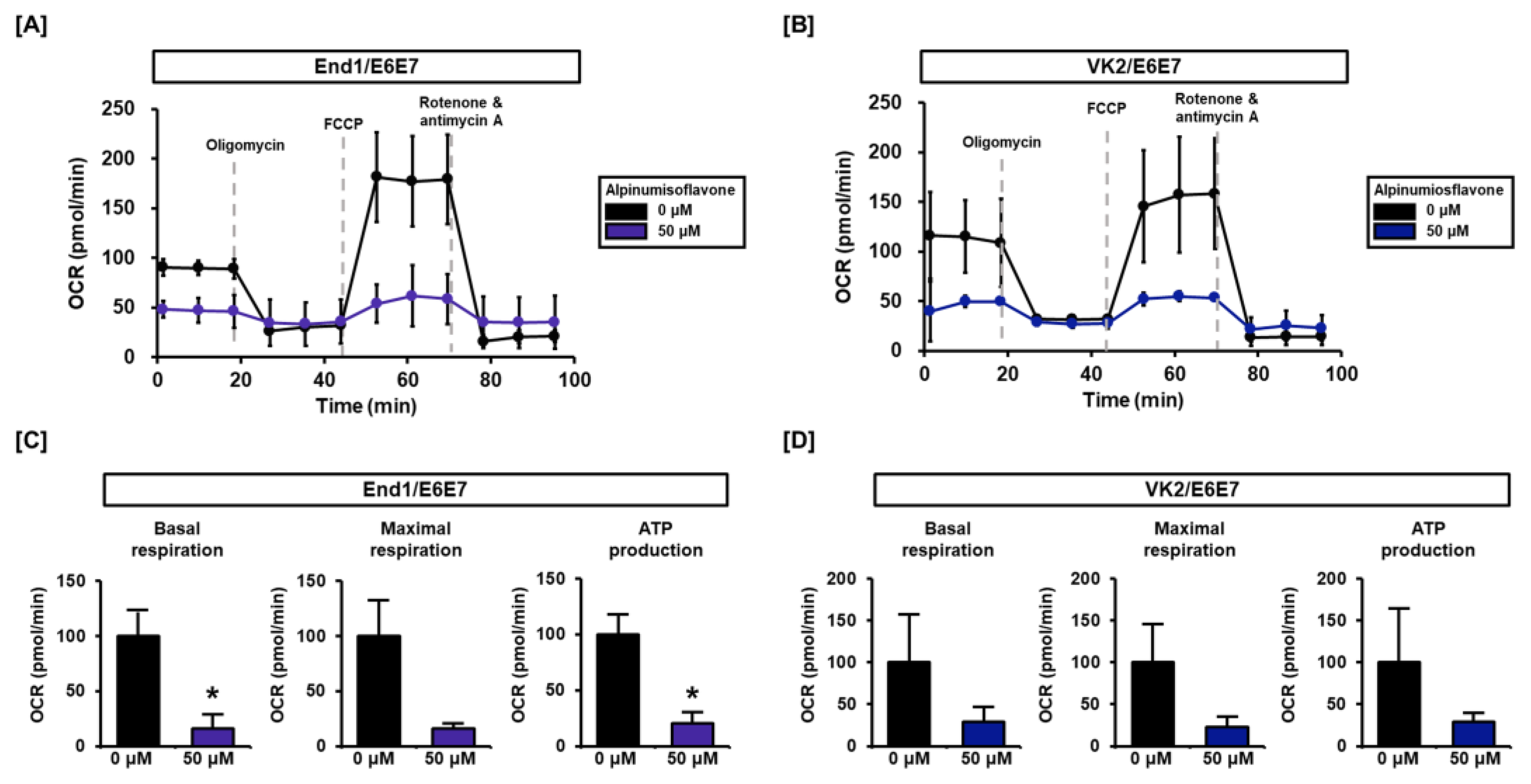
3.4. Alpinumisoflavone Regulates Mitochondrial Respiration in End1/E6E7 and VK2/E6E7 Cells
3.5. Alpinumisoflavone Downregulates the Intracellular Signaling Pathways Like PI3K/AKT and MAPK in Human Endometriosis-like Cells
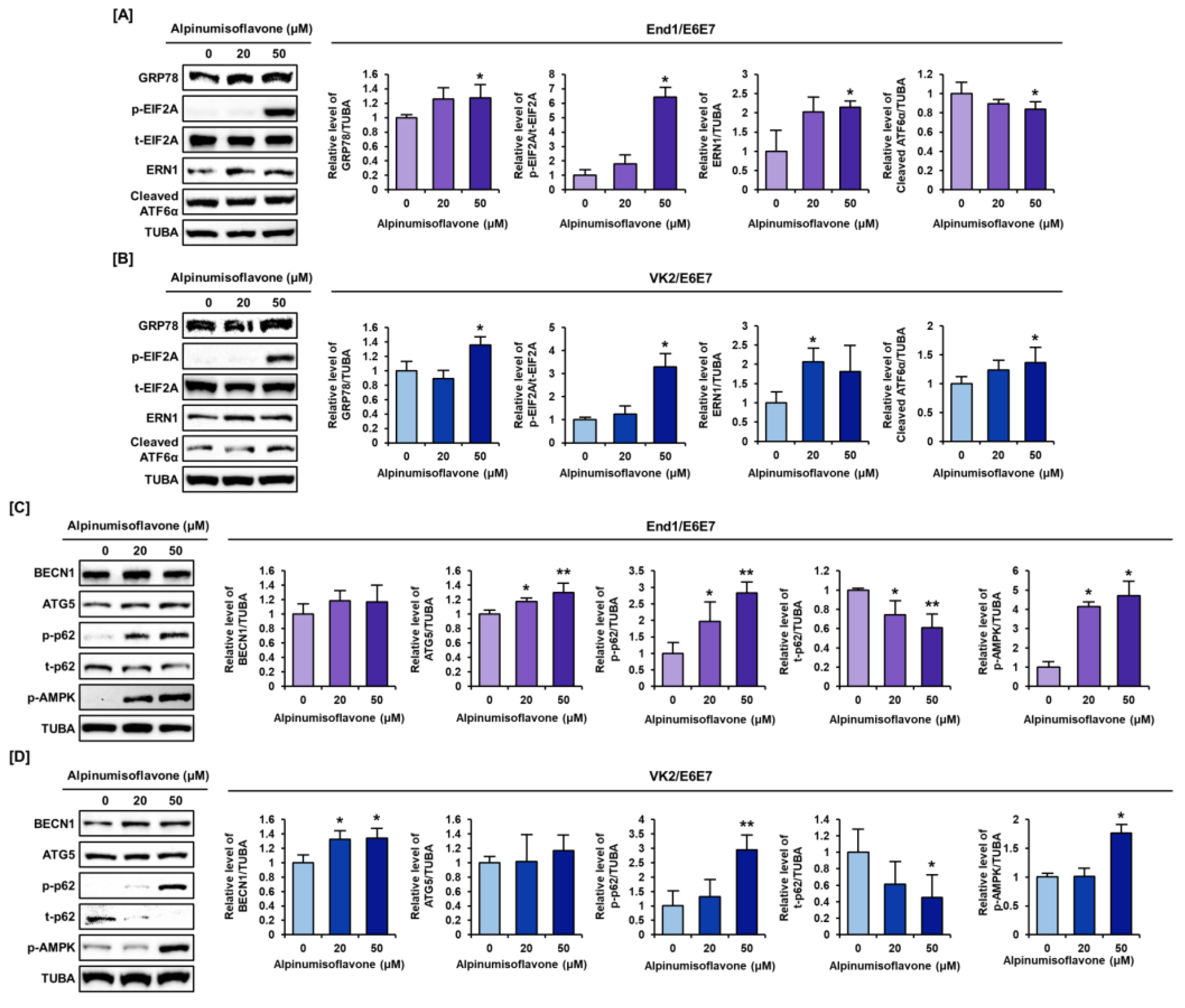
3.6. Alpinumisoflavone Regulated the ER Stress and Autophagy Signaling Pathway in End1/E6E7 and VK2/E6E7 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kanda, K.; Seto, M.; Takahashi, M.; Goto, K.; Nakazawa, H. The relationship of the quantity of dust particles with bacterial count or with the volume of traffic in a ward of internal medicine. Kango Gijutsu 1986, 32, 1824–1831. [Google Scholar] [PubMed]
- Park, S.; Ham, J.; Yang, C.; Park, W.; Park, H.; An, G.; Song, J.; Hong, T.; Park, S.J.; Kim, H.S.; et al. Melatonin inhibits endometriosis development by disrupting mitochondrial function and regulating tiRNAs. J. Pineal. Res. 2023, 74, e12842. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Christ, J.P.; Yu, O.; Schulze-Rath, R.; Grafton, J.; Hansen, K.; Reed, S.D. Incidence, prevalence, and trends in endometriosis diagnosis: A United States population-based study from 2006 to 2015. Am. J. Obstet. Gynecol. 2021, 225, 500.e1–500.e9. [Google Scholar] [CrossRef]
- Jang, H.; Ham, J.; Song, J.; Song, G.; Lim, W. Alpinumisoflavone Impairs Mitochondrial Respiration via Oxidative Stress and MAPK/PI3K Regulation in Hepatocellular Carcinoma Cells. Antioxidants 2022, 11, 1929. [Google Scholar] [CrossRef]
- Hong, T.; Ham, J.; Song, G.; Lim, W. Alpinumisoflavone Disrupts Endoplasmic Reticulum and Mitochondria Leading to Apoptosis in Human Ovarian Cancer. Pharmaceutics 2022, 14, 564. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Sun, M.; He, T.; Liu, Y.; Yang, X.; Shi, X.; Liu, X. Alpinumisoflavone suppresses hepatocellular carcinoma cell growth and metastasis via NLRP3 inflammasome-mediated pyroptosis. Pharmacol. Rep. 2020, 72, 1370–1382. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Y.; Chu, L.; Wu, T.; You, J. Alpinumisoflavone suppresses tumour growth and metastasis of clear-cell renal cell carcinoma. Am. J. Cancer Res. 2017, 7, 999–1015. [Google Scholar]
