The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health
Abstract
1. Introduction
2. Characteristics of Hydrogen Peroxide as a Chemical Compound and Oxidizing Agent
3. Bacterial Hydrogen Peroxide Production
3.1. Hydrogen Peroxide Production in the Vaginal Microbiota
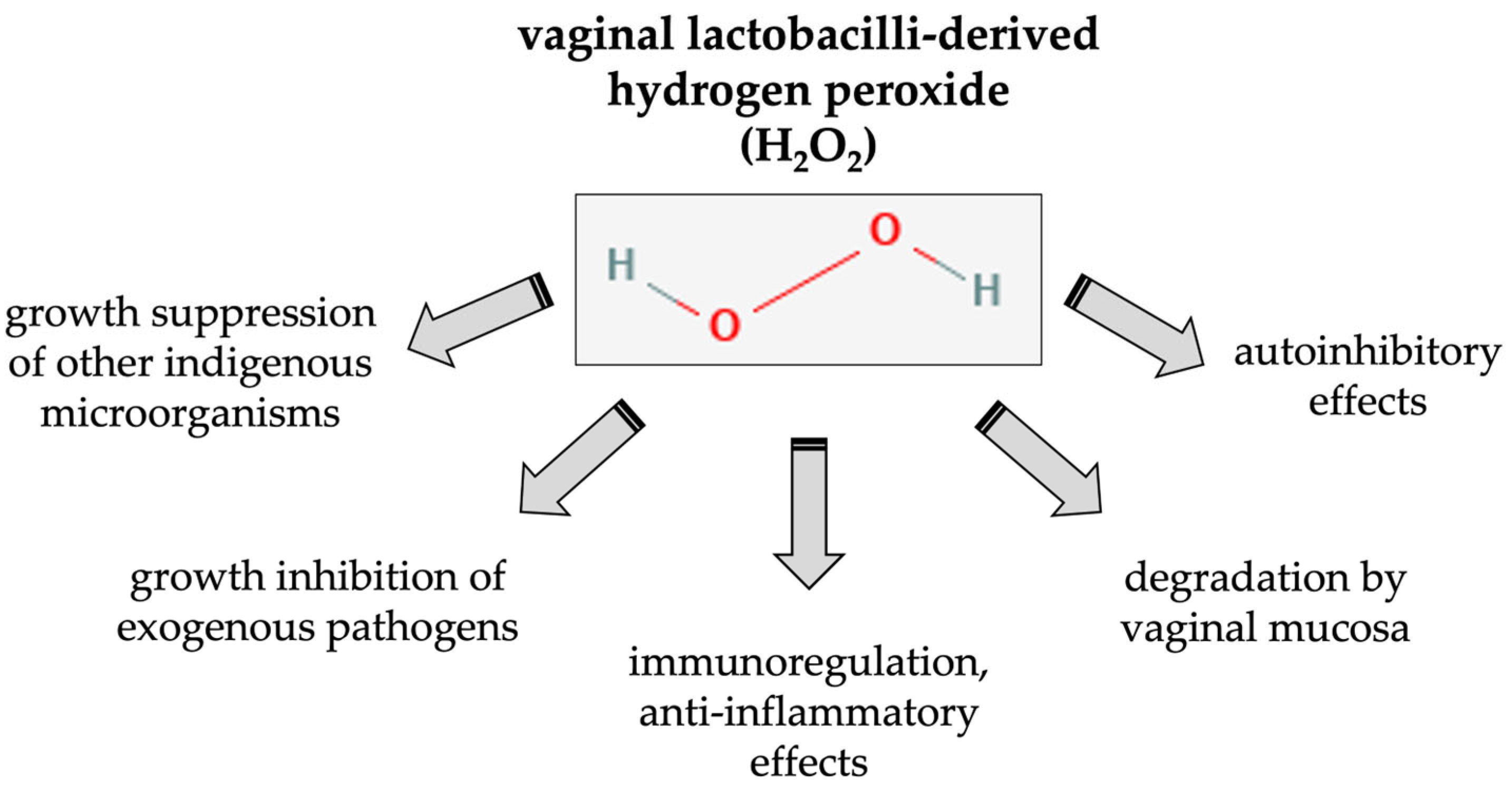
3.2. Effects of Lactobacillus-Derived H2O2 on the Host
4. The Possible Contribution of Lactobacillus-Derived Hydrogen Peroxide to Vaginal Health: Pros and Cons
4.1. Antimicrobial Effects of H2O2: Epidemiological Studies on Bacterial Vaginosis
4.2. Antimicrobial Effects of H2O2: Epidemiological Studies on Vulvovaginal Candidiasis (VVC)
4.3. Antimicrobial Effects of H2O2: Epidemiological Studies on STI Pathogens
4.4. Antimicrobial Effects of H2O2: Experimental Studies
4.5. Impact of H2O2-Producing Lactobacilli on Fertility and Pregnancy Outcome
5. H2O2-Producing Lactobacilli as Therapeutic Agents in Vaginal Probiotics
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.K.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal Microbiome of Reproductive-Age Women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a Deeper Understanding of the Vaginal Microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal Microbiome: Rethinking Health and Disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef]
- Antonio, M.A.; Hawes, S.E.; Hillier, S.L. The Identification of Vaginal Lactobacillus Species and the Demographic and Microbiologic Characteristics of Women Colonized by These Species. J. Infect. Dis. 1999, 180, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Bent, S.J.; Schneider, M.G.; Davis, C.C.; Islam, M.R.; Forney, L.J. Characterization of Vaginal Microbial Communities in Adult Healthy Women Using Cultivation-Independent Methods. Microbiology 2004, 150, 2565–2573. [Google Scholar] [CrossRef]
- Galask, R.P.; Larsen, B.; Ohm, M.J. Vaginal Flora and Its Role in Disease Entities. Clin. Obstet. Gynecol. 1976, 19, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Freitas, A.C.; Chaban, B.; Bocking, A.; Rocco, M.; Yang, S.; Hill, J.E.; Money, D.M.; Hemmingsen, S.; Reid, G.; Dumonceaux, T.; et al. The Vaginal Microbiome of Pregnant Women Is Less Rich and Diverse, with Lower Prevalence of Mollicutes, Compared to Non-Pregnant Women. Sci. Rep. 2017, 7, 9212. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, M.P.; Goyal, K. Diversity of Vaginal Microbiome in Pregnancy: Deciphering the Obscurity. Front. Public Health 2020, 8, 326. [Google Scholar] [CrossRef]
- Aagaard, K.; Riehle, K.; Ma, J.; Segata, N.; Mistretta, T.A.; Coarfa, C.; Raza, S.; Rosenbaum, S.; van den Veyver, I.; Milosavljevic, A.; et al. A Metagenomic Approach to Characterization of the Vaginal Microbiome Signature in Pregnancy. PLoS ONE 2012, 7, e36466. [Google Scholar] [CrossRef]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The Composition and Stability of the Vaginal Microbiota of Normal Pregnant Women Is Different from That of Non-Pregnant Women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef]
- Jakobsson, T.; Forsum, U. Lactobacillus Iners: A Marker of Changes in the Vaginal Flora? J. Clin. Microbiol. 2007, 45, 3145. [Google Scholar] [CrossRef] [PubMed]
- Verstraelen, H.; Verhelst, R.; Claeys, G.; de Backer, E.; Temmerman, M.; Vaneechoutte, M. Longitudinal Analysis of the Vaginal Microflora in Pregnancy Suggests That L. Crispatus Promotes the Stability of the Normal Vaginal Microflora and That L. Gasseri and/or L. Iners Are More Conducive to the Occurrence of Abnormal Vaginal Microflora. BMC Microbiol. 2009, 9, 116. [Google Scholar] [CrossRef]
- Klebanoff, S.J.; Hillier, S.L.; Eschenbach, D.A.; Waltersdorph, M. Control of the Microbial Flora of the Vagina by H202-Generating Lactobacilli. J. Infect. Dis. 1991, 164, 94–100. [Google Scholar] [CrossRef]
- Eschenbach, D.A.; Davick, P.R.; Williams, B.L.; Klebanoff, S.J.; Young-Smith, K.; Critchlow, C.M.; Holmes, K.K. Prevalence of Hydrogen Peroxide-Producing Lactobacillus Species in Normal Women and Women with Bacterial Vaginosis. J. Clin. Microbiol. 1989, 27, 251–256. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen Peroxide in the Human Body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Castagna, R.; Eiserich, J.P.; Budamagunta, M.S.; Stipa, P.; Cross, C.E.; Proietti, E.; Voss, J.C.; Greci, L. Hydroxyl Radical from the Reaction between Hypochlorite and Hydrogen Peroxide. Atmos. Environ. 2008, 42, 6551–6554. [Google Scholar] [CrossRef]
