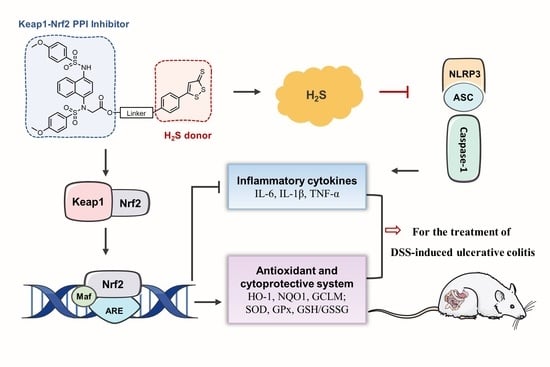
Novel Hydrogen Sulfide Hybrid Derivatives of Keap1-Nrf2 Protein–Protein Interaction Inhibitor Alleviate Inflammation and Oxidative Stress in Acute Experimental Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemistry Section
2.1.1. General Chemistry Methods
2.1.2. Synthesis of DDO-1636
2.1.3. Synthesis of ADT-OH from Anethole Trithione
2.1.4. General Procedure for the Synthesis of Compounds 1b~1d and 2b~2d
2.1.5. General Procedure for the Synthesis of Compounds DDO-1901~DDO-1908
2.2. Hydrogen Sulfide Release Evaluation (Methylene Blue Assay)
2.3. Esterase Triggering DDO-1636 Release as Monitored by HPLC
2.4. Cell Culture
2.5. Fluorescence Probe Studies of H2S Release in Cells
2.6. Cell Viability Study
2.7. Western Blotting
2.8. Detection of SOD, GPx, and MDA Activities and the Ratio of GSH/GSSG
2.9. IL-6, IL-1β, and TNF-α Production
2.10. Animal Experiments
2.10.1. Animal
2.10.2. DSS-induced Acute Colitis
2.10.3. IL-6, IL-1β, and TNF-α Production
2.10.4. Histopathological Examination
3. Results and Discussion
3.1. Chemistry
3.2. Hybrid Compounds Are Capable of Releasing H2S
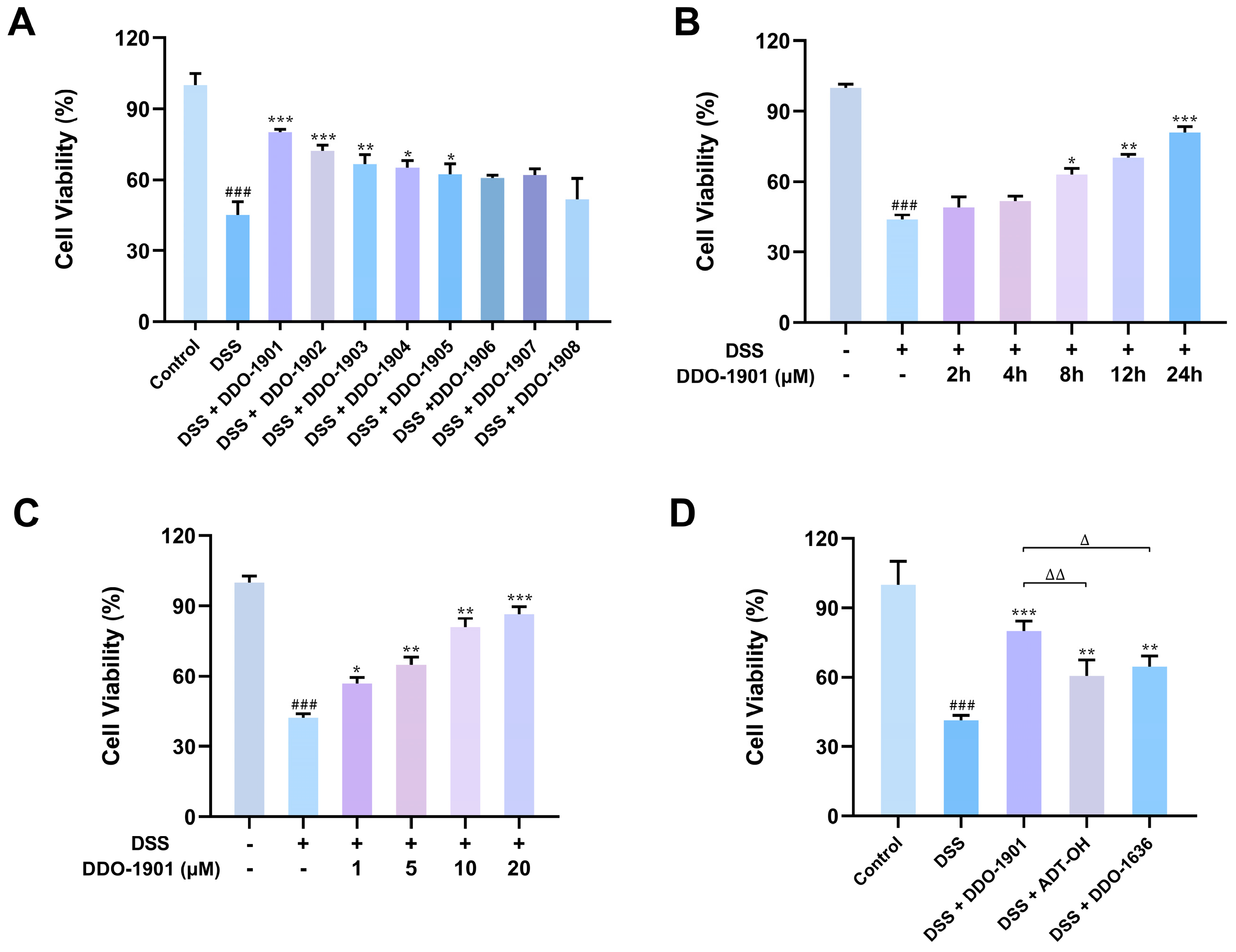
3.3. DDO-1901 Protects NCM460 Cells from DSS-Induced Injury
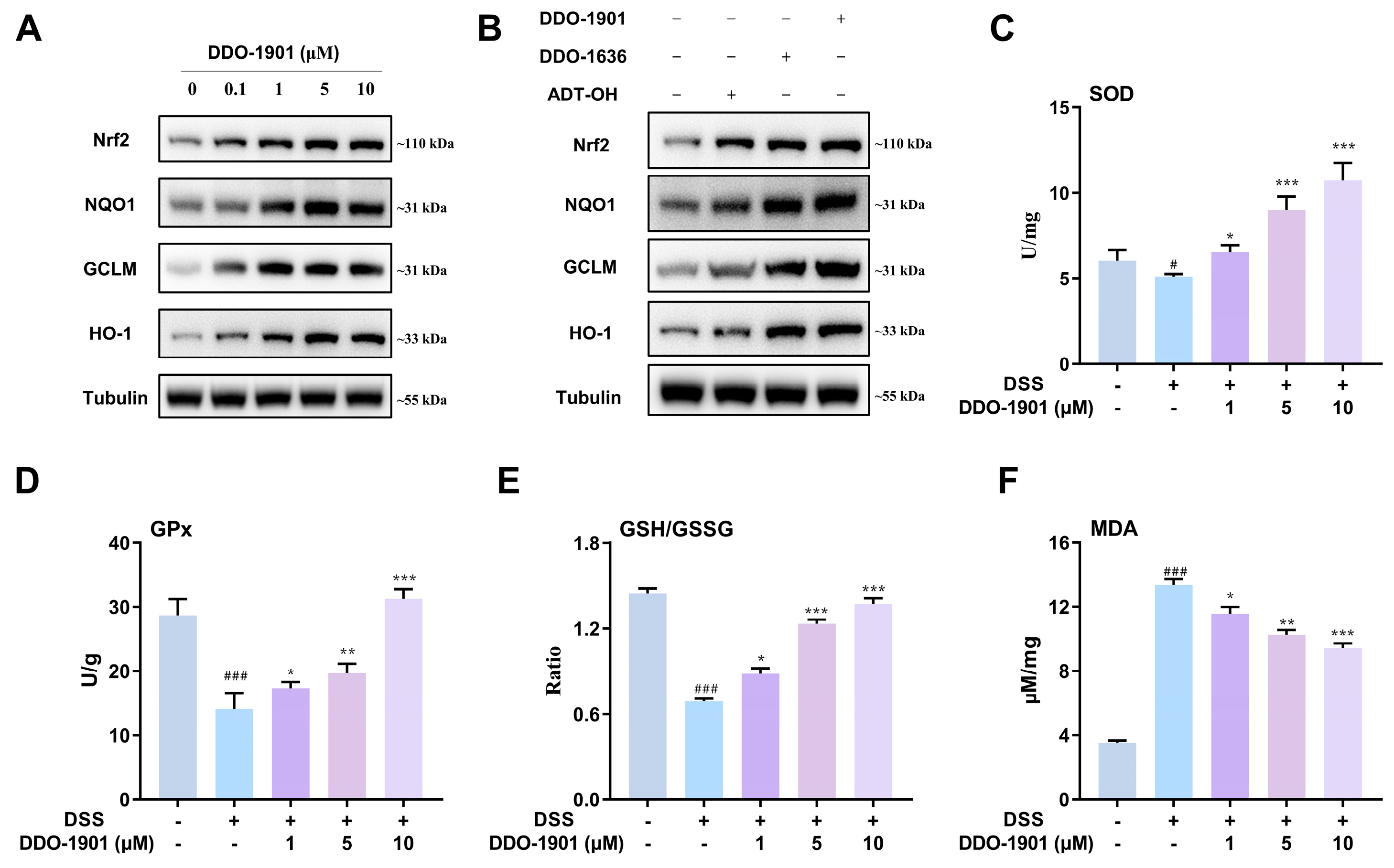
3.4. DDO-1901 Activates Nrf2-regulated Antioxidant System in NCM460 Cells
3.5. DDO-1901 Represses DSS-Induced NLRP3 Inflammasome Activation and Pro-Inflammatory Cytokines Production in NCM460 Cells
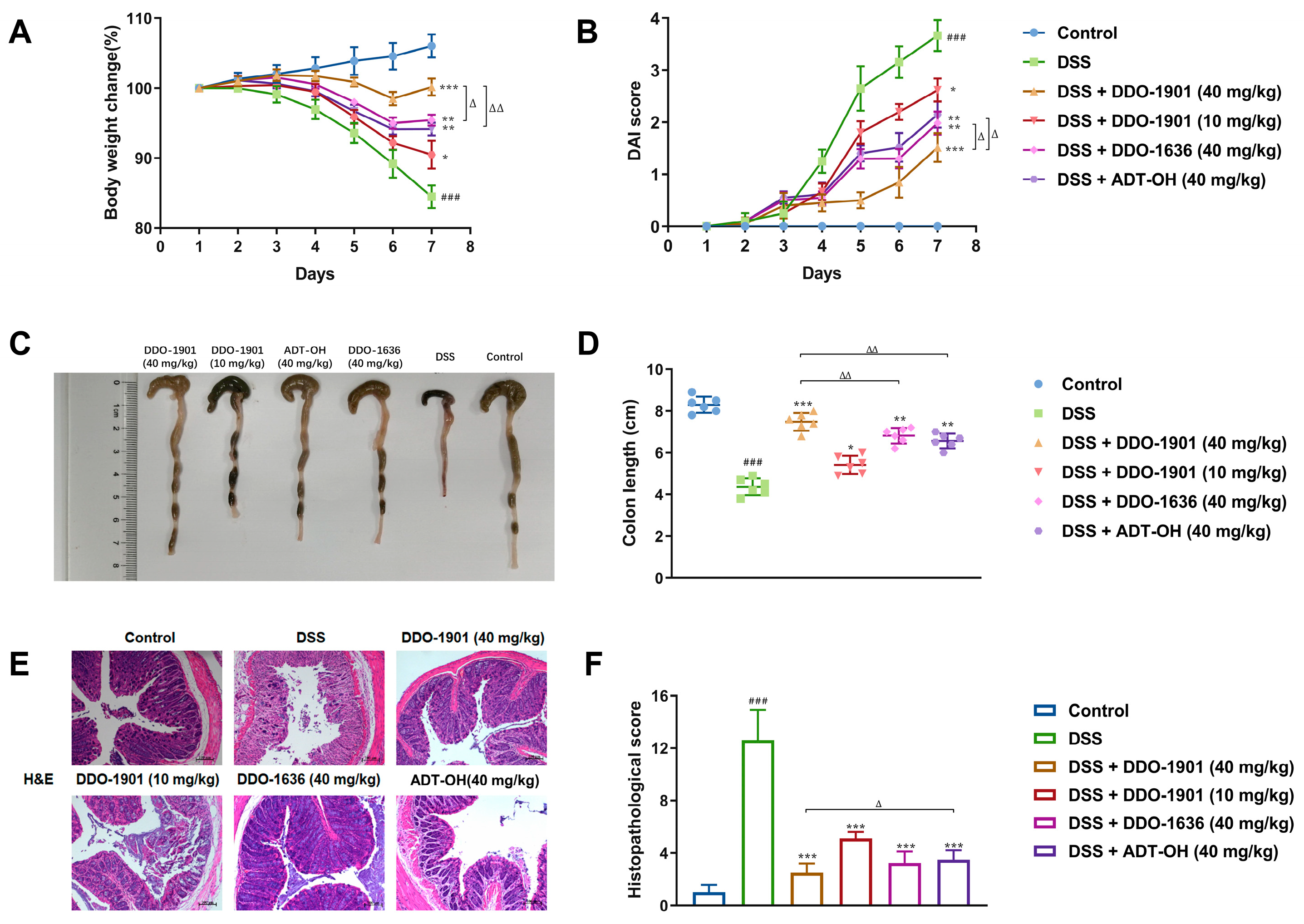
3.6. DDO-1901 Alleviates the Pathological Symptoms of DSS-Induced Colitis in Mice
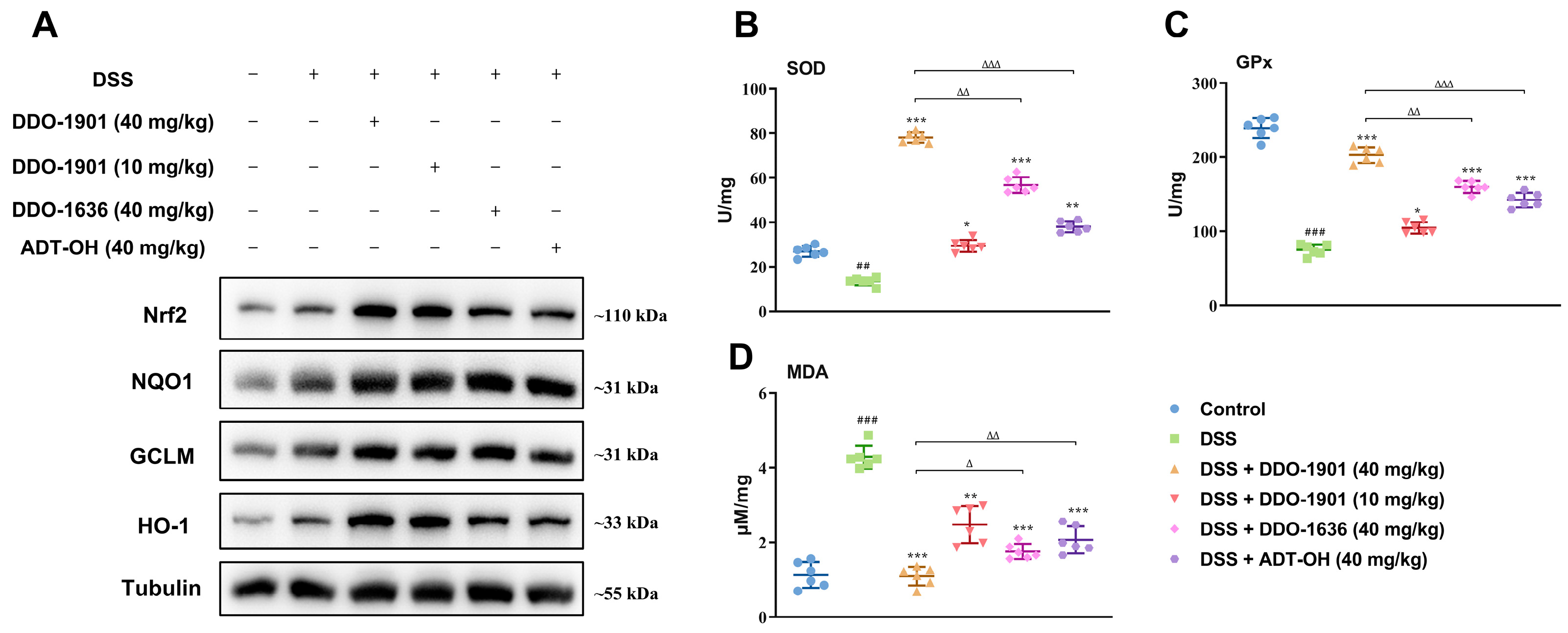
3.7. DDO-1901 Enhances the Antioxidant Defenses via Activation of Nrf2 against DSS-Induced Colitis in Mice
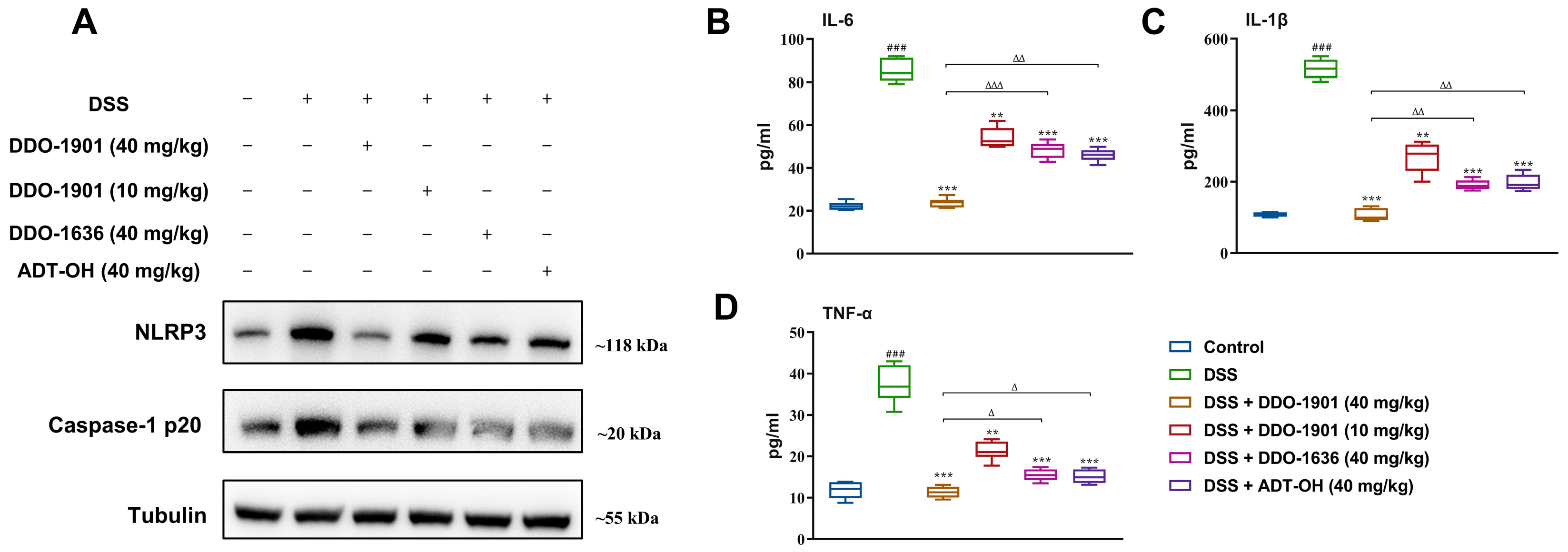
3.8. DDO-1901 Relieves the Inflammation Conditions in the DSS-Induced Colitis in Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eisenstein, M. Ulcerative Colitis: Towards Remission. Nature 2018, 563, S33. [Google Scholar] [CrossRef] [PubMed]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative Colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of Disease: Pathogenesis of Crohn’s disease and Ulcerative Colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef]
