Abstract
Oxidative stress is considered one of the main determinants in the pathophysiology of non-alcoholic fatty liver disease (NAFLD) and obesity. The alterations of oxidant/antioxidant balance are related to chronic impairment of metabolism leading to mitochondrial dysfunction. Increased oxidative stress also triggers hepatocytes stress pathways, leading to inflammation and contributing to the progression of non-alcoholic steatohepatitis (NASH). Currently, the first-line therapeutic treatment of NAFLD is based on lifestyle interventions, suggesting the Mediterranean Diet (MD) as a preferable nutritional approach due to its antioxidant properties. However, it is still debated if adherence to MD could have a role in determining the risk of developing NAFLD directly or indirectly through its effect on weight. We enrolled 336 subjects (aged 35.87 ± 10.37 years; BMI 31.18 ± 9.66 kg/m2) assessing anthropometric parameters, lifestyle habits, metabolic parameters (fasting plasma glucose, fasting plasma insulin, triglycerides (TG), total cholesterol, low-density (LDL) and high-density lipoprotein (HDL) cholesterol, alanine transaminase (ALT), aspartate aminotransferase (AST), and γ-glutamyltransferase (γGT), cardio-metabolic indices [Homeostatic Model Assessment Insulin Resistance (HoMA-IR), visceral adipose index (VAI) and fatty liver index (FLI)] and adherence to MD [with the PREvención con DIetaMEDiterránea (PREDIMED) questionnaire]. Subjects with NAFLD had significantly higher anthropometric parameters, cardio-metabolic indices and lower adherence to MD than subjects without NAFLD. In a multiple regression analysis, PREDIMED score was the main predictor of FLI (p < 0.001) and came in first, followed by HoMA-IR, while VAI was not a predictor. A PREDIMED score value of <6 could serve as a threshold to identify patients who are more likely to have NAFLD (p < 0.001). In conclusion, high adherence to MD resulted in a lower risk of having NAFLD. Adherence to MD could have a direct role on the risk of developing NAFLD, regardless of visceral adipose tissue.
Keywords:
Mediterranean diet; non-alcoholic liver disease; obesity; diet; nutrition; VAI; FLI; HoMA-IR 1. Introduction
The prevalence of non-alcoholic fatty liver disease (NAFLD) has increased from 25% (1990–2006) to 38% (2016–2019), becoming a growing epidemic condition [1,2]. NAFLD encompasses the entire spectrum of fatty liver disease, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), characterized by inflammation with hepatocyte lesions and with or without varying degrees of fibrosis, to cirrhosis and hepatocellular carcinoma (HCC) [3,4].
NAFLD is considered a multifactorial disease in which environmental factors, such as nutrient intake and exposure, physical activity, genetics, epigenetics, and gut microbiota composition have been shown to interact with each other contributing to its development [5,6]. In addition, oxidative stress represents an important key mediator in low-grade inflammation in metabolic syndrome and mostly in the progression of NAFLD into NASH.
Lifestyle management, such as diet and physical activity, aiming at controlling body weight and cardio-metabolic risk factors related to metabolic syndrome, is the basis of NAFLD treatment [7]. In this regard, the EASL-EASD-EASO clinical Practice Guidelines recommend as current treatment for NAFLD the Mediterranean diet (MD), an antioxidant nutritional approach rich in compounds, such as polyphenols, carotenoids, fiber, polyunsaturated fatty acids, low-refined and low-sugar foods, together with constant physical activity [4]. These recommendations are based on evidence that highlight the association between adherence to MD and NAFLD. Kontogianni et al. carried out a study on 73 subjects with obesity or overweight, of which 34 underwent liver biopsies [8]. Interestingly, a higher adherence to MD (as determined by the MedDiet Score) was associated with a lower likelihood to develop NAFLD [8]. This finding was also confirmed by Aller et al. who found subjects that more highly adhered to MD (as determined by the 14-item MD assessment tool) were less likely to develop NAFLD with severe histological features, as well as having insulin resistance [9]. Very recently, Baratta et al. found that adherence to MD was inversely associated with NAFLD prevalence (as evaluated by ultrasound) in a population of subjects with obesity or overweight at high risk of cardio-metabolic diseases [10]. In addition, a high adherence to MD was related to an increased chance to improve cardio-metabolic risk factors. However, low adherence to MD has also been detected in subjects with obesity [11].
