Dietary Vitamin E and/or Hydroxytyrosol Supplementation to Sows during Late Pregnancy and Lactation Modifies the Lipid Composition of Colostrum and Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals, Experimental Diets and Sample Collection
2.3. Laboratory Analysis
2.3.1. Lactose Concentrations of the Colostrum and Milk
2.3.2. Fat Quantifications of the Colostrum and Milk
2.3.3. Tocopherol and Retinol Quantifications of the Colostrum and Milk
2.3.4. Fatty Acid Compositions of the Colostrum and Milk
2.3.5. Oxidative Statuses of the Milk Samples, Sows and Piglets
2.4. Statistical Analysis
3. Results
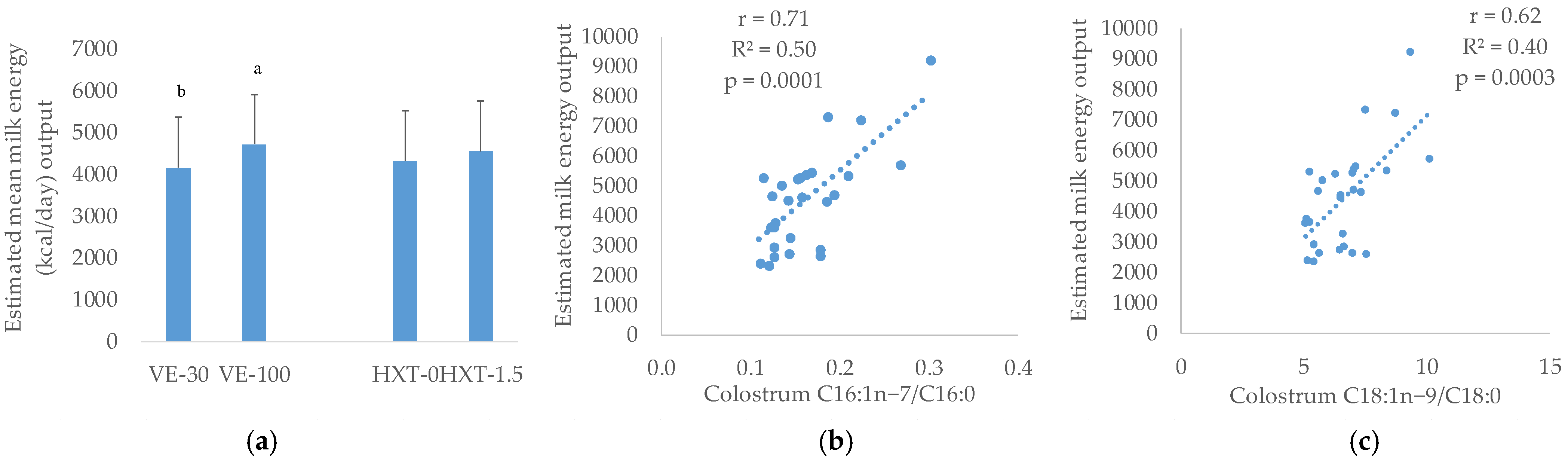
3.1. General Compositions of the Colostrum and Milk
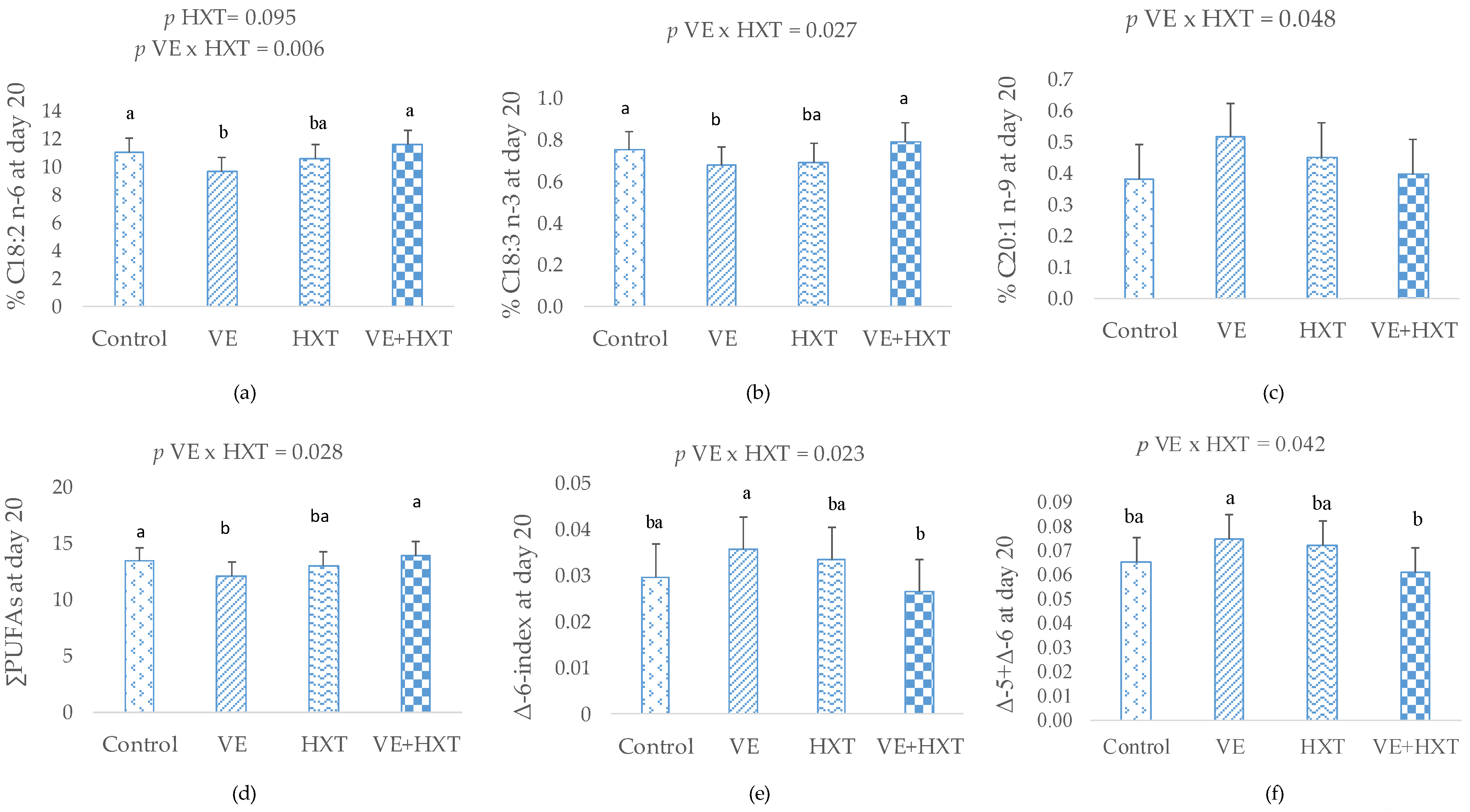
3.2. Fatty Acid Composition of Colostrum and Milk
3.3. Oxidative Stability of Milk and Oxidative Status of Sows and Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Major Nutrients 1 | Control | VE | HXT 2 | HXT+VE |
|---|---|---|---|---|
| Dry matter, % | 90.8 | 89.8 | 91.1 | 91.2 |
| Crude protein, % | 13.1 | 14.0 | 15.0 | 14.5 |
| Fat, % | 4.3 | 3.6 | 3.8 | 3.7 |
| Ash, % | 6.5 | 6.4 | 6.9 | 6.0 |
| Fiber, % | 4.3 | 4.4 | 4.3 | 4.4 |
| Starch,% | 49.0 | 46.1 | 41.1 | 43.0 |
| Energy (kcal GE/kg) | 4339.6 | 4251.7 | 4264.3 | 4313.3 |
| Vitamin E, mg/kg | 70.5 | 103.6 | 95.4 | 114.1 |
| Fatty Acid Composition | ||||
| C14:0 | 0.60 | 0.66 | 0.56 | 0.60 |
| C16:0 | 19.80 | 21.48 | 19.10 | 20.11 |
| C16:1n−9 | 0.12 | 0.15 | 0.12 | 0.12 |
| C16:1n−7 | 0.79 | 0.87 | 0.73 | 0.78 |
| C18:0 | 4.77 | 4.80 | 4.45 | 4.54 |
| C18:1n−9 | 28.97 | 25.26 | 30.33 | 26.94 |
| C18:1n−7 | 1.92 | 1.49 | 1.57 | 1.60 |
| C18:2n−6 | 38.97 | 40.81 | 38.97 | 40.89 |
| C18:3n−3 | 3.04 | 3.44 | 3.15 | 3.36 |
| C20:0 | 0.30 | 0.26 | 0.30 | 0.31 |
| C20:1n−9 | 0.52 | 0.58 | 0.55 | 0.58 |
| ∑SAT | 25.47 | 27.20 | 24.41 | 25.55 |
| ∑MUFAs | 32.33 | 28.35 | 33.29 | 30.02 |
| ∑PUFAs | 42.01 | 44.25 | 42.11 | 44.25 |
References
