Antioxidant and Anti-Inflammatory Properties of Walnut Constituents: Focus on Personalized Cancer Prevention and the Microbiome
Abstract
1. Introduction
2. Walnut Constituents
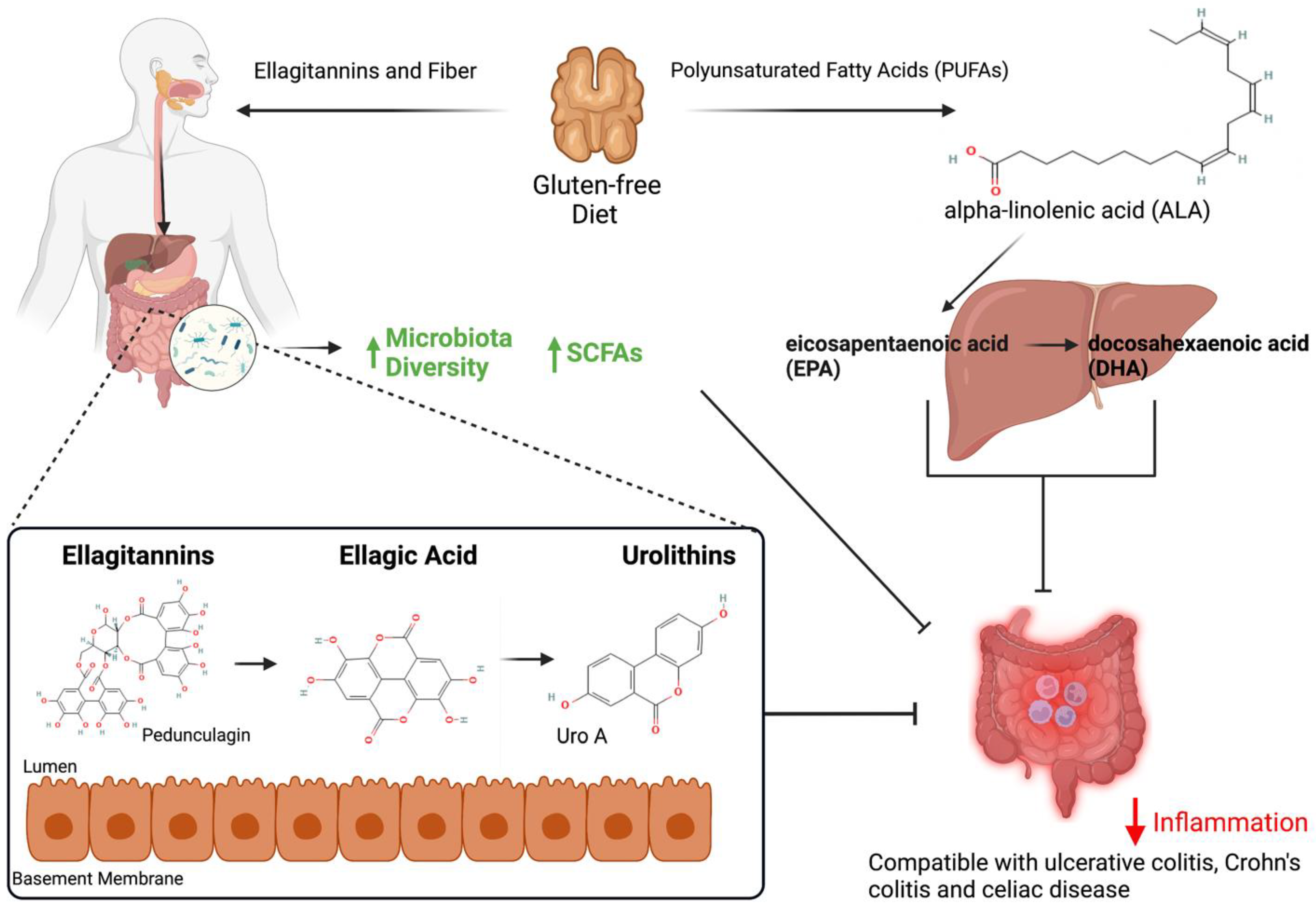
2.1. Alpha-Linolenic Acid
2.2. Polyphenols
3. Methods
Search Strategy and Study Selection
4. How Do Walnuts Influence Cancer Risk?
4.1. Animal Studies
| Author(s), Year, Reference | Animal Model(s) | Experimental Design | Key Findings |
|---|---|---|---|
| Hardman et al., 2011 [41] | C3(1) TAg murine breast cancer model | Maternal diet: Corn oil vs. walnut diet Offspring diet: Corn oil/Corn oil; Corn oil/Walnut Walnut/Walnut; Walnut/Corn Oil
|
|
| Koh et al., 2019 [50] | Acute (DSS) colitis; chronic colitis (IL-10-KO) and inflammation-associated CRC (AOM + DSS) mouse models | Walnut Phenolic Extract (WPE) (20 mg/kg) via oral gavage for 2 weeks Acute colitis: C57BL/6 mice given 4% DSS for 5 days Chronic colitis: IL-10 (-/-) mice Inflammation-cancer model: AOM + 2% DSS × 3 cycles |
|
| Hardman and Ion, 2008 [40] | Athymic nude mice (nu/nu) + MDA-MB 231 human breast cancer cells | After tumors reached 3–5 mm in diameter, mice were placed on the following diets: Corn oil (control) vs. Walnut diet (18% of total calories) |
|
| Guan et al., 2018 [49] |
| Apc1638N/+: 1. Control low-fat diet (LFD) (n = 11) 2. LFD + walnuts (6% by weight) (n = 12) 3. Control high-fat diet (HFD) (n = 18) 4. HFD + walnuts (7.7% by weight) (n = 23) ApcΔ14 1. The western diet (TWD) + 0% walnuts (males n = 12, females n = 10) 2. TWD +7% walnuts (males n = 11, females n = 9) C57BL/6J male (n = 48) 1. LFD (n = 12) 2. LFD + walnuts (n = 12) 3. HFD (n = 12) 4. HFD + walnuts (n = 12) |
|
| Nakanishi et al., 2016 [45] | A/J—AOM mouse CRC model | AIN-76A diet (4 weeks old) 0%, 9.4%, 14.1%, or 18.8% of walnuts by weight TWD (Total western diet) (4 weeks old) 0% 3.5%, 7%, or 14% of walnut by weight Six weekly injections of AOM (i.p.) at 5 weeks of age. |
|
| Tsoukas et al., 2015 [44] | HT-29 CRC explant model in athymic nude (nu/nu) mice | Control diet (n = 16) AIN-79 + corn oil (100 mg/kg) Walnut diet (n = 16) AIN-79 + Walnut (18.8% of calories) + Corn oil (100 mg/kg) |
|
| Davis et al., 2012 [42] | High-fat diet in TRAMP mouse model for prostate cancer | Mice are placed on diets at 8 weeks of age AIN-93M + whole walnut 20.5% of energy from fat AIN-93M + soybean oil (HFD) 100 g/kg) 20.7 % of energy from fat AIN-93M + soybean oil (LFD) 40 g/100 kg) 8.7% of energy from fat Mice were sacrificed at 9, 18 and 24 weeks after feeding |
|
| Nagel et al., 2012 [43] | HT-29 CRC explant model in athymic nude (nu/nu) mice | Mice were placed on diets at 7 weeks old (n = 16/group) Corn oil diet (control) AIN-76 Walnut diet 18.8% of a 2000 calorie per day diet Flaxseed oil diet 18.8% of a 2000 calorie per day diet |
