ARAG, an Antioxidant-Rich Gel, Shows Superiority to Mepilex Ag in the Treatment of Deep Partial Thickness Burns without Sacrificing Antimicrobial Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. ARAG Formulation
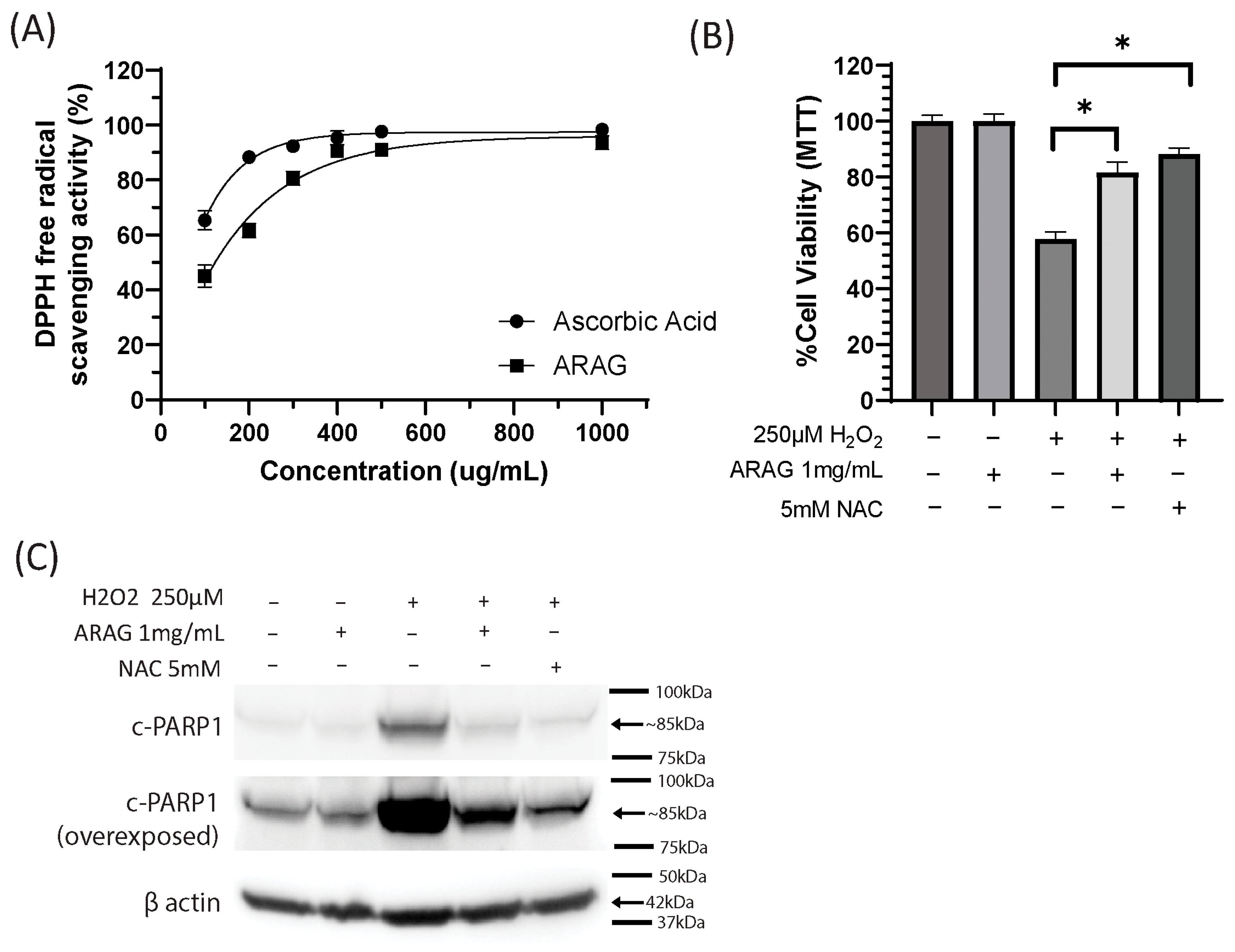
2.2. DPPH Measurement of Antioxidant Activity
2.3. Cell Culture, Viability Assays, and Western Blotting
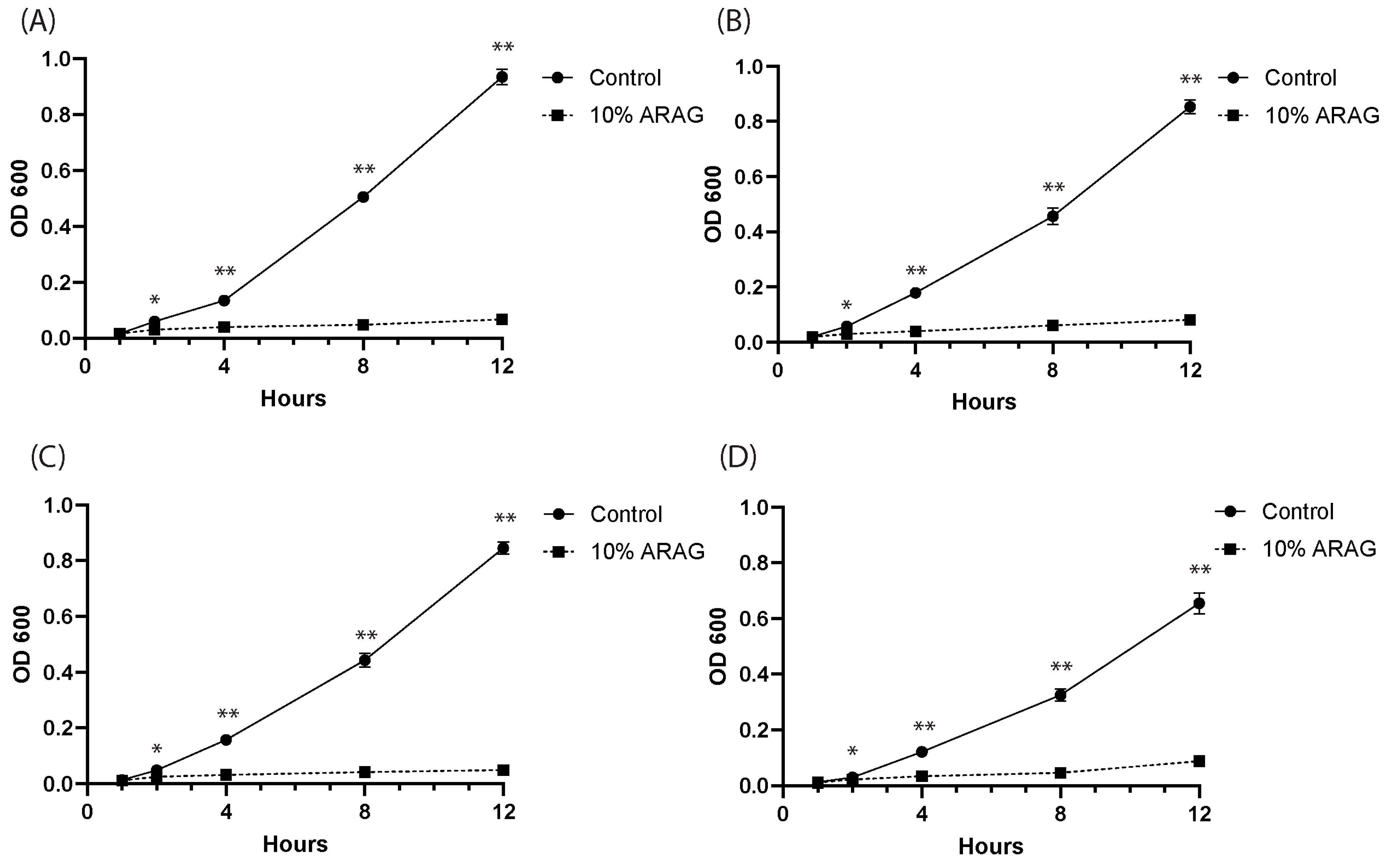
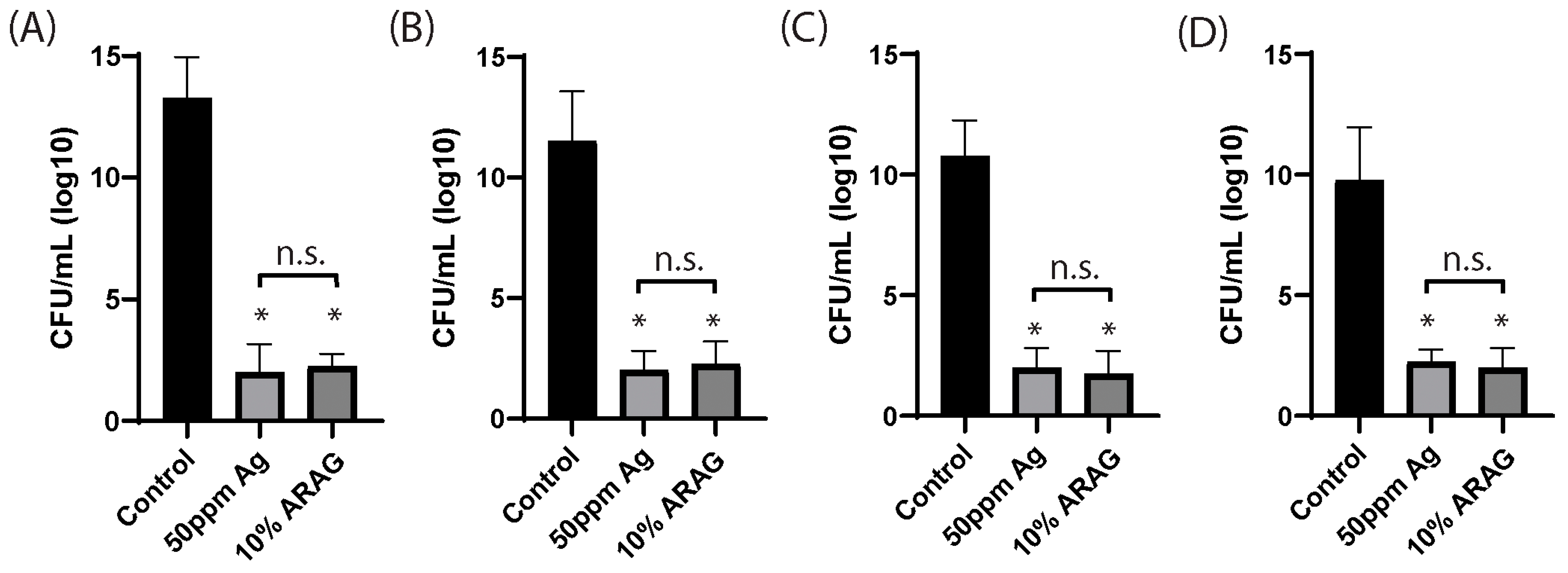
2.4. Planktonic Growth and Colony Formation Assays
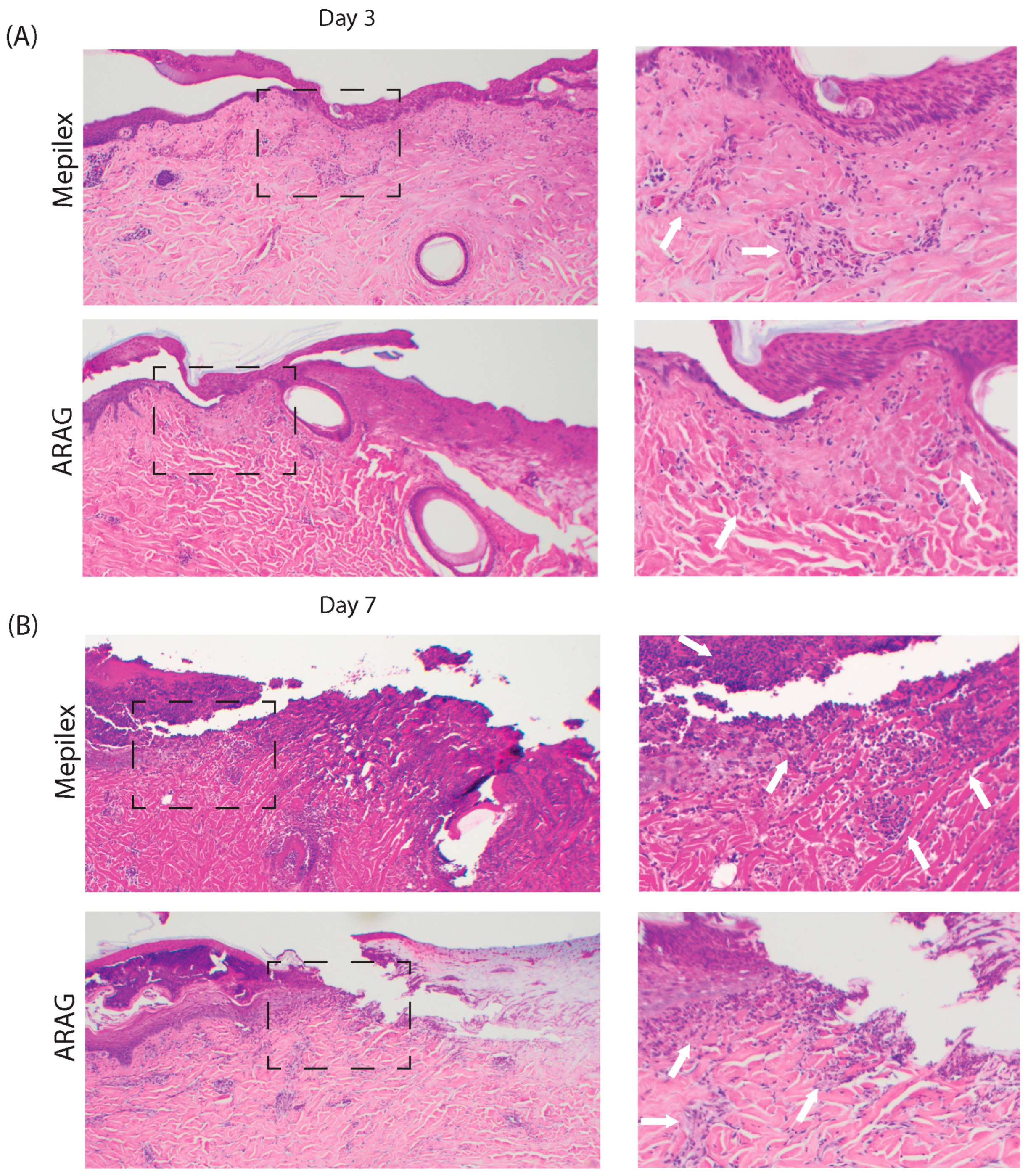
2.5. Deep Partial Thickness Burns
2.6. Statistical Analysis
3. Results
3.1. ARAG Is a Potent Antioxidant Capable of Preventing Oxidative Damage-Induced Cell Death
3.2. ARAG Is Antimicrobial and Possesses Similar Efficacy to Ionized Silver
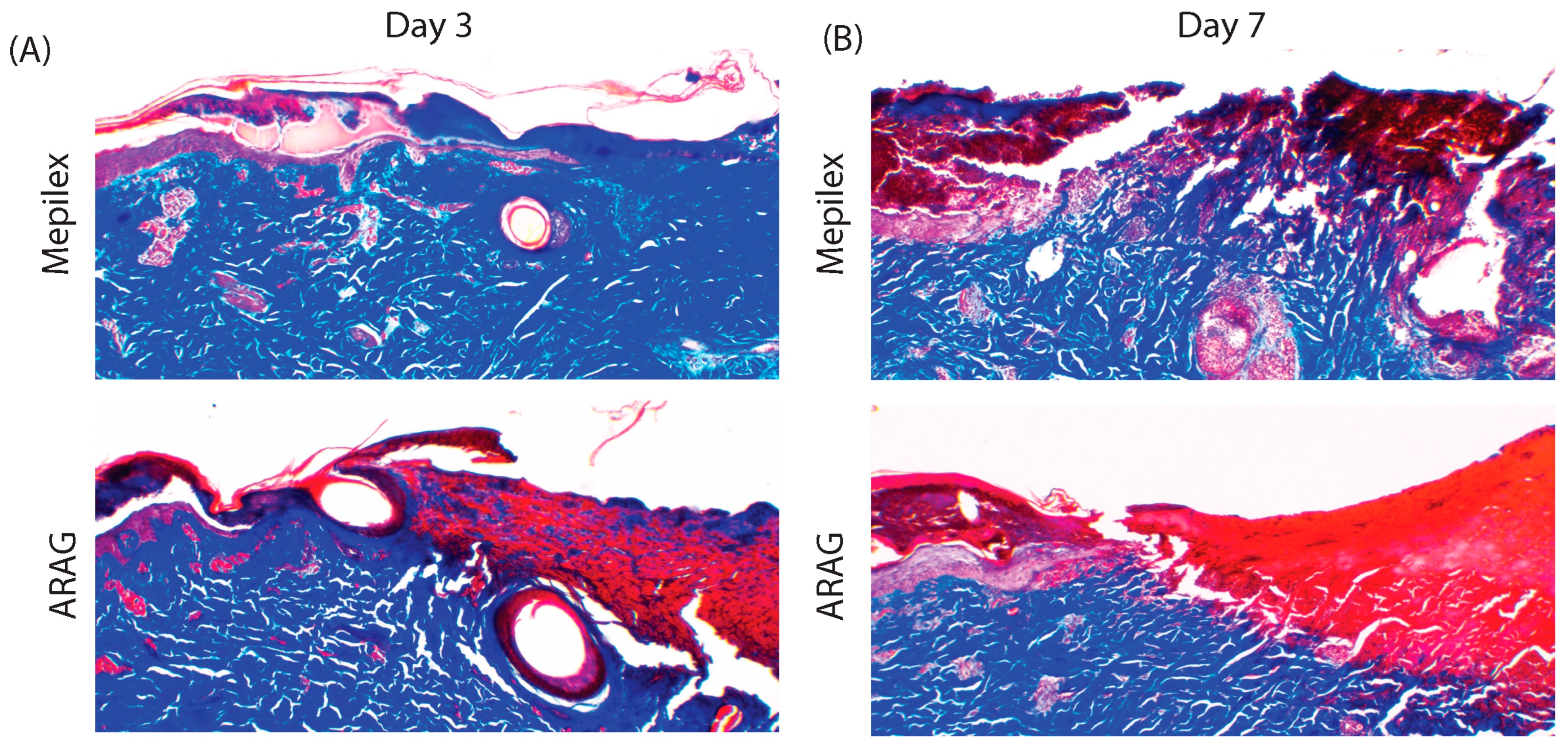
3.3. ARAG Increases the Healing Rate of Deep Partial Thickness Burns
3.4. ARAG Dampens the Late Inflammatory Response Leading to Organized Wound Debridement and Healing
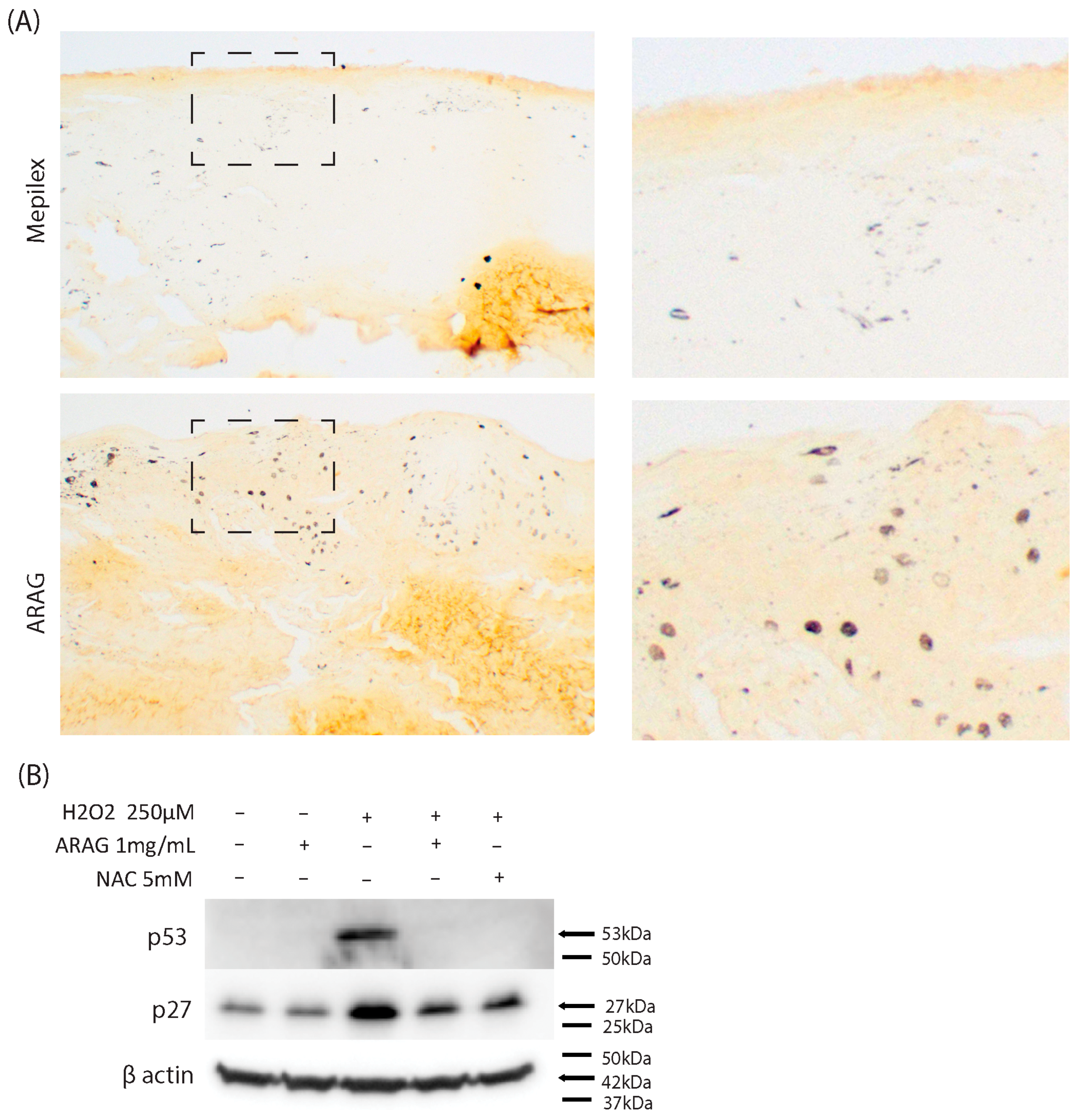
3.5. ARAG Maintains Cellular Proliferation In Vivo and Decreases Markers of Oxidative Stress-Induced Senescence In Vitro
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barillo, D.J.; Goode, R. Fire fatality study: Demographics of fire victims. Burns 1996, 22, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Snell, J.A.; Loh, N.H.; Mahambrey, T.; Shokrollahi, K. Clinical review: The critical care management of the burn patient. Crit. Care 2013, 17, 241. [Google Scholar] [CrossRef]
