Abstract
Villalva et al. evaluated the potential utility of an Achillea millefolium (yarrow) extract in the control of H. pylori infection. The agar-well diffusions bioassay was applied to determine the antimicrobial activity of yarrow extracts. The supercritical anti-solvent fractionation process of yarrow extract was made to give two different fractions with polar phenolic compounds and monoterpenes and sesquiterpenes, respectively. Phenolic compounds were identified by HPLC-ESIMS by using the accurate masses of [M−H]− ions and the characteristic product ions. However, some of the reported product ions seem to be disputable, as described below.
Eradication of Helicobacter pylori has become a serious challenge due to increasing antimicrobial resistance. That Gram-negative, microaerophilic bacterium that is known to affect over 50% of the worldwide population occurs initially during childhood but, if left untreated, could persist for life, resulting in a broad spectrum of gastropathies. It is particularly important because H. pylori is involved in the development of 80% of gastric cancers and 5.5% of all malignant conditions worldwide [1,2,3].
The most effective conventional therapies for H. pylori infection require a minimum of two antibiotics (commonly, amoxicillin and clarithromycin) in combination with a gastric acid inhibitor or bismuth to guarantee high eradication rates. That is why nowadays, much research work is concentrated on the search for new potential anti-H. pylori candidates, among others, vegetables and plant extracts showing antibacterial properties [4,5]. They act by inhibiting bacterial enzymes, suppressing nuclear factor-κB, adhesions with gastric mucosa, and by inhibiting oxidative stress [6,7]. Organic extracts (ethanol, methanol, acetone, chloroform, petroleum ether, and mixtures of mentioned) of Acacia nilotica, Alchornea triplinervia, Arrabidaea chica, Bridelia micrantha, Calotropis procera, Camellia sinensis, Carum carvi, Cocculus hirsutus, Derris trifoliate, Geranium wilfordii, Hydrastis canadensis, Myristica fragrans, Xanthium brasilicum, and Trachyspermum copticum and many others have demonstrated antibacterial activity against clinical isolates of H. pylori [8,9,10,11,12,13,14,15]. In the study by Villalva and coworkers, the potential utility of an Achillea millefolium (yarrow) extract in the control of H. pylori infection was evaluated [16]. The supercritical anti-solvent fractionation process of yarrow extract was made to give two different fractions with polar phenolic compounds and monoterpenes and sesquiterpenes.
Yarrow (Achillea millefolium L.) is one of the most commonly used medicinal herbs, in both folk and conventional medicine, for over 3000 years, growing wild and as a cultivated plant in the region of Eurasia and North America [17]. According to the conducted studies, Achillea millefolium L. is a biologically active plant that demonstrates multiple beneficial effects, including antioxidant, anti-inflammatory, spasmolytic, diaphoretic, hepatoprotective, choleretic, antipyretic, analgesic, antimicrobial and anticancer properties [18,19,20]. Achillea millefolium L. helps eliminate toxins from the body, controls bleeding, lowers blood pressure, relieves menstrual pain, and is used in the treatment of various diseases such as diabetes, tuberculosis, Alzheimer’s, and Parkinson’s disease [21,22,23]. Moreover, as indicated by Tilwani and coworkers, the yarrow-treated SARS-nCoV-2 cell exhibits the disintegration of the virus membrane [24].
Different extracts (hexane, petroleum ether, and methanol) of A. millefolium aerial parts were found to be active toward the following pathogens: Bacillus cereus, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella enteritidis, Yersinia enterocolitica, Aspergillus niger, and Candida albicans [17,25,26]. As the literature indicates, the Gram-positive bacteria were more susceptible than the Gram-negative ones, whereas S. aureus and B. cereus were the most susceptible Gram-positive bacteria [25,27].