- Mahaseth, T.; Kuzminov, A. Potentiation of Hydrogen Peroxide Toxicity: From Catalase Inhibition to Stable DNA-Iron Complexes. Mutat. Res. Rev. Mutat. Res. 2017, 773, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. The Antioxidants of Human Extracellular Fluids. Arch. Biochem. Biophys. 1990, 280, 1–8. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Role of Free Radicals and Catalytic Metal Ions in Human Disease: An Overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and Foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Wang, X.; Martindale, J.L.; Liu, Y.; Holbrook, N.J. The Cellular Response to Oxidative Stress: Influences of Mitogen-Activated Protein Kinase Signalling Pathways on Cell Survival. Biochem. J. 1998, 333 Pt 2, 291–300. [Google Scholar] [CrossRef]
- Griendling, K.K.; Harrison, D.G. Dual Role of Reactive Oxygen Species in Vascular Growth. Circ. Res. 1999, 85, 562–563. [Google Scholar] [CrossRef]
- Abe, J.I.; Berk, B.C. Fyn and JAK2 Mediate Ras Activation by Reactive Oxygen Species. J. Biol. Chem. 1999, 274, 21003–21010. [Google Scholar] [CrossRef]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of Gene Expression by Reactive Oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- D’Autréaux, B.; Toledano, M.B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R.H.; Gill, V.; Alliangana, D. Hydrogen Peroxide in Relation to Proliferation and Apoptosis in BHK-21 Hamster Fibroblasts. Free Radic. Res. 1996, 24, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Parola, M. Oxidative Damage and Fibrogenesis. Free Radic. Biol. Med. 1997, 22, 287–305. [Google Scholar] [CrossRef]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive Oxygen Intermediates as Apparently Widely Used Messengers in the Activation of the NF-Kappa B Transcription Factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Hertzberger, R.; Arents, J.; Dekker, H.L.; Pridmore, R.D.; Gysler, C.; Kleerebezem, M.; de Mattos, M.J.T. H(2)O(2) Production in Species of the Lactobacillus Acidophilus Group: A Central Role for a Novel NADH-Dependent Flavin Reductase. Appl. Environ. Microbiol. 2014, 80, 2229–2239. [Google Scholar] [CrossRef]
- Condon, S. Responses of Lactic Acid Bacteria to Oxygen. FEMS Microbiol. Lett. 1987, 46, 269–280. [Google Scholar] [CrossRef]
- Kawasaki, S.; Satoh, T.; Todoroki, M.; Niimura, Y. B-Type Dihydroorotate Dehydrogenase Is Purified as a H2O2-Forming NADH Oxidase from Bifidobacterium Bifidum. Appl. Environ. Microbiol. 2009, 75, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Marty-Teysset, C.; de la Torre, F.; Garel, J. Increased Production of Hydrogen Peroxide by Lactobacillus Delbrueckii Subsp. Bulgaricus upon Aeration: Involvement of an NADH Oxidase in Oxidative Stress. Appl. Environ. Microbiol. 2000, 66, 262–267. [Google Scholar] [CrossRef]
- Martín, R.; Soberón, N.; Vaneechoutte, M.; Flórez, A.B.; Vázquez, F.; Suárez, J.E. Characterization of Indigenous Vaginal Lactobacilli from Healthy Women as Probiotic Candidates. Int. Microbiol. 2008, 11, 261–266. [Google Scholar] [CrossRef]
- Hillier, S.L.; Krohn, M.E.; Klebanoff, S.J.; Eschenbach, D.A. The Relationship of Hydrogen Peroxide-Producing Lactobacilli to Bacterial Vaginosis and Genital Microflora in Pregnant Women. Obstet. Gynecol. 1992, 79, 369–373. [Google Scholar] [CrossRef]
- Kaewsrichan, J.; Peeyananjarassri, K.; Kongprasertkit, J. Selection and Identification of Anaerobic Lactobacilli Producing Inhibitory Compounds against Vaginal Pathogens. FEMS Immunol. Med. Microbiol. 2006, 48, 75–83. [Google Scholar] [CrossRef]
- Strus, M.; Brzychczy-Włoch, M.; Gosiewski, T.; Kochan, P.; Heczko, P.B. The in Vitro Effect of Hydrogen Peroxide on Vaginal Microbial Communities. FEMS Immunol. Med. Microbiol. 2006, 48, 56–63. [Google Scholar] [CrossRef]
- Strus, M.; Kucharska, A.; Kukla, G.; Brzychczy-Włoch, M.; Maresz, K.; Heczko, P.B. The in Vitro Activity of Vaginal Lactobacillus with Probiotic Properties against Candida. Infect. Dis. Obstet. Gynecol. 2005, 13, 69–75. [Google Scholar] [CrossRef]