- Bernstein, C.N. Treatment of IBD: Where We Are and Where We Are Going. Am. J. Gastroenterol. 2015, 110, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; DuBois, R.N. The Role of Anti-inflammatory Drugs in Colorectal Cancer. Annu. Rev. Med. 2013, 64, 131–144. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.-F. Ulcerative Colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Kwapisz, L.; Bruining, D.H.; Pardi, D.S.; Tremaine, W.J.; Kane, S.V.; Papadakis, K.A.; Coelho-Prabhu, N.; Kisiel, J.B.; Heron, V.; Faubion, W.A.; et al. Combination Biologic Therapy in Inflammatory Bowel Disease: Experience from a Tertiary Care Center. Clin. Gastroenterol. Hepatol. 2021, 19, 616–617. [Google Scholar] [CrossRef]
- Stalgis, C.; Deepak, P.; Mehandru, S.; Colombel, J.-F. Rational Combination Therapy to Overcome the Plateau of Drug Efficacy in Inflammatory Bowel Disease. Gastroenterology 2021, 161, 394–399. [Google Scholar] [CrossRef]
- Hu, A.; Tan, W.; Jess, A.; Li, P.S.; Kotze, P.G.; Burgevin, A.; Kroeker, K.; Halloran, B.; Panaccione, R.; Peyrin-Biroulet, L.; et al. Combination Therapy Does Not Improve Rate of Clinical or Endoscopic Remission in Patients with Inflammatory Bowel Diseases Treated with Vedolizumab or Ustekinumab. Clin. Gastroenterol. Hepatol. 2021, 19, 1366–1376. [Google Scholar] [CrossRef]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019, 62, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.d.O.; Duarte da Cruz, R.M.; Viana, J.d.O.; Olimpio de Moura, R.; Ishiki, H.M.; Barbosa Filho, J.M.; Diniz, M.F.F.M.; Scotti, M.T.; Scotti, L.; Mendonca, F.J.B., Jr. Hybrid Compounds as Direct Multitarget Ligands: A Review. Curr. Top. Med. Chem. 2017, 17, 1044–1079. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleki, A.; Ghasemi, J.B. Dual-acting of Hybrid Compounds-A New Dawn in the Discovery of Multi-target Drugs: Lead Generation Approaches. Curr. Top. Med. Chem. 2017, 17, 1096–1114. [Google Scholar] [CrossRef]
- Wallace, J.L.; Vong, L.; McKnight, W.; Dicay, M.; Martin, G.R. Endogenous and Exogenous Hydrogen Sulfide Promotes Resolution of Colitis in Rats. Gastroenterology 2009, 137, 569–578. [Google Scholar] [CrossRef]
- Wang, R. Physiological Implications of Hydrogen Sulfide: A Whiff Exploration that Blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Wallace, J.L.; Motta, J.P.; Buret, A.G. Hydrogen Sulfide: An Agent of Stability at the Microbiome-Mucosa Interface. Am. J. Physiol. 2018, 314, 143–149. [Google Scholar] [CrossRef]