|
| Chen et al., 2020 [51] | A/J—AOM mouse CRC model | Six weekly injections of AOM (i.p.) at 5 weeks old; control mice were injected with vehicle (0.9% NaCl) AOM group diets (n = 20/group, 10 males, 10 females) 1. 0% walnut level 2. 3.5% walnut level 3. 7% walnut level 4. 14% walnut level Control group diets (n = 10/group, 5 males, 5 females) 1. 0% walnut level 2. 7% walnut level Fecal samples collected at 6, 11, 13, 16, and 20 weeks of age. |
|
| Byerley et al., 2017 [52] | Fischer 344 rats | Walnut group (n = 10) 11% by weight ground walnuts Replacement group (n = 10) corn oil, alphacel fiber, and casein added to replace walnut
|
|
4.2. Human Studies
| Author(s), Year, Reference | Patient Population | Experimental Design | Key Findings |
|---|---|---|---|
| Hardman et al., 2019 [54] |
| Data/sample collected
|
|
| Soriano-Hernandez et al., 2015 [53] |
| Data/sample collected
|
|
| Hashemian et al., 2018 [56] |
| Data/sample collected
|
|
| Sui et al., 2019 [59] | Nurses’ Health Study,
| Data/sample collected
|
|
| Fang et al., 2021 [60] | Nurses’ Health Study
| Data/sample collected
|
|
| Fadelu et al., 2018 [61] |
| Data/sample collected
|
|
| Provatas et al., 2021 [62] |
| Data/sample collected
Three-weeks study design using walnut diet (2 oz/day) |
|
| Bamberger et al., 2018 [12] |
| Data/sample collected
Four-week cross-over design using a nut-free western control diet, (50% carbohydrates, 35% fat, and 15% protein) and walnut diet (43 g/day) |
|
| Bamberger et al., 2017 [68] |
| Data/sample collected
Four-weeks cross-over design using a nut-free western control diet (50% carbohydrate, 35% fat, and 15% protein) and walnut diet (43g/day) |
|
| Holscher et al., 2018 [66] |
| Data/sample collected
Three weeks cross over design using nut-free control diet and walnut diet (42g/day) |
|
| Garcia-Mantrana et al., 2019 [69] |
| Data/sample collected
3-day study design using walnut diet (33 g/day)Groups Subjects were placed in one of three groups:
|
|
| Tindall et al., 2020 [70] |
| Data/sample collected
Six-weeks cross-over design using a walnut diet, a walnut- fatty-acid-matched diet or a oleic acid diet (all 48% carbohydrate, 35% fat, and 17% protein) |
|
4.3. Cell Culture Studies
| Author(s), Year, Reference | Cell Line/Compound Analyzed | Methods Used | Key Findings |
|---|---|---|---|
| Nunez-Sanchez et al., 2016 [79] |
| Cells were treated with one of the following mixtures: Mixture 1 (MPhA) 85% Uro-A, 10% Uro-C, 5% EA mixture Mixture 2 (MPhB) 30% Uro-A, 50% IsoUro-A, 10% Uro-B, 5% Uro-C, 5% EA mixture |
|
| Wu et al., 2021 [39] | 37 phenolic compounds from walnut kernels | Extraction of free, esterified, and bound forms of the 37 targeted walnut phenolics (WPs) using organic solvent/water solvent |
