Carbon Nanodots Attenuate Lipid Peroxidation in the LDL Receptor Knockout Mouse Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Synthesis and Characterization of Carbon Nanodots
2.3. Elevated Plus Maze
2.4. Brain Tissue Collection
2.5. Protein Analysis
2.6. Trace Metal Analysis
2.7. Thiobarbituric Acid Reactive Substances (TBARS) Assay
2.8. Statistical Analyses
3. Results
3.1. Body Weight
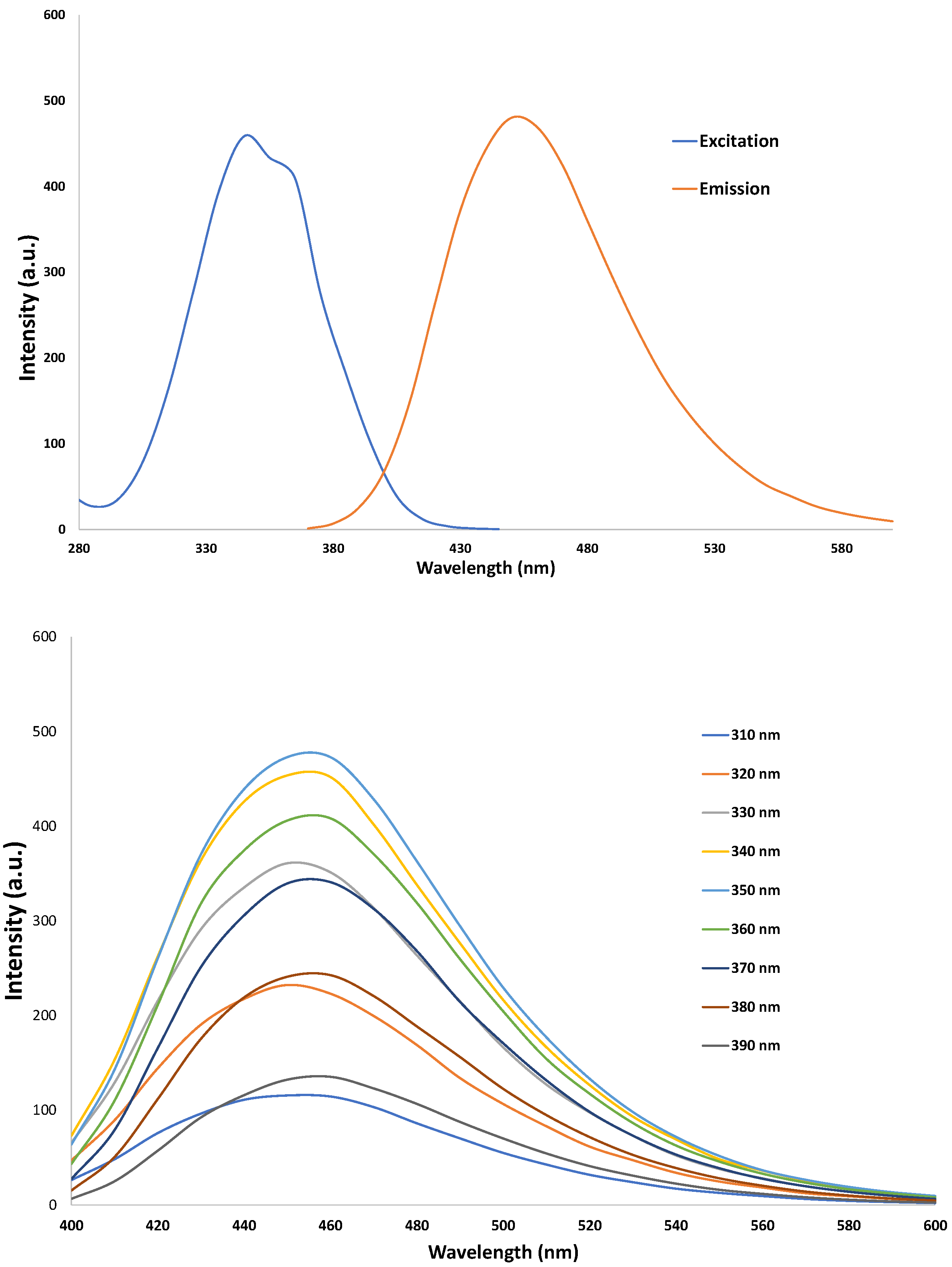
3.2. Characterization of CNDs: UV-VIS
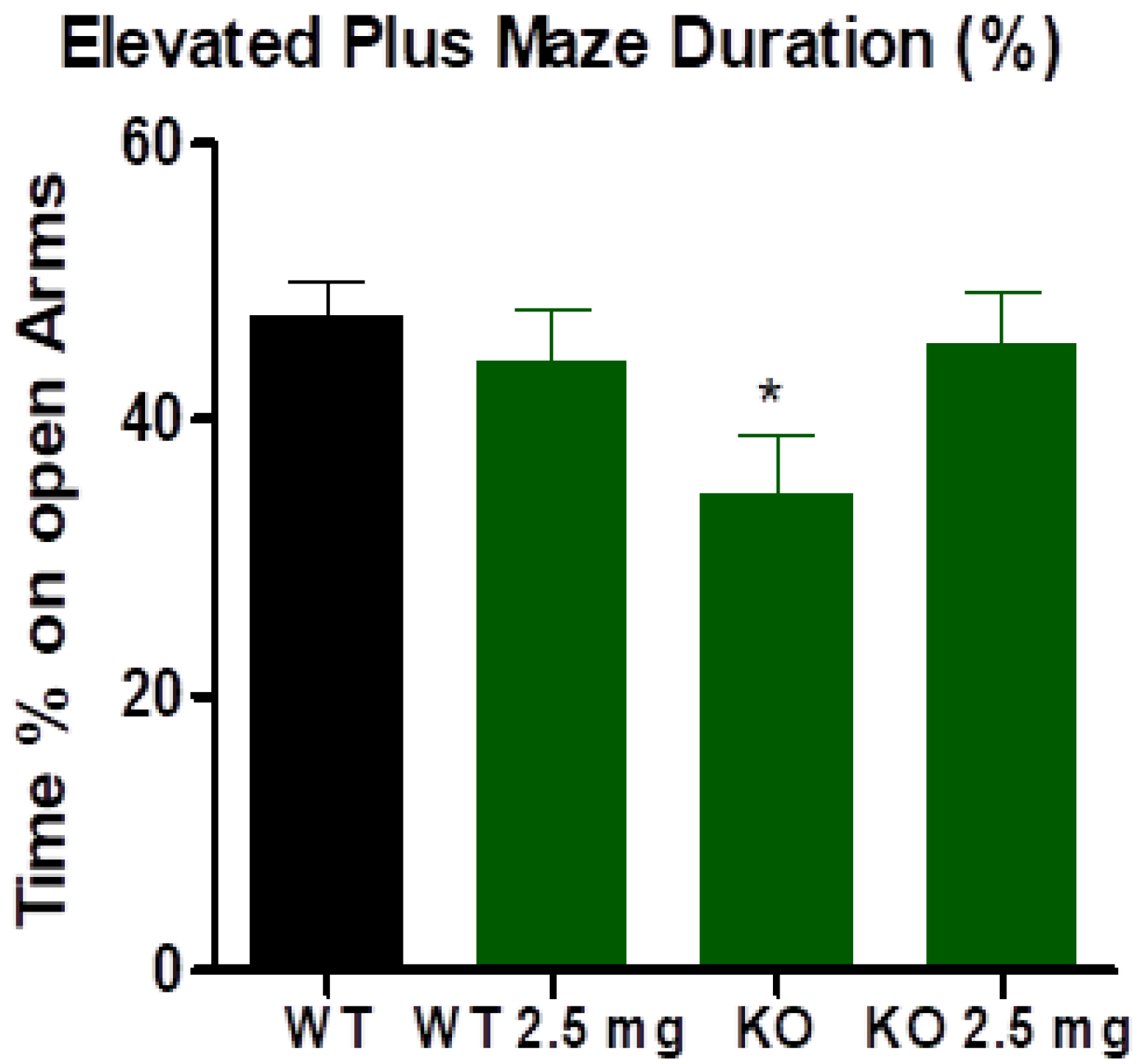
3.3. Elevated Plus Maze
3.4. Iron and Copper
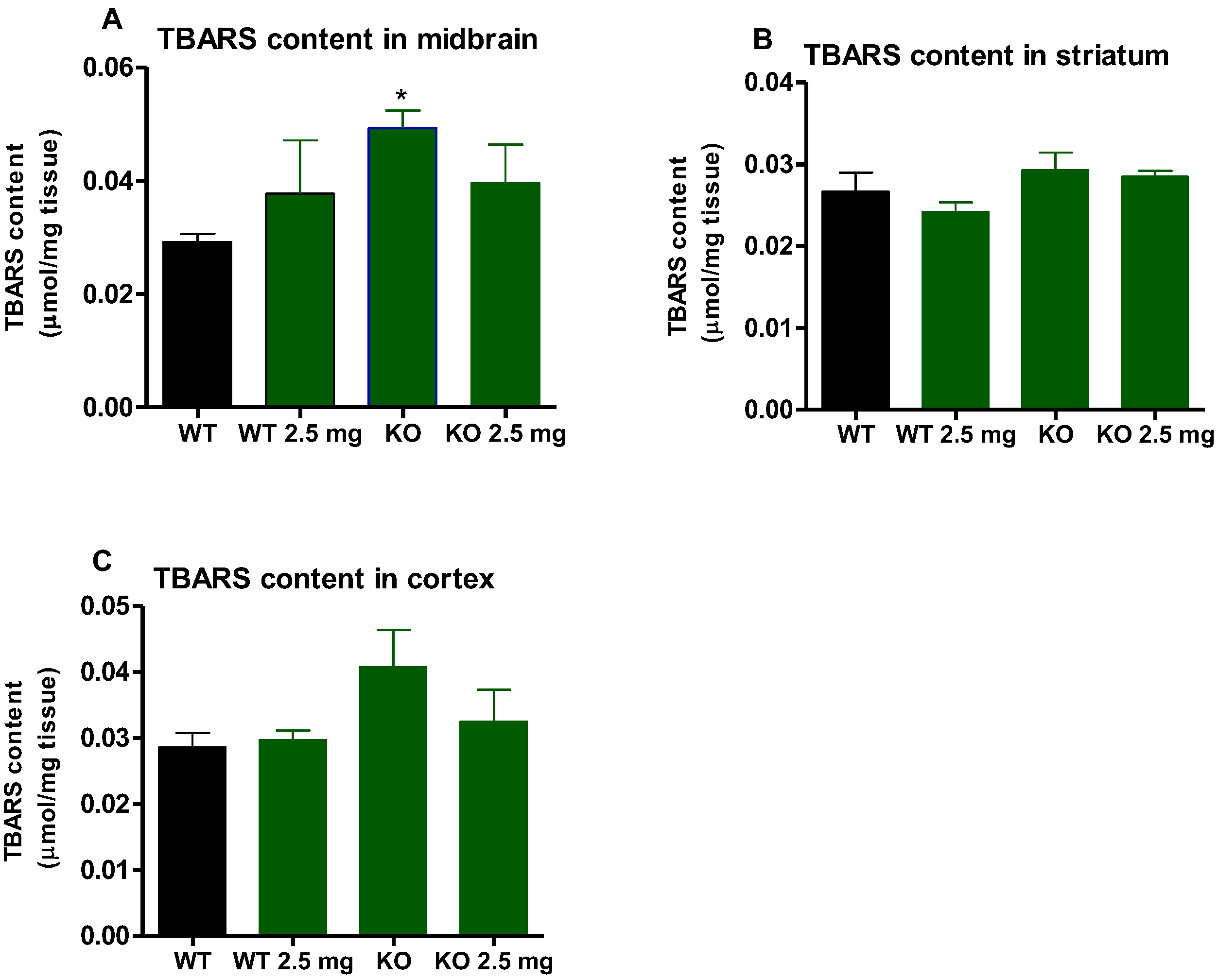
3.5. Malondialdehyde Levels Expressed as TBARS Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Iadecola, C. Atherosclerosis and neurodegeneration: Unexpected conspirators in Alzheimer’s dementia. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1951–1953. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Esh, C.; Kokjohn, T.A.; Kalback, W.; Luehrs, D.C.; Seward, J.D.; Sue, L.I.; Beach, T.G. Circle of willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Getz, G.S.; Reardon, C.A. Do the Apoe−/− and Ldlr−/− Mice Yield the Same Insight on Atherogenesis? Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Zhu, X.; Rong, S.; Shewale, S.; Seo, J.; Boudyguina, E.; Gebre, A.K.; Alexander-Miller, M.A.; Parks, J.S. Omega-3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A. Essential trace metals and brain function. Yakugaku Zasshi 2004, 124, 577–585. [Google Scholar] [CrossRef]
- Bartnicka, J.J.; Blower, P.J. Insights into Trace Metal Metabolism in Health and Disease from PET: “PET Metallomics”. J. Nucl. Med. 2018, 59, 1355–1359. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Kindy, M.C. Overdosing on iron: Elevated iron and degenerative brain disorders. Exp. Biol. Med. 2020, 245, 1444–1473. [Google Scholar] [CrossRef]
