Ceratonia siliqua L. Pod Extract: From Phytochemical Characterization to Liposomal Formulation and Evaluation of Behaviour in Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Extract Preparation
2.3. High-Resolution HPLC-ESI-QToF-MS/MS and HPLC-PDA Analysis
2.4. Preparation of Liposomes
2.5. Characterization of Liposomes
2.6. Small-Angle X-ray Scattering
2.7. Antioxidant Activity: DPPH and FRAP Assays
2.8. Liposomes’ Biocompatibility: Hemolytic Activity and Cell Viability
2.9. Antioxidant Activity in Cell Lines
2.10. Statistical Analysis
3. Results
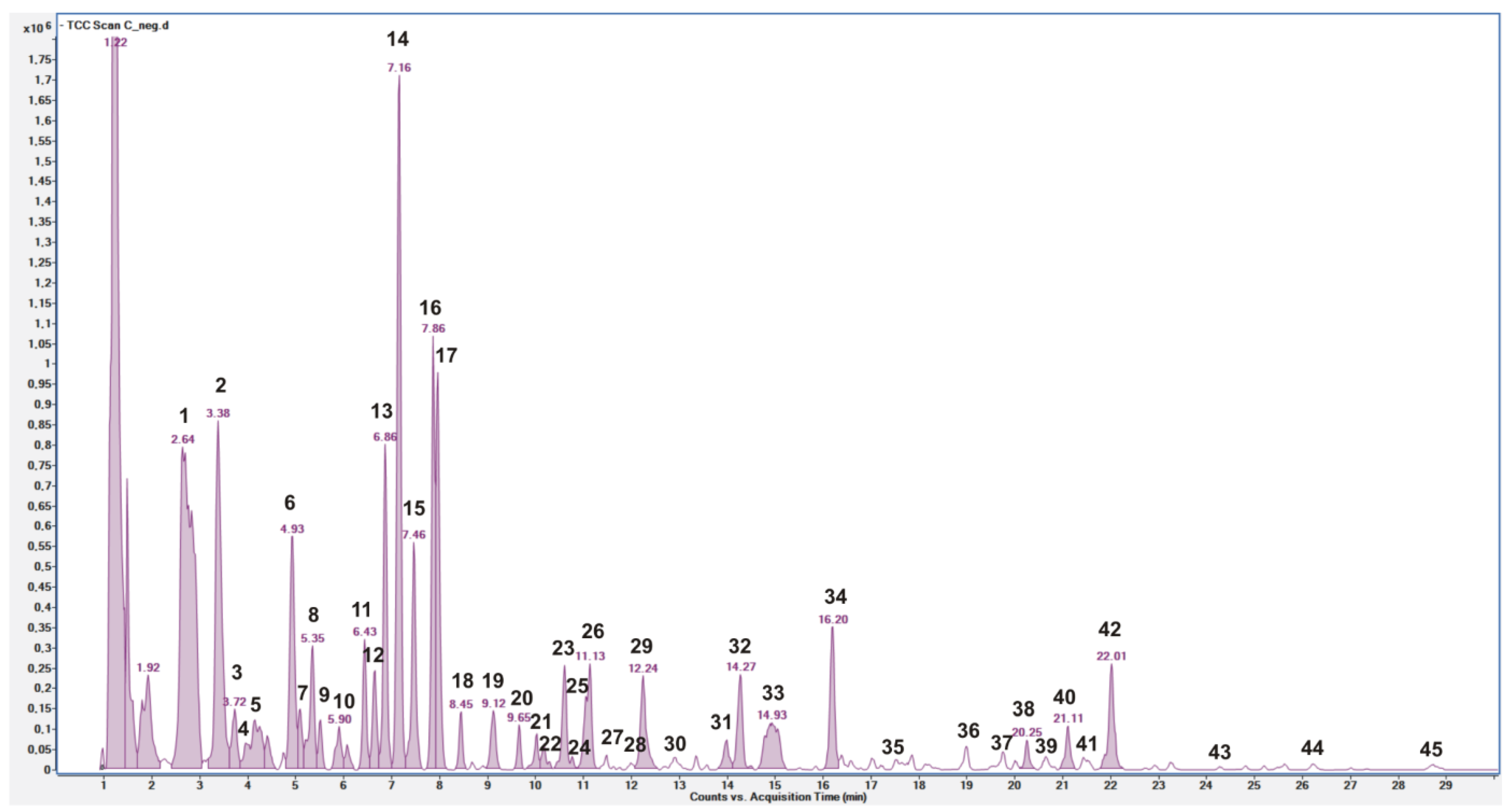
3.1. Quali–Quantitative Phenolic Profile of C. siliqua Pod Extract
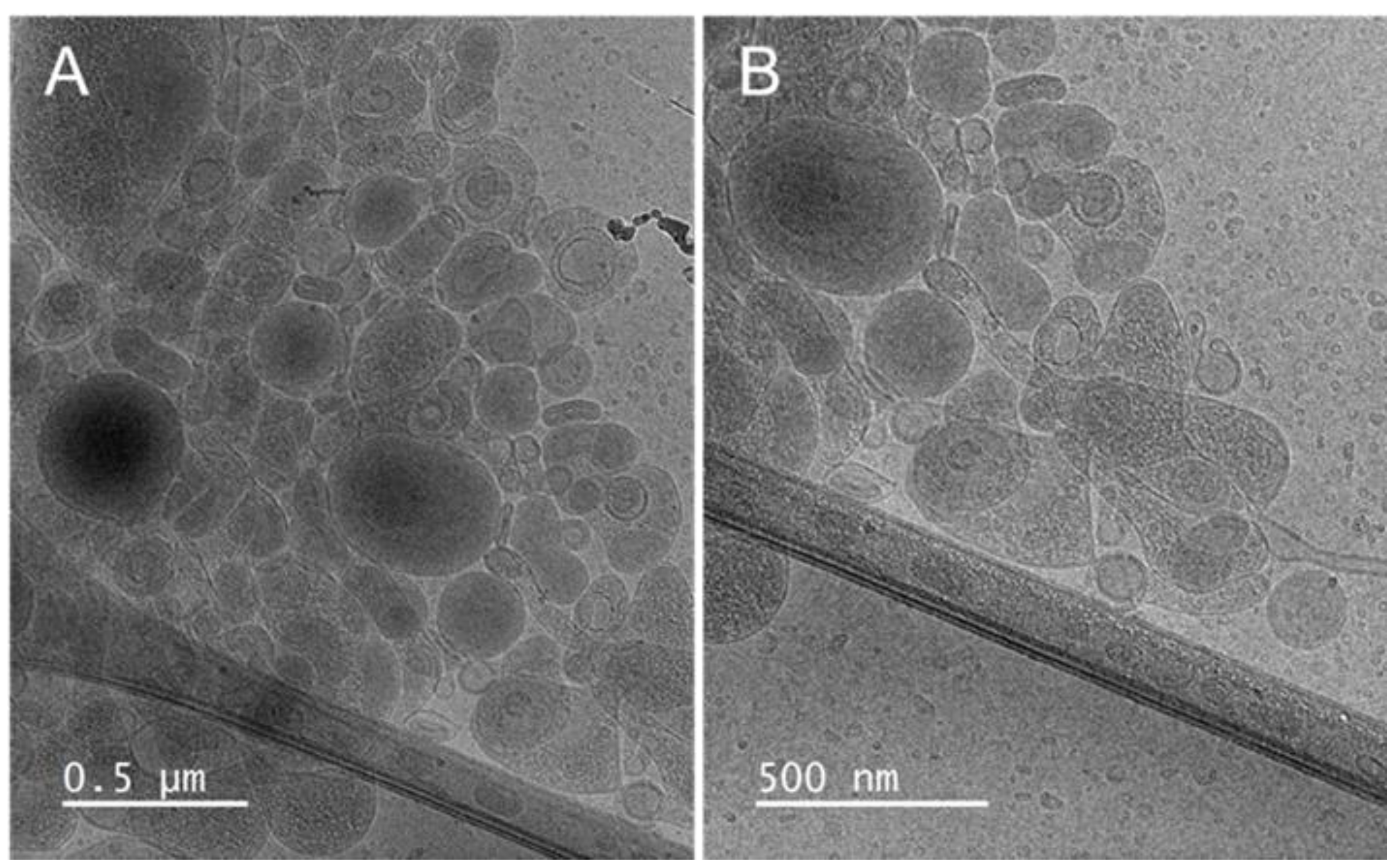
3.2. Liposomes’ Characteristics
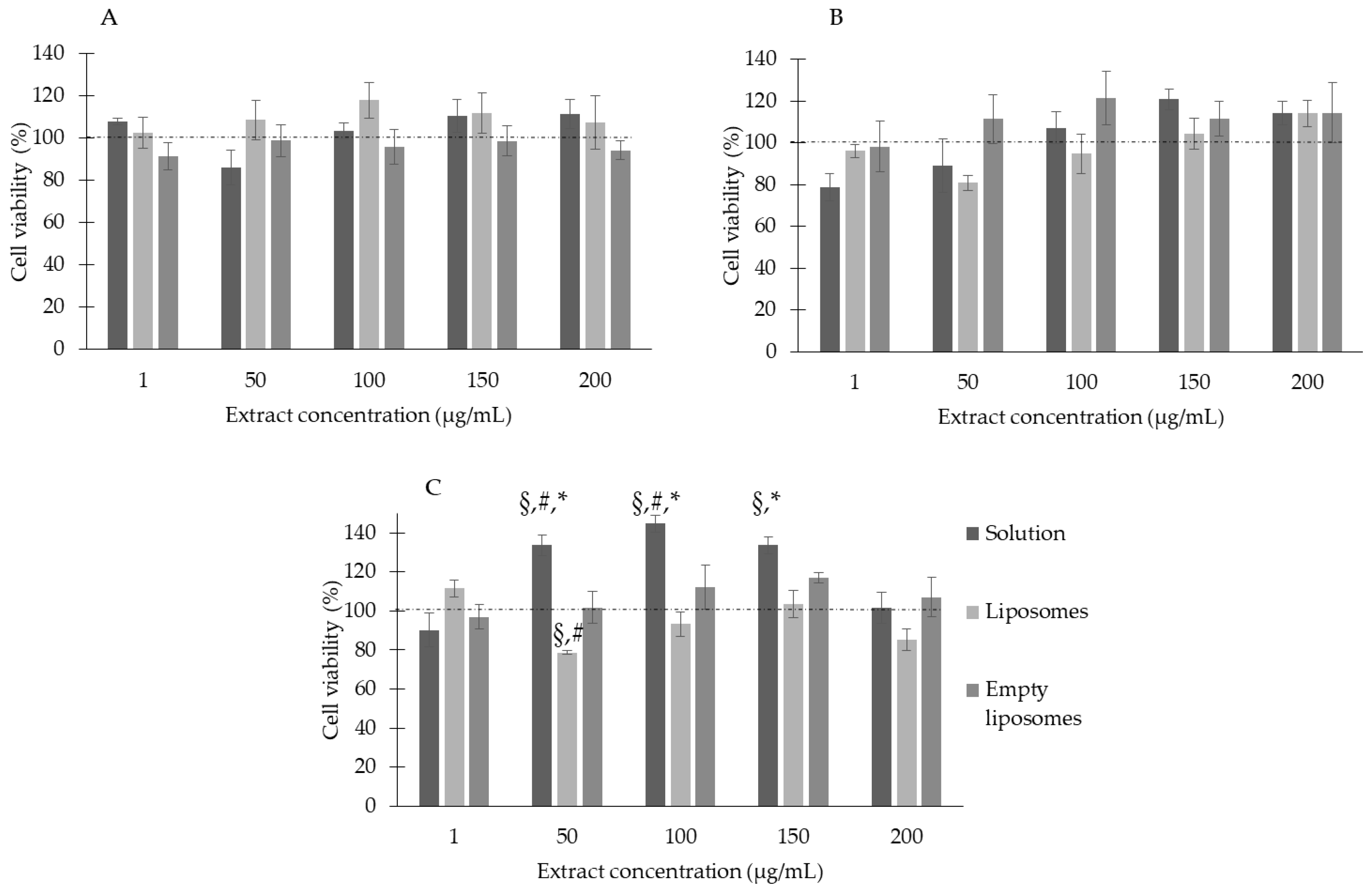
3.3. Liposomes’ Biocompatibility
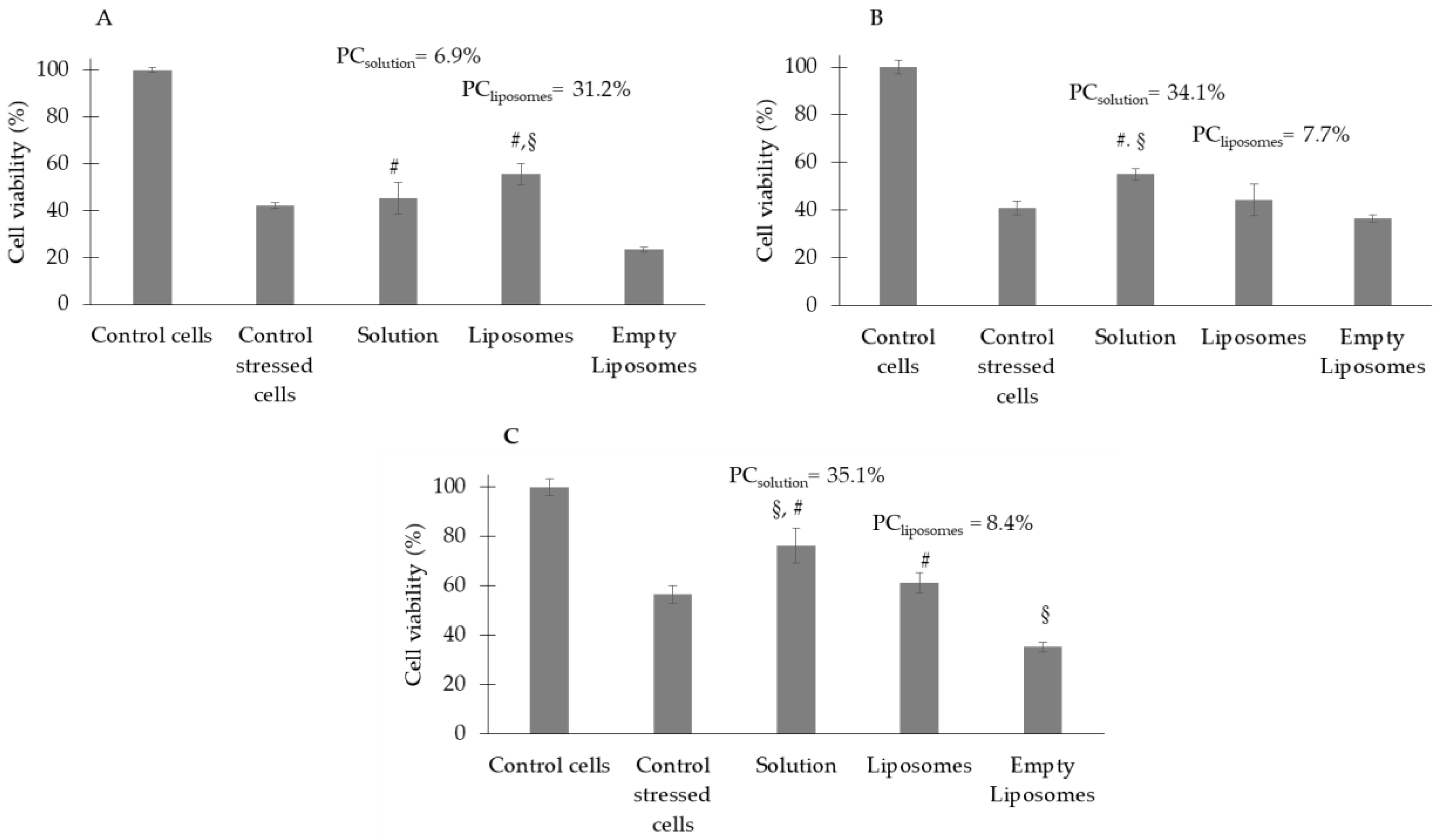
3.4. Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farag, M.A.; El-Kersh, D.M. Volatiles profiling in Ceratonia siliqua (Carob bean) from Egypt and in response to roasting as analyzed via solid-phase microextraction coupled to chemometrics. J. Adv. Res. 2017, 8, 379–385. [Google Scholar] [CrossRef]
- Gioxari, A.; Amerikanou, C.; Nestoridi, I.; Gourgari, E.; Pratsinis, H.; Kalogeropoulos, N.; Andrikopoulos, N.K.; Kaliora, A.C. Carob: A Sustainable Opportunity for Metabolic Health. Foods 2022, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Darwish, W.S.; Khadr, A.E.S.; Kamel, M.A.E.N.; Abd Eldaim, M.A.; El Sayed, I.E.T.; Abdel-Bary, H.M.; Ullah, S.; Ghareeb, D.A. Phytochemical characterization and evaluation of biological activities of egyptian carob pods (Ceratonia siliqua L.) aqueous extract: In vitro study. Plants 2021, 10, 2626. [Google Scholar] [CrossRef]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Çavuşoğlu, K.; Kurt, D.; Yalçın, E. A versatile model for investigating the protective effects of Ceratonia siliqua pod extract against 1,4-dioxane toxicity. Environ. Sci. Pollut. Res. 2020, 27, 27885–27892. [Google Scholar] [CrossRef]
- Peng, Z.T.; Xia, Y.J.; Yashiro, T.; Gao, X.; Dong, T.T.X.; Tsim, K.W.K.; Wang, H.Y. Novel phenylpropanoids and isoflavone glycoside are isolated and identified from the carob pods (Ceratonia siliqua L.). Nat. Prod. Res. 2022, 2022, 1–7. [Google Scholar] [CrossRef]
- Ben Ayache, S.; Behija Saafi, E.; Emhemmed, F.; Flamini, G.; Achour, L.; Muller, C.D. Biological activities of aqueous extracts from carob plant (Ceratonia siliqua L.) by antioxidant, analgesic and proapoptotic properties evaluation. Molecules 2020, 25, 3120. [Google Scholar] [CrossRef] [PubMed]