- Rashad, A.L.; Toffler, W.L.; Wolf, N.; Thornburg, K.; Kirk, E.P.; Ellis, G.; Whitehead, W.E. Vaginal PO2 in Healthy Women and in Women Infected with Trichomonas Vaginalis: Potential Implications for Metronidazole Therapy. Am. J. Obstet. Gynecol. 1992, 166, 620–624. [Google Scholar] [CrossRef] [PubMed]
- St Amant, D.C.; Valentin-Bon, I.E.; Jerse, A.E. Inhibition of Neisseria Gonorrhoeae by Lactobacillus Species That Are Commonly Isolated from the Female Genital Tract. Infect. Immun. 2002, 70, 7169–7171. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia Trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. Vaginal PH and Microbicidal Lactic Acid When Lactobacilli Dominate the Microbiota. PLoS ONE 2013, 8, e80074. [Google Scholar] [CrossRef]
- Cadieux, P.A.; Burton, J.P.; Devillard, E.; Reid, G. Lactobacillus By-Products Inhibit the Growth and Virulence of Uropathogenic Escherichia coli. J. Physiol. Pharmacol. 2009, 60 (Suppl. S6), 13–18. [Google Scholar] [PubMed]
- Atassi, F.; Servin, A.L. Individual and Co-Operative Roles of Lactic Acid and Hydrogen Peroxide in the Killing Activity of Enteric Strain Lactobacillus Johnsonii NCC933 and Vaginal Strain Lactobacillus Gasseri KS120.1 against Enteric, Uropathogenic and Vaginosis-Associated Pathogens. FEMS Microbiol. Lett. 2010, 304, 29–38. [Google Scholar] [CrossRef]
- Hickey, D.K.; Patel, M.V.; Fahey, J.V.; Wira, C.R. Innate and Adaptive Immunity at Mucosal Surfaces of the Female Reproductive Tract: Stratification and Integration of Immune Protection against the Transmission of Sexually Transmitted Infections. J. Reprod. Immunol. 2011, 88, 185–194. [Google Scholar] [CrossRef]
- Reis Machado, J.; da Silva, M.V.; Cavellani, C.L.; Antônia Dos Reis, M.; Monteiro, M.L.G.D.R.; Teixeira, V.D.P.A.; Rosa Miranda Corrêa, R. Mucosal Immunity in the Female Genital Tract, HIV/AIDS. Biomed. Res. Int. 2014, 2014, 350195. [Google Scholar] [CrossRef]
- Adapen, C.; Réot, L.; Menu, E. Role of the Human Vaginal Microbiota in the Regulation of Inflammation and Sexually Transmitted Infection Acquisition: Contribution of the Non-Human Primate Model to a Better Understanding? Front. Reprod. Health 2022, 4, 992176. [Google Scholar] [CrossRef]
- Strbo, N.; Alcaide, M.L.; Romero, L.; Bolivar, H.; Jones, D.; Podack, E.R.; Fischl, M.A. Loss of Intra-Epithelial Endocervical Gamma Delta (GD) 1 T Cells in HIV-Infected Women. Am. J. Reprod. Immunol. 2016, 75, 134–145. [Google Scholar] [CrossRef]
- Gibbs, A.; Leeansyah, E.; Introini, A.; Paquin-Proulx, D.; Hasselrot, K.; Andersson, E.; Broliden, K.; Sandberg, J.K.; Tjernlund, A. MAIT Cells Reside in the Female Genital Mucosa and Are Biased towards IL-17 and IL-22 Production in Response to Bacterial Stimulation. Mucosal Immunol. 2017, 10, 35–45. [Google Scholar] [CrossRef]
- Benjelloun, F.; Quillay, H.; Cannou, C.; Marlin, R.; Madec, Y.; Fernandez, H.; Chrétien, F.; le Grand, R.; Barré-Sinoussi, F.; Nugeyre, M.T.; et al. Activation of Toll-Like Receptors Differentially Modulates Inflammation in the Human Reproductive Tract: Preliminary Findings. Front. Immunol. 2020, 11, 1655. [Google Scholar] [CrossRef]
- Trifonova, R.T.; Lieberman, J.; van Baarle, D. Distribution of Immune Cells in the Human Cervix and Implications for HIV Transmission. Am. J. Reprod. Immunol. 2014, 71, 252–264. [Google Scholar] [CrossRef]
- Sullivan, D.A.; Richardson, G.S.; MacLaughlin, D.T.; Wira, C.R. Variations in the Levels of Secretory Component in Human Uterine Fluid during the Menstrual Cycle. J. Steroid Biochem. 1984, 20, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The Role of Sex Hormones in Immune Protection of the Female Reproductive Tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Fredricks, D.; Agnew, K.; Hitti, J. Hydrogen Peroxide-Producing Lactobacilli Are Associated with Lower Levels of Vaginal Interleukin-1β, Independent of Bacterial Vaginosis. Sex. Transm. Dis. 2015, 42, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Erttmann, S.F.; Gekara, N.O. Hydrogen Peroxide Release by Bacteria Suppresses Inflammasome-Dependent Innate Immunity. Nat. Commun. 