RNA-Seq and 16S rRNA Reveals That Tian–Dong–Tang–Gan Powder Alleviates Environmental Stress-Induced Decline in Immune and Antioxidant Function and Gut Microbiota Dysbiosis in Litopenaeus vannami
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Herbs
2.2. Experimental Diets
2.3. Stress Experiment
2.4. Sample Collection and Processing Methods
2.5. Immunological Parameter Analysis
2.6. Hepatopancreas Transcriptome Analysis
2.7. Gut Microbiome Analysis
2.8. Quantitative Real-Time PCR Validation
2.9. Calculations and Statistical Analysis
3. Results
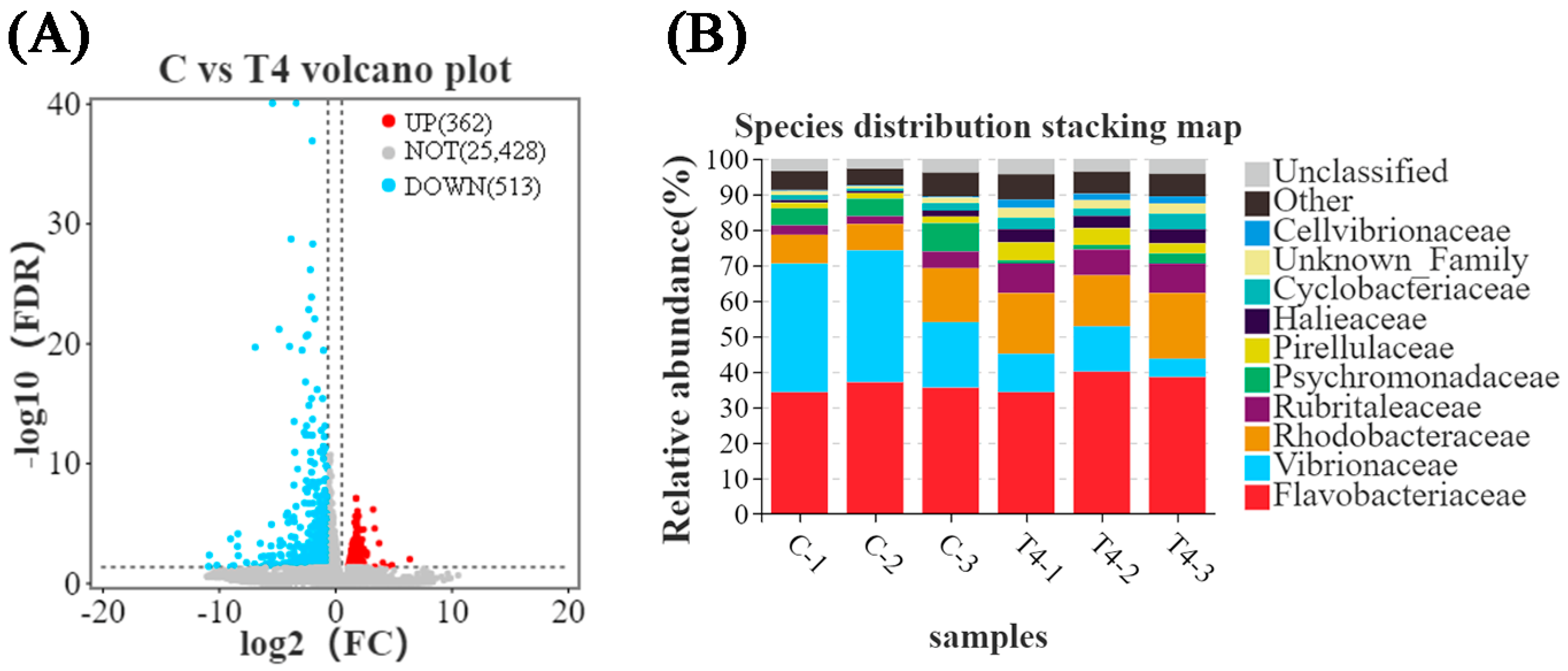
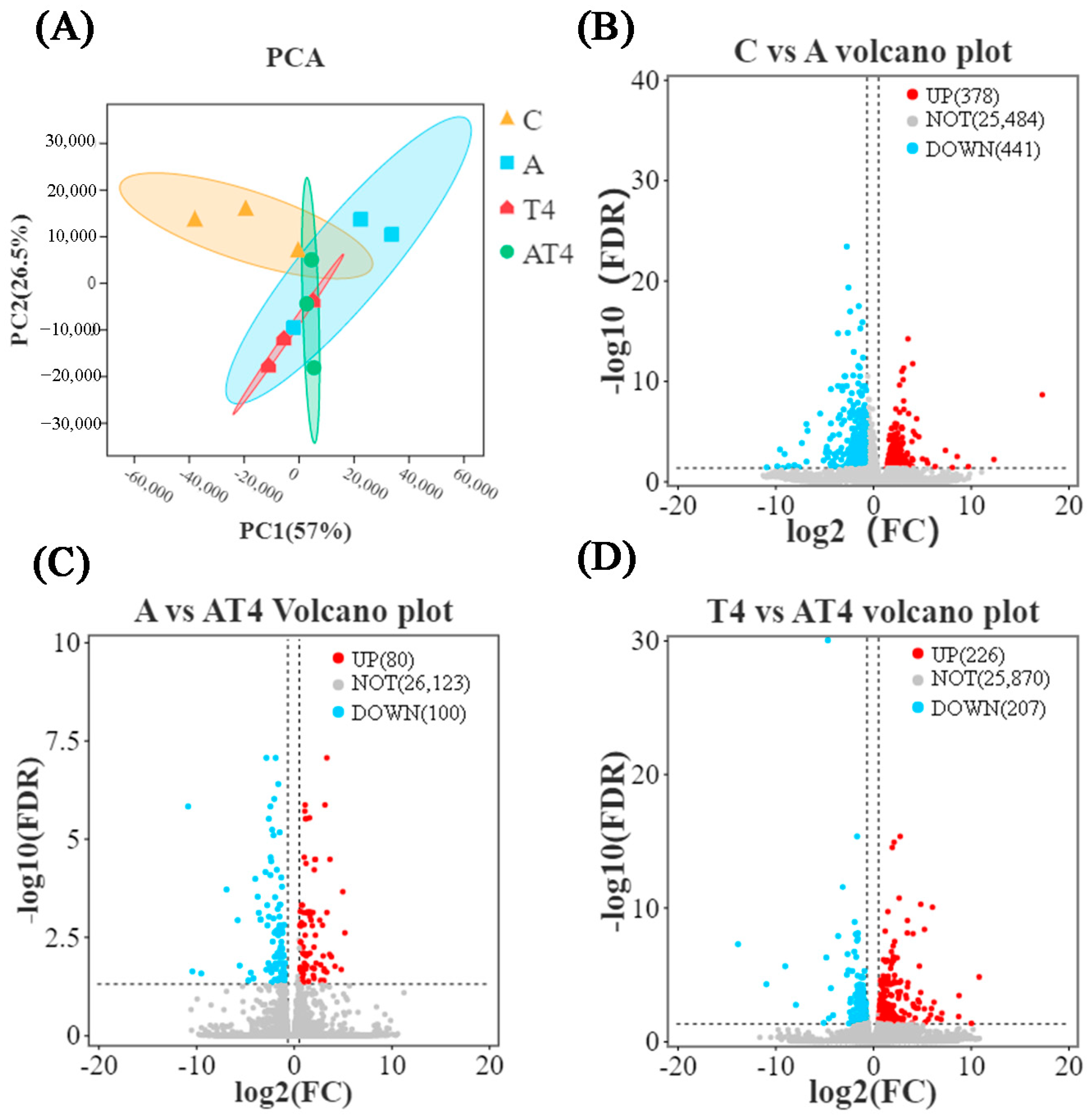
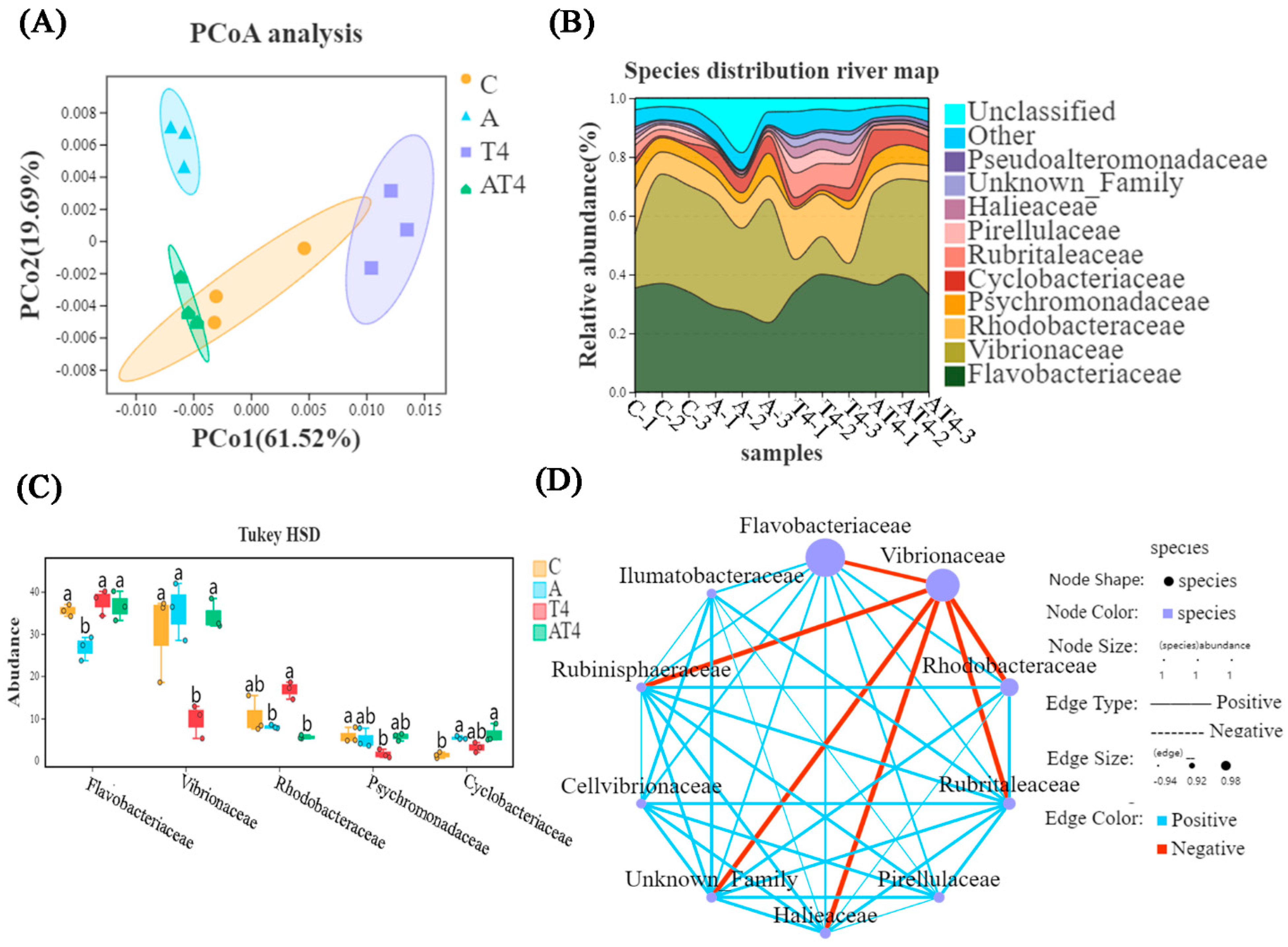
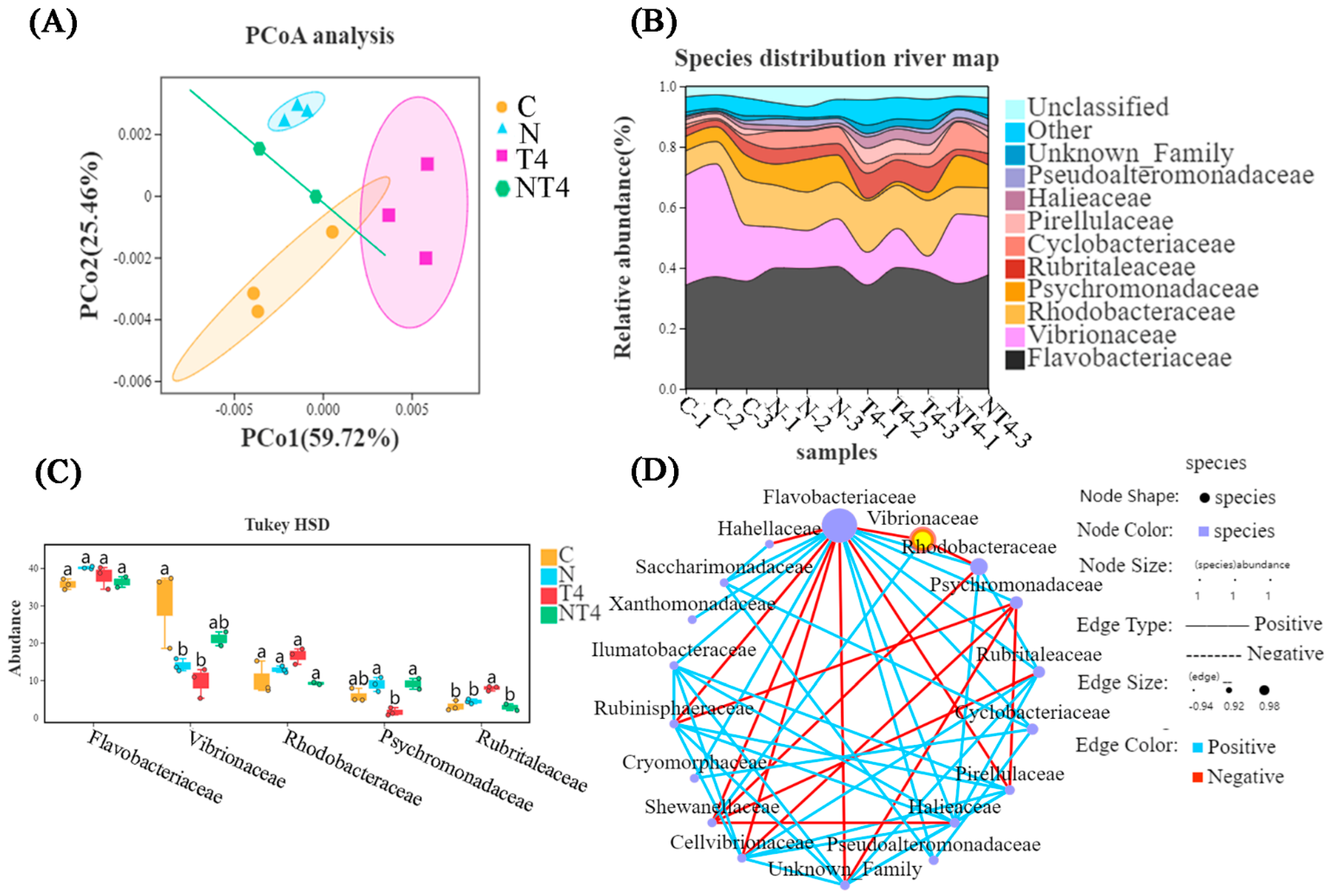
3.1. Transcriptomic and 16S rRNA Analysis in Feeding Experiments
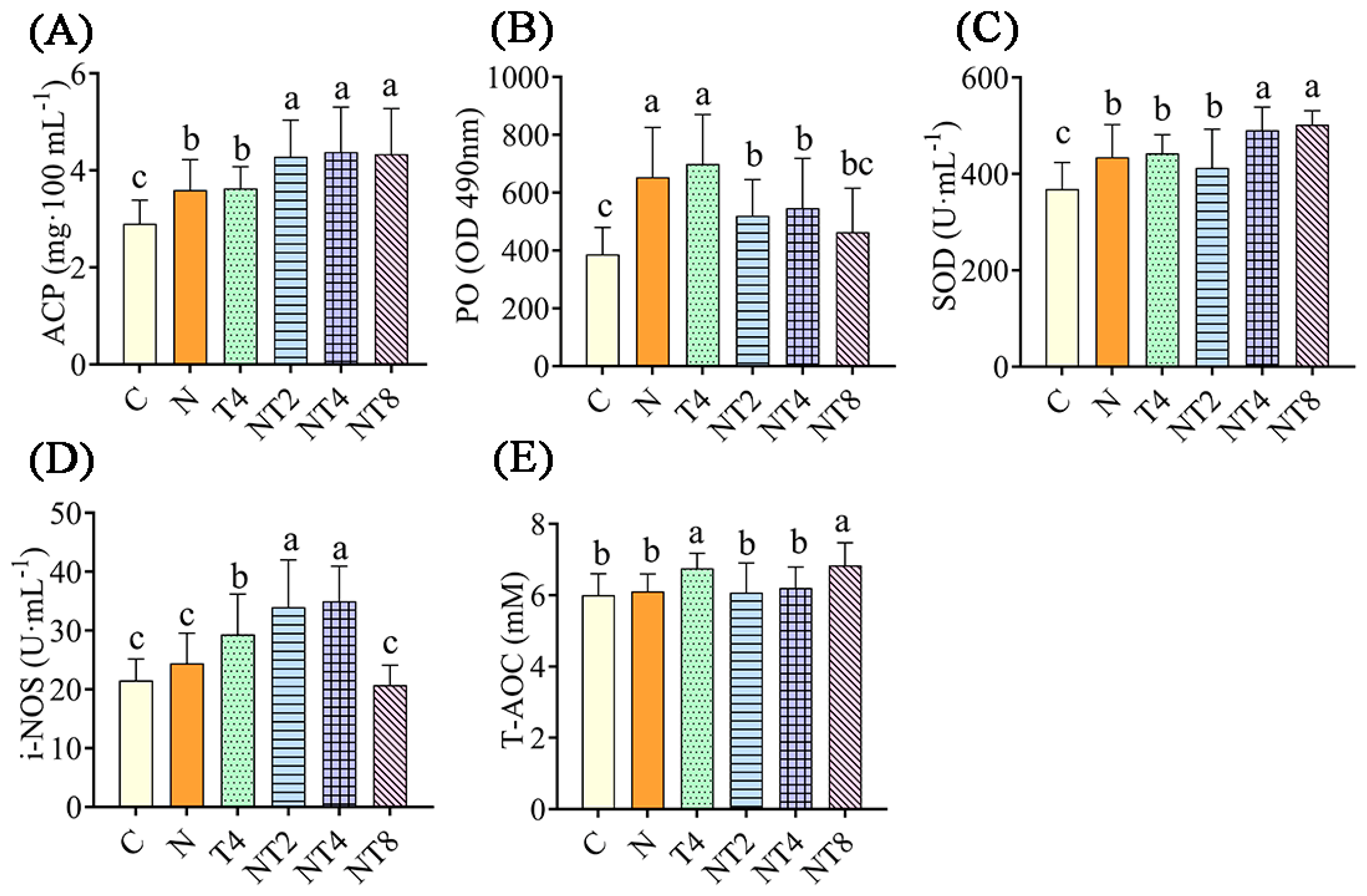
3.2. Ammonia Exposure Test
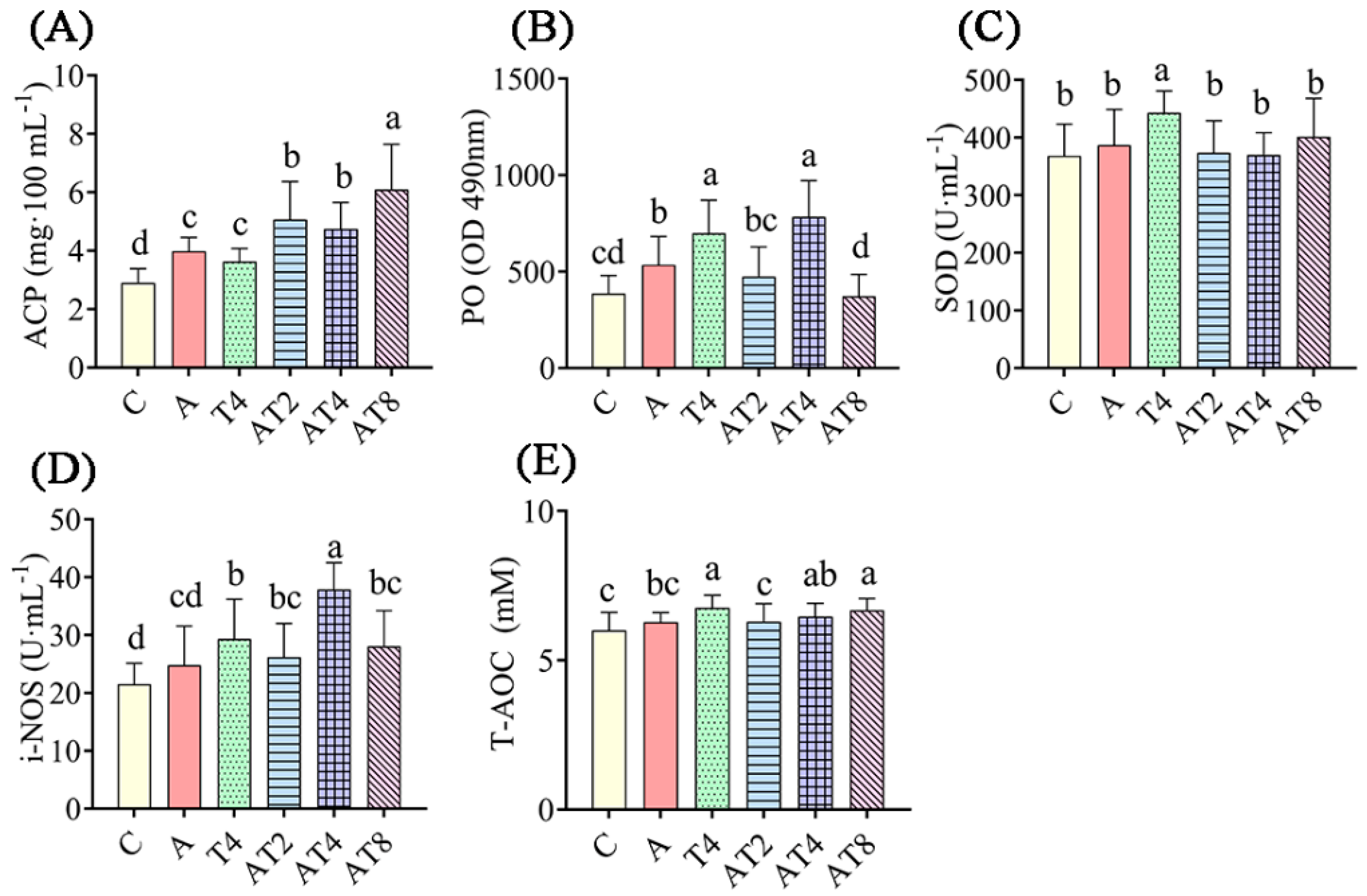
3.2.1. The Change in Hemolymph Factors in the Ammonia Exposure Test
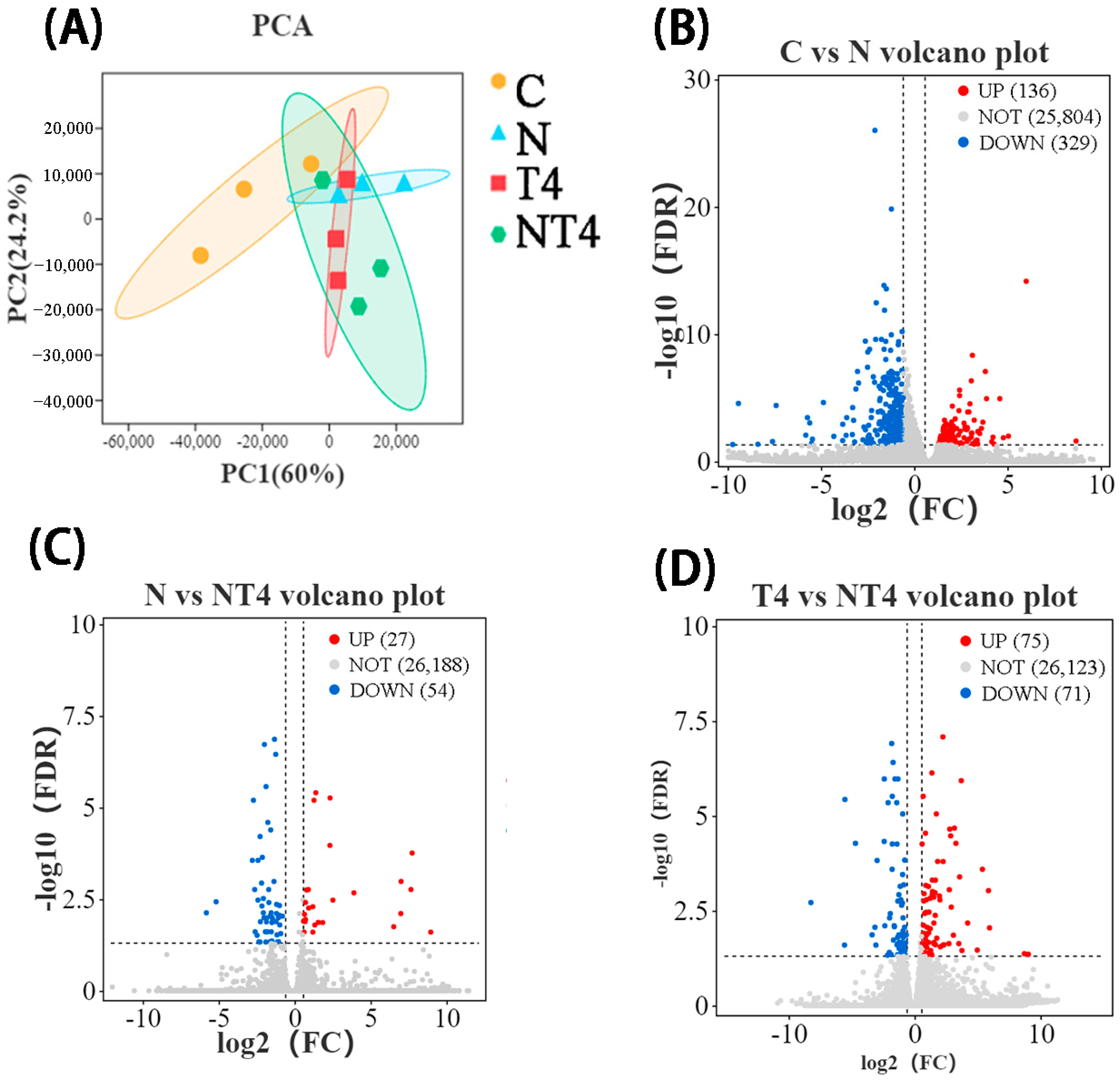
3.2.2. Hepatopancreas RNA-Seq Transcriptome Analysis in the Ammonia Exposure Test
3.2.3. Gut Microbial Composition in the Ammonia Exposure Test
3.3. Nitrite Exposure Test
3.3.1. Changes in the Hemolymph Factors in Nitrite Exposure Test
3.3.2. RNA-Seq Transcriptome Analysis of the Hepatopancreas in a Nitrite Exposure Test
3.3.3. Intestinal Microbial Composition in Nitrite Exposure Test
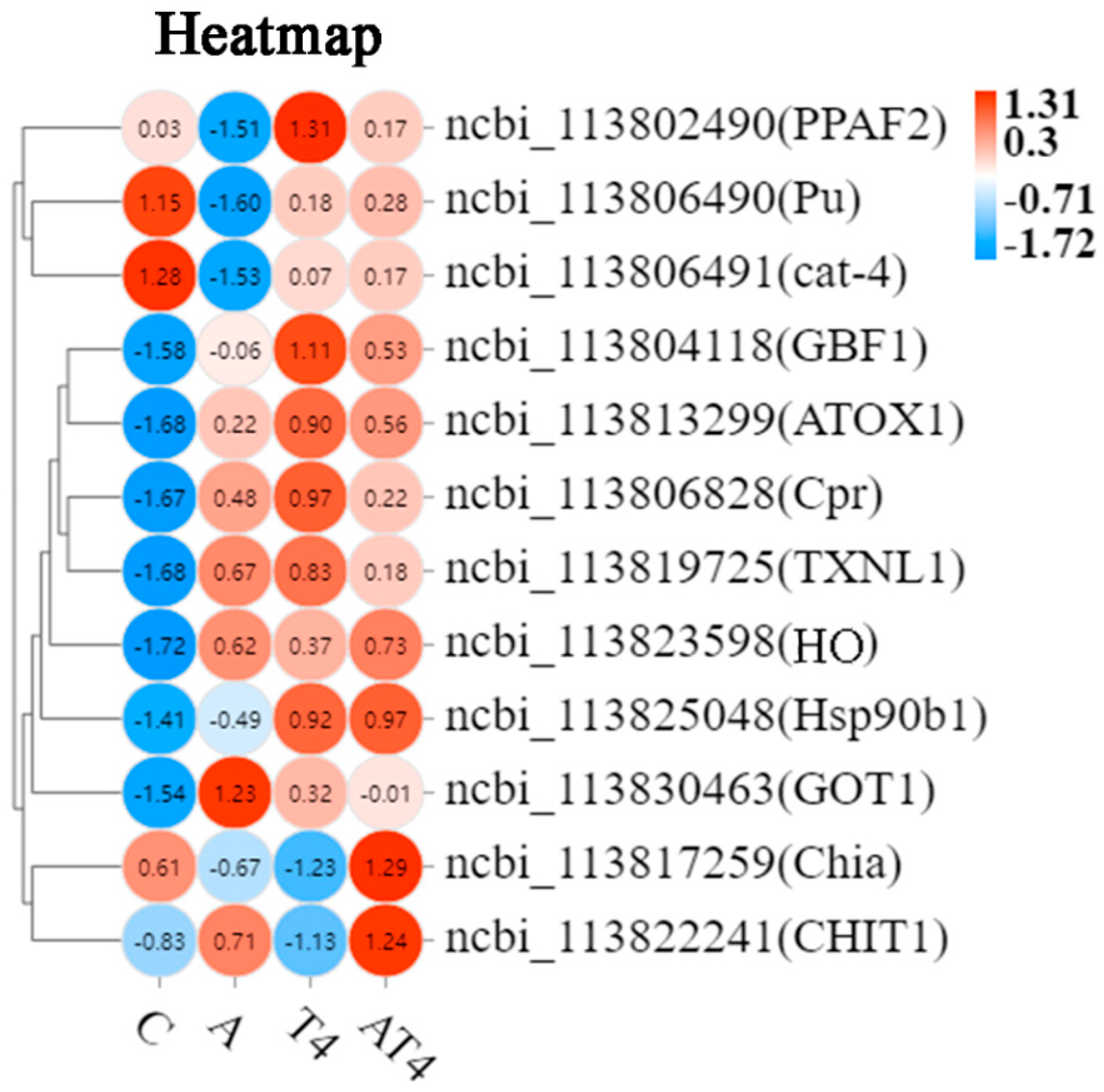
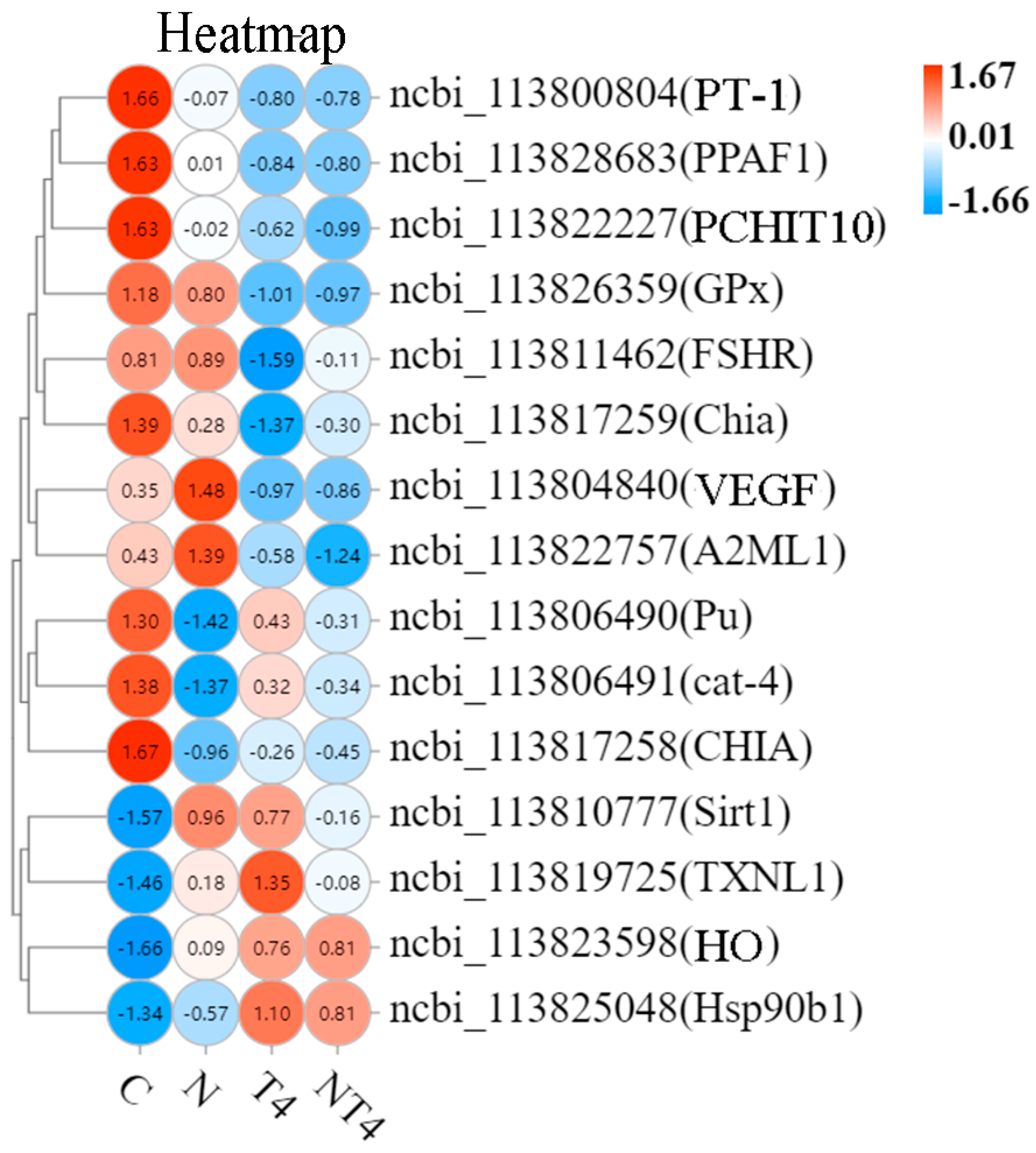
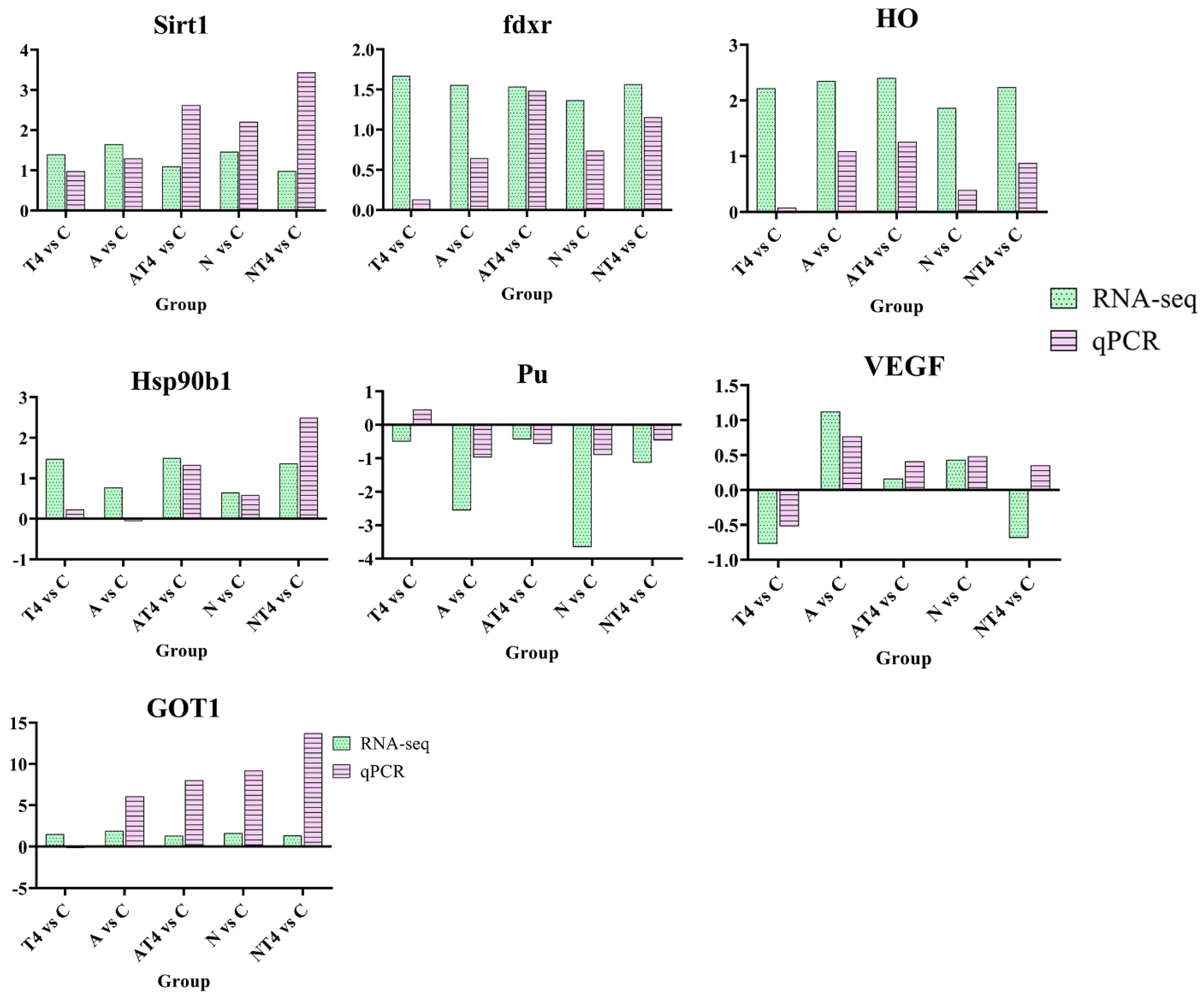
3.4. Quantitative Real-Time PCR Validation
4. Discussion
4.1. Modulation of Immune and Antioxidant Indicators by the TDTGP in the L. vannamei
4.2. Modulatory Effects of TDTGP on Immune and Antioxidant-Related Genes Expression