- Wallace, J.L.; Ferraz, J.G.P.; Muscara, M.N. Hydrogen Sulfide: An Endogenous Mediator of Resolution of Inflammation and Injury. Antioxid. Redox Signal. 2012, 17, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.V.; Wallace, J.L. Hydrogen Sulfide-based Therapeutics and Gastrointestinal Giseases: Translating Physiology to Treatments. Am. J. Physiol. 2013, 305, G467–G473. [Google Scholar]
- Medani, M.; Collins, D.; Docherty, N.G.; Baird, A.W.; O’Connell, P.R.; Winter, D.C. Emerging Role of Hydrogen Sulfide in Colonic Physiology and Pathophysiology. Inflamm. Bowel Dis. 2011, 17, 1620–1625. [Google Scholar] [CrossRef]
- Motta, J.-P.; Flannigan, K.L.; Agbor, T.A.; Beatty, J.K.; Blackler, R.W.; Workentine, M.L.; Da, S.G.J.; Wang, R.; Buret, A.G.; Wallace, J.L. Hydrogen Sulfide Protects from Colitis and Restores Intestinal Microbiota Biofilm and Mucus Production. Inflamm. Bowel Dis. 2015, 21, 1006–1017. [Google Scholar] [CrossRef]
- Ke, B.W.; Wu, W.X.; Liu, W.; Liang, H.; Gong, D.Y.; Hu, X.T.; Li, M.Y. Bioluminescence Probe for Detecting Hydrogen Sulfide in Vivo. Anal. Chem. 2016, 88, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Long, F.; Wu, W.j.; Yang, D.; Huang, M.w.; Xiao, C.x.; Chen, X.; Liu, X.h.; Zhu, Y.Z. Hydrogen Sulfide Protects Against DSS-induced Colitis by Inhibiting NLRP3 Inflammasome. Free Radic. Biol. Med. 2019, 137, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Hirata, I.; Naito, Y.; Takagi, T.; Mizushima, K.; Suzuki, T.; Omatsu, T.; Handa, O.; Ichikawa, H.; Ueda, H.; Yoshikawa, T. Endogenous Hydrogen Sulfide Is an Anti-inflammatory Molecule in Dextran Sodium Sulfate-Induced Colitis in Mice. Dig. Dis. Sci. 2011, 56, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Physiological and Pathophysiological Roles of Hydrogen Sulfide in the Gastrointestinal Tract. Antioxid. Redox Signal. 2010, 12, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.G.; Qiu, M.Y.; Lv, S.Y.; Zheng, H.; Niu, B.H.; Liu, H.Y.; Shi, X.Z. Hydrogen Sulfide Plays an Important Role by Influencing NLRP3 inflammasome. Int. J. Biol. Sci. 2020, 16, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.Z.; Liu, Y.; Bian, J.S. Hydrogen Sulfide and Cellular Redox Homeostasis. Oxid. Med. Cell. Longev. 2016, 2016, 6043038. [Google Scholar] [CrossRef] [PubMed]