|
| Schlormann et al., 2017 [72] | LT97 colon adenoma cells | LT97 cells were treated with fermentation supernatants (FS) from both raw and roasted walnuts |
|
| Lee et al., 2016 [71] | CD133+ CD44+ isolated from HCT116 human colon cancer cell line | CD133+ CD44+ HCT116 were treated with walnut phenolic extract (WPE) (0, 10, 20, and 40 ug/mL) |
|
| Koh et al., 2019 [50] | COLO205 human colonic epithelial cell line | Cells were pretreated with WPE and stimulated with tumor necrosis factor (TNF-α) |
|
| Ho et al., 2020 [77] |
| Cells were treated with 16 phenolic compounds to test for antioxidant activity, anticancer activities, and antioxidant response element |
|
| Choi et al., 2019 [75] | CD133+ CD44+ HCT116 colon cancer cell line | Cells were treated with 40 ug/mL of WPE for 6 days |
|
| Batirel et al., 2018 [73] | OE19 esophageal adenocarcinoma cell line | Cells were treated with walnut oil (0–40 mg/mL) |
|
| Park et al., 2020 [76] | RGM-1gastric muscosal cells infected with H. pylori | Cells were treated with WPE (20 ug/mL) |
|
| Park et al., 2021 [78] | AGS human gastric adenocarcinoma cells infected with H.pylori | Cells were pretreated with WPE for 1 h and then stimulated with H. pylori for 48 h |
|
| Calcabrini et al., 2017 [74] | Keratinocyte cell line NCTC 2544 | Antioxidant Juglans regia ethanolic extract (walnut kernel) DNA damage Induced by Thiol/Fe3+/O2 mixed function, tert-butyl hydroperoxide, or UVC radiations |
|
5. Walnut Effects on the Microbiome
5.1. Human Studies
5.2. Animal Studies
6. Walnuts and Inflammation
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) Chemical Composition and Research in Human Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guasch-Ferre, M.; Tobias, D.K.; Li, Y. Association of Walnut Consumption with Total and Cause-Specific Mortality and Life Expectancy in U.S. Adults. Nutrients 2021, 13, 2699. [Google Scholar] [CrossRef] [PubMed]
- Steffen, L.M.; Yi, S.Y.; Duprez, D.; Zhou, X.; Shikany, J.M.; Jacobs, D.R., Jr. Walnut consumption and cardiac phenotypes: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Cofan, M.; Sala-Vila, A.; Haddad, E.; Serra-Mir, M.; Bitok, E.; Roth, I.; Freitas-Simoes, T.M.; Kaur, A.; Valls-Pedret, C.; et al. Effects of Walnut Consumption for 2 Years on Lipoprotein Subclasses Among Healthy Elders: Findings From the WAHA Randomized Controlled Trial. Circulation 2021, 144, 1083–1085. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. Available online: https://www.dietaryguidelines.gov (accessed on 9 November 2022).
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. Available online: https://fdc.nal.usda.gov (accessed on 9 November 2022).