In the study by Villalva et al., the agar-well diffusions bioassay was applied to determine the antimicrobial activity of yarrow extracts (YE). YE was significantly (p < 0.05) effective as an antibacterial agent against all H. pylori strains tested (Hp48, Hp53, and Hp59) in the range of CFU reduction between 4.8 and 7.1 log [16]. Moreover, even better results were obtained for the fraction enriched in volatile compounds. In turn, in the experiment with the inflammatory response induced by H. pylori in AGS cells, interleukin 8 (IL-8) has been used as a biomarker. It is a well-documented fact that H. pylori infection is associated with an increase in IL-8 concentration, both in vitro and in vivo; it was among the first cytokines described to be produced by infected gastric epithelium; and leads to the recruitment of leukocytes in the gastric mucosa, representing a major step in the regulation of immune-inflammatory responses [2]. In the study by Villalva et al., the application of YE decreased IL-8 synthesis by 53% to 64% in human gastric epithelial cells, with a suggestion that the two types of compounds could contribute to this inhibition—not only phenolic compounds but also some essential oils. In addition, the authors analyzed the antioxidant activity of YE and its fractions against intracellular reactive oxygen species (ROS) synthesis in H. pylori-infected AGS cells. The inhibition effect of YE on ROS production depended on the examined H. pylori strain and ranged from 16% to 29%, while the fraction enriched in phenolic compounds was more active, regardless of the strain used, with about 40% intracellular ROS inhibition.
In the study by Villalva et al., the volatile compounds have been identified by GC-MS, and only retention times have been provided for them (Table 2 of [16]). Phenolic compounds have been identified by HPLC-ESIMS in the negative ion mode, and aside from the retention times, the accurate masses of [M−H]− ions and the characteristic product ions (MS/MS ions) have also been provided (Supplementary Material, Table S1 of [16]). However, some of the product ions seem to be disputable, as described below.
The most disputable is the product ion at m/z 112 reported for apigenin and diosmetin. This is the only product ion reported for these two compounds and has 100% ri (Supplementary Material, Table S1 of [16]). According to the published data, apigenin should yield characteristic product ions at m/z 225, 151, 149, 117, and 107, although the relative abundances of these ions may be different, depending on the instrumental conditions used [28,29,30,31]. Diosmetin should yield an abundant product ion at m/z 284 as a result of methyl radical loss—a characteristic feature of methoxylated flavonoids [32,33]. The other characteristic diosmetin product ions should be at m/z 256, 227, 151, and 107 [34]. Besides diosmetin, Villalva et al. have found one other methoxylated flavonoid, named ‘methoxyquercetin isomer’ (the third most abundant flavonoid in yarrow’s precipitated fraction, Table 1 of [16]); however, taking into account the m/z of [M−H]− at 315, it should be O-methyl quercetin. Villalva et al. claim only the detection of a product ion at m/z 301, thus the loss of mass 14 (elimination of a CH2 moiety). As mentioned above, the characteristic feature of the fragmentation of [M−H]− ions of methoxylated flavonoids is the loss of mass 15 (loss of a methyl radical). Furthermore, other product ions should be observed as well, enabling at least tentative identification of this compound (isorhamnetin glycosides have already been found in the Achillea millefolium L. [35,36]). It has to be stressed that the two other methoxylated flavonoids, centaureidin and casticin, have been observed to have methyl radical losses by Villalva et al. [16]. The authors claim the detection of one biflavonoid, namely amentoflavone ([M−H]− at m/z 537), for which they have reported two product ions at m/z 519 and 495. However, the characteristic amentoflavone product ions are at m/z 443, 417, 375 (the most abundant), and 331 [37,38].