- Song, J.; Song, G.; Park, S.; Lim, W. Inhibitory Effects of 6,8-Diprenylorobol on Endometriosis Progression in Humans by Disrupting Calcium Homeostasis and Mitochondrial Function. Antioxidants 2022, 11, 171. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, S.; Song, G.; Lim, W.; Han, Y.S. Antigrowth effects of Kaempferia parviflora extract enriched in anthocyanidins on human ovarian cancer cells through Ca2+-ROS overload and mitochondrial dysfunction. Mol. Cell. Toxicol. 2022, 18, 383–391. [Google Scholar] [CrossRef]
- Kim, L.; Hong, T.; Ham, J.; Lim, W. Effects of Agarum clathratum extract on cell death and calcium ion levels of ovarian cancer cell. Mol. Cell. Toxicol. 2022, 19, 303–310. [Google Scholar] [CrossRef]
- Yang, C.; An, G.; Song, J.; Song, G.; Lim, W. Palmitic acid induces inflammatory cytokines and regulates tRNA-derived stress-induced RNAs in human trophoblasts. J. Anim. Reprod. Biotechnol. 2022, 37, 218–225. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int J Mol Sci 2020, 21, 2815. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Estrogenic flavonoids and their molecular mechanisms of action. J. Nutr. Biochem. 2023, 114, 109250. [Google Scholar] [CrossRef]
- Ateba, S.B.; Mvondo, M.A.; Djiogue, S.; Zingue, S.; Krenn, L.; Njamen, D. A Pharmacological Overview of Alpinumisoflavone, a Natural Prenylated Isoflavonoid. Front. Pharmacol. 2019, 10, 952. [Google Scholar] [CrossRef]
- Djiogue, S.; Halabalaki, M.; Alexi, X.; Njamen, D.; Fomum, Z.T.; Alexis, M.N.; Skaltsounis, A.L. Isoflavonoids from Erythrina poeppigiana: Evaluation of their binding affinity for the estrogen receptor. J. Nat. Prod. 2009, 72, 1603–1607. [Google Scholar] [CrossRef]
- Mvondo, M.A.; Njamen, D.; Tanee Fomum, S.; Wandji, J. Effects of alpinumisoflavone and abyssinone V-4′-methyl ether derived from Erythrina lysistemon (Fabaceae) on the genital tract of ovariectomized female Wistar rat. Phytother. Res. 2012, 26, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Mvondo, M.A.; Njamen, D.; Fomum, S.T.; Wandji, J.; Vollmer, G. A postmenopause-like model of ovariectomized Wistar rats to identify active principles of Erythrina lysistemon (Fabaceae). Fitoterapia 2011, 82, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Apigenin induces ROS-dependent apoptosis and ER stress in human endometriosis cells. J. Cell Physiol. 2018, 233, 3055–3065. [Google Scholar] [CrossRef]