- Saratsi, K.; Hoste, H.; Voutzourakis, N.; Tzanidakis, N.; Stefanakis, A.; Thamsborg, S.M.; Mueller-Harvey, I.; Hadjigeorgiou, I.; Sotiraki, S. Feeding of carob (Ceratonia siliqua) to sheep infected with gastrointestinal nematodes reduces faecal egg counts and worm fecundity. Vet. Parasitol. 2020, 284, 109200. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Alibbini, S.; Khabour, O.F.; El-Elimat, T.; Al-zubi, M.; Alali, F.Q. Carob (Ceratonia siliqua L.) Prevents Short-Term Memory Deficit Induced by Chronic Stress in Rats. J. Mol. Neurosci. 2018, 66, 314–321. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Martínez-Villaluenga, C.; Aguirre, L.; Silván, J.M.; Dueñas, M.; De Luis, D.A.; Lasa, A. In vitro approach for evaluation of carob by-products as source bioactive ingredients with potential to attenuate metabolic syndrome (MetS). Heliyon 2019, 5, e01175. [Google Scholar] [CrossRef]
- Valero-Muñoz, M.; Ballesteros, S.; Ruiz-Roso, B.; Pérez-Olleros, L.; Martín-Fernández, B.; Lahera, V.; de las Heras, N. Supplementation with an insoluble fiber obtained from carob pod (Ceratonia siliqua L.) rich in polyphenols prevents dyslipidemia in rabbits through SIRT1/PGC-1α pathway. Eur. J. Nutr. 2019, 58, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Rtibi, K.; Selmi, S.; Grami, D.; Saidani, K.; Sebai, H.; Amri, M.; Eto, B.; Marzouki, L. Ceratonia siliqua L. (immature carob bean) inhibits intestinal glucose absorption, improves glucose tolerance and protects against alloxan-induced diabetes in rat. J. Sci. Food Agric. 2017, 97, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Qasem, M.A.; Noordin, M.I.; Arya, A.; Alsalahi, A.; Jayash, S.N. Evaluation of the glycemic effect of Ceratonia siliqua pods (Carob) on a streptozotocin-nicotinamide induced diabetic rat model. PeerJ. 2018, 2018, e4788. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, F.E.; Silva, J.C.G.E.; Cacciola, F.; Asraoui, F.; Tayeq, H.; Ben Amar, Y.M.; Lovillo, M.P.; Chouaibi, N.; Brigui, J. Evaluation of Different Extraction Methods on the Phenolic Profile and the Antioxidant Potential of Ceratonia siliqua L. Pods Extracts. Molecules 2022, 27, 6163. [Google Scholar] [CrossRef]
- Michalak, M. Plant-Derived Antioxidants: Significance in Skin Health and the Ageing Process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- De Luca, M.; Tuberoso, C.I.G.; Pons, R.; García, M.T.; Morán, M.d.C.; Ferino, G.; Vassallo, A.; Martelli, G.; Caddeo, C. Phenolic Fingerprint, Bioactivity and Nanoformulation of Prunus spinosa L. Fruit Extract for Skin Delivery. Pharmaceutics 2023, 15, 1063. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A rapid tool for turning tandem mass spectra into metabolite structure information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Nothias, L.F.; Ludwig, M.; Fleischauer, M.; Gentry, E.C.; Witting, M.; Dorrestein, P.C.; Dührkop, K.; Böcker, S. High-confidence structural annotation of metabolites absent from spectral libraries. Nat. Biotechnol. 2022, 40, 411–421. [Google Scholar] [CrossRef]
- De Luca, M.; Lucchesi, D.; Tuberoso, C.I.G.; Fernàndez-Busquets, X.; Vassallo, A.; Martelli, G.; Fadda, A.M.; Pucci, L.; Caddeo, C. Liposomal Formulations to Improve Antioxidant Power of Myrtle Berry Extract for Potential Skin Application. Pharmaceutics 2022, 14, 910. [Google Scholar] [CrossRef]
- Heftberger, P.; Kollmitzer, B.; Heberle, F.A.; Pan, J.; Rappolt, M.; Amenitsch, H.; Kučerka, N.; Katsaras, J.; Pabst, G. Global small-angle X-ray scattering data analysis for multilamellar vesicles: The evolution of the scattering density profile model. J. Appl. Crystallogr. 2014, 47, 173–180. [Google Scholar] [CrossRef]
- Pabst, G.; Rappolt, M.; Amenitsch, H.; Laggner, P. Structural information from multilamellar liposomes at full hydration: Full q-range fitting with high quality X-ray data. Phys. Rev. E-Stat. Phys. Plasmas Fluids Relat. Interdiscip. Top. 2000, 62, 4000–4009. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, J.S. Analysis of small-angle scattering data from colloids and polymer solutions: Modeling and least-squares fitting. Adv. Colloid Interface Sci. 1997, 70, 171–210. [Google Scholar] [CrossRef]
- Caddeo, C.; Pucci, L.; Gabriele, M.; Carbone, C.; Fernàndez-Busquets, X.; Valenti, D.; Pons, R.; Vassallo, A.; Fadda, A.M.; Manconi, M. Stability, biocompatibility and antioxidant activity of PEG-modified liposomes containing resveratrol. Int. J. Pharm. 2018, 538, 40–47. [Google Scholar] [CrossRef]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Topical Anti-Inflammatory Potential of Quercetin in Lipid-Based Nanosystems: In Vivo and In Vitro Evaluation. Pharm. Res. 2014, 31, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.; Pinazo, A.; Morán, M.C.; Pons, R. Aggregation Behavior, Antibacterial Activity and Biocompatibility of Catanionic Assemblies Based on Amino Acid-Derived Surfactants. Int. J. Mol. Sci. 2020, 21, 8912. [Google Scholar] [CrossRef]
- KNApSAcK Core System. Available online: http://www.knapsackfamily.com/knapsack_core/top.php (accessed on 11 February 2023).