Villalva et al. have also detected a number of flavone C-glycosides. Among them are three isomers, namely apigenin-C-hexoside-C-pentoside, schaftoside, and schaftoside isomer ([M−H]− at m/z 563). For all these three compounds, Villalva et al. have obtained two identical product ions with identical relative abundances (m/z 473 and 443, 10 and 20% ri, respectively). The loss of mass 90 and 120 is a characteristic feature of flavone C-glycosides fragmentation [39]; thus, the product ions at m/z 473 and 443 are typical of these compounds. However, other product ions should also be detected, e.g., at m/z 383 and 353, and at least minor differences in relative ion abundances should be observed for these three isomers detected [40,41]. Villalva et al. claim the detection of luteolin-6,8-di-C-glucoside ([M−H]− at m/z 609), for which they have detected two product ions at m/z 489 and 325 [16]. The first one (loss of mass 120) is a characteristic product ion of luteolin-6,8-di-C-glucoside, often having 100% ri; however, at m/z 325 it is not the characteristic one. Other characteristic product ions which should be observed for this compound are at m/z 591, 399, 369, and 327 [41,42]. Villalva et al. claim the detection of vicenin 2 (apigenin 6,8-di-C-glucoside); however, no product ions have been reported for this compound [16]. For the two last flavone C-glycosides, namely homoorientin (luteolin 6-C-glucoside) and vitexin (apigenin 8-C-glucoside), the reported by Villalva et al. product ions match perfectly with those, the most abundant ones, reported elsewhere (although vitexin cannot be classified as flavonols) [43,44].
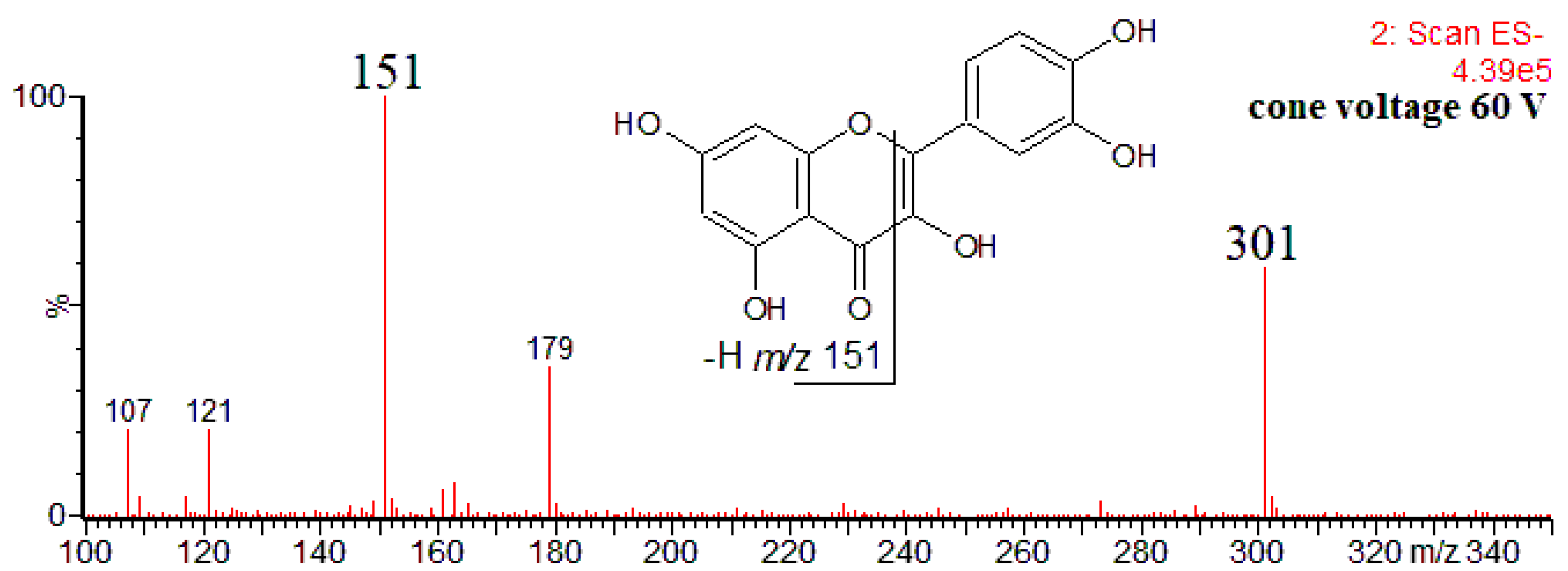
The product ions detected by Villalva et al. for other flavonoids are in agreement with those reported in the literature, at least as concerns the most characteristic ones. For example, for quercetin, the authors have detected only the product ion at m/z 151 [16]. Although deprotonated quercetin molecule yields a few other product ions (Figure 1), that at m/z 151 (formally 1,3A− product ion) is the most abundant, formed through the retro-Diels–Alder reaction, and can be regarded as a diagnostic ion for 5,7-dihydroxyflavonoids [28,45,46].