- White, C.E.; Renz, E.M. Advances in surgical care: Management of severe burn injury. Crit. Care Med. 2008, 36, S318–S324. [Google Scholar] [CrossRef] [PubMed]
- Cancio, L.C. Topical Antimicrobial Agents for Burn Wound Care: History and Current Status. Surg. Infect. 2021, 22, 3–11. [Google Scholar] [CrossRef]
- Liu, H.F.; Zhang, F.; Lineaweaver, W.C. History and Advancement of Burn Treatments. Ann. Plast. Surg. 2017, 78, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.N.; Beyene, T.J.; Lehman, K.; VerLee, S.N.; Schwartz, D.; Fabia, R.; Thakkar, R.K. Evaluating effects of burn injury characteristics on quality of life in pediatric burn patients and caregivers. Burns, 2023; in press. [Google Scholar] [CrossRef]
- Zhu, H.J.; Wang, S.J.; Yang, H.; Li, D.J.; Chi, Y.F.; Li, J. Cross-sectional survey of life quality of patients with deep partial-thickness and above burns on head and face at discharge and analysis of its influencing factors. Zhonghua Shao Shang Za Zhi Zhonghua Shaoshang Zazhi Chin. J. Burn. 2019, 35, 292–297. [Google Scholar] [CrossRef]
- Chokshi, S.N.; Powell, C.M.; Gavrilova, Y.; Wolf, S.E.; Ozhathil, D.K. A Narrative Review of the History of Burn-Related Depression and Stress Reactions. Medicina 2022, 58, 1395. [Google Scholar] [CrossRef]
- D’Abbondanza, J.A.; Shahrokhi, S. Burn Infection and Burn Sepsis. Surg. Infect. 2021, 22, 58–64. [Google Scholar] [CrossRef]
- Savetamal, A. Infection in Elderly Burn Patients: What Do We Know? Surg. Infect. 2021, 22, 65–68. [Google Scholar] [CrossRef]