- Corsello, T.; Komaravelli, N.; Casola, A. Role of Hydrogen Sulfide in NRF2- and Sirtuin-dependent Maintenance of Cellular Redox Balance. Antioxidants 2018, 7, 129. [Google Scholar] [CrossRef]
- Yang, W.; Tao, K.; Wang, Y.; Huang, Y.; Duan, C.; Wang, T.; Li, C.; Zhang, P.; Yin, Y.; Gao, J.; et al. Necrosulfonamide ameliorates intestinal inflammation via inhibiting GSDMD-medicated pyroptosis and MLKL-mediated necroptosis. Biochem. Pharmacol. 2022, 206, 115338. [Google Scholar] [CrossRef]
- Li, L.; Rose, P.; Moore, P.K. Hydrogen Sulfide and Cell Signaling. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 169–187. [Google Scholar] [CrossRef]
- Fan, H.N.; Wang, H.J.; Yang-Dan, C.R.; Ren, L.; Wang, C.; Li, Y.F.; Deng, Y. Protective effects of hydrogen sulfide on oxidative stress and fibrosis in hepatic stellate cells. Mol. Med. Rep. 2013, 7, 247–253. [Google Scholar] [CrossRef]
- Hourihan, J.M.; Kenna, J.G.; Hayes, J.D. The Gasotransmitter Hydrogen Sulfide Induces Nrf2-Target Genes by Inactivating the Keap1 Ubiquitin Ligase Substrate Adaptor Through Formation of a Disulfide Bond Between Cys-226 and Cys-613. Antioxid. Redox Signal. 2013, 19, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a Novel, Water-soluble Hydrogen Sulfide-releasing Molecule (GYY4137): New Insights into the Biology of Hydrogen Sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and Gastrointestinal Effects of a Novel Diclofenac Derivative. Free Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Lougiakis, N.; Papapetropoulos, A.; Gikas, E.; Toumpas, S.; Efentakis, P.; Wedmann, R.; Zoga, A.; Zhou, Z.; Iliodromitis, E.K.; Skaltsounis, A.-L.; et al. Synthesis and Pharmacological Evaluation of Novel Adenine-Hydrogen Sulfide Slow Release Hybrids Designed as Multitarget Cardioprotective Agents. J. Med. Chem. 2016, 59, 1776–1790. [Google Scholar] [CrossRef]
- Yu, B.C.; Kang, T.; Xu, Y.; Liu, Y.Q.; Ma, Y.R.; Ke, B.W. Prodrugs of Persulfide and Sulfide: Is There a Pharmacological Difference between the Two in the Context of Rapid Exchanges among Various Sulfur Species In Vivo? Angew. Chem.-Int. Edit. 2022, 61. [Google Scholar]
