Green Tea Catechins as Therapeutic Antioxidants for Glaucoma Treatment
Abstract
1. Green Tea Catechins: Chemistry and Pharmacokinetics
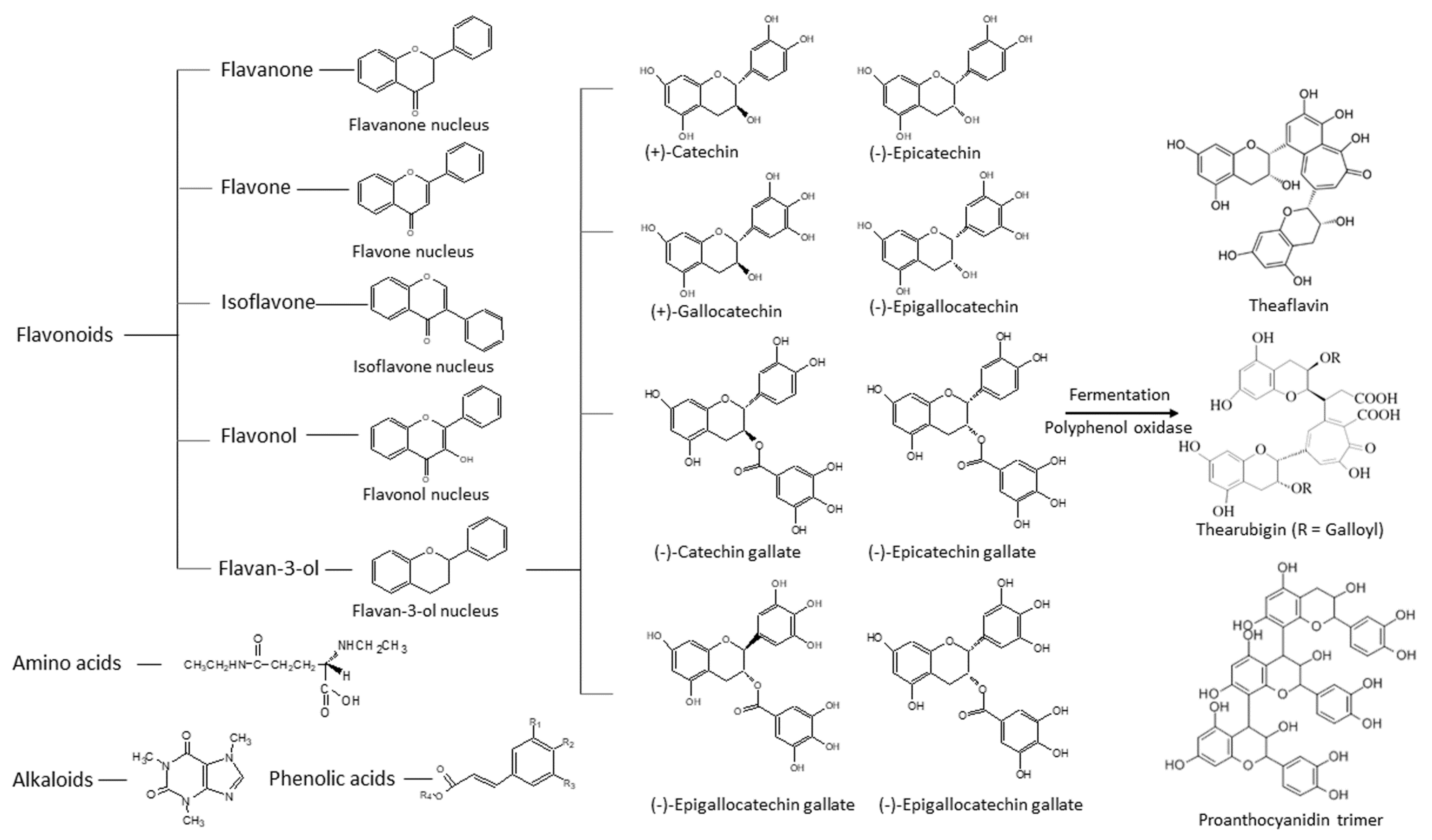
1.1. Chemistry of Green Tea Constituents
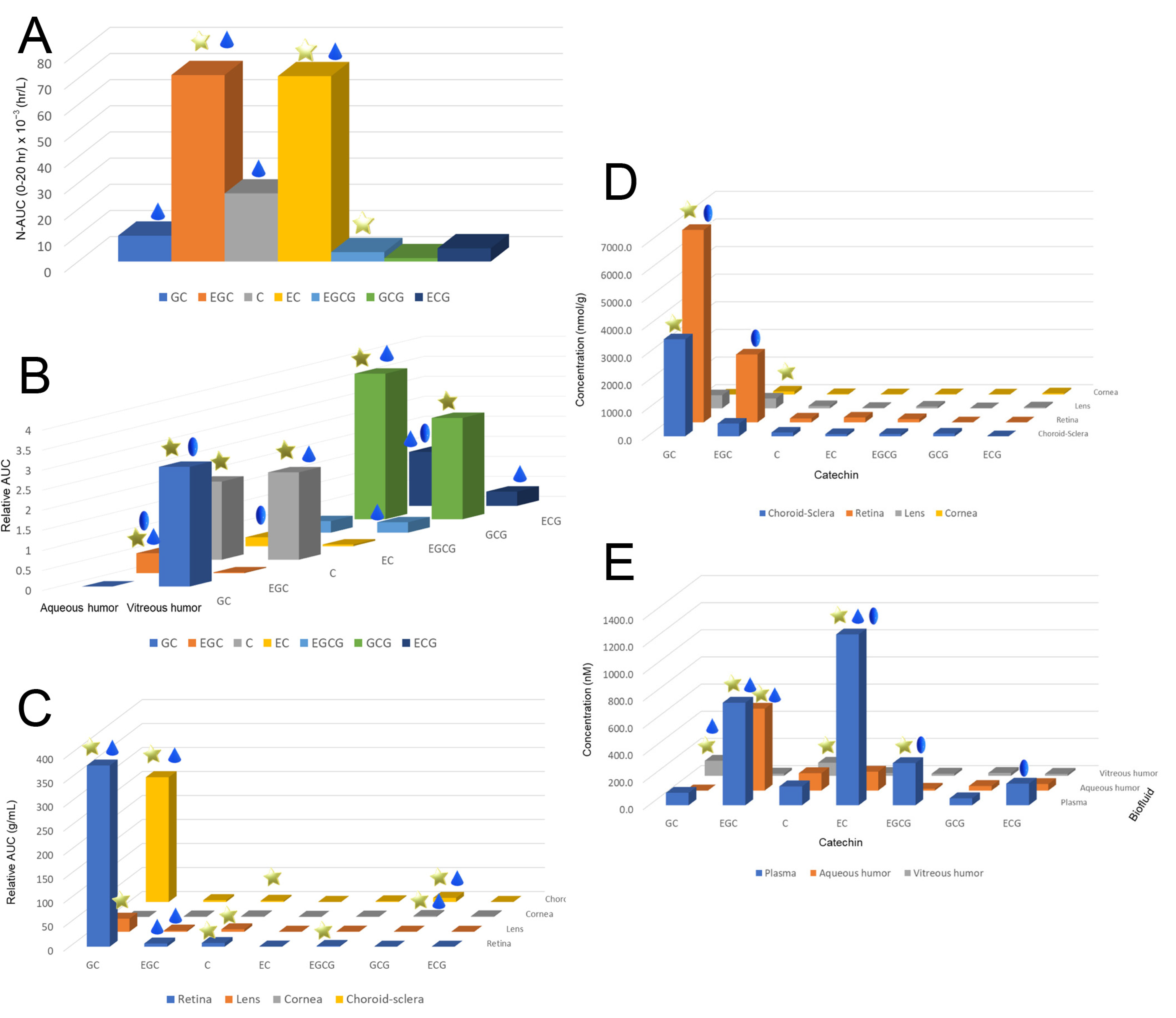
1.2. Pharmacokinetics of Catechins in the Eye
2. Therapeutic Properties of Green Tea Catechins: Antioxidation and Anti-Inflammation in the Eye
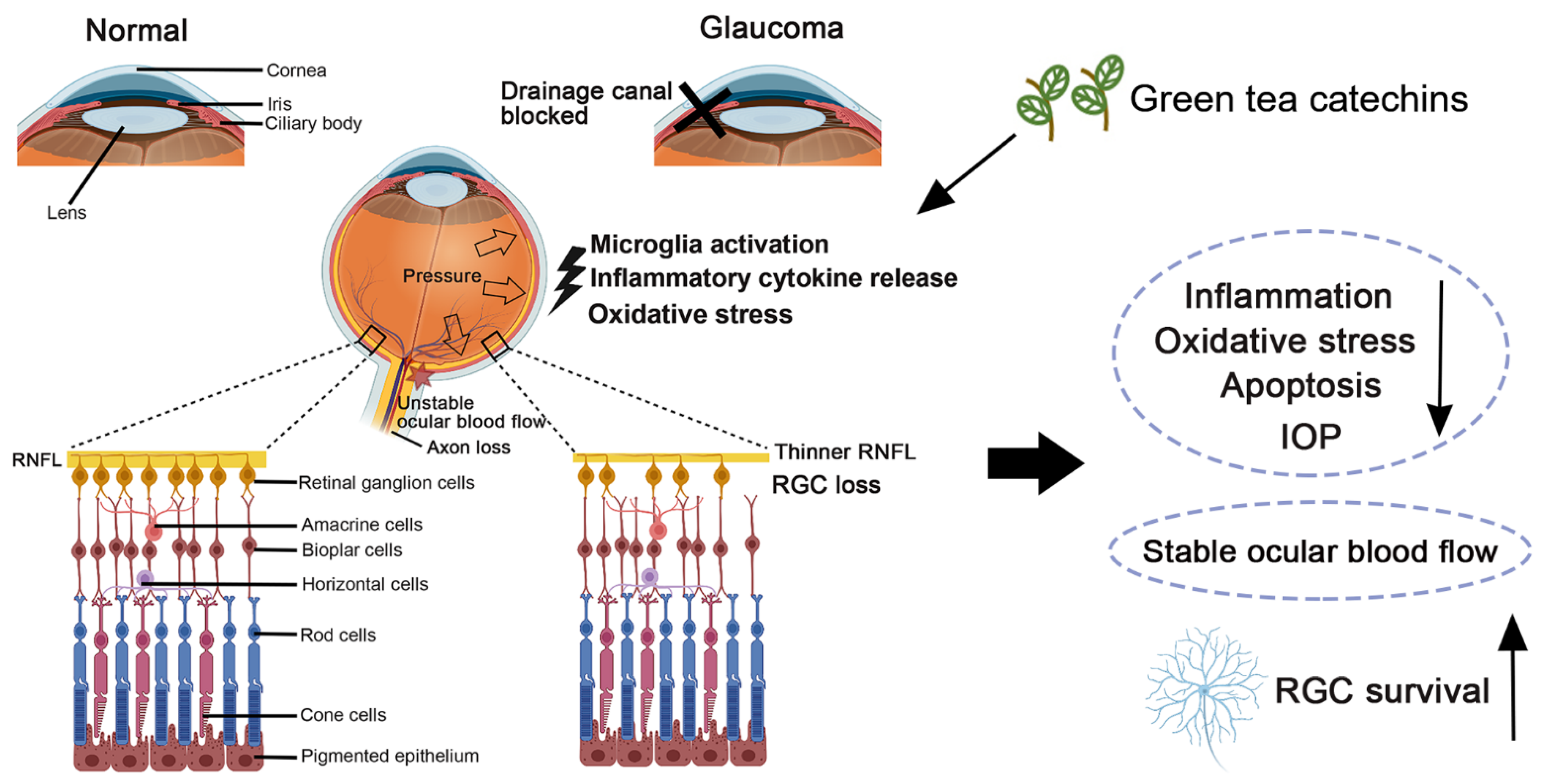
3. Pathophysiological Conditions in Glaucoma: Oxidative Stress and Inflammation
4. Green Tea Catechins in Experimental Cellular Models of Glaucoma
5. Green Tea Catechins in Experimental Animal Models of Glaucoma
6. Clinical Applications of Green Tea Catechins for Glaucoma Treatments
7. Summary, Challenges, and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramadan, G.; El-Beih, N.M.; Talaat, R.M.; Abd El-Ghffar, E.A. Anti-inflammatory activity of green versus black tea aqueous extract in a rat model of human rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Santamarina, A.B.; Carvalho-Silva, M.; Gomes, L.M.; Okuda, M.H.; Santana, A.A.; Streck, E.L.; Seelaender, M.; Do Nascimento, C.; Ribeiro, E.B.; Lira, F.S.; et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. J. Nutr. Biochem. 2015, 26, 1348–1356. [Google Scholar] [CrossRef]
- Turkozu, D.; Sanlier, N. L-theanine, unique amino acid of tea and its metabolism, health effects, and safety. Crit. Rev. Food Sci. Nutr. 2017, 57, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Huang, M.J.; Qin, C.Q.; Lv, B.Y.; Mao, Q.L.; Liu, Z.H. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Shi, S.S.; Bao, B.; Li, X.J.; Wang, S.C. Structure characterization of an arabinogalactan from green tea and its anti-diabetic effect. Carbohyd. Polym. 2015, 124, 98–108. [Google Scholar] [CrossRef]
- Song, C.W.; Yu, Q.S.; Li, X.H.; Jin, S.N.; Li, S.; Zhang, Y.; Jia, S.L.; Chen, C.; Xiang, Y.; Jiang, H.L. The hypolipidemic effect of total saponins from kuding tea in high-fat diet-induced hyperlipidemic mice and its composition characterized by UPLC-QTOF-MS/MS. J. Food Sci. 2016, 81, H1313–H1319. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Luca, V.S.; Stan, A.M.; Trifan, A.; Miron, A.; Aprotosoaie, A.C. Catechins profile, caffeine content and antioxidant activity of Camellia sinensis teas commercialized in romania. Med. Sur. J. Revista Med.-Chirurgicala 2016, 120, 457–463. [Google Scholar]
- Xu, Y.; Zhang, M.; Wu, T.; Dai, S.D.; Xu, J.L.; Zhou, Z.K. The anti-obesity effect of green tea polysaccharides, polyphenols and caffeine in rats fed with a high-fat diet. Food Funct. 2015, 6, 297–304. [Google Scholar] [CrossRef]
- Tang, G.Y.; Zhao, C.N.; Xu, X.Y.; Gan, R.Y.; Cao, S.Y.; Liu, Q.; Shang, A.; Mao, Q.Q.; Li, H.B. Phytochemical composition and antioxidant capacity of 30 Chinese teas. Antioxidants 2019, 8, 180. [Google Scholar] [CrossRef]
- Yang, H.; Xue, X.J.; Li, H.; Apandi, S.N.; Tay-Chan, S.C.; Ong, S.P.; Tian, E.F. The relative antioxidant activity and steric structure of green tea catechins—A kinetic approach. Food Chem. 2018, 257, 399–405. [Google Scholar] [CrossRef]
- Pastore, R.L.; Fratellone, P. Potential health benefits of green tea (Camellia sinensis): A narrative review. Explore 2006, 2, 531–539. [Google Scholar] [CrossRef]
- Yang, C.S.; Lambert, J.D.; Ju, J.; Lu, G.; Sang, S. Tea and cancer prevention: Molecular mechanisms and human relevance. Toxicol. Appl. Pharmacol. 2007, 224, 265–273. [Google Scholar] [CrossRef]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Metodiewa, D.; Jaiswal, A.K.; Genas, N.; Dickancaite, E.; Segora-Aguilar, J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999, 26, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Juneja, L.R.; Chu, D.C.; Kim, M. Chemistry and Application of Green Tea; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 1997; pp. 1–5. [Google Scholar]
- Baranowska, M.; Suliborska, K.; Chrzanowski, W.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. The relationship between standard reduction potentials of catechins and biological activities involved in redox control. Redox Biol. 2018, 17, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.B.; Kim, W.S.; Sung, N.Y.; Byun, E.H. Epigallocatechin-3-Gallate Regulates Anti-Inflammatory Action Through 67-kDa Laminin Receptor-Mediated Tollip Signaling Induction in Lipopolysaccharide-Stimulated Human Intestinal Epithelial Cells. Cell. Physiol. Biochem. 2018, 46, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Blayney, A.; Liu, X.R.; Gandy, L.; Jin, W.H.; Yan, L.F.; Ha, J.H.; Canning, A.J.; Connelly, M.; Yang, C.; et al. EGCG binds intrinsically disordered N-terminal domain of p53 and disrupts p53-MDM2 interaction. Nat. Commun. 2021, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Yang, C.S.; Kim, J.Y.; MacNevin, C.J.; Hahn, K.M.; Park, D.; Ginsberg, M.H.; Kim, C.H. Epigallocatechin gallate has pleiotropic effects on transmembrane signaling by altering the embedding of transmembrane domains. J. Biol. Chem. 2017, 292, 9858–9864. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of Epigallocatechin-3-Gallate on EGFR Signaling and Migration in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef]