- Rezaei, E.; Safari, H.; Naderinasab, M.; Aliakbarian, H. Common pathogens in burn wound and changes in their drug sensitivity. Burns 2011, 37, 805–807. [Google Scholar] [CrossRef]
- Tchakal-Mesbahi, A.; Abdouni, M.A.; Metref, M. Prevalence of Multidrug-Resistant Bacteria Isolated from Burn Wounds in Algeria. Ann Burn. Fire Disasters 2021, 34, 150–156. [Google Scholar]
- Norton, R.; Finley, P.J. Clinically isolated bacteria resistance to silver-based wound dressings. J. Wound Care 2021, 30, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Terzioğlu, E.; Arslan, M.; Balaban, B.G.; Çakar, Z.P. Microbial silver resistance mechanisms: Recent developments. World J. Microbiol. Biotechnol. 2022, 38, 158. [Google Scholar] [CrossRef] [PubMed]
- Strudwick, X.L.; Cowin, A.J. The Role of the Inflammatory Response in Burn Injury. In Hot Topics in Burn Injuries; InTech: London, UK, 2017. [Google Scholar]
- Bergquist, M.; Hästbacka, J.; Glaumann, C.; Freden, F.; Huss, F.; Lipcsey, M. The time-course of the inflammatory response to major burn injury and its relation to organ failure and outcome. Burns 2019, 45, 354–363. [Google Scholar] [CrossRef]
- Laggner, M.; Lingitz, M.-T.; Copic, D.; Direder, M.; Klas, K.; Bormann, D.; Gugerell, A.; Moser, B.; Radtke, C.; Hacker, S.; et al. Severity of thermal burn injury is associated with systemic neutrophil activation. Sci. Rep. 2022, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.; Valdera, F.; Varon, D.; Kankuri, E.; Nuutila, K. The Immune and Regenerative Response to Burn Injury. Cells 2022, 11, 3073. [Google Scholar] [CrossRef]