- Lal, A. Iron in Health and Disease: An Update. Indian J. Pediatr. 2020, 87, 58–65. [Google Scholar] [CrossRef]
- Belperain, S.; Kang, Z.Y.; Dunphy, A.; Priebe, B.; Chiu, N.H.L.; Jia, Z. Anti-Inflammatory Effect and Cellular Uptake Mechanism of Carbon Nanodots in in Human Microvascular Endothelial Cells. Nanomaterials 2021, 11, 1247. [Google Scholar] [CrossRef]
- Khan, S.; Dunphy, A.; Anike, M.S.; Belperain, S.; Patel, K.; Chiu, N.H.L.; Jia, Z. Recent Advances in Carbon Nanodots: A Promising Nanomaterial for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6786. [Google Scholar] [CrossRef]
- Dunphy, A.; Patel, K.; Belperain, S.; Pennington, A.; Chiu, N.H.L.; Yin, Z.; Zhu, X.; Priebe, B.; Tian, S.; Wei, J.; et al. Modulation of Macrophage Polarization by Carbon Nanodots and Elucidation of Carbon Nanodot Uptake Routes in Macrophages. Nanomaterials 2021, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chavez, J.; Zhu, X.; Chiu, N.H.L.; Zhang, W.; Yin, Z.; Han, J.; Yang, J.; Sigler, R.; Tian, S.; et al. Carbon Nanodots Inhibit Oxidized Low Density Lipoprotein-Induced Injury and Monocyte Adhesion to Endothelial Cells Through Scavenging Reactive Oxygen Species. J. Biomed. Nanotechnol. 2021, 17, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- Totten, M.S.; Pierce, D.M.; Erikson, K.M. The influence of sex and strain on trace element dysregulation in the brain due to diet-induced obesity. J. Trace Elem. Med. Biol. 2021, 63, 126661. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Dadhich, P.; Pal, P.; Srivas, P.K.; Bankoti, K.; Dhara, S. Carbon nanodots from date molasses: New nanolights for the in vitro scavenging of reactive oxygen species. J. Mater. Chem. B 2014, 2, 6839–6847. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, Z.; Wei, J. Electrochemical study of DPPH radical scavenging for evaluating the antioxidant capacity of carbon nanodots. J. Phys. Chem. C 2017, 121, 18635–18642. [Google Scholar] [CrossRef]