Thus, the aim of our study was to investigate the association between adherence to MD and the development of NAFLD, while also considering the potential impact of visceral adiposity and insulin resistance on this association. By exploring the interplay between MD, visceral adiposity, and insulin resistance, our study aimed to provide a deeper understanding of the complex mechanisms underlying NAFLD development and potentially identify new avenues for prevention and treatment strategies.
2. Materials and Methods
This was a cross-sectional observational study carried out at the Department of Clinical Medicine and Surgery, Unit of Endocrinology, University Federico II, Naples (Italy). The study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, and was approved by the Ethical Committee of the University of Naples “Federico II” Medical School (n. 05/14). The purpose of the protocol was explained to all the study participants, and written informed consent was obtained.
2.1. Population Study
Recruitment strategies included a sample of 336 subjects (age 35.87 ± 10.37 years; BMI 31.18 ± 9.66 kg/m2) of both genders consecutively enrolled among patients of the outpatient clinic at the Department of Clinical Medicine and Surgery, Unit of Endocrinology, University Federico II, Naples (Italy). The recruitment began in January 2022 and ended in January 2023. A full medical history, including drug use, was collected. To increase the homogeneity of the subject sample, we included only adults of both genders with the following criteria of exclusion:
- Impaired renal function (estimated glomerular filtration rate ≥ 90 mL/min/1.73 m2 calculated according to Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [12];
- Chronic liver diseases, viral hepatitis patients, hemochromatosis, hepatic malignancy;
- Presence of type 2 diabetes (T2DM) (according to the criteria of the American Diabetes Association (ADA) as follows: basal blood glucose ≥ 126 mg/dL on two occasions, or glycated haemoglobin (HbA1c) ≥ 6.5% (≥48 mmoL/moL) on two occasions, or both at the same time) [13]. Furthermore, participants on antidiabetic medication were considered to have T2DM;
- Clinical atherosclerosis (coronary artery disease, peripheral vascular disease);
- User of antibiotics or probiotics within two months of recruitment;
- Specific nutritional regimens, including vegan or vegetarian diets;
- Vitamin/mineral or antioxidant supplementation;
- Alcohol abuse according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) V diagnostic criteria [14].
2.2. Anthropometric Measurements
Anthropometric measurements were carried out in the morning between 8 and 10 am, after the participants fasted overnight. A trained nutritionist carried out the measurements using standardized procedures. During the visit, the participants were asked to wear light clothes and no shoes. The height and weight of each participant were measured to calculate their body mass index (BMI) (weight in kg divided by height squared in m2, kg/m2). Height was measured with a wall-mounted stadiometer (Seca 711; Seca, Hamburg, Germany) to the nearest 0.5 cm, while body weight was determined using a calibrated balance beam scale (Seca 711; Seca, Hamburg, Germany) to the nearest 0.1 kg. BMI was classified according to the World Health Organization (WHO)’s criteria, which defines a normal weight as 18.5–24.9 kg/m2, overweight as 25.0–29.9 kg/m2, grade I obesity as 30.0–34.9 kg/m2, grade II obesity as 35.0–39.9 kg/m2, and grade III obesity as ≥40.0 kg/m2. The waist circumference (WC) was measured to the nearest 0.1 cm using a non-stretchable measuring tape at the natural indentation or at a midway level between the lower edge of the rib cage and iliac crest, if no natural indentation was visible [15]. In accordance with NCEP ATP III criteria we considered WC cut-offs indicative of abdominal obesity as 102 cm for men and 88 cm for women [16].
2.3. Adherence to Mediterranean Diet
Adherence to MD was assessed using the 14-item Prevención con Dieta Mediterránea (PREDIMED) questionnaire, which has been validated before [17]. As previously reported, a trained nutritionist administered the questionnaire to all study participants during an in-person interview [18,19]. Each item was assigned either a score of 1 or 0, and the PREDIMED score was calculated based on the total scores. A score of 0–5 indicated the lowest adherence to MD, a score of 6–9 indicated average adherence, and a score of ≥10 indicated the highest adherence to MD.
2.4. Physical Activity and Smoking Habits
Physical activity levels were assessed using a standardized questionnaire that asked participants whether they engaged in at least 30 minutes of aerobic exercise per day on a regular basis (yes or no responses). Similarly, smoking habits were also evaluated using a standard questionnaire (yes or no responses). Participants who had quit smoking at least one year prior to the interview were classified as “former smokers”, while those who smoked at least one cigarette per day were classified as “current smokers”. Participants who did not currently smoke and had not smoked in the past year were considered “non-current smokers”. For the purposes of the analysis, former and non-current smokers were grouped together as “non-smokers”. These methods have been used in previous studies [20,21,22,23].