- Hateet, R.R. Isolation and Identification of Some Bacteria Contemn in Burn Wounds in Misan, Iraq. Arch. Razi Inst. 2021, 76, 1665–1670. [Google Scholar] [CrossRef]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; van Duin, D. Bacterial Infections After Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 2130–2136. [Google Scholar] [CrossRef]
- Gaines, C.; Poranki, D.; Du, W.; Clark, R.A.; Van Dyke, M. Development of a porcine deep partial thickness burn model. Burns 2013, 39, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Böttrich, J.G.; Braunwarth, H.; Dissemond, J.; Münter, K.C.; Schümmelfeder, F.; Wilken, P. Best practice recommendations for silver wound dressings: Results of an expert survey aiming to reach a consensus. Wound Med. 2019, 27, 100167. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Hsu, W.-S.; Chung, W.-Y.; Ko, T.-H.; Lin, J.-H. Evaluation of various silver-containing dressing on infected excision wound healing study. J. Mater. Sci. Mater. Med. 2014, 25, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care-Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surgery. Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef] [PubMed]
- Lasry, A.; Ben-Neriah, Y. Senescence-associated inflammatory responses: Aging and cancer perspectives. Trends Immunol. 2015, 36, 217–228. [Google Scholar] [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Koiwa, M.; Yonemura, A.; Miyake, K.; Kariya, R.; Kubota, S.; Yokomizo-Nakano, T.; Yasuda-Yoshihara, N.; Uchihara, T.; Itoyama, R.; et al. Inflammation-driven senescence-associated secretory phenotype in cancer-associated fibroblasts enhances peritoneal dissemination. Cell Rep. 2021, 34, 108779. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Sheekey, E.; Narita, M. p53 in senescence—It’s a marathon, not a sprint. FEBS J. 2023, 290, 1212–1220. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Ibrahim, N.; Naina Mohamed, I. Interdependence of Anti-Inflammatory and Antioxidant Properties of Squalene-Implication for Cardiovascular Health. Life 2021, 11, 103. [Google Scholar] [CrossRef]
- Cárdeno, A.; Aparicio-Soto, M.; Montserrat-de la Paz, S.; Bermudez, B.; Muriana, F.J.G.; Alarcón-de-la-Lastra, C. Squalene targets pro- and anti-inflammatory mediators and pathways to modulate over-activation of neutrophils, monocytes and macrophages. J. Funct. Foods 2015, 14, 779–790. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; López-Biedma, A.; Toledo, E.; Gaforio, J.J. Squalene Stimulates a Key Innate Immune Cell to Foster Wound Healing and Tissue Repair. Evid.-Based Complement. Altern. Med. Ecam 2018, 2018, 9473094. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.A.; Park, P.J.; Kim, S.B.; Bin, B.H.; Jang, D.J.; Kim, S.T. Topical Delivery of Coenzyme Q10-Loaded Microemulsion for Skin Regeneration. Pharmaceutics 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Zahid, S.; Khalid, H.; Ikram, F.; Iqbal, H.; Samie, M.; Shahzadi, L.; Shah, A.T.; Yar, M.; Chaudhry, A.A.; Awan, S.J.; et al. Bi-layered α-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater. Sci. Eng. C 2019, 101, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Yang, S.J.; Lim, Y. Gamma-tocopherol