2019, 10, 3493. [Google Scholar] [CrossRef]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen Peroxide Sensing and Signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Choi, S.E.; Min, S.H.; Shin, H.C.; Kim, H.E.; Jung, M.W.; Kang, Y. Involvement of Calcium-Mediated Apoptotic Signals in H2O2-Induced MIN6N8a Cell Death. Eur. J. Pharmacol. 2006, 547, 1–9. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shaded, E.; Levine, M. Pharmacologic Ascorbic Acid Concentrations Selectively Kill Cancer Cells: Action as a pro-Drug to Deliver Hydrogen Peroxide to Tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Sablina, A.A.; Budanov, A.V.; Ilyinskaya, G.V.; Agapova, L.S.; Kravchenko, J.E.; Chumakov, P.M. The Antioxidant Function of the P53 Tumor Suppressor. Nat. Med. 2005, 11, 1306–1313. [Google Scholar] [CrossRef]
- Voltan, S.; Martines, D.; Elli, M.; Brun, P.; Longo, S.; Porzionato, A.; Macchi, V.; D’Incà, R.; Scarpa, M.; Palù, G.; et al. Lactobacillus Crispatus M247-Derived H2O2 Acts as a Signal Transducing Molecule Activating Peroxisome Proliferator Activated Receptor-Gamma in the Intestinal Mucosa. Gastroenterology 2008, 135, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Pybus, V.; Onderdonk, A.B. Microbial Interactions in the Vaginal Ecosystem, with Emphasis on the Pathogenesis of Bacterial Vaginosis. Microbes Infect. 1999, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Fredricsson, B.; Englund, K.; Weintraub, L.; Ölund, A.; Nord, C.E. Ecological Treatment of Bacterial Vaginosis. Lancet 1987, 1, 276. [Google Scholar] [CrossRef]
- Hillier, S.L.; Nugent, R.P.; Eschenbach, D.A.; Krohn, M.A.; Gibbs, R.S.; Martin, D.H.; Cotch, M.F.; Edelman, R.; Pastorek, J.G.; Rao, A.V.; et al. Association between Bacterial Vaginosis and Preterm Delivery of a Low-Birth-Weight Infant. N. Engl. J. Med. 1995, 333, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.E.; Lamont, R.F.; Taylor-Robinson, D.; Morgan, D.J.; Ison, C.; Pearson, J. Abnormal Bacterial Colonisation of the Genital Tract and Subsequent Preterm Delivery and Late Miscarriage. BMJ 1994, 308, 295. [Google Scholar] [CrossRef]
- Armstrong, E.; Kaul, R. Beyond bacterial vaginosis: Vaginal lactobacilli and HIV risk. Microbiome 2021, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Hillier, S.L. Diagnostic Microbiology of Bacterial Vaginosis. Am. J. Obstet. Gynecol. 1993, 169, 455–459. [Google Scholar] [CrossRef]
- Hillier, S.L.; Krohn, M.A.; Rabe, L.K.; Klebanoff, S.J.; Eschenbach, D.A. The Normal Vaginal Flora, H2O2-Producing Lactobacilli, and Bacterial Vaginosis in Pregnant Women. Clin. Infect. Dis. 1993, 16, S273–S281. [Google Scholar] [CrossRef]
- Hawes, S.E.; Hillier, S.L.; Benedetti, J.; Stevens, C.E.; Koutsky, L.A.; Wølner-Hanssen, P.; Holmes, K.K. Hydrogen Peroxide-Producing Lactobacilli and Acquisition of Vaginal Infections. J. Infect. Dis. 1996, 174, 1058–1063. [Google Scholar] [CrossRef]
- Vallor, A.C.; Antonio, M.A.D.; Hawes, S.E.; Hillier, S.L. Factors Associated with Acquisition of, or Persistent Colonization by, Vaginal Lactobacilli: Role of Hydrogen Peroxide Production. J. Infect. Dis. 2001, 184, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Antonio, M.A.D.; Rabe, L.K.; Hillier, S.L. Colonization of the Rectum by Lactobacillus Species and Decreased Risk of Bacterial Vaginosis. J. Infect. Dis. 2005, 192, 394–398. [Google Scholar] [CrossRef]
- Baeten, J.M.; Hassan, W.M.; Chohan, V.; Richardson, B.A.; Mandaliya, K.; Ndinya-Achola, J.O.; Jaoko, W.; McClelland, R.S. Prospective Study of Correlates of Vaginal Lactobacillus Colonisation among High-Risk HIV-1 Seronegative Women. Sex. Transm. Infect. 2009, 85, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Al-Mushrif, S.; Jones, B.M. A Study of the Prevalence of Hydrogen Peroxide Generating Lactobacilli in Bacterial Vaginosis: The Determination of H2O2 Concentrations Generated, in Vitro, by Isolated Strains and the Levels Found in Vaginal Secretions of Women with and without Infection. J. Obstet. Gynaecol. 1998, 18, 63–67. [Google Scholar] [CrossRef]
- Rosenstein, U.; Fontaine, E.A.; Morgan, D.J.; Sheehan, M.; Lament, R.F.; Taylor-Robinson, D. Relationship between Hydrogen Peroxide-Producing Strains of Lactobacilli and Vaginosis-Associated Bacterial Species in Pregnant Women. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 517–522. [Google Scholar] [CrossRef]