2.5. Assay Methods
Blood specimens were collected in the morning between 8 and 10 am after the participants fasted for at least 8 h and were stored at a temperature of −80 °C until they were processed. All biochemical analyses, including fasting plasma glucose, total cholesterol, fasting plasma triglycerides (TG), alanine transaminase (ALT), aspartate aminotransferase (AST), and γ-glutamyltransferase (γGT), were conducted using a Roche Modular Analytics System in the Central Biochemistry Laboratory of the institution. The levels of low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol were determined using a direct method that uses a homogeneous enzymatic assay for the quantitative determination of LDL and HDL cholesterol. Fasting plasma insulin levels were measured using commercially available kits and a solid-phase chemiluminescent enzyme immunoassay. The intra-assay coefficients of variation were less than 5.5%, which has been previously reported [20,21,22,23].
2.6. Non-Alcoholic Fatty Liver Disease
The presence of NAFLD was assessed by means of the ‘fatty liver index’ (FLI). FLI was calculated using a specific validated formula and according to Bedogni’s criterion, an FLI ≥ 60 was considered as the cut-off value for indicating the presence of NAFLD [24].
FLI = eL/(1 + eL) × 100, L = 0.953 × loge TG + 0.139 BMI + 0.718 × logeγGT + 0.053 × WC − 15.745
FLI is an accurate and easy-to-use index, as BMI, WC, TG and γGT are routine measures in clinical practice.
2.7. Cardio-Metabolic Indices
Visceral adipose index (VAI) score was calculated separately for males and females using a sex-specific formula, and age-specific VAI cut-off values were applied based on Amato et al.’s research [25,26].
For males, the VAI score was calculated using the formula:
VAI = [WC/39.68 + (1.88 × BMI)] × (TG/1.03) × (1.31/HDL)
For females, the VAI score was calculated using the formula:
VAI = [WC/36.58 + (1.89 × BMI)] × (TG/0.81) × (1.52/HDL)
Homeostatic Model Assessment Insulin Resistance (HoMA IR) was calculated according to Matthews et al. and a value of HoMA-IR > 2.5 was used as cut-off of insulin resistance [27].
HoMA-IR = (fasting plasma glucose × fasting plasma insulin)/405
2.8. Power Size Justification
The power of the sample was calculated by the difference of means ± standard deviation (SD) of adherence to MD between subjects with FLI < 60 and subjects with FLI ≥ 60 (9.97 ± 2.33 vs. 6.01 ± 2.44, respectively). The number of cases required was 18 individuals for the two groups.
The calculated power size was 95%, with a type I (alpha) error of 0.05 (95%), and a type II (beta) of 0.05. The calculations of sample size and power were performed while using a sample size calculator Clinical Calc (https://clincalc.com/stats/samplesize.aspx, accessed on 1 January 2022), as previously reported [20,21,22,23].
2.9. Statistical Analysis
Continuous variables were expressed as mean ± SD whereas categorical variables were reported as numbers (n) and percentage (%). Kolmogorov–Smirnov test was used to test data distribution.
Differences in age, anthropometric measurements, metabolic parameters, and cardio-metabolic indices in PREDIMED categories were analyzed by ANOVA test, with the Bonferroni test as a post-hoc test. Differences between FLI cutoff (<60 and ≥60) were analyzed by Student’s independent t-test. Correlations between study variables were performed using Pearson’s r correlation coefficients (continuous variables). A partial correlation analyses was used to determine the relationship between FLI and age, anthropometric measurements, metabolic parameters, and cardio-metabolic indices controlling for HoMA-IR and VAI. A multiple linear regression analysis models (stepwise method), expressed as R2, Beta (β), and t, with PREDIMED score as dependent variables were used to estimate the predictive value of cardio-metabolic indices. Receiver operator characteristic (ROC) curve analysis was performed to determine sensitivity and specificity, area under the curve (AUC), as well as cut-off values of PREDIMED score in detecting NAFLD. A p value < 0.05 was considered significant. Statistical analysis was performed according to standard methods using the Statistical Package for Social Sciences software 26.0 (SPSS/PC; SPSS, Chicago, IL, USA).