- Magne Nde, C.B.; Njamen, D.; Tanee Fomum, S.; Wandji, J.; Simpson, E.; Clyne, C.; Vollmer, G. In vitro estrogenic activity of two major compounds from the stem bark of Erythrina lysistemon (Fabaceae). Eur. J. Pharmacol. 2012, 674, 87–94. [Google Scholar] [CrossRef]
- Han, Y.; Yang, X.; Zhao, N.; Peng, J.; Gao, H.; Qiu, X. Alpinumisoflavone induces apoptosis in esophageal squamous cell carcinoma by modulating miR-370/PIM1 signaling. Am. J. Cancer Res. 2016, 6, 2755–2771. [Google Scholar] [PubMed]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Machaca, K. Ca(2+) signaling, genes and the cell cycle. Cell Calcium. 2010, 48, 243–250. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Kass, G.E.; Orrenius, S. Calcium signaling and cytotoxicity. Environ. Health Perspect. 1999, 107 (Suppl. 1), 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G. Mitochondrial control of apoptosis: An overview. Biochem. Soc. Symp. 1999, 66, 1–15. [Google Scholar] [CrossRef]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef]
- Gorlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef]
- Iwasaki, H.; Mori, Y.; Hara, Y.; Uchida, K.; Zhou, H.; Mikoshiba, K. 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Recept. Channels 2001, 7, 429–439. [Google Scholar]
- Maruyama, T.; Kanaji, T.; Nakade, S.; Kanno, T.; Mikoshiba, K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997, 122, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, M.; Skibinska, I.; Kotwicka, M. Mitochondria: Target organelles for estrogen action. Postepy Hig. Med. Dosw. 2017, 71, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, Y.; Hu, C.; Wang, Y.; Yan, Z.; Li, Z.; Wu, R. Mitochondria and oxidative stress in ovarian endometriosis. Free Radic. Biol. Med. 2019, 136, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.L.; Lee, Y.C.; Tzeng, C.R.; Wang, Y.P.; Chang, H.Y.; Lin, Y.F.; Kao, S.H. Mitochondrial translocation of estrogen receptor beta affords resistance to oxidative insult-induced apoptosis and contributes to the pathogenesis of endometriosis. Free Radic. Biol. Med. 2019, 134, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Osaki, M.; Oshimura, M.; Ito, H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis 2004, 9, 667–676. [Google Scholar] [CrossRef]