Figure 1.
ESI mass spectrum (CID in-source) of quercetin obtained upon HPLC-MS(−) analysis of Prunus persica bark extract [46].
Villalva et al. have also detected a number of hydroxycinnamic acids and their conjugates, and the detection of most of them does not raise any doubts. The only disputable ones are the product ions reported for chlorogenic acid (trans-5-O-caffeoylquinic acid) and its isomer, cryptochlorogenic acid (trans-4-O-caffeoylquinic acid). For chlorogenic acid and cryptochlorogenic acid, Villalva et al. have obtained two identical product ions of almost identical relative abundances, namely at m/z 191 (100% ri) and 161 (10–11% ri) [16]. However, the fragmentation patterns of these compounds should be significantly different. Chlorogenic acid yields abundant product ions at m/z 191, and other product ions have very low abundances (practically are not detectable), whereas cryptochlorogenic acid, except the product ion at m/z 191, yields abundant product ions at m/z 179, 173, and 135 [47,48,49,50]. On the other hand, it has to be stressed that the product ions detected by Villalva et al. for neochlorogenic acid (trans-3-O-caffeoylquinic acid) perfectly match those reported elsewhere with respect to their m/z and ri values [47,48,49]. The detection of other hydroxycinnamic acids and their conjugates also does not raise any doubts, although the accurate m/z of ferulic acid [M−H]− ion should be 193.0504, not 103.0504 (minor typos error). It is also worth adding that the elution order of the mono-O-caffeoylquinic acid isomers ([M−H]− at m/z 353) and di-O-caffeoylquinic acid isomers ([M−H]− at m/z 515) reported by Villalva et al. perfectly matches that obtained elsewhere during reversed-phase liquid chromatographic analysis [51].
It should be emphasized that our very specific comments concerning the product ions do not affect the paper by Villalva et al., the paper is characterized by a high scientific level, and the authors finding may imply the next practical application of Achillea millefolium L. extracts. On the other hand, the correction of Table S1 is desirable, maybe as a reply to our comment or as a corrigendum to their paper since it surely would improve the quality of the paper.
Author Contributions
Conceptualization, R.F. and M.B.-B.; writing—original draft preparation, R.F. and M.B.-B.; writing—review and editing, R.F. and M.B.-B. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardos, I.A.; Zaha, D.C.; Sindhu, R.K.; Cavalu, S. Revisiting therapeutic strategies for H. pylori treatment in the context of antibiotic resistance: Focus on alternative and complementary therapies. Molecules 2021, 26, 6078. [Google Scholar] [CrossRef] [PubMed]
- Dincă, A.L.; Melit, L.E.; Mărginean, C.O. Old and new aspects of H. pylori-associated Inflammation and gastric cancer. Children 2022, 9, 1083. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, S.; Papastergiou, V. An update on current and advancing pharmacotherapy options for the treatment of H. pylori infection. Expert Opin. Pharmacother. 2021, 22, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Wang, M.; Xu, Y.L.; Zhu, Y.; Lu, N.H.; Hu, Y. Amoxicillin-vonoprazan dual therapy for Helicobacter pylori eradication: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2022, 37, 1666–1672. [Google Scholar] [CrossRef]
- Safavi, M.; Shams-Ardakani, M.; Foroumadi, A. Medicinal plants in the treatment of Helicobacter pylori infections. Pharmac. Biol. 2015, 53, 939–960. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in Helicobacter pylori infections: What are we doing now? Int. J. Mol. Sci. 2018, 19, 2361. [Google Scholar] [CrossRef]