- Zhang, H.H.; Cao, D.; Cui, W.; Ji, M.J.; Qian, X.H.; Zhong, L.W. Molecular bases of thioredoxin and thioredoxin reductase-mediated prooxidant actions of (−)-epigallocatechin-3-gallate. Free Radic. Biol. Med. 2010, 49, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Aribisala, J.O.; Sabiu, S. Redox Impact on Bacterial Macromolecule: A Promising Avenue for Discovery and Development of Novel Antibacterials. Biomolecules 2022, 12, 1545. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.; Wessler, S.; Follmann, E.; Michaelis, W.; Dusterhoft, T.; Baumann, G.; Stangl, K.; Stangl, V. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J. Biol. Chem. 2004, 279, 6190–6195. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.; Campos-Toimil, M.; Justiniano-Basaran, H.; Lugnier, C.; Orallo, F. Study of the mechanisms involved in the vasorelaxation induced by (−)-epigallocatechin-3-gallate in rat aorta. Br. J. Pharmacol. 2006, 147, 269–280. [Google Scholar] [CrossRef]
- Collins, Q.F.; Liu, H.Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5’-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef]
- Reiter, C.E.; Kim, J.A.; Quon, M.J. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: Roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology 2010, 151, 103–114. [Google Scholar] [CrossRef]
- Jang, H.J.; Ridgeway, S.D.; Kim, J.A. Effects of the green tea polyphenol, epigallocatechin-3-gallate (EGCG), on high fat diet-induced insulin resistance and endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1444–E1451. [Google Scholar] [CrossRef]
- Lee, M.J.; Wang, Z.Y.; Li, H.; Chen, L.; Sun, Y.; Gobbo, S.; Balentine, D.A.; Yang, C.S. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomark. Prev. 1995, 4, 393–399. [Google Scholar]
- Hong, J.; Lambert, J.D.; Lee, S.H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, M.J.; Li, H.; Yang, C.S. Absorption, distribution, elimination of tea polyphenols in rats. Drug. Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar] [PubMed]
- Lambert, J.D.; Lee, M.J.; Lu, H.; Meng, X.; Hong, J.J.; Seril, D.N.; Sturgill, M.G.; Yang, C.S. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J. Nutr. 2003, 133, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Jodoin, J.; Demeule, M.; Beliveau, R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta. 2002, 1542, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef] [PubMed]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Kirkpatrick, N.J.; Polagruto, J.A.; Ensunsa, J.L.; Schmitz, H.H.; Keen, C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003, 73, 857–869. [Google Scholar] [CrossRef]
- Chu, K.O.; Chan, K.P.; Wang, C.C.; Chu, C.Y.; Li, W.Y.; Choy, K.W.; Rogers, M.S.; Pang, C.P. Green tea catechins and their oxidative protection in the rat eye. J. Agric. Food Chem. 2010, 58, 1523–1534. [Google Scholar] [CrossRef]
- Chu, K.O.; Chan, K.P.; Yang, Y.; Qin, Y.J.; Li, W.Y.; Chan, S.O.; Wang, C.C.; Pang, C.P. Effects of EGCG content in green tea extract on pharmacokinetics, oxidative status and expression of inflammatory and apoptotic genes in the rat ocular tissues. J. Nutr. Biochem. 2015, 26, 1357–1367. [Google Scholar] [CrossRef]
- Qin, Y.J.; Chu, K.O.; Yip, Y.W.; Li, W.Y.; Yang, Y.P.; Chan, K.P.; Ren, J.L.; Chan, S.O.; Pang, C.P. Green tea extract treatment alleviates ocular inflammation in a rat model of endotoxin-induced uveitis. PLoS ONE 2014, 9, e103995. [Google Scholar] [CrossRef]
- Chu, K.O.; Wang, C.C.; Chu, C.Y.; Choy, K.W.; Pang, C.P.; Rogers, M.S. Uptake and distribution of catechins in fetal organs following in utero exposure in rats. Hum. Reprod. 2007, 22, 280–287. [Google Scholar] [CrossRef]