- Datta, S.R.; Brunet, A.; Greenberg, M.E. Cellular survival: A play in three Akts. Genes Dev. 1999, 13, 2905–2927. [Google Scholar] [CrossRef]
- Mezynski, M.J.; Farrelly, A.M.; Cremona, M.; Carr, A.; Morgan, C.; Workman, J.; Armstrong, P.; McAuley, J.; Madden, S.; Fay, J.; et al. Targeting the PI3K and MAPK pathways to improve response to HER2-targeted therapies in HER2-positive gastric cancer. J. Transl. Med. 2021, 19, 184. [Google Scholar] [CrossRef]
- Castel, P.; Toska, E.; Engelman, J.A.; Scaltriti, M. The present and future of PI3K inhibitors for cancer therapy. Nat. Cancer 2021, 2, 587–597. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef]
- Yagyu, T.; Tsuji, Y.; Haruta, S.; Kitanaka, T.; Yamada, Y.; Kawaguchi, R.; Kanayama, S.; Tanase, Y.; Kurita, N.; Kobayashi, H. Activation of mammalian target of rapamycin in postmenopausal ovarian endometriosis. Int. J. Gynecol. Cancer 2006, 16, 1545–1551. [Google Scholar] [CrossRef]
- Driva, T.S.; Schatz, C.; Sobocan, M.; Haybaeck, J. The Role of mTOR and eIF Signaling in Benign Endometrial Diseases. Int. J. Mol. Sci. 2022, 23, 3416. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, C.G.; Altuna, S.C.; Habeeb, B.S.; Trapani, D.; Khan, S.Z. The Potential of PI3K/AKT/mTOR Signaling as a Druggable Target for Endometrial and Ovarian Carcinomas. Curr. Drug Targets 2020, 21, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Leconte, M.; Nicco, C.; Ngo, C.; Chereau, C.; Chouzenoux, S.; Marut, W.; Guibourdenche, J.; Arkwright, S.; Weill, B.; Chapron, C.; et al. The mTOR/AKT inhibitor temsirolimus prevents deep infiltrating endometriosis in mice. Am. J. Pathol. 2011, 179, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhou, Y.; Qiu, J.; Wang, X.; Xia, Y.; Sui, L. Low expression of TUG1 promotes cisplatin sensitivity in cervical cancer by activating the MAPK pathway. JBUON 2019, 24, 1020–1026. [Google Scholar]
- Chomlamay, N.; Poorahong, W.; Innajak, S.; Watanapokasin, R. Apoptosis Induction Associated with Enhanced ER Stress Response and Up-Regulation of c-Jun/p38 MAPK Proteins in Human Cervical Cancer Cells by Colocasia esculenta var. aquatilis Hassk Extract. Sci. Pharm. 2022, 90, 45. [Google Scholar] [CrossRef]
- Sasaki, K.; Yoshida, H. Organelle autoregulation-stress responses in the ER, Golgi, mitochondria and lysosome. J. Biochem. 2015, 157, 185–195. [Google Scholar] [CrossRef]