- Zhou, S.C.; Mou, Y.J.; Liu, M.; Du, Q.; Ali, B.; Ramprasad, J.; Qiao, C.H.; Hu, L.F.; Ji, X.Y. Insights into the Mechanism of Thiol-Triggered COS/H2S Release from N-Dithiasuccinoyl Amines. J. Org. Chem. 2020, 85, 8352–8359. [Google Scholar] [CrossRef]
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286. [Google Scholar] [CrossRef]
- Kashfi, K.; Olson, K.R. Biology and Therapeutic Potential of Hydrogen Sulfide and Hydrogen Sulfide-releasing Chimeras. Biochem. Pharmacol. 2013, 85, 689–703. [Google Scholar] [CrossRef]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G.; Fiorucci, S. Gastrointestinal Safety and Anti-inflammatory Effects of a Hydrogen Sulfide-releasing Diclofenac Derivative in the Rat. Gastroenterology 2007, 132, 261–271. [Google Scholar] [CrossRef]
- Wallace, J.L.; Caliendo, G.; Santagada, V.; Cirino, G. Markedly Reduced Toxicity of a Hydrogen Sulphide-releasing Derivative of Naproxen (ATB-346). Br. J. Pharmacol. 2010, 159, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Orlandi, S.; Mencarelli, A.; Caliendo, G.; Santagada, V.; Distrutti, E.; Santucci, L.; Cirino, G.; Wallace, J.L. Enhanced Activity of a Hydrogen Sulphide-releasing Derivative of Mesalamine (ATB-429) in a Mouse Model of Colitis. Br. J. Pharmacol. 2007, 150, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Zhang, D.D. Mechanistic Studies of the Nrf2-Keap1 Signaling Pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Molecular Mechanisms Activating the Nrf2-Keap1 Pathway of Antioxidant Gene Regulation. Antioxid. Redox Signal. 2005, 7, 385–394. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, L.J.; Chen, H.H.; Zhang, W.N.A.; Xing, C.G.; Qu, Z.; Yu, J.Q.; Zhuang, C.L. Structure-based molecular hybridization design of Keap1-Nrf2 inhibitors as novel protective agents of acute lung injury. Eur. J. Med. Chem. 2021, 222. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, J.X.; Chen, Y.F.; Shang, H.; Zhang, W.N.; Yu, J.Q.; He, L.; Xing, C.G.; Zhuang, C.L. Direct inhibition of Keap1-Nrf2 Protein-Protein interaction as a potential therapeutic strategy for Alzheimer’s disease. Bioorg. Chem. 2020, 103. [Google Scholar] [CrossRef]
- Khor, T.O.; Huang, M.T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.N. Nrf2-Deficient Mice Have an Increased Susceptibility to Dextran Sulfate Sodium-Induced Colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef]