supplementation ameliorated hyper-inflammatory response during the early cutaneous wound healing in alloxan-induced diabetic mice. Exp. Biol. Med. 2017, 242, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Nasole, E.; Nicoletti, C.; Yang, Z.J.; Girelli, A.; Rubini, A.; Giuffreda, F.; Di Tano, A.; Camporesi, E.; Bosco, G. Effects of alpha lipoic acid and its R+ enantiomer supplemented to hyperbaric oxygen therapy on interleukin-6, TNF-α and EGF production in chronic leg wound healing. J. Enzym. Inhib. Med. Chem. 2014, 29, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Vehapi, M.; Özçimen, D. Antimicrobial and bacteriostatic activity of surfactants against B. subtilis for microbial cleaner formulation. Arch. Microbiol. 2021, 203, 3389–3397. [Google Scholar] [CrossRef] [PubMed]
- Falk, N.A. Surfactants as Antimicrobials: A Brief Overview of Microbial Interfacial Chemistry and Surfactant Antimicrobial Activity. J. Surfactants Deterg. 2019, 22, 1119–1127. [Google Scholar] [CrossRef]
- Richardson, K.E.; Xue, Z.; Huang, Y.; Seo, Y.; Lapitsky, Y. Physicochemical and antibacterial properties of surfactant mixtures with quaternized chitosan microgels. Carbohydr. Polym. 2013, 93, 709–717. [Google Scholar] [CrossRef]
- Coiffard, L.; Couteau, C. Soap and syndets: Differences and analogies, sources of great confusion. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11432–11439. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Vijayasarathy, P.; Sin, A.; Nam, H.; Khan, S.; Parambath, J.B.M.; Mohamed, A.A.; Han, C. Antimicrobial activity of quaternary ammonium salts: Structure-activity relationship. Med. Chem. Res. 2022, 31, 1663–1678. [Google Scholar] [CrossRef]