- Bhat, T.A.; Chaudhary, A.K.; Kumar, S.; O’Malley, J.; Inigo, J.R.; Kumar, R.; Yadav, N.; Chandra, D. Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1867, 58–66. [Google Scholar] [CrossRef]
- Hoyer-Hansen, M.; Bastholm, L.; Szyniarowski, P.; Campanella, M.; Szabadkai, G.; Farkas, T.; Bianchi, K.; Fehrenbacher, N.; Elling, F.; Rizzuto, R.; et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol. Cell 2007, 25, 193–205. [Google Scholar] [CrossRef]
- Gozuacik, D.; Bialik, S.; Raveh, T.; Mitou, G.; Shohat, G.; Sabanay, H.; Mizushima, N.; Yoshimori, T.; Kimchi, A. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008, 15, 1875–1886. [Google Scholar] [CrossRef]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell Biol. 2001, 21, 1249–1259. [Google Scholar] [CrossRef]
- Szegezdi, E.; Macdonald, D.C.; Ni Chonghaile, T.; Gupta, S.; Samali, A. Bcl-2 family on guard at the ER. Am. J. Physiol. Cell Physiol. 2009, 296, C941–C953. [Google Scholar] [CrossRef]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Yorimitsu, T.; Klionsky, D.J. Autophagy: Molecular machinery for self-eating. Cell Death Differ. 2005, 12 (Suppl. 2), 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Klionsky, D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell 2004, 6, 463–477. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, I.J.; Hernandez-Tiedra, S.; Lorente, M.; Egia, A.; Vazquez, P.; Blazquez, C.; Torres, S.; Garcia, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef]
- Neufeld, T.P. Autophagy and cell growth—The yin and yang of nutrient responses. J. Cell Sci. 2012, 125, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.K.; Bialik, S.; Levin-Zaidman, S.; Levin-Salomon, V.; Merrill, A.H., Jr.; Futerman, A.H.; Kimchi, A. Signalome-wide RNAi screen identifies GBA1 as a positive mediator of autophagic cell death. Cell Death Differ 2017, 24, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Liu, W.; Nakamura, K.; Yoshida, K.; Ikeda, Y.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; et al. Plasma-activated medium promotes autophagic cell death along with alteration of the mTOR pathway. Sci. Rep. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, C.H.; Seo, M.; Otto, N.M.; Grunwald, D.; Kim, K.H.; Moriarity, B.; Kim, Y.M.; Starker, C.; Nho, R.S.; et al. The ULK1 complex mediates MTORC1 signaling to the autophagy initiation machinery via binding and phosphorylating ATG14. Autophagy 2016, 12, 547–564. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of AMPK in autophagy. Front. Physiol. 2022, 13, 1015500. [Google Scholar] [CrossRef]