3. Results
Three hundred and thirty-six subjects (37.5% males and 62.5% females; aged 35.87 ± 10.37 years) were enrolled. In Table 1, sex, age, lifestyle habits, anthropometric measurements, metabolic parameters, and cardio-metabolic indices are reported. Normal weight was present in most (43.2%) of the enrolled subjects. Overweight was detected in 145 (13.4%) subjects of cohort, while grade I obesity was found in 38 subjects (11.3%), grade II obesity in 40 subjects (11.9%) and grade III obesity in 68 individuals (20.2%). The mean FLI was 53.77 ± 5.43. One hundred eighty-three (54.5%) subjects had FLI < 60, while 153 (45.6%) subjects had FLI ≥ 60.

Table 1.
Sex, age, lifestyle habits, anthropometric measurements, metabolic parameters and cardio-metabolic indices of the entire study population.
In Table 2, response frequency of dietary components included in PREDIMED questionnaire and adherence to MD of the entire study population are reported. Extra virgin olive oil was the most consumed food item (83.3%), followed by red processed meats with poultry in third position. Seventy-six subjects (22.6%) reported low adherence to MD, 152 subjects (45.2%) reported average adherence to MD, while 108 subjects (32.2%) reported high adherence to MD.

Table 2.
Response frequency of dietary components included in PREDIMED questionnaire and adherence to MD of the entire study population.
Study participants’ characteristics grouped according to the degree of adherence to MD are summarized in Table 3. As shown, subjects with low adherence to MD presented significant higher values of BMI and WC than subjects with average and high adherence to MD. In addition, subjects with low adherence to MD had significantly higher fasting plasma glucose, fasting plasma insulin, LDL cholesterol, TG, AST ALT, and γGT than subjects with average and high adherence to MD. In addition, cardio-metabolic indices such as HoMA-IR, VAI and FLI were significantly higher in subjects with low adherence to MD than subjects with average and high adherence to MD. HDL cholesterol was significantly lower in subjects with low adherence to MD compared to the other two groups. Interestingly, subjects with average adherence to MD had significantly higher BMI and WC than subjects with high adherence to MD. In the same manner, they had significantly higher fasting plasma glucose, fasting plasma insulin, LDL cholesterol, TG, AST, ALT, γGT than subjects with high adherence to MD. No differences were observed in age and male-to-female ratio. Cardio-metabolic indices, such as HoMA-IR, VAI and FLI, were significantly higher in subjects with average adherence to MD than subjects with high adherence to MD. HDL cholesterol was significantly lower in subjects with average adherence to MD compared to subjects with high adherence to MD. No differences were detected in terms of age among the three groups.

Table 3.
Anthropometric measurements, metabolic parameters and cardio-metabolic indices according to the adherence to MD.
Subjects with NAFLD (FLI ≥ 60) had significantly higher BMI and WC values compared to subjects without NAFLD (FLI < 60) (Table 4). Metabolic parameters such as fasting plasma glucose, fasting plasma insulin, LDL cholesterol, HDL cholesterol, TG, AST, ALT, γGT were significantly higher in subjects with NAFLD than subjects without NAFLD. Cardio-metabolic indices such as HoMA-IR, VAI and FLI were significantly higher in subjects with NAFLD than subjects without NAFLD. In addition, subjects with NAFLD (FLI ≥ 60) had significantly lower PREDIMED scores and a higher percentage of subjects with average adherence to the MD. No differences between the two groups were detected regarding age.

Table 4.
Anthropometric measurements, metabolic parameters, cardio-metabolic indices and PREDIMED score of the entire study population above and below the cut off of FLI.
The correlations of FLI with anthropometric measurements, metabolic parameters, cardio-metabolic indices and PREDIMED score are summarized in Table 5. FLI showed significant positive correlations with all anthropometric measurements, metabolic parameters and cardio-metabolic indices. Significant negative correlations of FLI with HDL cholesterol and PREDIMED score were observed. These correlations remained significant even after adjustment for VAI and HOMA-IR.

Table 5.
Correlations of FLI with anthropometric measurements, metabolic parameters, cardio-metabolic indices and PREDIMED score.
To compare the relative predictive power of the cardio-metabolic indices associated with PREDIMED score, we performed a multiple linear regression analysis using a model that included as HoMA-IR, VAI, and FLI (Table 6). Using this model, FLI entered at the first step (p < 0.001), while HoMA-IR and VAI were excluded. To compare the relative predictive power of FLI and the cardio-metabolic indices associated with PREDIMED score, we performed a second multiple linear regression analysis model. Using this second model, FLI entered at the first step (p < 0.001), while VAI were excluded.

Table 6.