- Osburn, W.O.; Karim, B.; Dolan, P.M.; Liu, G.; Yamamoto, M.; Huso, D.L.; Kensler, T.W. Increased Colonic Inflammatory Injury and Formation of Aberrant Cryptfoci in Nrf2-deficient Mice upon Dextran Sulfate Treatment. Int. J. Cancer 2007, 121, 1883–1891. [Google Scholar] [CrossRef]
- Kim, W.; Lee, H.; Kim, S.; Joo, S.; Jeong, S.; Yoo, J.W.; Jung, Y. Sofalcone, a gastroprotective drug, covalently binds to KEAP1 to activate Nrf2 resulting in anti-colitic activity. Eur. J. Pharmacol. 2019, 865. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; Ji, J.A.; Jiang, Y.L.; Chen, Z.Y.; Yuan, Z.W.; You, Q.D.; Jiang, Z.Y. An Inhibitor of the Keap1-Nrf2 Protein-protein Interaction Protects NCM460 Colonic Cells and Alleviates Experimental Colitis. Sci. Rep. 2016, 6, 26585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Yan, T.T.; Sun, D.X.; Xie, C.; Wang, T.X.; Liu, X.Y.; Wang, J.; Wang, Q.; Luo, Y.H.; Wang, P.; et al. Rutaecarpine Inhibits KEAP1-NRF2 Interaction to Activate NRF2 and Ameliorate Dextran Sulfate Sodium-induced Colitis. Free Radic. Biol. Med. 2020, 148, 33–41. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant Signaling Attenuates NFκB-Inflammatory Response and Elicits Apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Aleksunes, L.M.; Manautou, J.E. Emerging Role of Nrf2 in Protecting Against Hepatic and Gastrointestinal Disease. Toxicol. Pathol. 2007, 35, 459–473. [Google Scholar] [CrossRef]
- Theiss, A.L.; Vijay-Kumar, M.; Obertone, T.S.; Jones, D.P.; Hansen, J.M.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Prohibitin is a Novel Regulator of Antioxidant Response that Attenuates Colonic Inflammation in Mice. Gastroenterology 2009, 137, 199–208. [Google Scholar] [CrossRef]
- Wagner, A.E.; Will, O.; Sturm, C.; Lipinski, S.; Rosenstiel, P.; Rimbach, G. DSS-induced Acute Colitis in C57BL/6 Mice is Mitigated by Sulforaphane Pre-treatment. J. Nutr. Biochem. 2013, 24, 2085–2091. [Google Scholar] [CrossRef]
- Li, Y.; Shen, L.; Luo, H. Luteolin Ameliorates Dextran Sulfate Sodium-induced Colitis in Mice possibly through Activation of the Nrf2 Signaling Pathway. Int. Immunopharmacol. 2016, 40, 24–31. [Google Scholar] [CrossRef]