- Xiao, K.; Jiang, J.; Guan, C.; Dong, C.; Wang, G.; Bai, L.; Sun, J.; Hu, C.; Bai, C. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J. Pharmacol. Sci. 2013, 123, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jo, M.; Lee, E.; Lee, D.Y.; Choi, D. Dienogest enhances autophagy induction in endometriotic cells by impairing activation of AKT, ERK1/2, and mTOR. Fertil. Steril. 2015, 104, 655–664.e651. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Nishida, Y.; Arakawa, S.; Fujitani, K.; Yamaguchi, H.; Mizuta, T.; Kanaseki, T.; Komatsu, M.; Otsu, K.; Tsujimoto, Y.; Shimizu, S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 2009, 461, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Yoshida, T.; Tsujioka, M.; Arakawa, S. Autophagic Cell Death and Cancer. Int. J. Mol. Sci. 2014, 15, 3145–3153. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Ham, J.; Park, S.; Park, S.J.; Kim, H.S.; Song, G.; Lim, W. Alpinumisoflavone Activates Disruption of Calcium Homeostasis, Mitochondria and Autophagosome to Suppress Development of Endometriosis. Antioxidants 2023, 12, 1324. https://doi.org/10.3390/antiox12071324
Song J, Ham J, Park S, Park SJ, Kim HS, Song G, Lim W. Alpinumisoflavone Activates Disruption of Calcium Homeostasis, Mitochondria and Autophagosome to Suppress Development of Endometriosis. Antioxidants. 2023; 12(7):1324. https://doi.org/10.3390/antiox12071324
Chicago/Turabian StyleSong, Jisoo, Jiyeon Ham, Sunwoo Park, Soo Jin Park, Hee Seung Kim, Gwonhwa Song, and Whasun Lim. 2023. "Alpinumisoflavone Activates Disruption of Calcium Homeostasis, Mitochondria and Autophagosome to Suppress Development of Endometriosis" Antioxidants 12, no. 7: 1324. https://doi.org/10.3390/antiox12071324
APA StyleSong, J., Ham, J., Park, S., Park, S. J., Kim, H. S., Song, G., & Lim, W. (2023). Alpinumisoflavone Activates Disruption of Calcium Homeostasis, Mitochondria and Autophagosome to Suppress Development of Endometriosis. Antioxidants, 12(7), 1324. https://doi.org/10.3390/antiox12071324