- Sanchez-Gonzalez, C.; Ciudad, C.; Noe, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- Ros, E.; Izquierdo-Pulido, M.; Sala-Vila, A. Beneficial effects of walnut consumption on human health: Role of micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef]
- Blondeau, N.; Lipsky, R.H.; Bourourou, M.; Duncan, M.W.; Gorelick, P.B.; Marini, A.M. α-linolenic acid: An omega-3 fatty acid with neuroprotective properties-ready for use in the stroke clinic? BioMed Res. Int. 2015, 2015, 519830. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits-A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Fischer, S.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients 2018, 10, 244. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, L.; Nandy, S.K.; Payal, N.; Yadav, S.; Vargas-De-La-Cruz, C.; Anwer, M.K.; Khan, H.; Behl, T.; Bungau, S.G. Molecular Aspects and Therapeutic Implications of Herbal Compounds Targeting Different Types of Cancer. Molecules 2023, 28, 750. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.; Chung, D.; Mahmoud, N.; Sepulveda, A.R.; Manafe, M.; Arch, J.; Adada, H.; van der Merwe, T. Why do African Americans get more colon cancer than Native Africans? J. Nutr. 2007, 137, 175S–182S. [Google Scholar] [CrossRef] [PubMed]
- Koller, K.R.; Wilson, A.; Normolle, D.P.; Nicholson, J.K.; Li, J.V.; Kinross, J.; Lee, F.R.; Flanagan, C.A.; Merculieff, Z.T.; Iyer, P.; et al. Dietary fibre to reduce colon cancer risk in Alaska Native people: The Alaska FIRST randomised clinical trial protocol. BMJ Open 2021, 11, e047162. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Bueno, J.; Baca-Bocanegra, B.; Hernandez-Hierro, J.M.; Garcia, R.; Barroso, J.M.; Heredia, F.J.; Rato, A.E. Assessment of Total Fat and Fatty Acids in Walnuts Using Near-Infrared Hyperspectral Imaging. Front. Plant Sci. 2021, 12, 729880. [Google Scholar] [CrossRef] [PubMed]
- Burdge, G.C.; Jones, A.E.; Wootton, S.A. Eicosapentaenoic and docosapentaenoic acids are the principal products of α-linolenic acid metabolism in young men. Br. J. Nutr. 2002, 88, 355–363. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of α-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef]
- Tyagi, A.; Kumar, U.; Reddy, S.; Santosh, V.S.; Mohammed, S.B.; Ehtesham, N.Z.; Ibrahim, A. Attenuation of colonic inflammation by partial replacement of dietary linoleic acid with α-linolenic acid in a rat model of inflammatory bowel disease. Br. J. Nutr. 2012, 108, 1612–1622. [Google Scholar] [CrossRef]
- Todorov, H.; Kollar, B.; Bayer, F.; Brandao, I.; Mann, A.; Mohr, J.; Pontarollo, G.; Formes, H.; Stauber, R.; Kittner, J.M.; et al. α-Linolenic Acid-Rich Diet Influences Microbiota Composition and Villus Morphology of the Mouse Small Intestine. Nutrients 2020, 12, 732. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Wen, J.; Khan, I.; Li, A.; Chen, X.; Yang, P.; Song, P.; Jing, Y.; Wei, J.; Che, T.; Zhang, C. α-linolenic acid given as an anti-inflammatory agent in a mouse model of colonic inflammation. Food Sci. Nutr. 2019, 7, 3873–3882. [Google Scholar] [CrossRef]
- Reifen, R.; Karlinsky, A.; Stark, A.H.; Berkovich, Z.; Nyska, A. α-Linolenic acid (ALA) is an anti-inflammatory agent in inflammatory bowel disease. J. Nutr. Biochem. 2015, 26, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Camuesco, D.; Galvez, J.; Nieto, A.; Comalada, M.; Rodriguez-Cabezas, M.E.; Concha, A.; Xaus, J.; Zarzuelo, A. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J. Nutr. 2005, 135, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, U.E.; Ong, J.S.; An, J.; Gharahkhani, P.; Law, M.H.; MacGregor, S. Mendelian Randomization Study for Genetically Predicted Polyunsaturated Fatty Acids Levels on Overall Cancer Risk and Mortality. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Aune, D.; Beyene, J.; Mobarak, S.; Asadi, M.; Sadeghi, O. Dietary intake and biomarkers of α linolenic acid and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of cohort studies. BMJ 2021, 375, n2213. [Google Scholar] [CrossRef]
- Yang, B.; Wang, F.L.; Ren, X.L.; Li, D. Biospecimen long-chain N-3 PUFA and risk of colorectal cancer: A meta-analysis of data from 60,627 individuals. PLoS ONE 2014, 9, e110574. [Google Scholar] [CrossRef]
- Hanson, S.; Thorpe, G.; Winstanley, L.; Abdelhamid, A.S.; Hooper, L. Omega-3, omega-6 and total dietary polyunsaturated fat on cancer incidence: Systematic review and meta-analysis of randomised trials. Br. J. Cancer 2020, 122, 1260–1270. [Google Scholar] [CrossRef]