- de Toledo, A.M.N.; Silva, N.C.C.; Sato, A.C.K.; Picone, C.S.F. A comprehensive study of physical, antimicrobial and emulsifying properties of self-assembled chitosan/lecithin complexes produced in aqueous media. Future Foods 2021, 4, 100083. [Google Scholar] [CrossRef]
- Cani, P.D.; Everard, A. Keeping gut lining at bay: Impact of emulsifiers. Trends Endocrinol. Metab. TEM 2015, 26, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.J.; Mohammed, J.J. Pharmaceutical Applications of Vitamin E TPGS. In Vitamin E in Health and Disease; Pınar, E., Júlia Scherer, S., Eds.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar]
- Mehata, A.K.; Setia, A.; Vikas; Malik, A.K.; Hassani, R.; Dailah, H.G.; Alhazmi, H.A.; Albarraq, A.A.; Mohan, S.; Muthu, M.S. Vitamin E TPGS-Based Nanomedicine, Nanotheranostics, and Targeted Drug Delivery: Past, Present, and Future. Pharmaceutics 2023, 15, 722. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; McNaught, C.-E. The physiology of wound healing. Surgery 2011, 29, 475–479. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Hudspith, J.; Rayatt, S. First aid and treatment of minor burns. BMJ 2004, 328, 1487–1489. [Google Scholar] [CrossRef] [PubMed]