Multiple regression analysis model (stepwise method) with the PREDIMED score as dependent variable to estimate the predictive value of FLI, VAI and HoMA-IR.
An ROC curve analysis was then performed to identify the cut off value of the PREDIMED score that was predictive of NAFLD (FLI ≥ 60). Specifically, PREDIMED scores < 6 (p < 0.001, AUC 0.804, standard error 0.0235, 95% CI 0.75 to 0.85 (Figure 1)) were identified as the thresholds for NAFLD (FLI ≥ 60).
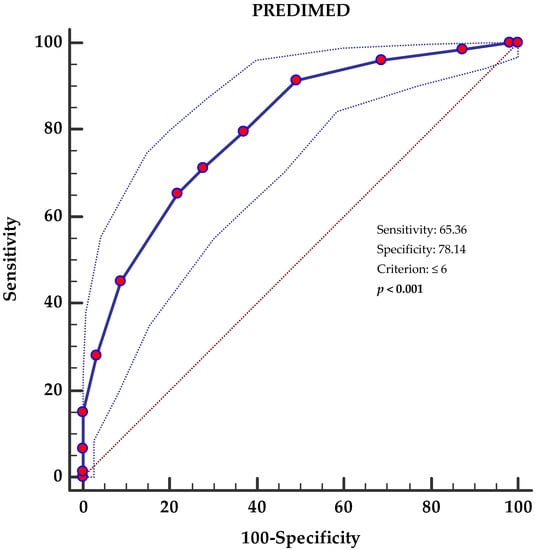
Figure 1.
Receiver operator characteristic (ROC) for NAFLD’s (FLI ≥ 60) predictive values of PREDIMED score. The 45° diagonal line serves as a reference line, as it is the ROC curve of the random classification. The line with the red dots represents the distribution of the data while the two dashed lines outside it represent the confidence intervals. The point furthest northwest of the ROC curve corresponds to the best cut-off, in the sense of maximizing the number of correctly classified subjects (and minimizing the number of subjects with a false diagnosis). A p-value in bold denotes a significant difference (p < 0.05). PREDIMED, PREvención con DIetaMEDiterránea.
4. Discussion
In this study, we demonstrated that both MD and insulin resistance might impact the risk of NAFLD. Specifically, we showed that increased HoMA-IR, together with low adherence to MD, were independently and significantly associated with NAFLD, while visceral adipose tissue calculated as VAI was not. Interestingly in our cohort, subjects with NAFLD showed higher anthropometric measurements, metabolic parameters and cardio-metabolic indices than subjects without NAFLD. Subjects with NAFLD had significantly lower values of PREDIMED score, i.e., lower adherence to MD than subjects without NAFLD.
The link between NAFLD and MD has been previously reported in 243 youths with overweight/obesity with and without NAFLD in which adherence to MD was assessed by the KIDMED score [28]. The key finding of this study was the existence of the negative association between the extent of liver damage and the level of adherence to MD, indicating that adherence to MD may play a role in the severity of liver damage in relation to NALFD.
Further, we identified a cut off of PREDIMED score < 6 as a tool to be used to screen subjects at high risk of having NAFLD. Our results are therefore consistent, but also bring up and reinforce the concept that adherence of MD independently of visceral adipose tissue influences NAFLD, as demonstrated by the results of our regression analysis. In agreement with our results, Trovato et al. carried out a study in 1199 subjects with overweight/obesity with and without ultrasound-diagnosed hepatic steatosis, finding that patients with NAFLD were less adherent to MD, but interestingly adherence to MD predicted the occurrence of NAFLD independently of BMI [29]. In addition, the same authors evaluated the effect of MD intervention on the Bright Liver Score at baseline and after 1, 3 and 6 months [30]. Over a 6-month period the reduction of fat liver content was greater the higher the adherence to MD, and interestingly, the effect of MD was independent of other lifestyle changes [30]. These finding are also reported by other similar studies [31,32].