- Fontaine, E.A.; Claydon, E.; Taylor-Robinson, D.; Fontainet, E.A.; Claydons, E.; Taylor-Robinsonts, D. Lactobacilli from Women with or without Bacterial Vaginosis and Observations on the Significance of Hydrogen Peroxide. Microb. Ecol. Health Dis. 2009, 9, 135–141. [Google Scholar] [CrossRef]
- Beigi, R.H.; Wiesenfeld, H.C.; Hillier, S.L.; Straw, T.; Krohn, M.A. Factors Associated with Absence of H2O2-Producing Lactobacillus among Women with Bacterial Vaginosis. J. Infect. Dis. 2005, 191, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Jeanmonod, R.; Jeanmonod, D. Vaginal Candidiasis; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sobel, J.D.; Chaim, W. Vaginal Microbiology of Women with Acute Recurrent Vulvovaginal Candidiasis. J. Clin. Microbiol. 1996, 34, 2497–2499. [Google Scholar] [CrossRef]
- Mijač, V.D.; Dukić, S.V.; Opavski, N.Z.; Dukić, M.K.; Ranin, L.T. Hydrogen Peroxide Producing Lactobacilli in Women with Vaginal Infections. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 129, 69–76. [Google Scholar] [CrossRef]
- Knežević, A.; Stepanović, S.; Ćupić, M.; Jevtović, D.; Ranin, J.; Jovanović, T. Reduced Quantity and Hydrogen-Peroxide Production of Vaginal Lactobacilli in HIV Positive Women. Biomed. Pharmacother. 2005, 59, 521–523. [Google Scholar] [CrossRef]
- Balkus, J.E.; Mitchell, C.; Agnew, K.; Liu, C.; Fiedler, T.; Cohn, S.E.; Luque, A.; Coombs, R.; Fredricks, D.N.; Hitti, J. Detection of Hydrogen Peroxide-Producing Lactobacillus Species in the Vagina: A Comparison of Culture and Quantitative PCR among HIV-1 Seropositive Women. BMC Infect. Dis. 2012, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.L.; Richardson, B.A.; Nyange, P.M.; Lavreys, L.; Hillier, S.L.; Chohan, B.; Mandaliya, K.; Ndinya-Achola, J.O.; Bwayo, J.; Kreiss, J. Vaginal Lactobacilli, Microbial Flora, and Risk of Human Immunodeficiency Virus Type 1 and Sexually Transmitted Disease Acquisition. J. Infect. Dis. 1999, 180, 1863–1868. [Google Scholar] [CrossRef]
- Petrin, D.; Delgaty, K.; Bhatt, R.; Garber, G. Clinical and Microbiological Aspects of Trichomonas Vaginalis. Clin. Microbiol. Rev. 1998, 11, 300–317. [Google Scholar] [CrossRef]
- Lovett, A.; Seña, A.C.; Macintyre, A.N.; Sempowski, G.D.; Duncan, J.A.; Waltmann, A. Cervicovaginal Microbiota Predicts Neisseria Gonorrhoeae Clinical Presentation. Front. Microbiol. 2022, 12, 4171. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Petterson, M.; MÅRdh, P.-A. Antibiosis between Bacteria Isolated from the Vagina of Women with and without Signs of Bacterial Vaginosis. Apmis 1991, 99, 739–744. [Google Scholar] [CrossRef] [PubMed]
- McLean, N.W.; McGroarty, J.A. Growth Inhibition of Metronidazole-Susceptible and Metronidazole-Resistant Strains of Gardnerella Vaginalis by Lactobacilli in Vitro. Appl. Environ. Microbiol. 1996, 62, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.Y.; Alcorn, T.M.; Cohen, M.S. Effects of H2O2-Producing Lactobacilli on Neisseria Gonorrhoeae Growth and Catalase Activity. J. Infect. Dis. 1994, 170, 1209–1215. [Google Scholar] [CrossRef]
- Conti, C.; Malacrino, C.; Mastromarino, P. Inhibition of Herpes Simplex Virus Type 2 by Vaginal Lactobacilli. J. Physiol. Pharmacol. 2009, 60 (Suppl. S6), 19–26. [Google Scholar]
- Klebanoff, S.J.; Coombs, R.W. Viricidal Effect of Lactobacillus Acidophilus on Human Immunodeficiency Virus Type 1: Possible Role in Heterosexual Transmission. J. Exp. Med. 1991, 174, 289–292. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Lanier, B.R.; Moench, T.R.; Cone, R.A. Cervicovaginal Fluid and Semen Block the Microbicidal Activity of Hydrogen Peroxide Produced by Vaginal Lactobacilli. BMC Infect. Dis. 2010, 10, 120. [Google Scholar] [CrossRef]
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In Vaginal Fluid, Bacteria Associated with Bacterial Vaginosis Can Be Suppressed with Lactic Acid but Not Hydrogen Peroxide. BMC Infect. Dis. 2011, 11, 200. [Google Scholar] [CrossRef]