- Wu, J.; Wilson, K.M.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E.L. A 24-year prospective study of dietary α-linolenic acid and lethal prostate cancer. Int. J. Cancer 2018, 142, 2207–2214. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, M.J.; Ortea, I.; Guil-Guerrero, J.L. α-Linolenic and gamma-linolenic acids exercise differential antitumor effects on HT-29 human colorectal cancer cells. Toxicol. Res. 2020, 9, 474–483. [Google Scholar] [CrossRef]
- Fan, H.; Huang, W.; Guo, Y.; Ma, X.; Yang, J. α-Linolenic Acid Suppresses Proliferation and Invasion in Osteosarcoma Cells via Inhibiting Fatty Acid Synthase. Molecules 2022, 27, 2741. [Google Scholar] [CrossRef]
- Wang, S.C.; Sun, H.L.; Hsu, Y.H.; Liu, S.H.; Lii, C.K.; Tsai, C.H.; Liu, K.L.; Huang, C.S.; Li, C.C. α-Linolenic acid inhibits the migration of human triple-negative breast cancer cells by attenuating Twist1 expression and suppressing Twist1-mediated epithelial-mesenchymal transition. Biochem. Pharmacol. 2020, 180, 114152. [Google Scholar] [CrossRef]
- Buckner, A.L.; Buckner, C.A.; Montaut, S.; Lafrenie, R.M. Treatment with flaxseed oil induces apoptosis in cultured malignant cells. Heliyon 2019, 5, e02251. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Singh, M.; Sammi, S.R.; Pandey, R.; Kaithwas, G. ALA-mediated biphasic downregulation of α-7nAchR/HIF-1α along with mitochondrial stress modulation strategy in mammary gland chemoprevention. J. Cell. Physiol. 2019, 234, 4015–4029. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Cai, Y. Nuts, especially walnuts, have both antioxidant quantity and efficacy and exhibit significant potential health benefits. Food Funct. 2012, 3, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lipinska, L.; Klewicka, E.; Sojka, M. The structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut polyphenolics inhibit in vitro human plasma and LDL oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shen, D.; Wang, R.; Li, Q.; Mo, R.; Zheng, Y.; Zhou, Y.; Liu, Y. Phenolic profiles and antioxidant activities of free, esterified and bound phenolic compounds in walnut kernel. Food Chem. 2021, 350, 129217. [Google Scholar] [CrossRef]
- Hardman, W.E.; Ion, G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr. Cancer 2008, 60, 666–674. [Google Scholar] [CrossRef]
- Hardman, W.E.; Ion, G.; Akinsete, J.A.; Witte, T.R. Dietary walnut suppressed mammary gland tumorigenesis in the C(3)1 TAg mouse. Nutr. Cancer 2011, 63, 960–970. [Google Scholar] [CrossRef]
- Davis, P.A.; Vasu, V.T.; Gohil, K.; Kim, H.; Khan, I.H.; Cross, C.E.; Yokoyama, W. A high-fat diet containing whole walnuts (Juglans regia) reduces tumour size and growth along with plasma insulin-like growth factor 1 in the transgenic adenocarcinoma of the mouse prostate model. Br. J. Nutr. 2012, 108, 1764–1772. [Google Scholar] [CrossRef]
- Nagel, J.M.; Brinkoetter, M.; Magkos, F.; Liu, X.; Chamberland, J.P.; Shah, S.; Zhou, J.; Blackburn, G.; Mantzoros, C.S. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition 2012, 28, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Chen, Y.; Qendro, V.; Miyamoto, S.; Weinstock, E.; Weinstock, G.M.; Rosenberg, D.W. Effects of Walnut Consumption on Colon Carcinogenesis and Microbial Community Structure. Cancer Prev. Res. 2016, 9, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Hintze, K.J.; Benninghoff, A.D.; Ward, R.E. Formulation of the Total Western Diet (TWD) as a basal diet for rodent cancer studies. J. Agric. Food Chem. 2012, 60, 6736–6742. [Google Scholar] [CrossRef]
- Benninghoff, A.D.; Hintze, K.J.; Monsanto, S.P.; Rodriguez, D.M.; Hunter, A.H.; Phatak, S.; Pestka, J.J.; Wettere, A.J.V.; Ward, R.E. Consumption of the Total Western Diet Promotes Colitis and Inflammation-Associated Colorectal Cancer in Mice. Nutrients 2020, 12, 544. [Google Scholar] [CrossRef]
- Huffman, D.M.; Augenlicht, L.H.; Zhang, X.; Lofrese, J.J.; Atzmon, G.; Chamberland, J.P.; Mantzoros, C.S. Abdominal obesity, independent from caloric intake, accounts for the development of intestinal tumors in Apc1638N/+ female mice. Cancer Prev. Res. 2013, 6, 177–187. [Google Scholar] [CrossRef]
- Guan, F.; Tabrizian, T.; Novaj, A.; Nakanishi, M.; Rosenberg, D.W.; Huffman, D.M. Dietary Walnuts Protect Against Obesity-Driven Intestinal Stem Cell Decline and Tumorigenesis. Front. Nutr. 2018, 5, 37. [Google Scholar] [CrossRef]