The direct effect of MD on NAFLD, both on pathogenesis and on risk of progression from NAFLD to NASH, could be explained by antinflammatory and antioxidant properties of MD [33,34]. Indeed, MD contains compounds such as polyphenols, vitamins and other molecules that play a role in this sense. Polyphenols are in whole-grain cereals, vegetables and fresh fruits, red wine, olive oil and nuts. They have a phenolic structure, and they are a heterogenic group consisting of flavonoid polyphenols and non-flavonoid polyphenols based on their chemical structure [34]. Flavonoids exert a hepatoprotective effect due to their antioxidant and anti-inflammatory properties [34,35]. In addition, resveratrol, a non-flavonoid mostly present in red wine, has been reported to interact with homeostasis of vessels, blood platelets and the clotting and fibrinolytic system of plasma, thus exerting hepato-protective properties [36,37]. Vitamins, such as vitamin E, D, and C, are components of MD and they have been shown to play antioxidant properties, too [38,39,40]. In addition, lycopene, a carotenoid found in several fruits and vegetables of the MD, also has been demonstrated to exert a preventive effect on experimental NASH through the reduction of steatosis, inflammation, and oxidative stress in animal studies [41]. High MUFA content along with a balanced PUFA omega 6-to-omega 3 ratio is another key player of MD in preventing the development of NAFLD by improving plasma lipid levels, decreasing body fat and reducing postprandial adiponectin expression [42,43].
The liver is closely related to the gut since it receives about 70% of its blood supply from the intestine though the portal vein [44,45]. The tight relationship between NAFLD and gut microbiota has been highlighted by several studies [44,45]. In this context, MD could also have a beneficial role on NAFLD acting through gut microbiota. Indeed, high dietary fiber intake contained in MD reduces Firmicutes and increased Bacteroidetes, thus promoting a microbial pattern that improves obesity, inflammation, and related metabolic alterations [46]. Moreover, polyphenols promote the increase of Bifidobacterium, that are well known for their properties in reducing plasma cholesterol and C-reactive protein [46]. Interestingly, we found an independent association of HoMA-IR with NAFLD. Insulin resistance is a well-established risk factor for the onset of NAFLD and its progression to NASH [47]. Indeed, in the insulin resistance state, there is an impaired uptake synthesis, export, and oxidation of fatty free acids that results in fat accumulation. Interestingly, this mechanism is independent of obesity, as we found, and as has been demonstrated in subjects without obesity [48].
Moreover, in the insulin resistant condition, the liver does not suppress hepatic glucose production in response to insulin and increases de novo lipogenesis, activating the Notch signaling pathway; [49] in fact, in subjects with NAFLD the increase in de novo lipogenesis is 5-fold higher than in healthy subjects [49].
One of the main limitations of our study was the cross-sectional design that provides information on associations of parameters, but not on causality. In addition, although the sample size could seem relatively small, it was calculated by using 95% statistical power to detect statistical significance of the results with adequate power. The study population from an outpatient clinic was another limitation of the study. However, it is important to study patients from an outpatient clinic, as studying this specific population can provide valuable insights into clinical aspects, disease management and treatment outcomes in a healthcare setting. However, further population-based or multicenter trial studies remain necessary to validate the results in more diverse populations, including individuals outside the outpatient setting. Finally, although we observed a significant association between adherence to MD and NAFLD, it is important to recognize the possibility of residual confounding of potential unmeasured variables in the study. However, to mitigate the impact of residual confounding, we used appropriate statistical techniques, such as multivariable regression models, to adjust for known confounders. To overcome this limitation, future studies might consider including a more complete set of confounding variables in their analyses.
The strength of our study lies on the accurate characterization of enrolled subjects by a trained team of Endocrinologists and Nutritionists. To ensure the homogeneity of the sample, all enrolled subjects came from the same geographical area with similar nutritional habits. Third, our study highlights the role of MD in the onset of NAFLD and it was the first to identify a cutoff of PREDIMED score as an easy tool to screen subjects at risk of having NAFLD.
5. Conclusions
In conclusion, this study shows that low adherence to MD and insulin resistance were associated with the risk of developing NAFLD. We found compelling evidence indicating that NAFLD was independently and significantly associated with both increased HoMA-IR and low adherence to MD. Notably, our findings revealed that the calculated measure of visceral adipose tissue known as VAI did not demonstrate a main association with NAFLD. Therefore, it might provide the rational basis for a personalized management of patients with NAFLD taking into account nutritional habits and insulin sensitivity state that may pose an increased risk of liver disease onset and progression. However, future randomized controlled trials that specifically address potential confounders and employ rigorous study designs can provide more robust evidence regarding the association between adherence to MD and NAFLD.