- Xu, H.Y.; Tian, W.H.; Wan, C.X.; Jia, L.J.; Wang, L.Y.; Yuan, J.; Liu, C.M.; Zeng, M.; Wei, H. Antagonistic Potential against Pathogenic Microorganisms and Hydrogen Peroxide Production of Indigenous Lactobacilli Isolated from Vagina of Chinese Pregnant Women. Biomed. Environ. Sci. 2008, 21, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Bracewell-Milnes, T.; Saso, S.; Nikolaou, D.; Norman-Taylor, J.; Johnson, M.; Thum, M.Y. Investigating the Effect of an Abnormal Cervico-Vaginal and Endometrial Microbiome on Assisted Reproductive Technologies: A Systematic Review. Am. J. Reprod. Immunol. 2018, 80, e13037. [Google Scholar] [CrossRef]
- Singer, M.; Borg, M.; Ouburg, S.; Morré, S.A. The Relation of the Vaginal Microbiota to Early Pregnancy Development during in Vitro Fertilization Treatment-A Meta-Analysis. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 223–229. [Google Scholar] [CrossRef]
- Hyman, R.W.; Herndon, C.N.; Jiang, H.; Palm, C.; Fukushima, M.; Bernstein, D.; Vo, K.C.; Zelenko, Z.; Davis, R.W.; Giudice, L.C. The Dynamics of the Vaginal Microbiome during Infertility Therapy with in Vitro Fertilization-Embryo Transfer. J. Assist. Reprod. Genet. 2012, 29, 105–115. [Google Scholar] [CrossRef]
- Bernabeu, A.; Lledo, B.; Díaz, M.C.; Lozano, F.M.; Ruiz, V.; Fuentes, A.; Lopez-Pineda, A.; Moliner, B.; Castillo, J.C.; Ortiz, J.A.; et al. Effect of the Vaginal Microbiome on the Pregnancy Rate in Women Receiving Assisted Reproductive Treatment. J. Assist. Reprod. Genet. 2019, 36, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Haahr, T.; Jensen, J.S.; Thomsen, L.; Duus, L.; Rygaard, K.; Humaidan, P. Abnormal Vaginal Microbiota May Be Associated with Poor Reproductive Outcomes: A Prospective Study in IVF Patients. Hum. Reprod. 2016, 31, 795–803. [Google Scholar] [CrossRef]
- Hütt, P.; Lapp, E.; Štšepetova, J.; Smidt, I.; Taelma, H.; Borovkova, N.; Oopkaup, H.; Ahelik, A.; Rööp, T.; Hoidmets, D.; et al. Characterisation of Probiotic Properties in Human Vaginal Lactobacilli Strains. Microb. Ecol. Health Dis. 2016, 27, 30484. [Google Scholar] [CrossRef] [PubMed]
- Martius, J.; Krohn, M.A.; Hillier, S.L.; Stamm, W.E.; Holmes, K.K.; Eschenbach, D.A. Relationships of Vaginal Lactobacillus Species, Cervical Chlamydia Trachomatis, and Bacterial Vaginosis to Preterm Birth. Obstet. Gynecol. 1988, 71, 89–95. [Google Scholar] [PubMed]
- Wilks, M.; Wiggins, R.; Whiley, A.; Hennessy, E.; Warwick, S.; Porter, H.; Corfield, A.; Millar, M. Identification and H2O2 Production of Vaginal Lactobacilli from Pregnant Women at High Risk of Preterm Birth and Relation with Outcome. J. Clin. Microbiol. 2004, 42, 713. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, A.; Aguilera, M. Vaginal Probiotics for Reproductive Health and Related Dysbiosis: Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.I.; Lievens, E.; Malik, S.; Imholz, N.; Lebeer, S. Lactobacillus Species as Biomarkers and Agents That Can Promote Various Aspects of Vaginal Health. Front. Physiol. 2015, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Cardone, A.; Zarcone, R.; Borrelli, A.; Di Cunzolo, A.; Russo, A.; Tartaglia, E. Utilisation of Hydrogen Peroxide in the Treatment of Recurrent Bacterial Vaginosis. Minerva Ginecol. 2003, 55, 483–492. [Google Scholar] [PubMed]
- Chaithongwongwatthana, S.; Limpongsanurak, S.; Sitthi-Amorn, C. Single Hydrogen Peroxide Vaginal Douching versus Single-Dose Oral Metronidazole for the Treatment of Bacterial Vaginosis: A Randomized Controlled Trial. J. Med. Assoc. Thail. 2003, 86 (Suppl. S2), S379–S384. [Google Scholar]
- Ocaña, V.S.; Pesce de Ruiz Holgado, A.A.; Nader-Macías, M.E. Selection of Vaginal H2O2-Generating Lactobacillus Species for Probiotic Use. Curr. Microbiol. 1999, 38, 279–284. [Google Scholar] [CrossRef]
- McLEAN, N.W.; Rosenstein, I.J. Characterisation and Selection of a Lactobacillus Species to Re-Colonise the Vagina of Women with Recurrent Bacterial Vaginosis. J. Med. Microbiol. 2000, 49, 543–552. [Google Scholar] [CrossRef]
- Martinez, R.C.R.; Franceschini, S.A.; Patta, M.C.; Quintana, S.M.; Candido, R.C.; Ferreira, J.C.; De Martinis, E.C.P.; Reid, G. Improved Treatment of Vulvovaginal Candidiasis with Fluconazole plus Probiotic Lactobacillus Rhamnosus GR-1 and Lactobacillus Reuteri RC-14. Lett. Appl. Microbiol. 2009, 48, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Brigidi, P.; Macchia, S.; Maggi, L.; Pirovano, F.; Trinchieri, V.; Conte, U.; Matteuzzi, D. Characterization and Selection of Vaginal Lactobacillus Strains for the Preparation of Vaginal Tablets. J. Appl. Microbiol. 2002, 93, 884–893. [Google Scholar] [CrossRef]