4.3. Effect of TDTGP on Gut Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ourth, D.D.; Renis, H.E. Antiviral melanization reaction of Heliothis virescens hemolymph against DNA and RNA viruses in vitro. Comp. Biochem. Physiol. Part B Comp. Biochem. 1993, 105, 719–723. [Google Scholar] [CrossRef]
- Jian-Chu Chen, P.-C.L.; Yao-Tsu, L. Super Intensive Culture of Red-Tailed Shrimp Penaeus penicillatus. World Aquac. Soc. 1988, 19, 127–131. [Google Scholar] [CrossRef]
- Tacon, A.G.J.; Cody, J.J.; Conquest, L.D.; Divakaran, S.; Forster, I.P.; Decamp, O.E. Effect of culture system on the nutrition and growth performance of Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquac. Nutr. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Duan, Y.; Xiong, D.; Wang, Y.; Li, H.; Dong, H.; Zhang, J. Toxic effects of ammonia and thermal stress on the intestinal microbiota and transcriptomic and metabolomic responses of Litopenaeus vannamei. Sci. Total Environ. 2021, 754, 141867. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Huang, L.; Liao, M.; Dong, W.; Liu, C.; Zhuang, X.; Liu, Y.; Yin, X.; Liang, Q.; Wang, W. Pva-miR-252 participates in ammonia nitrogen-induced oxidative stress by modulating autophagy in Penaeus vannamei. Ecotoxicol. Environ. Saf. 2021, 225, 112774. [Google Scholar] [CrossRef]
- Fulde, M.; Hornef, M.W. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol. Rev. 2014, 260, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Núñez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Dai, W.-F.; Zhang, J.-J.; Qiu, Q.-F.; Chen, J.; Yang, W.; Ni, S.; Xiong, J.-B. Starvation stress affects the interplay among shrimp gut microbiota, digestion and immune activities. Fish Shellfish Immunol. 2018, 80, 191–199. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, S.; Xiong, J.; Hou, D.; Zhou, R.; Xing, C.; Wei, D.; Deng, X.; Yu, L.; Wang, H.; et al. Microecological Koch’s postulates reveal that intestinal microbiota dysbiosis contributes to shrimp white feces syndrome. Microbiome 2020, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Palanikumar, P.; Daffni Benitta, D.J.; Lelin, C.; Thirumalaikumar, E.; Michaelbabu, M.; Citarasu, T. Effect of Argemone mexicana active principles on inhibiting viral multiplication and stimulating immune system in Pacific white leg shrimp Litopenaeus vannamei against white spot syndrome virus. Fish Shellfish. Immunol. 2018, 75, 243–252. [Google Scholar] [CrossRef]
- Su, C.; Liu, X.; Lu, Y.; Pan, L.; Zhang, M. Effect of dietary Xiao-Chaihu-Decoction on growth performance, immune response, detoxification and intestinal microbiota of pacific white shrimp (Litopenaeus vannamei). Fish Shellfish. Immunol. 2021, 114, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-D.; Cao, M.-X.; Chen, Q.; Yu, M.-L.; Liu, Q.-Y.; Zhao, Y.-Z.; Zhang, L.; Hu, T.-J. Effect of medical herbs in Tian-Dong-Tang-Gan powder on the oxidative stress induced by ammonia and nitrite in Litopenaeus vannamei. Aquaculture 2022, 548, 737584. [Google Scholar] [CrossRef]
- Xie, X.-D.; Zhou, S.-M.; Cheng, J.; Yu, M.-L.; Wei, Y.-Y.; Mo, M.-L.; Hu, T.-J. Effects of medical herbs in Tian-Dong-Tang-Gan powder on non-specific immune responses and resistance to acute ammonia stress in Litopenaeus vannamei. Aquac. Res. 2021, 52, 3360–3370. [Google Scholar] [CrossRef]
- Mason, H.S. Structures and Functions of the Phenolase Complex. Nature 1956, 177, 79–81. [Google Scholar] [CrossRef]