Author Contributions
Conceptualization, L.B. and G.M.; methodology, L.B. and G.M.; writing—original draft preparation, G.M.; writing—review and editing, L.V. and G.M.; supervision, S.S. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Committee of the University of Naples “Federico II” Medical School (n. 05/14).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Juanola, O.; Martinez-Lopez, S.; Frances, R.; Gomez-Hurtado, I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int. J. Environ. Res. Public Health 2021, 18, 5227. [Google Scholar] [CrossRef]
- Schattenberg, J.M.; Allen, A.M.; Jarvis, H.; Zelber-Sagi, S.; Cusi, K.; Dillon, J.F.; Caussy, C.; Francque, S.M.; Younossi, Z.; Alkhouri, N.; et al. A multistakeholder approach to innovations in NAFLD care. Commun. Med. 2023, 3, 1. [Google Scholar] [CrossRef]
- Promrat, K.; Kleiner, D.E.; Niemeier, H.M.; Jackvony, E.; Kearns, M.; Wands, J.R.; Fava, J.L.; Wing, R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010, 51, 121–129. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Tileli, N.; Margariti, A.; Georgoulis, M.; Deutsch, M.; Tiniakos, D.; Fragopoulou, E.; Zafiropoulou, R.; Manios, Y.; Papatheodoridis, G. Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin. Nutr. 2014, 33, 678–683. [Google Scholar] [CrossRef]
- Aller, R.; Izaola, O.; de la Fuente, B.; De Luis Roman, D.A. Mediterranean Diet Is Associated with Liver Histology in Patients with Non Alcoholic Fatty Liver Disease. Nutr. Hosp. 2015, 32, 2518–2524. [Google Scholar] [CrossRef]
- Baratta, F.; Pastori, D.; Polimeni, L.; Bucci, T.; Ceci, F.; Calabrese, C.; Ernesti, I.; Pannitteri, G.; Violi, F.; Angelico, F.; et al. Adherence to Mediterranean Diet and Non-Alcoholic Fatty Liver Disease: Effect on Insulin Resistance. Am. J. Gastroenterol. 2017, 112, 1832–1839. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Verde, L.; Sulu, C.; Katsiki, N.; Hassapidou, M.; Frias-Toral, E.; Cucalon, G.; Pazderska, A.; Yumuk, V.D.; Colao, A.; et al. Mediterranean Diet and Obesity-related Disorders: What is the Evidence? Curr. Obes. Rep. 2022, 11, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- National Cholesterol Education Program. ATP III Guidelines At-A-Glance Quick Desk Reference. Available online: https://www.nhlbi.nih.gov/files/docs/guidelines/atglance.pdf (accessed on 1 January 2022).
- Martinez-Gonzalez, M.A.; Garcia-Arellano, A.; Toledo, E.; Salas-Salvado, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schroder, H.; Aros, F.; Gomez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; de Alteriis, G.; Porcelli, T.; Vetrani, C.; Verde, L.; Aprano, S.; Fonderico, F.; Troncone, G.; Colao, A.; et al. Adherence to the Mediterranean Diet as a Modifiable Risk Factor for Thyroid Nodular Disease and Thyroid Cancer: Results From a Pilot Study. Front. Nutr. 2022, 9, 944200. [Google Scholar] [CrossRef]
- Verde, L.; Dalamaga, M.; Capo, X.; Annunziata, G.; Hassapidou, M.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G.; Barrea, L. The Antioxidant Potential of the Mediterranean Diet as a Predictor of Weight Loss after a Very Low-Calorie Ketogenic Diet (VLCKD) in Women with Overweight and Obesity. Antioxidants 2022, 12, 18. [Google Scholar] [CrossRef]
- Barrea, L.; Di Somma, C.; Macchia, P.E.; Falco, A.; Savanelli, M.C.; Orio, F.; Colao, A.; Savastano, S. Influence of nutrition on somatotropic axis: Milk consumption in adult individuals with moderate-severe obesity. Clin. Nutr. 2017, 36, 293–301. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Annunziata, G.; Megna, M.; Falco, A.; Balato, A.; Colao, A.; Savastano, S. Coffee consumption, metabolic syndrome and clinical severity of psoriasis: Good or bad stuff? Arch. Toxicol. 2018, 92, 1831–1845. [Google Scholar] [CrossRef]