- Antonio, M.A.D.; Meyn, L.A.; Murray, P.J.; Busse, B.; Hillier, S.L. Vaginal Colonization by Probiotic Lactobacillus Crispatus CTV-05 Is Decreased by Sexual Activity and Endogenous Lactobacilli. J. Infect. Dis. 2009, 199, 1506–1513. [Google Scholar] [CrossRef]
- Hallén, A.; Jarstrand, C.; Påhlson, C. Treatment of Bacterial Vaginosis with Lactobacilli. Sex. Transm. Dis. 1992, 19, 146–148. [Google Scholar] [CrossRef]
- Parent, D.; Bossens, M.; Bayot, D.; Kirkpatrick, C.; Graf, F.; Wilkinson, F.E.; Kaiser, R.R. Therapy of bacterial vaginosis using exogenously-applied Lactobacilli acidophili and a low dose of estriol: A placebo-controlled multicentric clinical trial. Arzneim.-Forsch. 1996, 46, 68–73. [Google Scholar]
- Bradshaw, C.S.; Pirotta, M.; de Guingand, D.; Hocking, J.S.; Morton, A.N.; Garland, S.M.; Fehler, G.; Morrow, A.; Walker, S.; Vodstrcil, L.A.; et al. Efficacy of Oral Metronidazole with Vaginal Clindamycin or Vaginal Probiotic for Bacterial Vaginosis: Randomised Placebo-Controlled Double-Blind Trial. PLoS ONE 2012, 7, e34540. [Google Scholar] [CrossRef]
- Bohbot, J.M.; Daraï, E.; Bretelle, F.; Brami, G.; Daniel, C.; Cardot, J.M. Efficacy and Safety of Vaginally Administered Lyophilized Lactobacillus Crispatus IP 174178 in the Prevention of Bacterial Vaginosis Recurrence. J. Gynecol. Obstet. Hum. Reprod. 2018, 47, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.; Carlsson, B.; Forsum, U.; Larsson, P.G. A Double-Blind Treatment Study of Bacterial Vaginosis with Normal Vaginal Lactobacilli after an Open Treatment with Vaginal Clindamycin Ovules. Acta Derm. Venereol. 2005, 85, 42–46. [Google Scholar] [CrossRef]
- Larsson, P.G.; Stray-Pedersen, B.; Ryttig, K.R.; Larsen, S. Human Lactobacilli as Supplementation of Clindamycin to Patients with Bacterial Vaginosis Reduce the Recurrence Rate; a 6-Month, Double-Blind, Randomized, Placebo-Controlled Study. BMC Womens Health 2008, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Cacciotti, F.; Masci, A.; Mosca, L. Antiviral Activity of Lactobacillus Brevis towards Herpes Simplex Virus Type 2: Role of Cell Wall Associated Components. Anaerobe 2011, 17, 334–336. [Google Scholar] [CrossRef]
- Reid, G.; Jass, J.; Sebulsky, M.T.; McCormick, J.K. Potential Uses of Probiotics in Clinical Practice. Clin. Microbiol. Rev. 2003, 16, 658–672. [Google Scholar] [CrossRef]
- Anukam, K.; Osazuwa, E.; Ahonkhai, I.; Ngwu, M.; Osemene, G.; Bruce, A.W.; Reid, G. Augmentation of Antimicrobial Metronidazole Therapy of Bacterial Vaginosis with Oral Probiotic Lactobacillus Rhamnosus GR-1 and Lactobacillus Reuteri RC-14: Randomized, Double-Blind, Placebo Controlled Trial. Microbes Infect. 2006, 8, 1450–1454. [Google Scholar] [CrossRef]
- Petricevic, L.; Witt, A. The Role of Lactobacillus Casei Rhamnosus Lcr35 in Restoring the Normal Vaginal Flora after Antibiotic Treatment of Bacterial Vaginosis. BJOG 2008, 115, 1369–1374. [Google Scholar] [CrossRef]
- Anukam, K.C.; Osazuwa, E.; Osemene, G.I.; Ehigiagbe, F.; Bruce, A.W.; Reid, G. Clinical Study Comparing Probiotic Lactobacillus GR-1 and RC-14 with Metronidazole Vaginal Gel to Treat Symptomatic Bacterial Vaginosis. Microbes Infect. 2006, 8, 2772–2776. [Google Scholar] [CrossRef]
- Marcone, V.; Rocca, G.; Lichtner, M.; Calzolari, E. Long-Term Vaginal Administration of Lactobacillus Rhamnosus as a Complementary Approach to Management of Bacterial Vaginosis. Int. J. Gynaecol. Obstet. 2010, 110, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, G.E.; Heinemann, C.; Bruce, A.W.; Beuerman, D.; Reid, G. Persistence of Lactobacillus Fermentum RC-14 and Lactobacillus Rhamnosus GR-1 but Not L. Rhamnosus GG in the Human Vagina as Demonstrated by Randomly Amplified Polymorphic DNA. Clin. Diagn. Lab. Immunol. 2002, 9, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, H.; Larsson, P.G.; Andersen, K.K.; Zuo, F.; Mikkelsen, L.S.; Brandsborg, E.; Gray, G.; Laher, F.; Otwombe, K. An Exploratory Pilot Study Evaluating the Supplementation of Standard Antibiotic Therapy with Probiotic Lactobacilli in South African Women with Bacterial Vaginosis. BMC Infect. Dis. 2019, 19, 824. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Seney, S.; McMillan, A.; Vongsa, R.; Koenig, D.; Wong, L.F.; Dvoracek, B.; Gloor, G.B.; Sumarah, M.; Ford, B.; et al. A Systems Biology Approach Investigating the Effect of Probiotics on the Vaginal Microbiome and Host Responses in a Double Blind, Placebo-Controlled Clinical Trial of Post-Menopausal Women. PLoS ONE 2014, 9, e104511. [Google Scholar] [CrossRef]