- Blaženović, I.; Kind, T.; Ji, J.; Fiehn, O. Software Tools and Approaches for Compound Identification of LC-MS/MS Data in Metabolomics. Metabolites 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Ghorbaninejad, Z.; Eghbali, A.; Ghorbaninejad, M.; Ayyari, M.; Zuchowski, J.; Kowalczyk, M.; Baharvand, H.; Shahverdi, A.; Eftekhari-Yazdi, P.; Esfandiari, F. Carob extract induces spermatogenesis in an infertile mouse model via upregulation of Prm1, Plzf, Bcl-6b, Dazl, Ngn3, Stra8, and Smc1b. J. Ethnopharmacol. 2023, 301, 115760. [Google Scholar] [CrossRef]
- Owen, R.W.; Haubner, R.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem. Toxicol. 2003, 41, 1727–1738. [Google Scholar] [CrossRef]
- Penner, N.; Ramanathan, R.; Zgoda Pols, J.; Chowdhury, S. Quantitative determination of hippuric and benzoic acids in urine by LC-MS/MS using surrogate standards. J. Pharm. Biomed. Anal. 2010, 52, 534–543. [Google Scholar] [CrossRef]
- Gao, S.; Zhan, Q.; Li, J.; Yang, Q.; Li, X.; Chen, W.; Sun, L. LC-MS/MS method for the simultaneous determination of ethyl gallate and its major metabolite in rat plasma. Biomed. Chromatogr. 2010, 24, 472–478. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente-Fernández, M.; de la Fuente-Muñoz, M.; Román-Carmena, M.; Amor, S.; García-Redondo, A.B.; Blanco-Rivero, J.; González-Hedström, D.; Espinel, A.E.; García-Villalón, Á.L.; Granado, M. Carob Extract Supplementation Together with Caloric Restriction and Aerobic Training Accelerates the Recovery of Cardiometabolic Health in Mice with Metabolic Syndrome. Antioxidants 2022, 11, 1803. [Google Scholar] [CrossRef] [PubMed]
- Lakkab, I.; Ouakil, A.; El Hajaji, H.; Lachkar, N.; Lefter, R.; Ciobica, A.; El Bali, B.; Dobrin, R.; Hritcu, L.D.; Lachkar, M. Carob Seed Peels Effect on Cognitive Impairment and Oxidative Stress Status in Methionine-Induced Mice Models of Schizophrenia. Brain Sci. 2022, 12, 1660. [Google Scholar] [CrossRef]
- Frühbauerová, M.; Červenka, L.; Hájek, T.; Pouzar, M.; Palarčík, J. Bioaccessibility of phenolics from carob (Ceratonia siliqua L.) pod powder prepared by cryogenic and vibratory grinding. Food Chem. 2022, 377, 131968. [Google Scholar] [CrossRef]
- Ayad, R.; Ayad, R.; Bourekoua, H.; Lefahal, M.; Makhloufi, E.H.; Akkal, S.; Medjroubi, K.; Nieto, G. Process Optimization of Phytoantioxidant and Photoprotective Compounds from Carob Pods (Ceratonia siliqua L.) Using Ultrasonic Assisted Extraction Method. Molecules 2022, 27, 8802. [Google Scholar] [CrossRef]
- Choubey, S.; Goyal, S.; Varughese, L.R.; Kumar, V.; Sharma, A.K.; Beniwal, V. Probing Gallic Acid for Its Broad Spectrum Applications. Mini-Rev. Med. Chem. 2018, 18, 1283–1293. [Google Scholar] [CrossRef]
- AL Zahrani, N.A.; El-Shishtawy, R.M.; Asiri, A.M. Recent developments of gallic acid derivatives and their hybrids in medicinal chemistry: A review. Eur. J. Med. Chem. 2020, 204, 112609. [Google Scholar] [CrossRef]