- Koh, S.J.; Choi, Y.I.; Kim, Y.; Kim, Y.S.; Choi, S.W.; Kim, J.W.; Kim, B.G.; Lee, K.L. Walnut phenolic extract inhibits nuclear factor kappaB signaling in intestinal epithelial cells, and ameliorates experimental colitis and colitis-associated colon cancer in mice. Eur. J. Nutr. 2019, 58, 1603–1613. [Google Scholar] [CrossRef]
- Chen, Y.; Nakanishi, M.; Bautista, E.J.; Qendro, V.; Sodergren, E.; Rosenberg, D.W.; Weinstock, G.M. Colon Cancer Prevention with Walnuts: A Longitudinal Study in Mice from the Perspective of a Gut Enterotype-like Cluster. Cancer Prev. Res. 2020, 13, 15–24. [Google Scholar] [CrossRef]
- Byerley, L.O.; Samuelson, D.; Blanchard, E.T.; Luo, M.; Lorenzen, B.N.; Banks, S.; Ponder, M.A.; Welsh, D.A.; Taylor, C.M. Changes in the gut microbial communities following addition of walnuts to the diet. J. Nutr. Biochem. 2017, 48, 94–102. [Google Scholar] [CrossRef]
- Soriano-Hernandez, A.D.; Madrigal-Perez, D.G.; Galvan-Salazar, H.R.; Arreola-Cruz, A.; Briseno-Gomez, L.; Guzman-Esquivel, J.; Dobrovinskaya, O.; Lara-Esqueda, A.; Rodriguez-Sanchez, I.P.; Baltazar-Rodriguez, L.M.; et al. The protective effect of peanut, walnut, and almond consumption on the development of breast cancer. Gynecol. Obstet. Investig. 2015, 80, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Primerano, D.A.; Legenza, M.T.; Morgan, J.; Fan, J.; Denvir, J. Dietary walnut altered gene expressions related to tumor growth, survival, and metastasis in breast cancer patients: A pilot clinical trial. Nutr. Res. 2019, 66, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.; Primerano, D.A.; Legenza, M.T.; Morgan, J.; Fan, J.; Denvir, J. mRNA expression data in breast cancers before and after consumption of walnut by women. Data Brief 2019, 25, 104050. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, M.; Murphy, G.; Etemadi, A.; Poustchi, H.; Sharafkhah, M.; Kamangar, F.; Pourshams, A.; Malekshah, A.F.; Khoshnia, M.; Gharavi, A.; et al. Nut consumption and the risk of oesophageal squamous cell carcinoma in the Golestan Cohort Study. Br. J. Cancer 2018, 119, 176–181. [Google Scholar] [CrossRef]
- Naghshi, S.; Sadeghian, M.; Nasiri, M.; Mobarak, S.; Asadi, M.; Sadeghi, O. Association of Total Nut, Tree Nut, Peanut, and Peanut Butter Consumption with Cancer Incidence and Mortality: A Comprehensive Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 793–808. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, C.; Zhou, L.; Li, Y.; Liu, K.; Deng, Y.J.; Li, N.; Zheng, Y.; Hao, Q.; Yang, S.; et al. Meta-analysis of the association between nut consumption and the risks of cancer incidence and cancer-specific mortality. Aging 2020, 12, 10772–10794. [Google Scholar] [CrossRef]
- Sui, J.; Yang, W.; Ma, Y.; Li, T.Y.; Simon, T.G.; Meyerhardt, J.A.; Liang, G.; Giovannucci, E.L.; Chan, A.T.; Zhang, X. A Prospective Study of Nut Consumption and Risk of Primary Hepatocellular Carcinoma in the U.S. Women and Men. Cancer Prev. Res. 2019, 12, 367–374. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, Y.; Li, Y.; Zhang, X.; Willett, W.C.; Eliassen, A.H.; Rosner, B.; Song, M.; Mucci, L.A.; Giovannucci, E.L. Association of nut consumption with risk of total cancer and 5 specific cancers: Evidence from 3 large prospective cohort studies. Am. J. Clin. Nutr. 2021, 114, 1925–1935. [Google Scholar] [CrossRef]
- Fadelu, T.; Zhang, S.; Niedzwiecki, D.; Ye, X.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.B.; et al. Nut Consumption and Survival in Patients with Stage III Colon Cancer: Results From CALGB 89803 (Alliance). J. Clin. Oncol. 2018, 36, 1112–1120. [Google Scholar] [CrossRef]
- Provatas, A.A.; Ayers, S.A.; Callas, A.A.; Birk, J.W.; Lacson, T.A.; Rosenberg, D.W. Quantitative determination of selected urolthin metabolites in human urine by simple sample preparation and UPLC-MS/MS analysis. Curr. Top. Anal. Chem. 2021, 13, 60–80. [Google Scholar]
- Garcia-Villalba, R.; Gimenez-Bastida, J.A.; Cortes-Martin, A.; Avila-Galvez, M.A.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C.; Gonzalez-Sarrias, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, 2101019. [Google Scholar] [CrossRef] [PubMed]
- Marafini, I.; Monteleone, G.; Stolfi, C. Association between Celiac Disease and Cancer. Int. J. Mol. Sci. 2020, 21, 4155. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Guetterman, H.M.; Swanson, K.S.; An, R.; Matthan, N.R.; Lichtenstein, A.H.; Novotny, J.A.; Baer, D.J. Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J. Nutr. 2018, 148, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Di Biase, A.R.; Schiumerini, R.; Eusebi, L.H.; Iughetti, L.; Ravaioli, F.; Scaioli, E.; Colecchia, A.; Festi, D. Gut Microbiota and Celiac Disease. Dig. Dis. Sci. 2016, 61, 1461–1472. [Google Scholar] [CrossRef]