- Macklaim, J.M.; Clemente, J.C.; Knight, R.; Gloor, G.B.; Reid, G. Changes in Vaginal Microbiota Following Antimicrobial and Probiotic Therapy. Microb. Ecol. Health Dis. 2015, 26, 27799. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, E.F.M.; Bellen, G.; Claes, I.; Henkens, T.; Allonsius, C.N.; Wittouck, S.; van den Broek, M.F.L.; Wuyts, S.; Kiekens, F.; Donders, G.G.G.; et al. Impact of a Lactobacilli-Containing Gel on Vulvovaginal Candidosis and the Vaginal Microbiome. Sci. Rep. 2020, 10, 7976. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.; Bellen, G.; Oerlemans, E.; Claes, I.; Ruban, K.; Henkens, T.; Kiekens, F.; Lebeer, S. The Use of 3 Selected Lactobacillary Strains in Vaginal Probiotic Gel for the Treatment of Acute Candida Vaginitis: A Proof-of-Concept Study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1551–1558. [Google Scholar] [CrossRef]
- Kovachev, S.M.; Vatcheva-Dobrevska, R.S. Local Probiotic Therapy for Vaginal Candida Albicans Infections. Probiotics Antimicrob. Proteins 2015, 7, 38–44. [Google Scholar] [CrossRef]
- Zeng, X.; An, R.; Li, H.; Zhang, Y. Improved Treatment of Vulvovaginal Candidiasis with Clotrimazole plus Probiotic Lacidophilin Vaginal Capsules: A Prospective, Real-World Study. Medicine 2023, 102, e32664. [Google Scholar] [CrossRef] [PubMed]
- Nouraei, S.; Amir Ali Akbari, S.; Jorjani, M.; Alavi Majd, H.; Afrakhteh, M.; Ghafoorian, A.; Tafazzoli Harandi, H. Comparison between Fluconazole with Oral Protexin Combination and Fluconazole in the Treatment of Vulvovaginal Candidiasis. ISRN Obstet. Gynecol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- De Seta, F.; Parazzini, F.; De Leo, R.; Banco, R.; Maso, G.P.; De Santo, D.; Sartore, A.; Stabile, G.; Inglese, S.; Tonon, M.; et al. Lactobacillus Plantarum P17630 for Preventing Candida Vaginitis Recurrence: A Retrospective Comparative Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 182, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Corbett, G.A.; Crosby, D.A.; McAuliffe, F.M. Probiotic Therapy in Couples with Infertility: A Systematic Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, Y.; Bar-Hava, I.; Fisch, B.; Ashkenazi, J.; Voliovitch, I.; Borkowski, T.; Orvieto, R. Does Intravaginal Probiotic Supplementation Increase the Pregnancy Rate in IVF-Embryo Transfer Cycles? Reprod. BioMed. Online 2005, 11, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, I.E.; Saxtorph, M.H.; Englund, A.L.M.; Petersen, K.B.; Wissing, M.L.M.; Hviid, T.V.F.; Macklon, N. Probiotic Treatment with Specific Lactobacilli Does Not Improve an Unfavorable Vaginal Microbiota Prior to Fertility Treatment-A Randomized, Double-Blinded, Placebo-Controlled Trial. Front. Endocrinol. 2022, 13, 1057022. [Google Scholar] [CrossRef] [PubMed]
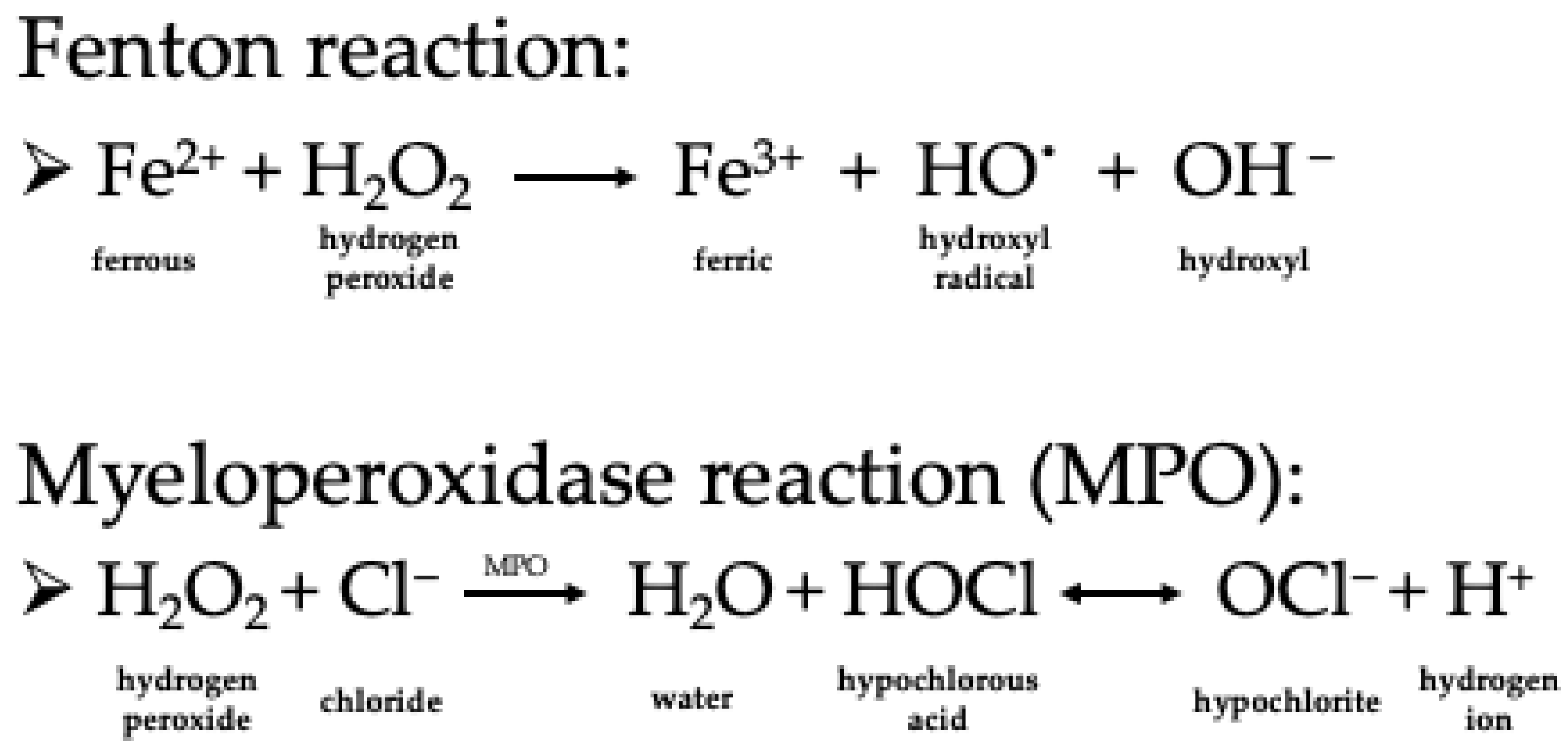
 |
|---|
| Lactic acid production |
|
| Hydrogene peroxide generation |
|
| Bacteriocin synthesis |
|
| Epithelial cell adhesion |
|
| S-layer protein expression |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miko, E.; Barakonyi, A. The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health. Antioxidants 2023, 12, 1055. https://doi.org/10.3390/antiox12051055
Miko E, Barakonyi A. The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health. Antioxidants. 2023; 12(5):1055. https://doi.org/10.3390/antiox12051055
Chicago/Turabian StyleMiko, Eva, and Aliz Barakonyi. 2023. "The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health" Antioxidants 12, no. 5: 1055. https://doi.org/10.3390/antiox12051055
APA StyleMiko, E., & Barakonyi, A. (2023). The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health. Antioxidants, 12(5), 1055. https://doi.org/10.3390/antiox12051055