- Kenneth Söderhäll, V.J.S. Separation of the haemocyte populations of CarcinusMaenas and other marine decapods, and prophenoloxidase distribution. Dev. Comp. Immunol. 1983, 7, 229–239. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Li, H.; Zhai, B.; Sun, J.; Fan, Y.; Zou, J.; Cheng, J.; Zhang, X.; Shi, Y.; Guo, D. Antioxidant, Anti-Aging and Organ Protective Effects of Total Saponins from Aralia taibaiensis. Drug Des. Devel. Ther. 2021, 15, 4025–4042. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef]
- Feng, N.N.; Sun, Y.M.; Wang, R.; Zhang, C.S.; Li, F.H. Analysis on the activity of immune related enzymes in survived Exopalaemon carinicauda from WSSV infection. Mar. Sci. 2014, 38, 75–79. [Google Scholar]
- Zhai, Q.; Li, J. Effectiveness of traditional Chinese herbal medicine, San-Huang-San, in combination with enrofloxacin to treat AHPND-causing strain of Vibrio parahaemolyticus infection in Litopenaeus vannamei. Fish Shellfish. Immunol. 2019, 87, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Chen, Y.; Ou, L.; Xu, Y.; Yu, X. Aqueous root extract of Asparagus cochinchinensis (Lour.) Merr. Has antioxidant activity in D-galactose-induced aging mice. BMC Complement Altern. Med. 2017, 17, 469. [Google Scholar] [CrossRef]
- Sun, Z.; Tan, X.; Ye, H.; Zou, C.; Ye, C.; Wang, A. Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish Shellfish. Immunol. 2018, 73, 234–244. [Google Scholar] [CrossRef]
- Guo, H.; Li, K.; Wang, W.; Wang, C.; Shen, Y. Effects of Copper on Hemocyte Apoptosis, ROS Production, and Gene Expression in White Shrimp Litopenaeus vannamei. Biol. Trace Elem. Res. 2017, 179, 318–326. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, K.J.; Zhang, F.Y.; Song, W.; Zhao, M.; Wei, H.Q.; Meng, Y.Y.; Ma, L.B. Characterization and expression analysis of the prophenoloxidase activating factor from the mud crab Scylla paramamosain. Genet. Mol. Res. 2015, 14, 8847–8860. [Google Scholar] [CrossRef]