- Fung, S.T.; Ho, C.K.; Choi, S.W.; Chung, W.Y.; Benzie, I.F.F. Comparison of catechin profiles in human plasma and urine after single dosing and regular intake of green tea (Camellia sinensis). Br. J. Nutr. 2013, 109, 2199–2207. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Auger, C.; Hara, Y.; Crozier, A. Bioavailability of polyphenon E flavan-3-ols in humans with an ileostomy. J. Nutr. 2008, 138, 1535S–1542S. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Ichikawa, T.; Minoda, K.; Kusaka, K.; Ito, S.; Suzuki, Y.; Akagawa, M.; Mochizuki, K.; Goda, T.; Nakayama, T. Human serum albumin as an antioxidant in the oxidation of (−)-epigallocatechin gallate: Participation of reversible covalent binding for interaction and stabilization. Biosci. Biotechnol. Biochem. 2011, 75, 100–106. [Google Scholar] [CrossRef]
- Ishii, T.; Minoda, K.; Bae, M.J.; Mori, T.; Uekusa, Y.; Ichikawa, T.; Aihara, Y.; Furuta, T.; Wakimoto, T.; Kan, T.; et al. Binding affinity of tea catechins for HSA: Characterization by high-performance affinity chromatography with immobilized albumin column. Mol. Nutr. Food Res. 2010, 54, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.L.; Crespy, V.; Oliveira, M.; Cooper, K.A.; Gibson, B.B.; Williamson, G. (+)-Catechin is more bioavailable then (−)-catechin: Relevance to the bioavailability of catechin from cocoa. Free Radic. Res. 2006, 40, 1029–1034. [Google Scholar] [CrossRef]
- Stalmach, A.; Troufflard, S.; Serafini, M.; Crozier, A. Absorption, metabolism and excretion of Choladi green tea flavan-3-ols by humans. Mol. Nutr. Food Res. 2009, 53 (Suppl. S1), S44–S53. [Google Scholar] [CrossRef]
- Lambert, J.D.; Elias, R.J. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Lee, L.S.; Kim, S.H.; Kim, Y.B.; Kim, Y.C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef]
- Martínez, V.; Ugartondo, V.; Vinardell, M.P.; Torres, J.L.; Mitjans, M. Grape epicatechin conjugates prevent erythrocyte membrane protein oxidation. J. Agric. Food Chem. 2012, 60, 4090–4095. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Imran, A.; Sharif, M.K.; Ahmad, R.S.; Xiao, H.; Imran, M.; Rsool, H.A. Black tea polyphenols: A mechanistic treatise. Crit. Rev. Food Sci. Nutr. 2014, 54, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Yokotani, K.; Umegaki, K. Evaluation of plasma antioxidant activity in rats given excess EGCg with reference to endogenous antioxidants concentrations and assay methods. Free Radic. Res. 2017, 51, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Chen, Y.K.; Hou, Z.; Xiao, H.; Jin, H.; Lu, G.; Lee, M.J.; Liu, B.; Guan, F.; Yang, Z.; et al. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: A comparative study in vivo and in vitro. Carcinogenesis 2010, 31, 902–910. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hasumi, K.; Woo, J.T.; Nagai, K.; Wachi, M. Generation of hydrogen peroxide primarily contributes to the induction of Fe (II)-dependent apoptosis in Jurkat cells by (−)-epigallocatechin gallate. Carcinogenesis 2004, 25, 1567–1574. [Google Scholar] [CrossRef]
- Nakagawa, H.; Wachi, M.; Woo, J.T.; Kato, M.; Kasai, S.; Takahashi, F.; Lee, I.S.; Nagai, K. Fenton reaction is primarily involved in a mechanism of (−)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem. Biophys. Res. Commun. 2002, 292, 94–101. [Google Scholar] [CrossRef]
- Ko, C.H.; Li, K.; Ng, P.C.; Fung, K.P.; Li, C.L.; Wong, R.P.O.; Chui, K.M.; Gu, G.J.S.; Yung, E.; Wang, C.C.; et al. Pro-oxidative effects of tea and polyphenols, epigallocatechin-3-gallate and epigallocatechin, on G6PD-deficient erythrocytes in vitro. Int. J. Mol. Med. 2006, 18, 987–994. [Google Scholar] [CrossRef]
- Elbling, L.; Herbacek, I.; Weiss, R.M.; Jantschitsch, C.; Micksche, M.; Gerner, C.; Pangratz, H.; Grusch, M.; Knasmuller, S.; Berger, W. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free Radic. Biol. Med. 2010, 49, 1444–1452. [Google Scholar] [CrossRef]
- Bae, Y.S.; Lee, J.H.; Choi, S.H.; Kim, S.; Almazan, F.; Witztum, J.L.; Miller, Y.I. Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: Toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2. Circ. Res. 2009, 104, 210–218. [Google Scholar] [CrossRef]
- Pullikotil, P.; Chen, H.; Muniyappa, R.; Greenberg, C.C.; Yang, S.; Reiter, C.E.; Lee, J.W.; Chung, J.H.; Quon, M.J. Epigallocatechin gallate induces expression of heme oxygenase-1 in endothelial cells via p38 MAPK and Nrf-2 that suppresses proinflammatory actions of TNF-alpha. J. Nutr. Biochem. 2012, 23, 1134–1145. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsu, M.C.; Hsieh, C.W.; Lin, J.B.; Lai, P.H.; Wung, B.S. Upregulation of heme oxygenase-1 by Epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006, 78, 2889–2897. [Google Scholar] [CrossRef]