- Noblet, J.; Le Dividich, J. Energy metabolism of the newborn pig during the first 24 hrs of life. Biol. Neonate 1981, 40, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, structure, and digestive dynamics of milk from different species—A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef] [PubMed]
- Aguinaga, M.A.; Gomez-Carballar, F.; Nieto, R.; Aguilera, J.F. Production and composition of Iberian sow’s milk and use of milk nutrients by the suckling Iberian piglet. Animal 2011, 5, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Boudry, G.; Charton, E.; Le Huerou-Luron, I.; Ferret-Bernard, S.; Le Gall, S.; Even, S.; Blat, S. The relationship between breast milk components and the infant gut microbiota. Front. Nutr. 2021, 8, 629740. [Google Scholar] [CrossRef]
- Hurley, W.L. Composition of sow colostrum and milk. In The Gestating and Lactating Sow; Farmer, C., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Wang, L.; Xiaodong, X.; Ge, S.; Baoming, S.; Anshan, S. High concentration of vitamin E supplementation in sow diet during the last week of gestation and lactation affects the immunological variables and antioxidative parameters in piglets. J. Dairy Res. 2017, 84, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef]
- Lauridsen, C.; Engel, H.; Jensen, S.K.; Craig, A.M.; Traber, M.G. Lactating sows and suckling piglets preferentially incorporate RRR- over all-rac-α-tocopherol into milk, plasma and tissues. J. Nutr. 2002, 132, 1258–1264. [Google Scholar] [CrossRef]
- Amazan, D.; Rey, A.I.; Fernandez, E.; López-Bote, C.J. Natural vitamin E (d-α-tocopherol) supplementation in drinking water prevents oxidative stress in weaned piglets. Livest. Sci. 2002, 145, 55–62. [Google Scholar] [CrossRef]
- Amazan, D.; Cordero, G.; López-Bote, C.J.; Lauridsen, C.; Rey, A.I. Effects of oral micellized natural vitamin E (d-α-tocopherol) vs. synthetic vitamin E (dl-α-tocopherol) in feed on α-tocopherol levels, stereoisomer distribution, oxidative stress and the immune response in piglets. Animal 2014, 8, 410–419. [Google Scholar] [CrossRef]