- Yet, S.-F.; Melo, L.G.; Layne, M.D.; Perrella, M.A. [16]—Heme Oxygenase 1 in Regulation of Inflammation and Oxidative Damage. In Methods in Enzymology 353; Sen, C.K., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 163–176. [Google Scholar]
- Aispuro-Hernandez, E.; Garcia-Orozco, K.D.; Muhlia-Almazan, A.; del-Toro-Sanchez, L.; Robles-Sanchez, R.M.; Hernandez, J.; Gonzalez-Aguilar, G.; Yepiz-Plascencia, G.; Sotelo-Mundo, R.R. Shrimp thioredoxin is a potent antioxidant protein. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 94–99. [Google Scholar] [CrossRef]
- Johnson, J.G.; Paul, M.R.; Kniffin, C.D.; Anderson, P.E.; Burnett, L.E.; Burnett, K.G. High CO2 alters the hypoxia response of the Pacific whiteleg shrimp (Litopenaeus vannamei) transcriptome including known and novel hemocyanin isoforms. Physiol. Genomics 2015, 47, 548–558. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Huang, L.; Dong, P.; Wang, S.; Chen, H.; Lu, Z.; Hou, D.; Zhang, D. Fine-scale succession patterns and assembly mechanisms of bacterial community of Litopenaeus vannamei larvae across the developmental cycle. Microbiome 2020, 8, 106. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Zhao, J.; Liu, S.; Zhang, L.; Zhao, Y.; Yang, H.; Sun, L. Quantitative microbiome profiling links microbial community variation to the intestine regeneration rate of the sea cucumber Apostichopus japonicus. Genomics 2020, 112, 5012–5020. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Q.; Liu, S.; Huo, D.; Zhao, J.; Zhang, L.; Zhao, Y.; Sun, L.; Yang, H. Genomic and Metagenomic Insights Into the Microbial Community in the Regenerating Intestine of the Sea Cucumber Apostichopus japonicus. Front. Microbiol. 2019, 10, 1165. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Panda, S.K.; Luyten, W. Anti-vibrio and immune-enhancing activity of medicinal plants in shrimp: A comprehensive review. Fish Shellfish. Immunol. 2021, 117, 192–210. [Google Scholar] [CrossRef]