- Chu, K.O.; Chan, S.O.; Pang, C.P.; Wang, C.C. Pro-oxidative and antioxidative controls and signaling modification of polyphenolic phytochemicals: Contribution to health promotion and disease prevention? J. Agric. Food Chem. 2014, 62, 4026–4038. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.L.; Yu, Q.X.; Liang, W.C.; Leung, P.Y.; Ng, T.K.; Chu, W.K.; Pang, C.P.; Chan, S.O. Green tea extract attenuates LPS-induced retinal inflammation in rats. Sci. Rep. 2018, 8, 429. [Google Scholar] [CrossRef]
- Chu, K.O.; Chan, K.P.; Yip, Y.W.Y.; Chu, W.K.; Wang, C.C.; Pang, C.P. Systemic and Ocular Anti-Inflammatory Mechanisms of Green Tea Extract on Endotoxin-Induced Ocular Inflammation. Front. Endocrinol. 2022, 13, 899271. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yip, Y.W.Y.; Ren, J.L.; Hui, W.K.; He, J.N.; Yu, Q.X.; Chu, K.O.; Ng, T.K.; Chan, S.O.; Pang, C.P.; et al. Green tea catechins alleviate autoimmune symptoms and visual impairment in a murine model for human chronic intraocular inflammation by inhibiting Th17-associated pro-inflammatory gene expression. Sci. Rep. 2019, 9, 2301. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals-An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Barro, L.; Tsai, S.T.; Feng, T.W.; Wu, X.Y.; Chao, C.W.; Yu, R.S.; Chin, T.Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.O.; Man, G.C.W.; Chan, K.P.; Chu, C.Y.; Chan, T.H.; Pang, C.P.; Wang, C.C. Determination of exogenous epigallocatechin gallate peracetate in mouse plasma using liquid chromatography with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2014, 37, 3473–3480. [Google Scholar] [CrossRef]
- Wang, C.C.; Xu, H.; Man, G.C.W.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.Y.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Ascenção, K.; Silva, F.A.; Sarmento, B.; Oliveira, M.B.; Andrade, J.C. Drug-delivery systems of green tea catechins for improved stability and bioavailability. Curr. Med. Chem. 2013, 20, 4744–4757. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Zheng, T.; Ho, C.T.; Huang, Q.R.; Wu, Q.L.; Zhang, M. Improving the stability and bioavailability of tea polyphenols by encapsulations: A review. Food Sci. Hum. Wellness 2022, 11, 537–556. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segrè, A.V.; Rouhana, J.M.; et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Gazzard, G.; Konstantakopoulou, E.; Garway-Heath, D.; Garg, A.; Vickerstaff, V.; Hunter, R.; Ambler, G.; Bunce, C.; Wormald, R.; Nathwani, N.; et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): A multicentre andomized controlled trial. Lancet 2019, 393, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.M.; Crawley, L.; Pahlitzsch, M.; Javaid, F.; Cordeiro, M.F. Glaucoma: The retina and beyond. Acta. Neuropathologica 2016, 132, 807–826. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, Y.; Liang, J.J.; Zhuang, X.; Ng, T.K. Longitudinal and simultaneous profiling of 11 modes of cell death in mouse retina post-optic nerve injury. Exp. Eye Res. 2022, 222, 109159. [Google Scholar] [CrossRef]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef]
- Baudouin, C.; Kolko, M.; Melik-Parsadaniantz, S.; Messmer, E.M. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog. Retin. Eye Res. 2021, 83, 100916. [Google Scholar] [CrossRef]
- Mozaffarieh, M.; Grieshaber, M.C.; Flammer, J. Oxygen and blood flow: Players in the pathogenesis of glaucoma. Mol. Vis. 2008, 14, 224–233. [Google Scholar] [PubMed]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, D.; Griffiths, H.R.; Hilton, E.J.; Cunliffe, I.A.; Hosking, S.L. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Gherghel, D.; Mroczkowska, S.; Qin, L. Reduction in blood glutathione levels occurs similarly in patients with primary-open angle or normal tension glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3333–3339. [Google Scholar] [CrossRef]
- Tezel, G.; Wax, M.B. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch. Ophthalmol. 2004, 122, 1348–1356. [Google Scholar] [CrossRef]
- Fan, W.; Huang, W.; Chen, J.; Li, N.; Mao, L.; Hou, S. Retinal microglia: Functions and diseases. Immunology 2022, 166, 268–286. [Google Scholar] [CrossRef]
- Tezel, G. Molecular regulation of neuroinflammation in glaucoma: Current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye Res. 2022, 87, 100998. [Google Scholar] [CrossRef]
- Yuan, L.; Neufeld, A.H. Activated microglia in the human glaucomatous optic nerve head. J. Neurosci. Res. 2001, 64, 523–532. [Google Scholar] [CrossRef]