- Jeong, S.; Kang, C.; Park, S.; Ju, S.; Yoo, J.W.; Yoon, I.S.; Yun, H.; Jung, Y. Eletrophilic Chemistry of Tranilast Is Involved in Its Anti-Colitic Activity via Nrf2-HO-1 Pathway Activation. Pharmaceuticals 2021, 14, 1092. [Google Scholar] [CrossRef]
- Lu, M.C.; Zhang, X.; Zhao, J.; You, Q.D.; Jiang, Z.Y. A Hydrogen Peroxide Responsive Prodrug of Keap1-Nrf2 Inhibitor for Improving Oral Absorption and Selective Activation in Inflammatory Conditions. Redox Biol. 2020, 34, 101565. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Arra, C.; Turco, M.C. Review of Molecular Mechanisms Involved in the Activation of the Nrf2-ARE Signaling Pathway by Chemopreventive Agents. Methods Mol. Biol. 2010, 647, 37–74. [Google Scholar] [PubMed]
- Lee, J.S.; Surh, Y.J. Nrf2 as a Novel Molecular Target for Chemoprevention. Cancer Lett. 2005, 224, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.L.; Wu, Z.L.; Xing, C.G.; Miao, Z.Y. Small molecules inhibiting Keap1-Nrf2 protein-protein interactions: A novel approach to activate Nrf2 function. Medchemcomm 2017, 8, 286–294. [Google Scholar] [CrossRef]
- Meng, N.; Tang, H.; Zhang, H.; Jiang, C.S.; Su, L.; Min, X.; Zhang, W.N.; Zhang, H.; Miao, Z.Y.; Zhang, W.; et al. Fragment-growing guided design of Keap1-Nrf2 protein-protein interaction inhibitors for targeting myocarditis. Free Radic. Biol. Med. 2018, 117, 228–237. [Google Scholar] [CrossRef]
- Zaki, M.H.; Lamkanfi, M.; Kanneganti, T.-D. The Nlrp3 Inflammasome: Contributions to Intestinal Homeostasis. Trends Immunol. 2011, 32, 171–179. [Google Scholar] [CrossRef]
- Bauer, C.; Duewell, P.; Mayer, C.; Lehr, H.A.; Fitzgerald, K.A.; Dauer, M.; Tschopp, J.; Endres, S.; Latz, E.; Schnurr, M. Colitis Induced in Mice with Dextran Sulfate Sodium (DSS) is Mediated by the NLRP3 Inflammasome. Gut 2010, 59, 1192–1199. [Google Scholar] [CrossRef]
- Shao, B.Z.; Wu, K.; Linghu, E.Q.; Wang, S.-L.; Pan, P.; Li, Z.S.; Bai, Y.; Yao, J. Targeting NLRP3 Inflammasome in Inflammatory Bowel Disease: Putting out the Fire of Inflammation. Inflammation 2019, 42, 1147–1159. [Google Scholar] [CrossRef]