- Sathianarayanan, S.; Aparna, V.; Biswas, R.; Anita, B.; Sukumaran, S.; Venkidasamy, B. A new approach against Helicobacter pylori using plants and its constituents: A review study. Microb. Pathog. 2022, 168, 105594. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Naz, F.; Mehmood, T.; Saariet, N. Anti-Helicobacter pylori and urease inhibition activities of some traditional medicinal plants. Molecules 2013, 18, 2135–2149. [Google Scholar] [CrossRef]
- He, C.; Chen, J.; Liu, J.; Li, Y.; Zhou, Y.; Mao, T.; Li, Z.; Qin, X.; Jin, S. Geranium wilfordii maxim.: A review of its traditional uses, phytochemistry, pharmacology, quality control and toxicology. J. Ethnopharmacol. 2022, 285, 114907. [Google Scholar] [CrossRef]
- Kaewkod, T.; Wangroongsarb, P.; Promputtha, I.; Tragoolpua, Y. Inhibitory efficacy of Camellia sinensis leaf and medicinal plant extracts on Helicobacter pylori standard and isolate strains growth, urease enzyme production and epithelial cell adhesion. Chiang Mai J. Sci. 2021, 48, 56–73. [Google Scholar]
- Mafioleti, L.; da Silva, I.F., Jr.; Colodel, E.M.; Flach, A.; de Oliveira Martins, D.T. Evaluation of the toxicity and antimicrobial activity of hydroethanolic extract of Arrabidaea chica (Humb. & Bonpl.) B. Verl. J. Ethnopharmacol. 2013, 150, 576–582. [Google Scholar] [PubMed]
- Okeleye, B.I.; Bessong, P.O.; Ndip, R.N. Preliminary phytochemical screening and in vitro anti-Helicobacter pylori activity of extracts of the stem bark of Bridelia micrantha (Hochst., Baill., Euphorbiaceae). Molecules 2011, 16, 6193–6205. [Google Scholar] [CrossRef]
- Poovendran, P.; Kalaigandhi, V.; Poongunran, E. Antimicrobial activity of the leaves of Cocculus hirsutus against gastric ulcer producing Helicobacter pylori. J. Pharm. Res. 2011, 4, 4294–4295. [Google Scholar]
- Suthisamphat, N.; Dechayont, B.; Phuaklee, P.; Prajuabjinda, O.; Vilaichone, R.K.; Itharat, A.; Prommee, N. Anti-helicobacter pylori, anti-inflammatory, cytotoxic, and antioxidant activities of mace extracts from Myristica fragrans. Evid. Based Complement. Alternat. Med. 2020, 2020, 7576818. [Google Scholar] [CrossRef] [PubMed]
- Uyub, A.M.; Nwachukwu, I.N.; Azlan, A.A.; Fariza, S.S. In-vitro antibacterial activity and cytotoxicity of selected medicinal plant extracts from Penang Island Malaysia on metronidazole-resistant-Helicobacter pylori and some pathogenic bacteria. Ethnobot. Res. Appl. 2010, 8, 95–106. [Google Scholar] [CrossRef]
- Villalva, M.; Silvan, J.M.; Alarcón-Cavero, T.; Villanueva-Bermejo, D.; Jaime, L.; Santoyo, S.; Martinez-Rodriguez, A.J. Antioxidant, anti-inflammatory, and antibacterial properties of an Achillea millefolium L. extract and its fractions obtained by supercritical anti-solvent fractionation against Helicobacter pylori. Antioxidants 2022, 11, 1849. [Google Scholar] [CrossRef]
- Grigore, A.; Colceru-Mihul, S.; Bazdoaca, C.; Yuksel, R.; Ionita, C.; Glava, L. Antimicrobial activity of an Achillea millefolium L. Proceedings 2020, 57, 34. [Google Scholar]
- Karaaslan Ayhan, N.; Karaaslan Tunc, M.G.; Noma, S.A.A.; Kurucay, A.; Ates, B. Characterization of the antioxidant activity, total phenolic content, enzyme inhibition, and anticancer properties of Achillea millefolium L.(yarrow). Instrum. Sci. Technol. 2022, 50, 654–667. [Google Scholar] [CrossRef]