- Gramlich, O.W.; Beck, S.; von Thun Und Hohenstein-Blaul, N.; Boehm, N.; Ziegler, A.; Vetter, J.M.; Pfeiffer, N.; Grus, F.H. Enhanced insight into the autoimmune component of glaucoma: IgG autoantibody accumulation and pro-inflammatory conditions in human glaucomatous retina. PLoS ONE 2013, 8, e57557. [Google Scholar] [CrossRef]
- Yang, X.; Luo, C.; Cai, J.; Powell, D.W.; Yu, D.; Kuehn, M.H.; Tezel, G. Neurodegenerative and inflammatory pathway components linked to TNF-α/TNFR1 signaling in the glaucomatous human retina. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8442–8454. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, C.; Chen, Y.; Liang, J.J.; Xu, Y.; Chen, S.L.; Huang, S.; Yang, Q.; Cen, L.P.; Pang, C.P.; et al. Green Tea Extract Ameliorates Ischemia-Induced Retinal Ganglion Cell Degeneration in Rats. Oxid. Med. Cell. Longev. 2019, 2019, 8407206. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, B.A.; Rahman, R.M.; Appleton, I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J. Nutr. Biochem. 2006, 17, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Mak, H.K.; Yung, J.S.Y.; Weinreb, R.N.; Ng, S.H.; Cao, X.; Ho, T.Y.C.; Ng, T.K.; Chu, W.K.; Yung, W.H.; Choy, K.W.; et al. MicroRNA-19a-PTEN Axis Is Involved in the Developmental Decline of Axon Regenerative Capacity in Retinal Ganglion Cells. Mol. Ther. Nucleic Acids 2020, 21, 251–263. [Google Scholar] [CrossRef]
- Ng, T.K.; Yung, J.S.; Choy, K.W.; Cao, D.; Leung, C.K.; Cheung, H.S.; Pang, C.P. Transdifferentiation of periodontal ligament-derived stem cells into retinal ganglion-like cells and its microRNA signature. Sci. Rep. 2015, 5, 16429. [Google Scholar] [CrossRef] [PubMed]
- Suen, H.C.; Qian, Y.; Liao, J.; Luk, C.S.; Lee, W.T.; Ng, J.K.W.; Chan, T.T.H.; Hou, H.W.; Li, I.; Li, K.; et al. Transplantation of Retinal Ganglion Cells Derived from Male Germline Stem Cell as a Potential Treatment to Glaucoma. Stem Cells Dev. 2019, 28, 1365–1375. [Google Scholar] [CrossRef]
- Cen, L.P.; Ng, T.K.; Liang, J.J.; Zhuang, X.; Yao, X.; Yam, G.H.; Chen, H.; Cheung, H.S.; Zhang, M.; Pang, C.P. Human Periodontal Ligament-Derived Stem Cells Promote Retinal Ganglion Cell Survival and Axon Regeneration After Optic Nerve Injury. Stem Cells 2018, 36, 844–855. [Google Scholar] [CrossRef]
- Maher, P.; Hanneken, A. The molecular basis of oxidative stress-induced cell death in an immortalized retinal ganglion cell line. Investig. Ophthalmol. Vis. Sci. 2005, 46, 749–757. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.R.; Clark, A.F.; Daudt, D.; Vishwanatha, J.K.; Yorio, T. A forensic path to RGC-5 cell line identification: Lessons learned. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5712–5719. [Google Scholar] [CrossRef]
- Zhang, B.; Safa, R.; Rusciano, D.; Osborne, N.N. Epigallocatechin gallate, an active ingredient from green tea, attenuates damaging influences to the retina caused by ischemia/reperfusion. Brain Res. 2007, 1159, 40–53. [Google Scholar] [CrossRef]
- Jin, J.; Ying, H.; Huang, M.; Du, Q. Bioactive compounds in green tea leaves attenuate the injury of retinal ganglion RGC-5 cells induced by H2O2 and ultraviolet radiation. Pak. J. Pharm. Sci. 2015, 28, 2267–2272. [Google Scholar]
- Zhang, B.; Osborne, N.N. Oxidative-induced retinal degeneration is attenuated by epigallocatechin gallate. Brain Res. 2006, 1124, 176–187. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.L.; Xu, Y.; Yao, Y.; Liang, J.J.; Wang, L.; Jhanji, V.; Sun, X.; Ma, D.; Ng, T.K. Green Tea Catechins Attenuate Human Primary Pterygium Cell Survival and Migration Via Modulation of ERK p42/p44 and p38 Pathways. J. Agric. Food Chem. 2021, 69, 12209–12218. [Google Scholar] [CrossRef]
- Cao, L.; Liu, H.; Lam, D.S.; Yam, G.H.; Pang, C.P. In vitro screening for angiostatic potential of herbal chemicals. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6658–6664. [Google Scholar] [CrossRef]
- Yoneyama, S.; Kawai, K.; Tsuno, N.H.; Okaji, Y.; Asakage, M.; Tsuchiya, T.; Yamada, J.; Sunami, E.; Osada, T.; Kitayama, J.; et al. Epigallocatechin gallate affects human dendritic cell differentiation and maturation. J. Allergy Clin. Immunol. 2008, 121, 209–214. [Google Scholar] [CrossRef]
- Yang, N.; Shang, Y.X. Epigallocatechin gallate ameliorates airway inflammation by regulating Treg/Th17 imbalance in an asthmatic mouse model. Int. Immunopharmacol. 2019, 72, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.; Amit, T.; Reznichenko, L.; Weinreb, O.; Youdim, M.B. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol. Nutr. Food Res. 2006, 50, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarieh, M.; Grieshaber, M.C.; Orgül, S.; Flammer, J. The potential value of natural antioxidative treatment in glaucoma. Surv. Ophthalmol. 2008, 53, 479–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Jiang, L.; Shen, C.; Wan, H.; Xu, L.; Wang, N.; Jonas, J.B. Neuroprotective effect of epigallocatechin-3-gallate against N-methyl-D-aspartate-induced excitotoxicity in the adult rat retina. Acta Ophthalmol. 2012, 90, e609–e615. [Google Scholar] [CrossRef]