- Zhang, W.; Chavez, J.; Zeng, Z.; Bloom, B.; Sheardy, A.; Ji, Z.; Yin, Z.; Waldeck, D.H.; Jia, Z.; Wei, J. Antioxidant Capacity of Nitrogen and Sulfur Codoped Carbon Nanodots. ACS Appl. Nano Mater. 2018, 1, 2699–2708. [Google Scholar] [CrossRef]
- Chen, P.; Totten, M.; Zhang, Z.; Bucinca, H.; Erikson, K.; Santamaria, A.; Bowman, A.B.; Aschner, M. Iron and manganese-related CNS toxicity: Mechanisms, diagnosis and treatment. Expert Rev. Neurother. 2019, 19, 243–260. [Google Scholar] [CrossRef]
- Lingor, P.; Carboni, E.; Koch, J.C. Alpha-synuclein and iron: Two keys unlocking Parkinson’s disease. J. Neural Transm. 2017, 124, 973–981. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simao, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Nnah, I.C.; Wessling-Resnick, M. Brain Iron Homeostasis: A Focus on Microglial Iron. Pharmaceuticals 2018, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.; El Khoury, J. beta-amyloid, microglia, and the inflammasome in Alzheimer’s disease. Semin. Immunopathol. 2015, 37, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.R.; Gahl, W.A. Disorders of metal metabolism. Transl. Sci. Rare Dis. 2017, 2, 101–139. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Heng, Y.; Yuan, Y.H.; Chen, N.H. Pathological alpha-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol. Lett. 2017, 265, 30–37. [Google Scholar] [CrossRef]
- Orr, J.S.; Kennedy, A.; Anderson-Baucum, E.K.; Webb, C.D.; Fordahl, S.C.; Erikson, K.M.; Zhang, Y.; Etzerodt, A.; Moestrup, S.K.; Hasty, A.H. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes 2014, 63, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.Y.; Lee, D.H.; Lee, J.Y.; Lee, E.C.; Park, S.W.; Lee, M.R.; Oh, J.S. Relationship between Brain Metabolic Disorders and Cognitive Impairment: LDL Receptor Defect. Int. J. Mol. Sci. 2022, 23, 8384. [Google Scholar] [CrossRef]