- Nemeth, E. Biological activities of yarrow species (Achillea spp.). Curr. Pharm. Des. 2008, 14, 3151–3167. [Google Scholar] [CrossRef]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflamatory potential in topical application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef]
- Mozafari, N.; Hassanshahi, J.; Ostadebrahimi, H.; Shamsizadeh, A.; Ayoobi, F.; Hakimizadeh, E.; Pak Hashemi, M.; Kaeidi, A. Neuroprotective effect of Achillea millefolium aqueous extract against oxidative stress and apoptosis induced by chronic morphine in rat hippocampal CA1 neurons. Acta Neurobiol. Exp. 2022, 82, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Noor, A.; Zargar, M.I.; Siddiqui, N.A. Ethnopharmacology, Phytochemistry, and Biological Activities of Achillea millefolium: A Comprehensive Review. In Edible Plants in Health and Diseases; Masoodi, M.H., Rehman, M.U., Eds.; Springer: Singapore, 2022; pp. 457–481. [Google Scholar]
- Zakeri, S. Achillea millefolium L. as a recommendation for the management of hysteria. Tradit. Integr. Med. 2020, 5, 19–25. [Google Scholar] [CrossRef]
- Tilwani, K.; Patel, A.; Parikh, H.; Thakker, D.J.; Dave, G. Investigation on anti-Corona viral potential of Yarrow tea. J. Biomol. Struct. Dyn. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Almadiy, A.A.; Nenaah, G.E.; Al Assiuty, B.A.; Moussa, E.A.; Mira, N.M. Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT-Food Sci. Technol. 2016, 69, 529–537. [Google Scholar] [CrossRef]
- Stojanovic, G.; Radulovic, N.; Hashimoto, T.; Palic, R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J. Ethnopharmacol. 2005, 101, 185–190. [Google Scholar] [CrossRef]
- El-Kalamouni, C.; Venskutonis, P.R.; Zebib, B.; Merah, O.; Raynaud, C.; Talou, T. Antioxidant and antimicrobial activities of the essential oil of Achillea millefolium L. grown in France. Medicines 2017, 4, 30. [Google Scholar] [CrossRef]
- Hughes, R.J.; Croley, T.R.; Metcalfe, C.D.; March, R.E. A tandem mass spectrometric study of selected characteristic flavonoids. Int. J. Mass Spectrom. 2001, 210, 371–385. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Beszterda, M.; Frański, R. Electrospray ionisation mass spectrometric behaviour of flavonoid 5-O-glucosides and their positional isomers detected in the extracts from the bark of Prunus cerasus L. and Prunus avium L. Phytochem. Anal. 2021, 32, 433–439. [Google Scholar] [CrossRef]
- Yang, W.Z.; Ye, M.; Qiao, X.; Wang, Q.; Bo, T.; Guo, D.A. Collision-induced dissociation of 40 flavonoid aglycones and differentiation of the common flavonoid subtypes using electrospray ionization ion-trap tandem mass spectrometry and quadrupole time-of-flight mass spectrometry. Eur. J. Mass Spectrom. 2012, 18, 493–503. [Google Scholar] [CrossRef]
- Frański, R.; Gierczyk, B.; Kozik, T.; Popenda, Ł.; Beszterda, M. Signals of diagnostic ions in the product ion spectra of [M−H]− ions of methoxylated flavonoids. Rapid Commun. Mass Spectrom. 2019, 33, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lech, K.; Witkoś, K.; Jarosz, M. HPLC-UV-ESI MS/MS identification of the color constituents of sawwort (Serratula tinctoria L.). Anal. Bioanal. Chem. 2014, 406, 3703–3708. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Benedek, B.; Rothwangl-Wiltschnigg, K.; Rozema, E.; Gjoncaj, N.; Reznicek, G.; Jurenitsch, J.; Kopp, B.; Glasl, S. Yarrow (Achillea millefolium L. sl): Pharmaceutical quality of commercial samples. Pharmazie 2008, 63, 23–26. [Google Scholar] [PubMed]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro and in vivo anticancer effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef]