- Peng, P.H.; Chiou, L.F.; Chao, H.M.; Lin, S.; Chen, C.F.; Liu, J.H.; Ko, M.L. Effects of epigallocatechin-3-gallate on rat retinal ganglion cells after optic nerve axotomy. Exp. Eye Res. 2010, 90, 528–534. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, L.; Zhang, T.; Jin, Y.; Yang, D.; Chen, F. Neuroprotective effects of Epigallocatechin-3-gallate (EGCG) in optic nerve crush model in rats. Neurosci. Lett. 2010, 479, 26–30. [Google Scholar] [CrossRef]
- Yuan, X.L.; Chen, S.L.; Xu, Y.Y.; Yao, Y.; Liang, J.J.; Zhuang, X.; Hald, E.S.; Ng, T.K. Green tea extract enhances retinal ganglion cell survival and axonal regeneration in rats with optic nerve injury. J. Nutr. Biochem. 2023, 117, 109333. [Google Scholar] [CrossRef]
- Shen, C.; Chen, L.; Jiang, L.; Lai, T.Y. Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neurosci. Lett. 2015, 600, 132–136. [Google Scholar] [CrossRef]
- Peng, P.H.; Ko, M.L.; Chen, C.F. Epigallocatechin-3-gallate reduces retinal ischemia/reperfusion injury by attenuating neuronal nitric oxide synthase expression and activity. Exp. Eye Res. 2008, 86, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Rusciano, D.; Osborne, N.N. Orally administered epigallocatechin gallate attenuates retinal neuronal death in vivo and light-induced apoptosis in vitro. Brain Res. 2008, 1198, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, J.; Martínez-Rosas, M.; Conde-Castañón, C.A.; Toscano-Garibay, J.D.; Ruiz-Pérez, N.J.; Flores, P.L.; Mera Jiménez, E.; Flores-Estrada, J. Epigallocatechin 3-Gallate Has a Neuroprotective Effect in Retinas of Rabbits with Ischemia/Reperfusion through the Activation of Nrf2/HO-1. Int. J. Mol. Sci. 2020, 21, 3716. [Google Scholar] [CrossRef]
- Atkinson-Leadbeater, K.; Hehr, C.L.; Johnston, J.; Bertolesi, G.; McFarlane, S. EGCG stabilizes growth cone filopodia and impairs retinal ganglion cell axon guidance. Dev. Dyn. 2016, 245, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Duran, M.D.; Shoaie-Nia, K.; Zanon-Moreno, V.; Sanz-Gonzalez, S.M.; Del Castillo, J.B.; Garcia-Medina, J.J. Strategies to Reduce Oxidative Stress in Glaucoma Patients. Curr. Neuropharmacol. 2018, 16, 903–918. [Google Scholar] [CrossRef]
- Sadowska-Krępa, E.; Domaszewski, P.; Pokora, I.; Żebrowska, A.; Gdańska, A.; Podgórski, T. Effects of medium-term green tea extract supplementation combined with CrossFit workout on blood antioxidant status and serum brain-derived neurotrophic factor in young men: A pilot study. J. Int. Soc. Sport Nutr. 2019, 16, 13. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Kang, J.H.; Ivey, K.L.; Boumenna, T.; Rosner, B.; Wiggs, J.L.; Pasquale, L.R. Prospective study of flavonoid intake and risk of primary open-angle glaucoma. Acta Ophthalmol. 2018, 96, e692–e700. [Google Scholar] [CrossRef]
- Chous, A.P.; Richer, S.P.; Gerson, J.D.; Kowluru, R.A. The Diabetes Visual Function Supplement Study (DiVFuSS). Br. J. Ophthalmol. 2016, 100, 227–234. [Google Scholar] [CrossRef]
- Bae, J.H.; Kim, J.M.; Lee, J.M.; Song, J.E.; Lee, M.Y.; Chung, P.W.; Park, K.H. Effects of consumption of coffee, tea, or soft drinks on open-angle glaucoma: Korea National Health and Nutrition Examination Survey 2010 to 2011. PLoS ONE 2020, 15, e0236152. [Google Scholar] [CrossRef]
- Ramdas, W.D.; Wolfs, R.C.; Kiefte-de Jong, J.C.; Hofman, A.; de Jong, P.T.; Vingerling, J.R.; Jansonius, N.M. Nutrient intake and risk of open-angle glaucoma: The Rotterdam Study. Eur. J. Epidemiol. 2012, 27, 385–393. [Google Scholar] [CrossRef]
- Falsini, B.; Marangoni, D.; Salgarello, T.; Stifano, G.; Montrone, L.; Di Landro, S.; Guccione, L.; Balestrazzi, E.; Colotto, A. Effect of epigallocatechin-gallate on inner retinal function in ocular hypertension and glaucoma: A short-term study by pattern electroretinogram. Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 1223–1233. [Google Scholar] [CrossRef]