- Allorto, N.; Atieh, B.; Bolgiani, A.; Chatterjee, P.; Cioffi, W.; Dziewulski, P.; de Jong, A.; Gibran, N.; Guerrero, L.; Hanumadass, M.; et al. ISBI Practice Guidelines for Burn Care, Part 2. Burns 2018, 44, 1617–1706. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.P.; Vasquez, S.A.; Granick, M.S.; Rhee, S.T. Topical antimicrobials in pediatric burn wound management. J. Craniofacial Surg. 2008, 19, 913–922. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartwright, B.M.; Fox, S.J.; Underdown, M.J.; Clark, W.A.; Molnar, J.A. ARAG, an Antioxidant-Rich Gel, Shows Superiority to Mepilex Ag in the Treatment of Deep Partial Thickness Burns without Sacrificing Antimicrobial Efficiency. Antioxidants 2023, 12, 1176. https://doi.org/10.3390/antiox12061176
Cartwright BM, Fox SJ, Underdown MJ, Clark WA, Molnar JA. ARAG, an Antioxidant-Rich Gel, Shows Superiority to Mepilex Ag in the Treatment of Deep Partial Thickness Burns without Sacrificing Antimicrobial Efficiency. Antioxidants. 2023; 12(6):1176. https://doi.org/10.3390/antiox12061176
Chicago/Turabian StyleCartwright, Brian Michael, Sean James Fox, Mary Jane Underdown, William Andrew Clark, and Joseph Andrew Molnar. 2023. "ARAG, an Antioxidant-Rich Gel, Shows Superiority to Mepilex Ag in the Treatment of Deep Partial Thickness Burns without Sacrificing Antimicrobial Efficiency" Antioxidants 12, no. 6: 1176. https://doi.org/10.3390/antiox12061176
APA StyleCartwright, B. M., Fox, S. J., Underdown, M. J., Clark, W. A., & Molnar, J. A. (2023). ARAG, an Antioxidant-Rich Gel, Shows Superiority to Mepilex Ag in the Treatment of Deep Partial Thickness Burns without Sacrificing Antimicrobial Efficiency. Antioxidants, 12(6), 1176. https://doi.org/10.3390/antiox12061176