- Michler, H.; Laakmann, G.; Wagner, H. Development of an LC-MS method for simultaneous quantitation of amentoflavone and biapigenin, the minor and major biflavones from Hypericum perforatum L., in human plasma and its application to real blood. Phytochem. Anal. 2011, 22, 42–50. [Google Scholar] [CrossRef]
- Beszterda, M.; Frański, R. Detection of flavone C-glycosides in the extracts from the bark of Prunus avium L. and Prunus cerasus L. Eur. J. Mass Spectrom. 2020, 26, 369–375. [Google Scholar] [CrossRef]
- Keskes, H.; Belhadj, S.; Jlail, L.; El Feki, A.; Sayadi, S.; Allouche, N. LC-MS-MS and GC-MS analyses of biologically active extracts of Tunisian Fenugreek (Trigonella foenum-graecum L.) Seeds. J. Food Meas. Charact. 2018, 12, 209–220. [Google Scholar] [CrossRef]
- Cao, J.; Yin, C.; Qin, Y.; Cheng, Z.; Chen, D. Approach to the study of flavone di-C-glycosides by high performance liquid chromatography-tandem ion trap mass spectrometry and its application to characterization of flavonoid composition in Viola yedoensis. J. Mass Spectrom. 2014, 49, 1010–1024. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G.; Bórquez, J.; Kennelly, E.J. The Passiflora tripartita (Banana Passion) fruit: A source of bioactive flavonoid C-glycosides isolated by HSCCC and characterized by HPLC-DAD-ESI/MS/MS. Molecules 2013, 18, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Salles, B.C.C.; da Silva, M.A.; Taniguthi, L.; Ferreira, J.N.; da Rocha, C.Q.; Vilegas, W.; Dias, P.H.; Pennacchi, P.C.; da Silveira Duarte, S.M.; Rodrigues, M.R.; et al. Passiflora edulis leaf extract: Evidence of antidiabetic and antiplatelet effects in rats. Biol. Pharm. Bull. 2020, 43, 169–174. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrum. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Śliwka-Kaszyńska, M.; Anusiewicz, I.; Skurski, P. The mechanism of a Retro-Diels-Alder fragmentation of luteolin: Theoretical studies supported by electrospray ionization tandem mass spectrometry results. Molecules 2022, 27, 1032. [Google Scholar] [CrossRef]
- Beszterda, M.; Frański, R. Seasonal qualitative variations of phenolic content in the stem bark of Prunus persica var. nucipersica-implication for the use of the bark as a source of bioactive compounds. ChemistrySelect 2022, 7, e202200418. [Google Scholar]
- Ramabulana, A.T.; Steenkamp, P.; Madala, N.; Dubery, I.A. Profiling of chlorogenic acids from Bidens pilosa and differentiation of closely related positional isomers with the aid of UHPLC-QTOF-MS/MS-based in-source collision-induced dissociation. Metabolites 2020, 10, 178. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef]
- Xie, C.; Yu, K.; Zhong, D.; Yuan, T.; Ye, F.; Jarrell, J.A.; Millar, A.; Chen, X. Investigation of isomeric transformations of chlorogenic acid in buffers and biological matrixes by ultraperformance liquid chromatography coupled with hybrid quadrupole/ion mobility/orthogonal acceleration time-of-flight mass spectrometry. J. Agric. Food Chem. 2011, 59, 11078–11087. [Google Scholar] [CrossRef]
- Han, B.; Xin, Z.; Ma, S.; Liu, W.; Zhang, B.; Ran, L.; Yi, L.; Ren, D. Comprehensive characterization and identification of antioxidants in Folium Artemisiae Argyi using high-resolution tandem mass spectrometry. J. Chromatogr. B 2017, 1063, 84–92. [Google Scholar] [CrossRef]
- Wianowska, D.; Gil, M. Recent advances in extraction and analysis procedures of natural chlorogenic acids. Phytochem. Rev. 2019, 18, 273–302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).