- Gasiunas, K.; Galgauskas, S. Green tea—A new perspective of glaucoma prevention. Int. J. Ophthalmol. 2022, 15, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Renno, W.M.; Khan, K.M.; Benov, L. Is there a role for neurotrophic factors and their receptors in augmenting the neuroprotective effect of (−)-epigallocatechin-3-gallate treatment of sciatic nerve crush injury? Neuropharmacology 2016, 102, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xu, J.; Shi, T.; Yu, H.; Bi, J.; Chen, G. Epigallocatechin-3-gallate augments therapeutic effects of mesenchymal stem cells in skin wound healing. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, Y.J.; Yip, Y.W.; Chan, K.P.; Chu, K.O.; Chu, W.K.; Ng, T.K.; Pang, C.P.; Chan, S.O. Green tea catechins are potent anti-oxidants that ameliorate sodium iodate-induced retinal degeneration in rats. Sci. Rep. 2016, 6, 29546. [Google Scholar] [CrossRef]
- Wang, J.; Man, G.C.W.; Chan, T.H.; Kwong, J.; Wang, C.C. A prodrug of green tea polyphenol (−)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer. Cancer Lett. 2018, 412, 10–20. [Google Scholar] [CrossRef]
- Mazzanti, G.; Menniti-Ippolito, F.; Moro, P.A.; Cassetti, F.; Raschetti, R.; Santuccio, C.; Mastrangelo, S. Hepatotoxicity from green tea: A review of the literature and two unpublished cases. Eur. J. Clin. Pharmacol. 2009, 65, 331–341. [Google Scholar] [CrossRef] [PubMed]
| Biological Properties | Mechanisms | References |
|---|---|---|
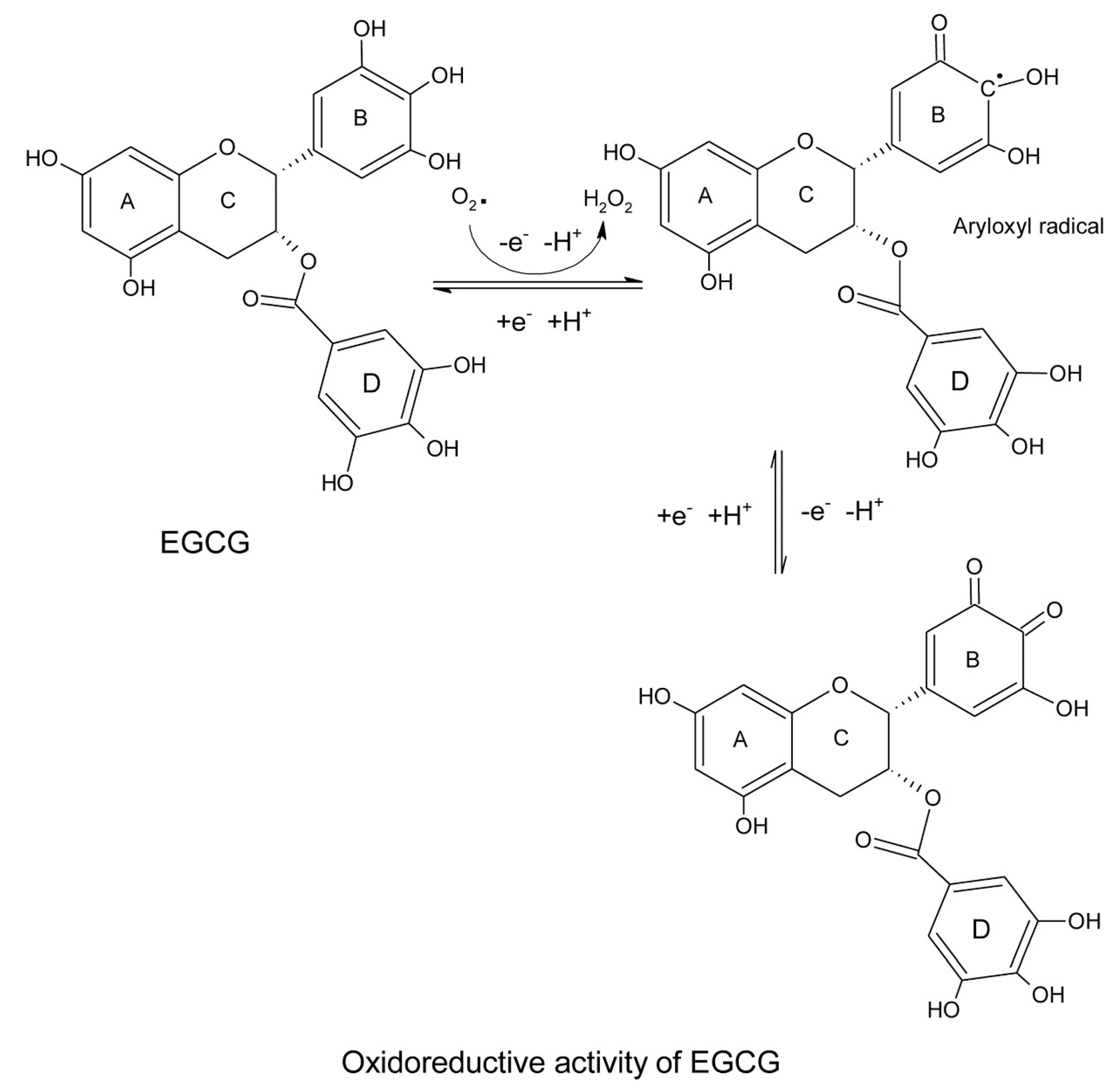
| Electron resonance within the phenolic moiety following abstraction of proton by ROS. | [15,16] |
| High reduction potential of catechins compared to endogenous antioxidants; reducing and recycling the oxidized endogenous molecules. | [17] |
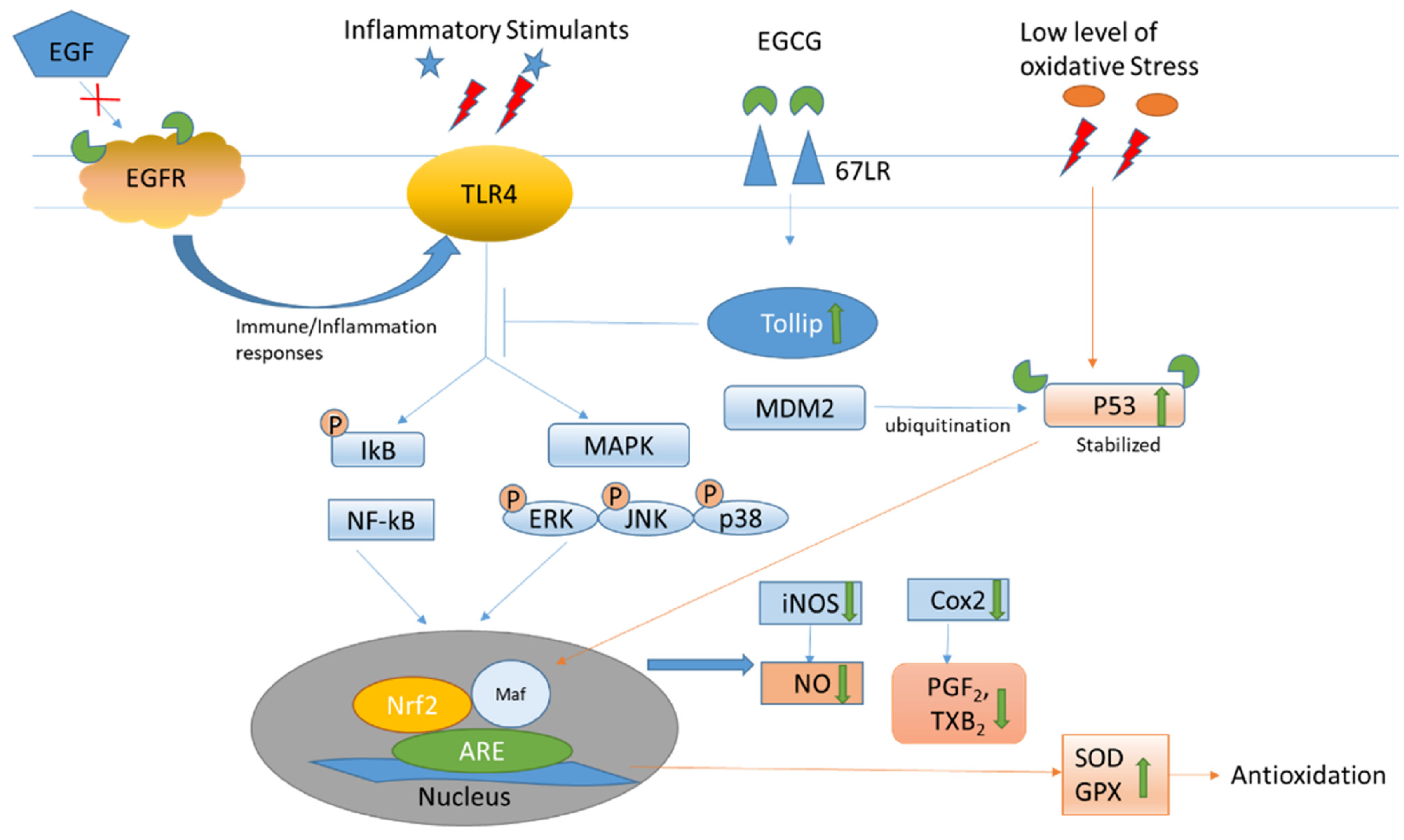
| Binding to 67LR to increase Tollip expression, which negatively regulates TLR signaling to suppress inflammatory mediators. | [18] |
| Binding to the active sites of p53 and changing the structural conformation to prevent ubiquitination by MDM2; retaining the biological level and activities of p53. Inhibiting the activation of the wild-type and some mutant EGF receptors in non-small cell lung cancer cell lines. | [19,21] |
| Binding to the EGF receptor to change the topology and block EGF to activate the receptor for subsequent inflammation activities. | [20] |
| Inhibiting anti-oxidative molecules, including the Trx/Trx receptor to increase ROS, which acts as a secondary messenger for various pathways Activating CaMKKβ to increase energy metabolism; elevating cytosolic calcium to increase nitric oxide production. Increasing cAMP to promote the phosphorylation of eNOS and vasodilator-stimulated phosphoprotein to cause vaso-relaxation. Activating AMPK to reduce endothelin-1 expression for vasodilation. | [22,24,25,26,27,28,29] |
| Maximum Concentration | GTE | GC | EGC | C | EC | EGCG | GCG | ECG |
|---|---|---|---|---|---|---|---|---|
| Cmax (nM) | ||||||||
| Plasma | Sunphenon DCF-1 | 91.5 ± 57.4 | 754.9 ± 235.8 | 139.0 ± 57.0 | 1258.4 ± 294.0 | 310.4 ± 59.9 | 50.8 ± 10.4 | 159.1 ± 33.9 |
| Theaphenon® E | 530.8 ± 200.2 | 13718.0 ± 4948.0 | 2990.0 ± 1990.0 | 9143.0 ± 1912.0 | 6687.0 ± 4437.0 | 131.3 ± 91.7 | 443.8 ± 352.3 | |
| Aqueous humor | Sunphenon DCF-1 | - | 602.9 ± 116.7 | 127.4 ± 62.8 | 138.9 ± 58.5 | 13.2 ± 5.1 | 33.5 ± 20.4 | 47.8 ± 8.1 |
| Theaphenon® E | 246.9 ± 34.9 | 911.3 ± 250.5 | 98.3 ± 19.2 | 708.1 ± 127.8 | 284.4 ± 58.4 | 0.57 ± 0.98 | 26.5 ± 10.3 | |
| Vitreous humor | Sunphenon DCF-1 | 110.6 ± 22.1 | 15.9 ± 7.0 | 96.5 ± 23.3 | 20.5 ± 10.6 | 15.4 ± 2.7 | 20.9 ± 9.9 | 14.0 ± 5.1 |
| Theaphenon® E | 4492.0 ± 443.5 | 404.1 ± 102.5 | 321.7 ± 69.5 | 436.8 ± 102.5 | 2224.4 ± 805.4 | 33.9 ± 31.0 | 369.6 ± 74.0 | |
| Cmax (ρmol/g) | ||||||||
| Choroid–sclera | Sunphenon DCF-1 | 11461.8 ± 5168.7 | 1506.3 ± 941.1 | 477.6 ± 346.9 | 283.5 ± 66.5 | 184.4 ± 39.0 | 220.5 ± 69.7 | 10.7 ± 4.3 |
| Theaphenon® E | 188.28 ± 111.3 | 542.2 ± 335.1 | 294.7 ± 32.8 | 1818.0 ± 563.0 | 1183.0 ± 611.0 | 59.0 ± 54.8 | 518.0 ± 292.0 | |
| Retina | Sunphenon DCF-1 | 22729.4 ± 4229.4 | 8020.8 ± 1658.5 | 492.7 ± 235.2 | 608.0 ± 112.0 | 259.1 ± 67.2 | 3.2 ± 1.9 | - |
| Theaphenon® E | 61.0 ± 43.5 | 118.2 ± 55.6 | 35.7 ± 15.0 | 174.5 ± 45.8 | 784.4 ± 195.9 | 59.0 ± 54.8 | 64.0 ± 16.0 | |
| Lens | Sunphenon DCF-1 | 1558.1 ± 318.4 | 1172.3 ± 207.8 | 300.0 ± 151.5 | 72.3 ± 19.1 | 149.1 ± 26.5 | 18.0 ± 6.6 | 90.3 ± 45.8 |
| Theaphenon® E | 1.9 ± 3.0 | 10.9 ± 8.9 | 4.1 ± 4.8 | 4.6 ± 6.9 | 43.9 ± 25.8 | 0.4 ± 0.6 | 1.0 ± 3.0 | |
| Cornea | Sunphenon DCF-1 | - | 359.4 ± 66.8 | 58.5 ± 15.4 | 30.6 ± 5.7 | 25.2 ± 15.5 | 10.7 ± 3.9 | 91.1 ± 18.7 |
| Theaphenon® E | 10.8 ± 16.7 | 59.5 ± 26.7 | 61.7 ± 17.5 | 536.4 ± 61.1 | 634.6 ± 122.9 | 18.8 ± 24.2 | 101.8 ± 43.1 | |
| Elimination | GTE | GC | EGC | C | EC | EGCG | GCG | ECG |
| λz (h−1) | ||||||||