- Bamberger, C.; Rossmeier, A.; Lechner, K.; Wu, L.; Waldmann, E.; Stark, R.G.; Altenhofer, J.; Henze, K.; Parhofer, K.G. A Walnut-Enriched Diet Reduces Lipids in Healthy Caucasian Subjects, Independent of Recommended Macronutrient Replacement and Time Point of Consumption: A Prospective, Randomized, Controlled Trial. Nutrients 2017, 9, 1097. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Calatayud, M.; Romo-Vaquero, M.; Espin, J.C.; Selma, M.V.; Collado, M.C. Urolithin Metabotypes Can Determine the Modulation of Gut Microbiota in Healthy Individuals by Tracking Walnuts Consumption over Three Days. Nutrients 2019, 11, 2483. [Google Scholar] [CrossRef] [PubMed]
- Tindall, A.M.; McLimans, C.J.; Petersen, K.S.; Kris-Etherton, P.M.; Lamendella, R. Walnuts and Vegetable Oils Containing Oleic Acid Differentially Affect the Gut Microbiota and Associations with Cardiovascular Risk Factors: Follow-up of a Randomized, Controlled, Feeding Trial in Adults at Risk for Cardiovascular Disease. J. Nutr. 2020, 150, 806–817. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.S.; Lee, J.; Heo, S.C.; Lee, K.L.; Choi, S.W.; Kim, Y. Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness. Nutrients 2016, 8, 439. [Google Scholar] [CrossRef]
- Schlormann, W.; Lamberty, J.; Ludwig, D.; Lorkowski, S.; Glei, M. In vitro-fermented raw and roasted walnuts induce expression of CAT and GSTT2 genes, growth inhibition, and apoptosis in LT97 colon adenoma cells. Nutr. Res. 2017, 47, 72–80. [Google Scholar] [CrossRef]
- Batirel, S.; Yilmaz, A.M.; Sahin, A.; Perakakis, N.; Kartal Ozer, N.; Mantzoros, C.S. Antitumor and antimetastatic effects of walnut oil in esophageal adenocarcinoma cells. Clin. Nutr. 2018, 37, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Calcabrini, C.; De Bellis, R.; Mancini, U.; Cucchiarini, L.; Stocchi, V.; Potenza, L. Protective Effect of Juglans regia L. Walnut Extract Against Oxidative DNA Damage. Plant Foods Hum. Nutr. 2017, 72, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shin, P.K.; Kim, Y.; Hong, C.P.; Choi, S.W. Metabolic influence of walnut phenolic extract on mitochondria in a colon cancer stem cell model. Eur. J. Nutr. 2019, 58, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; An, J.M.; Han, Y.M.; Surh, Y.J.; Hwang, S.J.; Kim, S.J.; Hahm, K.B. Walnut polyphenol extracts inhibit Helicobacter pylori-induced STAT3(Tyr705) phosphorylation through activation of PPAR-gamma and SOCS1 induction. J. Clin. Biochem. Nutr. 2020, 67, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.V.; Roy, A.; Foote, S.; Vo, P.H.; Lall, N.; Lin, C.H. Profiling Anticancer and Antioxidant Activities of Phenolic Compounds Present in Black Walnuts (Juglans nigra) Using a High-Throughput Screening Approach. Molecules 2020, 25, 4516. [Google Scholar] [CrossRef]
- Park, J.M.; Han, Y.M.; Lee, H.J.; Hwang, S.J.; Kim, S.J.; Hahm, K.B. Transcriptome profiling analysis of the response to walnut polyphenol extract in Helicobacter pylori-infected cells. J. Clin. Biochem. Nutr. 2021, 68, 201–214. [Google Scholar] [CrossRef]
- Nunez-Sanchez, M.A.; Karmokar, A.; Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Brown, K.; Espin, J.C. In vivo relevant mixed urolithins and ellagic acid inhibit phenotypic and molecular colon cancer stem cell features: A new potentiality for ellagitannin metabolites against cancer. Food Chem. Toxicol. 2016, 92, 8–16. [Google Scholar] [CrossRef]
- Inan, M.S.; Rasoulpour, R.J.; Yin, L.; Hubbard, A.K.; Rosenberg, D.W.; Giardina, C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology 2000, 118, 724–734. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Sebastian, C.; Mostoslavsky, R. Untangling the fiber yarn: Butyrate feeds Warburg to suppress colorectal cancer. Cancer Discov. 2014, 4, 1368–1370. [Google Scholar] [CrossRef]
- Wang, L.; Shannar, A.A.F.; Wu, R.; Chou, P.; Sarwar, M.S.; Kuo, H.C.; Peter, R.M.; Wang, Y.; Su, X.; Kong, A.N. Butyrate Drives Metabolic Rewiring and Epigenetic Reprogramming in Human Colon Cancer Cells. Mol. Nutr. Food Res. 2022, 66, e2200028. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Husted, A.S.; Trauelsen, M.; Rudenko, O.; Hjorth, S.A.; Schwartz, T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017, 25, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Matz, A.; Klemashevich, C.; Rosenberg, D.W. Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration. Nutrients 2019, 11, 1118. [Google Scholar] [CrossRef]