- Barrea, L.; Tarantino, G.; Somma, C.D.; Muscogiuri, G.; Macchia, P.E.; Falco, A.; Colao, A.; Savastano, S. Adherence to the Mediterranean Diet and Circulating Levels of Sirtuin 4 in Obese Patients: A Novel Association. Oxidative Med. Cell. Longev. 2017, 2017, 6101254. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Di Somma, C.; Altieri, B.; Vecchiarini, M.; Orio, F.; Spinosa, T.; Colao, A.; Savastano, S. Patient empowerment and the Mediterranean diet as a possible tool to tackle prediabetes associated with overweight or obesity: A pilot study. Hormones 2019, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C. Visceral adiposity index: An indicator of adipose tissue dysfunction. Int. J. Endocrinol. 2014, 2014, 730827. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off points of the visceral adiposity index (VAI) identifying a visceral adipose dysfunction associated with cardiometabolic risk in a Caucasian Sicilian population. Lipids Health Dis. 2011, 10, 183. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Della Corte, C.; Mosca, A.; Vania, A.; Alterio, A.; Iasevoli, S.; Nobili, V. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: The results of an Italian Study. Nutrition 2017, 39–40, 8–14. [Google Scholar] [CrossRef]
- Trovato, F.M.; Martines, G.F.; Brischetto, D.; Trovato, G.; Catalano, D. Neglected features of lifestyle: Their relevance in non-alcoholic fatty liver disease. World J. Hepatol. 2016, 8, 1459–1465. [Google Scholar] [CrossRef]
- Trovato, F.M.; Catalano, D.; Martines, G.F.; Pace, P.; Trovato, G.M. Mediterranean diet and non-alcoholic fatty liver disease: The need of extended and comprehensive interventions. Clin. Nutr. 2015, 34, 86–88. [Google Scholar] [CrossRef]
- Pinto, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bullo, M.; et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ramiro, I.; Vauzour, D.; Minihane, A.M. Polyphenols and non-alcoholic fatty liver disease: Impact and mechanisms. Proc. Nutr. Soc. 2016, 75, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Van De Wier, B.; Koek, G.H.; Bast, A.; Haenen, G.R. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2017, 57, 834–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yoneda, M.; Nakamura, K.; Makino, I.; Terano, A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: A pilot study. Aliment. Pharmacol. Ther. 2001, 15, 1667–1672. [Google Scholar] [CrossRef]
- Potter, J.J.; Liu, X.; Koteish, A.; Mezey, E. 1,25-dihydroxyvitamin D3 and its nuclear receptor repress human alpha1 (I) collagen expression and type I collagen formation. Liver Int. 2013, 33, 677–686. [Google Scholar] [CrossRef]
- Valdecantos, M.P.; Perez-Matute, P.; Quintero, P.; Martinez, J.A. Vitamin C, resveratrol and lipoic acid actions on isolated rat liver mitochondria: All antioxidants but different. Redox Rep. 2010, 15, 207–216. [Google Scholar] [CrossRef]
- Bahcecioglu, I.H.; Kuzu, N.; Metin, K.; Ozercan, I.H.; Ustundag, B.; Sahin, K.; Kucuk, O. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet. Vet. Med. Int. 2010, 2010, 262179. [Google Scholar] [CrossRef]
- Eccel Prates, R.; Beretta, M.V.; Nascimento, F.V.; Bernaud, F.R.; de Almeira, J.C.; Rodrigues, T.C. Saturated fatty acid intake decreases serum adiponectin levels in subjects with type 1 diabetes. Diabetes Res. Clin. Pract. 2016, 116, 205–211. [Google Scholar] [CrossRef]
- Paniagua, J.A.; de la Sacristana, A.G.; Sanchez, E.; Romero, I.; Vidal-Puig, A.; Berral, F.J.; Escribano, A.; Moyano, M.J.; Perez-Martinez, P.; Lopez-Miranda, J.; et al. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J. Am. Coll. Nutr. 2007, 26, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Comelli, E.M.; Arendt, B.M.; Bonengel, J.; Fung, S.K.; Fischer, S.E.; McGilvray, I.D.; Allard, J.P. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013, 58, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Tse, C.H.; Lam, T.T.; Wong, G.L.; Chim, A.M.; Chu, W.C.; Yeung, D.K.; Law, P.T.; Kwan, H.S.; Yu, J.; et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis--a longitudinal study. PLoS ONE 2013, 8, e62885. [Google Scholar] [CrossRef]
- Queipo-Ortuno, M.I.; Boto-Ordonez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andres-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.J.; Miotto, P.M.; De Nardo, W.; Montgomery, M.K. The Liver as an Endocrine Organ-Linking NAFLD and Insulin Resistance. Endocr. Rev. 2019, 40, 1367–1393. [Google Scholar] [CrossRef] [PubMed]
- Vogelberg, K.H.; Gries, F.A.; Moschinski, D. Hepatic production of VLDL-triglycerides. Dependence of portal substrate and insulin concentration. Horm. Metab. Res. 1980, 12, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).