- Daglia, M.; Lorenzo, A.; Nabavi, S.; Talas, Z.; Nabavi, S. Polyphenols: Well Beyond The Antioxidant Capacity: Gallic Acid and Related Compounds as Neuroprotective Agents: You are What You Eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Klenow, S.; Jahns, F.; Pool-Zobel, B.L.; Glei, M. Does an extract of carob (Ceraionia siliqua L.) have chemopreventive potential related to oxidative stress and drug metabolism in human colon cells? J. Agric. Food Chem. 2009, 57, 2999–3004. [Google Scholar] [CrossRef]
- Ünal, E.; Sulukan, E.; Şenol, O.; Baran, A.; Nadaroğlu, H.; Kankaynar, M.; Kızıltan, T.; Ceyhun, S.B. Antioxidant/protective effects of carob pod (Ceratonia siliqua L.) water extract against deltamethrin-induced oxidative stress/toxicity in zebrafish larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 267, 109584. [Google Scholar] [CrossRef] [PubMed]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Alajmi, R.A.; Othman, M.S.; Bauomy, A.A.; Ibrahim, S.R.; Abdel Moneim, A.E. Ceratonia siliqua pod extract ameliorates Schistosoma mansoni-induced liver fibrosis and oxidative stress. BMC Complement. Altern. Med. 2016, 16, 434. [Google Scholar] [CrossRef] [PubMed]

| P90G | Extract | Water | |
|---|---|---|---|
| Liposomes | 150 mg | 20 mg | 1 mL |
| Empty liposomes | 150 mg | 1 mL |
| Peak No. | Rt Min | Identity | [M-H]− * m/z | Molecular Formula | Δ ppm | MS/MS § m/z | References | Confidence Level # |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.64 | Gallic acid glucoside | 331.0677 | C13H16O10 | 0.3297 | 271.0456(14)/211.0230(13)/169.0132(100) | [5,29] | 2 |
| 2 | 3.38 | Gallic acid | 169.0144 | C7H6O5 | 0.1532 | 125.0243(100) | [5,29] | 1 |
| 3 | 3.72 | (iso)butyryl-dihexose | 457.1567 [FA] | C16H28O12 | −1.1088 | 411.1508(9)/342.1085(88)/87.046(12) | [29] | 3 |
| 4 | 4.15 | Gallic acid glucoside | 331.06709 | C13H16O10 | 0.3297 | 271.0443(65)/211.0243(72)/169.0134(100) | [29] | 2 |
| 5 | 4.26 | Digalloyl glucose | 483.0783 | C20H20O14 | 0.4729 | 331.0661(47)/313.0562(27)/169.0144(100) | [5,29] | 2 |
| 6 | 4.93 | Monogalloyl dihexoside | 493.1201 | C19H26O15 | 0.8063 | 313.0564(100)/283.0431(72)/169.0133(54) | [5,29,30] | 3 |
| 7 | 5.09 | Unknown | 417.1172 | C17H30O14 | 4 | |||
| 8 | 5.35 | Monogalloyl dihexoside | 493.1201 | C19H26O15 | 0.3063 | 313.0565(100)/271.0438(65)/169.0129(60) | [29] | 3 |
| 9 | 5.51 | Monogalloyl dihexoside | 493.1201 | C19H26O15 | 1.0063 | 313.0571(100)/283.0454(63)/169.0135(87) | [29] | 2 |