| Plasma | Sunphenon DCF-1 | 0.107 ± 0.010 | 0.213 ± 0.015 | 0.104 ± 0.038 | 0.371 ± 0.000 | 0.236 ± 0.007 | 0.171 ± 0.013 | 0.211 ± 0.010 |
| Theaphenon® E | 0.270 ± 0.030 | 0.390 ± 0.040 | 0.370 ± 0.080 | 0.400 ± 0.050 | 0.230 ± 0.020 | 1.250 ± 0.380 | 0.210 ± 0.040 | |
| Aqueous humor | Sunphenon DCF-1 | - | 0.045 ± 0.001 | 0.209 ± 0.012 | 0.093 ± 0.062 | 0.304 ± 0.012 | 0.111 ± 0.033 | 0.124 ± 0.043 |
| Theaphenon® E | 0.110 ± 0.020 | 0.240 ± 0.020 | 0.130 ± 0.030 | 0.210 ± 0.040 | 0.090 ± 0.020 | - | 0.130 ± 0.120 | |
| Vitreous humor | Sunphenon DCF-1 | 0.166 ± 0.010 | 0.041 ± 0.001 | 0.106 ± 0.030 | 0.067 ± 0.004 | 0.058 ± 0.012 | 0.042 ± 0.006 | 0.224 ± 0.035 |
| Theaphenon® E | 0.020 ± 0.010 | 0.110 ± 0.090 | 0.110 ± 0.060 | 0.100 ± 0.030 | 0.080 ± 0.020 | - | - | |
| Choroid–sclera | Sunphenon DCF-1 | 0.057 ± 0.001 | 0.461 ± 0.015 | 0.220 ± 0.014 | 0.488 ± 0.007 | 0.267 ± 0.019 | 0.929 ± 0.049 | - |
| Theaphenon® E | - | 0.250 ± 0.090 | 0.220 ± 0.090 | 0.370 ± 0.060 | 0.080 ± 0.040 | - | 0.150 ± 0.070 | |
| Retina | Sunphenon DCF-1 | 0.188 ± 0.045 | 0.203 ± 0.050 | 0.245 ± 0.010 | 2.432 ± 0.154 | 0.413 ± 0.040 | - | - |
| Theaphenon® E | - | 0.040 ± 0.030 | 0.040 ± 0.010 | 0.060 ± 0.020 | 0.040 ± 0.020 | - | 0.090 ± 0.030 | |
| Lens | Sunphenon DCF-1 | 0.302 ± 0.049 | 0.084 ± 0.020 | 0.234 ± 0.032 | 0.049 ± 0.004 | 0.269 ± 0.011 | 3.160 ± 0.130 | - |
| Theaphenon® E | - | - | - | - | 0.130 ± 0.060 | - | - | |
| Cornea | Sunphenon DCF-1 | - | 0.170 ± 0.031 | 0.116 ± 0.007 | 0.043 ± 0.012 | 0.125 ± 0.001 | 0.372 ± 0.006 | 0.477 ± 0.021 |
| Theaphenon® E | - | - | 0.220 ± 0.100 | 0.220 ± 0.100 | 0.090 ± 0.020 | - | 0.100 ± 0.090 |
| Identifier. | Country | Status | Phase | Enrollment | Targeted Eye Diseases or Conditions | Intervention | Dosage | Duration |
|---|---|---|---|---|---|---|---|---|
| NCT00476138 | Italy | Unknown | Phase I/II | 40 | Primary open angle glaucoma Ocular hypertension | Oral EGCG treatment | 200 mg/day | 3 months |
| NCT00718653 | United States | Completed | Not Applicable | 40 | Eye health | Lutein plus green tea extract | Lutein (12 mg/day) Green tea extract (200 mg/day) | Unknown |
| NCT01646047 | United States | Completed [118] | Not Applicable | 70 | Diabetes Mellitus—Type 1 Diabetes Mellitus—Type 2 Non-proliferative diabetic retinopathy | Multi-component nutritional supplement capsules (vitamin C, mixed tocopherols/tocotrienols, vitamin D, fish oil, lutein, zeaxanthin, pine bark extract, benfotiamine, curcumin, and green tea extract) | 2 capsules/day | 6 months |
| NCT02984813 | United States | Terminated | Phase I | 21 | Open-angle glaucoma Diabetic retinopathy | Nutritional supplements capsules (alpha lipoic acid, citicoline, Co-enzyme Q10, Ginkgo biloba extract, grape seed extract, N-acetyl-cysteine, curcumin, and green tea extract) | 2 capsules/day | 3 months |
| NCT03866005 | United States | Unknown | Not Applicable | 150 | Center-involved diabetic macular edema | Multi-component nutritional supplement capsules (macular carotenoids lutein, zeaxanthin, vitamins B1, B12, C, D, E, lipoic acid, coenzyme Q10, resveratrol, patented extract of French maritime pine bark grape seed, curcumin, and green tea extract) | 2 or 4 capsules/day | Study duration |
| NCT04117022 | United States | Recruiting | Not Applicable | 45 | Diabetes Diabetic Retinopathy | Multi-component nutritional supplement capsules (vitamins C, D3 and E (d-α tocopherol), zinc oxide, eicosapentaenoic acid, docosahexaenoic acid, α-lipoic acid, coenzyme Q10, mixed tocotrienols/tocopherols, zeaxanthin, lutein, benfotiamine, N-acetyl cysteine, grape seed extract, resveratrol, turmeric root extract, Pycnogeno, and green tea leaf) | 2 capsules/day | 6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, T.K.; Chu, K.O.; Wang, C.C.; Pang, C.P. Green Tea Catechins as Therapeutic Antioxidants for Glaucoma Treatment. Antioxidants 2023, 12, 1320. https://doi.org/10.3390/antiox12071320
Ng TK, Chu KO, Wang CC, Pang CP. Green Tea Catechins as Therapeutic Antioxidants for Glaucoma Treatment. Antioxidants. 2023; 12(7):1320. https://doi.org/10.3390/antiox12071320
Chicago/Turabian StyleNg, Tsz Kin, Kai On Chu, Chi Chiu Wang, and Chi Pui Pang. 2023. "Green Tea Catechins as Therapeutic Antioxidants for Glaucoma Treatment" Antioxidants 12, no. 7: 1320. https://doi.org/10.3390/antiox12071320
APA StyleNg, T. K., Chu, K. O., Wang, C. C., & Pang, C. P. (2023). Green Tea Catechins as Therapeutic Antioxidants for Glaucoma Treatment. Antioxidants, 12(7), 1320. https://doi.org/10.3390/antiox12071320