- Grivennikov, S.I. Inflammation and colorectal cancer: Colitis-associated neoplasia. Semin. Immunopathol. 2013, 35, 229–244. [Google Scholar] [CrossRef]
- Mangerich, A.; Knutson, C.G.; Parry, N.M.; Muthupalani, S.; Ye, W.; Prestwich, E.; Cui, L.; McFaline, J.L.; Mobley, M.; Ge, Z.; et al. Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc. Natl. Acad. Sci. USA 2012, 109, E1820–E1829. [Google Scholar] [CrossRef]
- Meira, L.B.; Bugni, J.M.; Green, S.L.; Lee, C.W.; Pang, B.; Borenshtein, D.; Rickman, B.H.; Rogers, A.B.; Moroski-Erkul, C.A.; McFaline, J.L.; et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Investig. 2008, 118, 2516–2525. [Google Scholar] [CrossRef]
- Marin, M.; Maria Giner, R.; Rios, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef]
- Zhang, S.; Al-Maghout, T.; Cao, H.; Pelzl, L.; Salker, M.S.; Veldhoen, M.; Cheng, A.; Lang, F.; Singh, Y. Gut Bacterial Metabolite Urolithin A (UA) Mitigates Ca2+ Entry in T Cells by Regulating miR-10a-5p. Front. Immunol. 2019, 10, 1737. [Google Scholar] [CrossRef] [PubMed]
- Bartoszek, A.; Makaro, A.; Bartoszek, A.; Kordek, R.; Fichna, J.; Salaga, M. Walnut Oil Alleviates Intestinal Inflammation and Restores Intestinal Barrier Function in Mice. Nutrients 2020, 12, 1302. [Google Scholar] [CrossRef]
- Cofan, M.; Rajaram, S.; Sala-Vila, A.; Valls-Pedret, C.; Serra-Mir, M.; Roth, I.; Freitas-Simoes, T.M.; Bitok, E.; Sabate, J.; Ros, E. Effects of 2-Year Walnut-Supplemented Diet on Inflammatory Biomarkers. J. Am. Coll. Cardiol. 2020, 76, 2282–2284. [Google Scholar] [CrossRef] [PubMed]
- Ou, F.; Hou, Z.; Wu, X.; Xiao, D. Analysis and Design of a Polygonal Oblique Beam for the Butterfly Vibratory Gyroscope with Improved Robustness to Fabrication Imperfections. Micromachines 2018, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.S.A.; Maghrabi, I.A. Diosmin protects against ethanol-induced gastric injury in rats: Novel anti-ulcer actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef]
- Liu, R.; Hao, Y.T.; Zhu, N.; Liu, X.R.; Kang, J.W.; Mao, R.X.; Hou, C.; Li, Y. The Gastroprotective Effect of Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) against Ethanol-Induced Gastric Mucosal Injury in Rats. Nutrients 2020, 12, 1138. [Google Scholar] [CrossRef]
- Park, J.M.; Han, Y.M.; Park, Y.J.; Hahm, K.B. Dietary intake of walnut prevented Helicobacter pylori-associated gastric cancer through rejuvenation of chronic atrophic gastritis. J. Clin. Biochem. Nutr. 2021, 68, 37–50. [Google Scholar] [CrossRef]
- Xian, W.; Yang, S.; Deng, Y.; Yang, Y.; Tan, Z.; Li, W.; Yang, R. Potential of Establishing the Corresponding Human Microbial Community in Pseudo Germ-Free Mice through Fecal Microbe Transfer from Three Urolithin Metabotypes. J. Agric. Food Chem. 2022, 70, 9388–9398. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Madhu Kumar Nair, R.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. Int. 2020, 27, 26025–26035. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, A.; Martorell, M.; Ilango, K.; Bungau, S.G.; Radu, A.F.; Behl, T.; Sharifi-Rad, J. Theoretical evaluation of Cleome species’ bioactive compounds and therapeutic potential: A literature review. Biomed. Pharmacol. 2022, 151, 113161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, N.; Fusco, J.L.; Rosenberg, D.W. Antioxidant and Anti-Inflammatory Properties of Walnut Constituents: Focus on Personalized Cancer Prevention and the Microbiome. Antioxidants 2023, 12, 982. https://doi.org/10.3390/antiox12050982
Fan N, Fusco JL, Rosenberg DW. Antioxidant and Anti-Inflammatory Properties of Walnut Constituents: Focus on Personalized Cancer Prevention and the Microbiome. Antioxidants. 2023; 12(5):982. https://doi.org/10.3390/antiox12050982
Chicago/Turabian StyleFan, Nuoxi, Jennifer L. Fusco, and Daniel W. Rosenberg. 2023. "Antioxidant and Anti-Inflammatory Properties of Walnut Constituents: Focus on Personalized Cancer Prevention and the Microbiome" Antioxidants 12, no. 5: 982. https://doi.org/10.3390/antiox12050982
APA StyleFan, N., Fusco, J. L., & Rosenberg, D. W. (2023). Antioxidant and Anti-Inflammatory Properties of Walnut Constituents: Focus on Personalized Cancer Prevention and the Microbiome. Antioxidants, 12(5), 982. https://doi.org/10.3390/antiox12050982