- Jayasree, L.; Janakiram, P.; Madhavi, R. Characterization of Vibrio spp. Associated with Diseased Shrimp from Culture Ponds of Andhra Pradesh (India). J. World Aquacult. Soc. 2006, 37, 523–532. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Zhang, Z.; Feng, Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics 2020, 10, 11302–11323. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Du, F.; Gao, X.; Ma, X.; Huang, Y.; Xu, F.; Niu, W.; Wang, F.; Mao, Y.; et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab. Dispos. 2009, 37, 2290–2298. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhu, L.; Li, Y.; Cui, Y.; Jiang, S.; Tao, N.; Chen, H.; Zhao, Z.; Xu, J.; Dong, C. A novel inulin-type fructan from Asparagus cochinchinensis and its beneficial impact on human intestinal microbiota. Carbohydr. Polym. 2020, 247, 116761. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhou, L.; Qu, Y.; Lu, K.; Han, F.; Li, E. Effects of Different Dietary β-Glucan Levels on Antioxidant Capacity and Immunity, Gut Microbiota and Transcriptome Responses of White Shrimp (Litopenaeus vannamei) under Low Salinity. Antioxidants 2022, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, L.; Huang, J.; Liang, J.; Wang, X.; Ren, Y.; Li, H.; Yue, T.; Gao, Z. Evaluation of chemical composition, antioxidant activity, and gut microbiota associated with pumpkin juice fermented by Rhodobacter sphaeroides. Food Chem. 2023, 401, 134122. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Kikuta, K.; Suzuki, A.; Katsuta, A.; Kasai, H.; Yasumoto-Hirose, M.; Matsuo, Y.; Misawa, N.; Takaichi, S. Rare carotenoids, (3R)-saproxanthin and (3R,2’S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl. Microbiol. Biotechnol. 2007, 74, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