- Kim, J.K.; Jin, H.S.; Suh, H.W.; Jo, E.-K. Negative Regulators and Their Mechanisms in NLRP3 Inflammasome Activation and SIgnaling. Immunol. Cell Biol. 2017, 95, 584–592. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Cao, Q.; Wang, Z.; Zhao, M.; Xu, L.; Zhuang, Q. Mitochondrial dysfunction in fibrotic diseases. Cell Death Discov. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Hennig, P.; Garstkiewicz, M.; Grossi, S.; Di Filippo, M.; French, L.E.; Beer, H.D. The Crosstalk between Nrf2 and Inflammasomes. Int. J. Mol. Sci. 2018, 19, 562. [Google Scholar] [CrossRef]
- Liu, X.t.; Zhou, W.; Zhang, X.; Lu, P.; Du, Q.m.; Tao, L.; Ding, Y.; Wang, Y.j.; Hu, R. Dimethyl Fumarate Ameliorates Dextran Sulfate Sodium-induced Murine Experimental Colitis by Activating Nrf2 and Suppressing NLRP3 Inflammasome Activation. Biochem. Pharmacol. 2016, 112, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Q.; Zhang, Y.; Shi, W.; Wang, H.; Zheng, Z.; Meng, L.; Xin, Y.; Jiang, X. Sulforaphane-Mediated Nrf2 Activation Prevents Radiation-Induced Skin Injury through Inhibiting the Oxidative-Stress-Activated DNA Damage and NLRP3 Inflammasome. Antioxidants 2021, 10, 1850. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Cui, K.; Wang, X.; Tong, Y.; Liu, C.; Zhu, Y.; You, Q.; Jiang, Z.; Guo, X. Novel Hydrogen Sulfide Hybrid Derivatives of Keap1-Nrf2 Protein–Protein Interaction Inhibitor Alleviate Inflammation and Oxidative Stress in Acute Experimental Colitis. Antioxidants 2023, 12, 1062. https://doi.org/10.3390/antiox12051062
Zhang X, Cui K, Wang X, Tong Y, Liu C, Zhu Y, You Q, Jiang Z, Guo X. Novel Hydrogen Sulfide Hybrid Derivatives of Keap1-Nrf2 Protein–Protein Interaction Inhibitor Alleviate Inflammation and Oxidative Stress in Acute Experimental Colitis. Antioxidants. 2023; 12(5):1062. https://doi.org/10.3390/antiox12051062
Chicago/Turabian StyleZhang, Xian, Keni Cui, Xiaolu Wang, Yuanyuan Tong, Chihong Liu, Yuechao Zhu, Qidong You, Zhengyu Jiang, and Xiaoke Guo. 2023. "Novel Hydrogen Sulfide Hybrid Derivatives of Keap1-Nrf2 Protein–Protein Interaction Inhibitor Alleviate Inflammation and Oxidative Stress in Acute Experimental Colitis" Antioxidants 12, no. 5: 1062. https://doi.org/10.3390/antiox12051062
APA StyleZhang, X., Cui, K., Wang, X., Tong, Y., Liu, C., Zhu, Y., You, Q., Jiang, Z., & Guo, X. (2023). Novel Hydrogen Sulfide Hybrid Derivatives of Keap1-Nrf2 Protein–Protein Interaction Inhibitor Alleviate Inflammation and Oxidative Stress in Acute Experimental Colitis. Antioxidants, 12(5), 1062. https://doi.org/10.3390/antiox12051062