- Rutkowsky, J.M.; Lee, L.L.; Puchowicz, M.; Golub, M.S.; Befroy, D.E.; Wilson, D.W.; Anderson, S.; Cline, G.; Bini, J.; Borkowski, K.; et al. Reduced cognitive function, increased blood-brain-barrier transport and inflammatory responses, and altered brain metabolites in LDLr -/-and C57BL/6 mice fed a western diet. PLoS ONE 2018, 13, e0191909. [Google Scholar] [CrossRef]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef]
- Saeed, S.A.; Shad, K.F.; Saleem, T.; Javed, F.; Khan, M.U. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp. Brain Res. 2007, 182, 1–10. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Wang, S.H.; Huang, Y.; Yuan, Y.; Xia, W.Q.; Wang, P.; Huang, R. LDL receptor knock-out mice show impaired spatial cognition with hippocampal vulnerability to apoptosis and deficits in synapses. Lipids Health Dis. 2014, 13, 175. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Ragnauth, A.; Dorr, N.; Franciosi, S.; Schmeidler, J.; Haroutunian, V.; Buxbaum, J.D. Increased locomotor activity in mice lacking the low-density lipoprotein receptor. Behav. Brain Res. 2008, 191, 256–265. [Google Scholar] [CrossRef]
- Hritcu, L.; Noumedem, J.A.; Cioanca, O.; Hancianu, M.; Postu, P.; Mihasan, M. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav. Brain Funct. 2015, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Ramesh, V.; Gozal, D. Cognitive Deficits Are Attenuated in Neuroglobin Overexpressing Mice Exposed to a Model of Obstructive Sleep Apnea. Front. Neurol. 2018, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Maaroufi, K.; Ammari, M.; Jeljeli, M.; Roy, V.; Sakly, M.; Abdelmelek, H. Impairment of emotional behavior and spatial learning in adult Wistar rats by ferrous sulfate. Physiol. Behav. 2009, 96, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Totten, M.S.; Wallace, C.W.; Pierce, D.M.; Fordahl, S.C.; Erikson, K.M. The impact of a high-fat diet on physical activity and dopamine neurochemistry in the striatum is sex and strain dependent in C57BL/6J and DBA/2J mice. Nutr. Neurosci. 2022, 25, 2601–2615. [Google Scholar] [CrossRef] [PubMed]