- Rey, A.I.; Amazan, D.; Cordero, G.; Olivares, A.; López-Bote, C.J. Lower Oral Doses of Micellized α-Tocopherol Compared to α-Tocopheryl Acetate in Feed Modify Fatty Acid Profiles and Improve Oxidative Status in Pigs. Int. J. Vitam. Nutr. Res. 2014, 84, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, K.A.; Nalini, S.; Cheesman, K.H.; Slater, T.F. Non esterified fatty acids inhibit iron dependent lipid peroxidation. Biochim. Biophys. Acta 1989, 1003, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Power, G.W.; Cake, M.H.; Newsholme, E.A. Influence of diet on the kinetic behavior of hepatic carnitine palmitoyltransferase I toward different acyl CoA esters. Lipids 1997, 32, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Terramoccia, S.; Bartocci, S.; Taticchi, A.; Di Giovanni, S.; Pauselli, M.; Mourvaki, E.; Urbani, S.; Servili, M. Use of dried stoned olive pomace in the feeding of lactating buffaloes: Effect on the quantity and quality of the milk produced. Asian Australas. J. Anim. Sci. 2013, 26, 971–980. [Google Scholar] [CrossRef]
- Rey, A.I.; de-Cara, A.; Calvo, L.; Puig, P.; Hechavarría, T. Changes in Plasma Fatty Acids, Free Amino Acids, Antioxidant Defense, and Physiological Stress by Oleuropein Supplementation in Pigs Prior to Slaughter. Antioxidants 2020, 9, 56–74. [Google Scholar] [CrossRef]
- Hadrich, F.; Garcia, M.; Maalej, A.; Modes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Theil, P.K.; Hansen, A.V.; Sørensen, M. Effects of gestation and transition diets on colostrum intake and piglet survival. In Book of Abstracts of the 61st Annual Meeting of the European Association for Animal Production, Heraklion, Greece, 23–27 August 2010; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010; p. 14. [Google Scholar]
- Kim, S.W. Sow milk. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, F.W., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- BOE. RD 53/2013, de 21 de octubre por la que se establecen las normas básicas aplicables para la protección de los animales utilizados en experimentación y otros fines científicos, incluyendo la docencia, Spain. Boletín Of. Estado 2013, 252, 34367–34391. [Google Scholar]