| Groups | ||||
|---|---|---|---|---|
| Blank Control Group and Stress Control Group | TDTGP-2 Group | TDTGP-4 Group | TDTGP-8 Group | |
| Ingredients | ||||
| Fish meal | 20.00 | 20.00 | 20.00 | 20.00 |
| Wheat gluten | 30.00 | 30.00 | 30.00 | 30.00 |
| Wheat meal | 20.00 | 20.00 | 20.00 | 20.00 |
| Cellulose | 18.00 | 17.80 | 17.60 | 17.20 |
| Fish oil | 2.50 | 2.50 | 2.50 | 2.50 |
| Soybean oil | 2.50 | 2.50 | 2.50 | 2.50 |
| Soybean phospholipids | 2.00 | 2.00 | 2.00 | 2.00 |
| Gelatin | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin mix | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral mix | 1.00 | 1.00 | 1.00 | 1.00 |
| TDTGP | 0.20 | 0.40 | 0.80 | |
| Proximate composition (as fed) | ||||
| Crude protein | 43.34 | 43.06 | 42.75 | 42.01 |
| Crude fat | 7.31 | 7.26 | 7.12 | 7.04 |
| Crude ash | 12.91 | 12.98 | 13.05 | 13.08 |
| Group | Richness | Chao | Shannon | Simpson | Chao (%) |
|---|---|---|---|---|---|
| Blank control group | 329.67 ± 55.97 | 353.93 ± 57.03 | 4.55 ± 0.49 b | 0.90 ± 0.04 b | 99.96 ± 0.01 |
| Ammonia stress group | 305.00 ± 82.15 | 349.18 ± 82.52 | 4.73 ± 0.14 b | 0.92 ± 0.01 ab | 99.96 ± 0.01 |
| TDTGP-4 group | 354.33 ± 7.51 | 379.18 ± 8.60 | 5.42 ± 0.13 a | 0.95 ± 0.01 a | 99.96 ± 0.001 |
| TDTGP-4 + ammonia stress group | 285.00 ± 39.89 | 317.27 ± 46.60 | 4.30 ± 0.03 b | 0.89 ± 0.001 b | 99.97 ± 0.02 |
| Group | Richness | Chao | Shannon | Simpson | Coverage (%) |
|---|---|---|---|---|---|
| Blank control group | 312.00 ± 39.89 ab | 326.12 ± 39.34 ab | 4.53 ± 0.49 b | 0.90 ± 0.04 | 99.97 ± 0.01 a |
| Nitrite stress group | 245.00 ± 6.56 c | 271.44 ± 4.97 b | 4.91 ± 0.08 ab | 0.94 ± 0.01 | 99.96 ± 0.01 ab |