- Mansukhani, N.A.; Wang, Z.; Shively, V.P.; Kelly, M.E.; Vercammen, J.M.; Kibbe, M.R. Sex Differences in the LDL Receptor Knockout Mouse Model of Atherosclerosis. Artery Res. 2017, 20, 8–11. [Google Scholar] [CrossRef]


| Treatment Group | Initial Body Weight (day 1, g) | After Treatment Body Weight (16 Weeks, g) |
|---|---|---|
| C57BL/6J Saline Control | 23.08 ± 0.44 a | 33.92 ± 1.03 a |
| C57BL/6J CND 2.5 mg/kg | 23.15 ± 0.63 a | 30.71 ± 0.81 a |
| LDLrKO CND 0 mg/kg | 21.80 ± 0.32 a | 42.79 ± 1.05 b |
| LDLrKO CND 2.5 mg/kg | 22.08 ± 0.33 a | 44.50 ± 1.27 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erikson, K.M.; El-Khouri, K.; Petric, R.; Tang, C.; Chen, J.; Vasquez, D.E.C.; Fordahl, S.C.; Jia, Z. Carbon Nanodots Attenuate Lipid Peroxidation in the LDL Receptor Knockout Mouse Brain. Antioxidants 2023, 12, 1081. https://doi.org/10.3390/antiox12051081
Erikson KM, El-Khouri K, Petric R, Tang C, Chen J, Vasquez DEC, Fordahl SC, Jia Z. Carbon Nanodots Attenuate Lipid Peroxidation in the LDL Receptor Knockout Mouse Brain. Antioxidants. 2023; 12(5):1081. https://doi.org/10.3390/antiox12051081
Chicago/Turabian StyleErikson, Keith M., Kristina El-Khouri, Radmila Petric, Chenhao Tang, Jinlan Chen, Delicia Esther Cardenas Vasquez, Steve C. Fordahl, and Zhenquan Jia. 2023. "Carbon Nanodots Attenuate Lipid Peroxidation in the LDL Receptor Knockout Mouse Brain" Antioxidants 12, no. 5: 1081. https://doi.org/10.3390/antiox12051081
APA StyleErikson, K. M., El-Khouri, K., Petric, R., Tang, C., Chen, J., Vasquez, D. E. C., Fordahl, S. C., & Jia, Z. (2023). Carbon Nanodots Attenuate Lipid Peroxidation in the LDL Receptor Knockout Mouse Brain. Antioxidants, 12(5), 1081. https://doi.org/10.3390/antiox12051081