| 10 | 5.90 | (iso)Btyryl-dihexose | 411.1518 | C16H28O12 | −1.0943 | 323.0981(28)/179.0558(9) | [29] | 3 |
| 11 | 6.43 | (iso)Butyryl-hexose-pentose | 427.1466 [FA] | C15H26O11 | −0.9178 | 381.1402(4)/125.0239(31)/87.0460(36) | [29] | 3 |
| 12 | 6.65 | Digalloyl glucose | 483.0783 | C20H20O14 | 0.6233 | 331.0672(26)/313.0565(20)/169.0141(100) | [5,29] | 2 |
| 13 | 6.86 | (iso)Butyryl-hexose-pentose | 427.1466 [FA] | C15H26O11 | −0.793 | 233.0627(8)/87.0450(37)/59.0144(65) | [29] | 3 |
| 14 | 7.16 | (iso)Butyryl-hexose-pentose | 427.1466 [FA] | C15H26O11 | −0.9045 | 149.0448(6)/87.0456(85)/59.0145(56) | [29] | 3 |
| 15 | 7.46 | (iso)Butyryl-dihexose | 457.1567 [FA] | C16H28O12 | −1.1092 | 341.1090(13)/323.0978(11)/87.0463(44) | [29] | 3 |
| 16 | 7.86 | (iso)Butyryl-dihexose | 411.1518 | C16H28O12 | −1.0699 | 341.1083(4)/87.046(35)/59.0145(14) | [29] | 3 |
| 17 | 7.96 | (iso)Butyryl-dihexose | 411.1518 | C16H28O12 | −1.1092 | 341.1087(6)/87.0457(34) | [29] | 3 |
| 18 | 8.45 | (iso)Butyryl-dihexose | 411.1518 | C16H28O12 | −1.1092 | 341.1086(5)/87.0468(25) | [29] | 3 |
| 19 | 9.12 | Methyl gallic acid | 183.0300 | C8H8O5 | 0.1031 | 168.0064(14)/124.0169(100) | [5] | 2 |
| 20 | 9.66 | Unknown | 443.1923 | C21H32O10 | −0.6477 | 89.0260(41)/71.0145(33)/59.0154(49) | [29] | 4 |
| 21 | 10.03 | Acylated hexose-pentose | 417.1144 [FA] | C16H28O11 | 0.2744 | 293.0878(29)/101.0614(100) | [18] | 3 |
| 22 | 10.17 | Acylated hexose-pentose | 441.1615 [FA] | C16H28O11 | −1.4446 | 101.0625(66)/71.0144(47)/59.0151(88) | [29] | 3 |
| 23 | 10.59 | Acylated hexose-pentose | 441.1615 [FA] | C16H28O11 | −1.4446 | 101.0613(49)/71.0123(24)/59.015(61) | [29] | 3 |
| 24 | 10.77 | Digalloyl glucose | 483.0783 | C20H20O14 | −1.22 | 331.0669(17)/313.0563(17)/169.0135(94) | [5,29] | 2 |
| 25 | 11.04 | Unknown | 261.0879 | C13H14N2O4 | 1.224 | 4 | ||
| 26 | 11.13 | Trigalloyl glucose | 635.0886 | C27H24O18 | 0.8457 | 465.0682(88)/169.0134(28) | [5,29] | 2 |
| 27 | 11.47 | Acylated hexose-pentose | 461.1296 [FA] | C18H24O11 | −0.7563 | 121.0299(100) | [29] | 3 |
| 28 | 12.21 | Acylated hexose-pentose | 461.1296 [FA] | C18H24O11 | −1.0071 | 267.0890(11)/121.0284(100) | [29] | 3 |
| 29 | 12.24 | Siliquapyranone | 595.1309 | C26H28O16 | 0.4416 | 483.0773(8)/331.0625(11)/169.0124(100) | [5,29] | 2 |
| 30 | 12.89 | Trigalloyl glucose isomer | 635.0888 | C27H24O18 | −0.5480 | 465.0675(94)/169.0126(13) | [5,29] | 2 |
| 31 | 13.94 | Unknown | 186.1140 | C9H17NO3 | −0.2786 | [29] | 4 | |
| 32 | 14.27 | Ethyl gallic acid | 197.046 | C9H10O5 | 0.453 | 169.0134(26)/125.0245(100)/124.0153(58) | [18] | 3 |