| TDTGP-4 group | 354.67 ± 10.02 a | 376.85 ± 13.49 a | 5.44 ± 0.14 a | 0.95 ± 0.01 | 99.96 ± 0.01 ab |
| TDTGP-4 + nitrite stress group | 289.50 ± 53.03 bc | 320.27 ± 46.64 ab | 4.92 ± 0.37 a | 0.94 ± 0.01 | 99.96 ± 0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.-D.; Zhou, Y.; Sun, Y.-B.; Yi, S.-L.; Zhao, Y.; Chen, Q.; Xie, Y.-H.; Cao, M.-X.; Yu, M.-L.; Wei, Y.-Y.; et al. RNA-Seq and 16S rRNA Reveals That Tian–Dong–Tang–Gan Powder Alleviates Environmental Stress-Induced Decline in Immune and Antioxidant Function and Gut Microbiota Dysbiosis in Litopenaeus vannami. Antioxidants 2023, 12, 1262. https://doi.org/10.3390/antiox12061262
Xie X-D, Zhou Y, Sun Y-B, Yi S-L, Zhao Y, Chen Q, Xie Y-H, Cao M-X, Yu M-L, Wei Y-Y, et al. RNA-Seq and 16S rRNA Reveals That Tian–Dong–Tang–Gan Powder Alleviates Environmental Stress-Induced Decline in Immune and Antioxidant Function and Gut Microbiota Dysbiosis in Litopenaeus vannami. Antioxidants. 2023; 12(6):1262. https://doi.org/10.3390/antiox12061262
Chicago/Turabian StyleXie, Xiao-Dong, Ying Zhou, Yu-Bo Sun, Shou-Li Yi, Yi Zhao, Qi Chen, Ying-Hong Xie, Mi-Xia Cao, Mei-Ling Yu, Ying-Yi Wei, and et al. 2023. "RNA-Seq and 16S rRNA Reveals That Tian–Dong–Tang–Gan Powder Alleviates Environmental Stress-Induced Decline in Immune and Antioxidant Function and Gut Microbiota Dysbiosis in Litopenaeus vannami" Antioxidants 12, no. 6: 1262. https://doi.org/10.3390/antiox12061262
APA StyleXie, X.-D., Zhou, Y., Sun, Y.-B., Yi, S.-L., Zhao, Y., Chen, Q., Xie, Y.-H., Cao, M.-X., Yu, M.-L., Wei, Y.-Y., Zhang, L., & Hu, T.-J. (2023). RNA-Seq and 16S rRNA Reveals That Tian–Dong–Tang–Gan Powder Alleviates Environmental Stress-Induced Decline in Immune and Antioxidant Function and Gut Microbiota Dysbiosis in Litopenaeus vannami. Antioxidants, 12(6), 1262. https://doi.org/10.3390/antiox12061262