| 33 | 14.93 | Trigalloyl glucose isomer | 635.0888 | C27H24O18 | 0.9724 | 465.0681(92)/169.0124(11) | [5,29] | 2 |
| 34 | 16.20 | Gallic acid derivative | 401.1095 | C17H22O11 | 0.5649 | 169.0138(100)/123.0084(65)/101.0616(50) | [5] | 2 |
| 35 | 17.51 | Benzoic acid | 121.02935 | C7H6O2 | −0.3030 | 77.0397(100) | [31] | 2 |
| 36 | 18.99 | Myricetin deoxyhexoside | 463.0876 | C21H20O12 | −0.5996 | 317.0272(16)/316.0207(100) | [5,29] | 2 |
| 37 | 19.76 | Quercetin hexoside | 463.0876 | C21H20O12 | 0.1004 | 301.0359(39)/300.0271(100) | [5,29] | 2 |
| 38 | 20.25 | p-Coumaroyl galloyl hexose | 477.10464 | C22H22O12 | −0.2197 | 331.0645(21)/169.0138(100)/125.0234(31) | [5] | 2 |
| 39 | 20.65 | Quercetin pentoside | 433.07735 | C20H18O11 | −1.1749 | 301.0337(59)/300.0283(100) | [5,29] | 2 |
| 40 | 21.11 | Gallic acid derivative | 507.11438 | C23H24O13 | −1.2278 | 235.0621(100)/169.0156(83) | [5] | 2 |
| 41 | 21.42 | Unknown | 435.09317 | C20H20O11 | −0.9888 | 4 | ||
| 42 | 22.01 | Quercetin-3-O-rhamnoside | 447.09389 | C21H20O11 | 0.9150 | 301.0336(40)\300.0273(100)\271.0248(16) | [5,29] | 1 |
| 43 | 24.81 | Kaempferol deoxyhexoside | 431.09883 | C21H20O10 | 1.7355 | 285.0404(19)/284.0294(22)/255.0280(31) | [5,29] | 2 |
| 44 | 26.23 | Tetrahydroxy flavanone | 287.05581 | C15H12O6 | −0.9017 | 151.0042(100)\135.0459(58) | [5] | 2 |
| 45 | 28.73 | Kaempferol | 285.0441 | C15H9O6 | 0.0384 | / | [5,29] | 1 |
| Compound | Peak No. § | C. siliqua Extract (mg/g dr) | |
|---|---|---|---|
| Mean | ±SD | ||
| Total Hydroxybenzoic acids | 7.20 | 0.37 | |
| Gallic acid hexose a | 1 | 1.51 | 0.00 |
| Gallic acid | 2 | 1.31 | 0.01 |
| Gallic acid hexose a | 4 | 0.06 | 0.00 |
| Digalloyl glucose a | 5 | 0.06 | 0.00 |
| Monogalloyl dihexoside a | 6 | 0.58 | 0.01 |
| Monogalloyl dihexoside a | 8 | 0.15 | 0.00 |
| Monogalloyl dihexoside a | 9 | 0.08 | 0.00 |
| Digalloyl glucose a | 12 | 0.22 | 0.00 |
| Methyl gallic acid a | 19 | 0.36 | 0.00 |
| Digalloyl glucose a | 24 | 0.15 | 0.00 |
| Trigalloyl glucose a | 26 | 0.14 | 0.00 |
| Siliquapyranone a | 29 | 0.15 | 0.00 |
| Trigalloyl glucose isomer a | 30 | 0.19 | 0.00 |
| Ethyl gallic acid a | 32 | 0.29 | 0.00 |
| Trigalloyl glucose isomer a | 33 | 0.11 | 0.00 |
| Gallic acid derivative a | 34 | 1.70 | 0.01 |
| Gallic acid derivative a | 40 | 0.14 | 0.00 |
| Total Flavonoids | 0.36 | 0.02 | |
| Myricetin deoxyhexoside b | 36 | 0.05 | 0.00 |
| Quercetin hexoside c | 37 | 0.04 | 0.00 |
| Quercetin pentoside c | 39 | 0.02 | 0.01 |
| Quercetin-3-O-ramnoside | 42 | 0.21 | 0.00 |
| Kaempferol deoxyhexoside d | 43 | 0.01 | 0.00 |
| Tetrahydroxy flavanone e | 44 | 0.02 | 0.00 |
| Kaempferol | 45 | 0.01 | 0.00 |
| Total polyphenols | 7.56 | 0.41 | |
| Liposomes | Empty Liposomes | |
|---|---|---|
| Mean diameter (nm ± SD) | * 107 ± 3.8 | 73 ± 2.0 |
| Polydispersity index (± SD) | 0.27 ± 0.01 | 0.31 ± 0.03 |
| Zeta potential (mV ± SD) | −13 ± 2.8 | −18 ± 2.7 |
| Liposomes | Empty Liposomes | |
|---|---|---|
| χ2 | 1.75 | 1.61 |
| N | 1.26 | 1.00 |
| d (Å) | 62.16 | / |
| η1 | 0.23 | / |
| ZH (Å) | 16.05 ± 0.20 | 15.60 ± 0.20 |
| σH (Å) | 3.44 ± 0.20 | 4.16 ± 0.20 |
| σC (Å) | 5.19 ± 0.50 | 7.48 ± 0.50 |
| Peak No. § | Compound | EE (% ± SD) |
|---|---|---|
| 33 | Trigalloyl glucose a | 97 ± 6.3 |
| 42 | Quercetin-3-O-rhamnoside | 91 ± 7.7 |
| Extract Concentration mg/mL | Hemolytic Activity (% ± SD) | |
|---|---|---|
| Solution | 1 | 1.7 ± 0.70 |
| Liposomes | 1 | 2.4 ± 2.89 |
| Empty liposomes | / | 1.4 ± 1.14 |
| Solution | 2 | 1.6 ± 1.07 |
| Liposomes | 2 | 0.9 ± 1.33 |
| Empty liposomes | / | 3.7 ± 1.92 |
| AA (%) | TE (µg Trolox Equivalents/mL) | |
|---|---|---|
| Solution | 92 ± 4.2 | 469 ± 14.1 |
| Liposomes | 95 ± 1.7 | * 486 ± 10.5 |
| Empty liposomes | 40 ± 4.1 | 220 ± 18.2 |
| TE (µg Fe2+ Equivalents/mL Solution) | |
|---|---|
| Solution | 2139 ± 257 |
| Liposomes | 1995 ± 253 |
| Empty liposomes | 687 ± 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, M.; Tuberoso, C.I.G.; Pons, R.; García, M.T.; Morán, M.d.C.; Martelli, G.; Vassallo, A.; Caddeo, C. Ceratonia siliqua L. Pod Extract: From Phytochemical Characterization to Liposomal Formulation and Evaluation of Behaviour in Cells. Antioxidants 2023, 12, 1209. https://doi.org/10.3390/antiox12061209
De Luca M, Tuberoso CIG, Pons R, García MT, Morán MdC, Martelli G, Vassallo A, Caddeo C. Ceratonia siliqua L. Pod Extract: From Phytochemical Characterization to Liposomal Formulation and Evaluation of Behaviour in Cells. Antioxidants. 2023; 12(6):1209. https://doi.org/10.3390/antiox12061209
Chicago/Turabian StyleDe Luca, Maria, Carlo Ignazio Giovanni Tuberoso, Ramon Pons, María Teresa García, María del Carmen Morán, Giuseppe Martelli, Antonio Vassallo, and Carla Caddeo. 2023. "Ceratonia siliqua L. Pod Extract: From Phytochemical Characterization to Liposomal Formulation and Evaluation of Behaviour in Cells" Antioxidants 12, no. 6: 1209. https://doi.org/10.3390/antiox12061209
APA StyleDe Luca, M., Tuberoso, C. I. G., Pons, R., García, M. T., Morán, M. d. C., Martelli, G., Vassallo, A., & Caddeo, C. (2023). Ceratonia siliqua L. Pod Extract: From Phytochemical Characterization to Liposomal Formulation and Evaluation of Behaviour in Cells. Antioxidants, 12(6), 1209. https://doi.org/10.3390/antiox12061209