- EC. Council Regulation (EC) No 2010/63/CE of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union. 2010, 276, 33–79. [Google Scholar]
- Nutrient Requirement of Swine, 11th ed.; National Research Council: Ottawa, ON, Canada; National Academy Press: Washington, DC, USA, 2012.
- Hansen, A.V.; Strathe, A.B.; Kebreab, E.; France, J.; Theil, P.K. Predicting milk yield and composition in lactating sows: A Bayesian approach. J. Anim. Sci. 2012, 90, 2285–2298. [Google Scholar] [CrossRef]
- Beutler, H.O. Lactose and D-Galactose. In Methods of Enzymatic Analysis, 3rd ed.; Bergmeyer, H.U., Ed.; VCH Publishers, Ltd.: Cambridge, UK, 1988; Volume VI, pp. 104–112. [Google Scholar]
- Fat in milk. In Methods of Test for Dairy Industry, 17th ed.; Official method 905.02; A.O.A.C.: Gaithersburg, MD, USA, 2000.
- Rey, A.I.; Daza, A.; López-Carrasco, C.; López-Bote, C.J. Quantitative study of the alpha- and gamma-tocopherols accumulation in muscle and backfat from Iberian pigs kept free-range as affected by time of free-range feeding or weight gain. Anim. Sci. 2006, 82, 901–908. [Google Scholar] [CrossRef]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty-acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Zhang, S.; Knight, T.J.; Stalder, K.J.; Goodwing, R.N.; Lonergan, S.M.; Beitz, D.C. Effects of breed, sex, and halothane genotype on fatty acid composition of pork longissimus muscle. J. Anim. Sci. 2007, 85, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Korniluk, K.; Gabryszuk, M.; Kowalczyk, I.; Czauderna, M. Effect of diet supplementation with selenium, zinc and α-tocopherol on fatty acid composition in the liver and loin muscle of lambs. Anim. Sci. Pap. Rep. 2008, 26, 59–70. [Google Scholar]
- Dal Bosco, A.; Mugnai, C.; Ruggeri, S.; Mattioli, S.; Castellini, C. Fatty acid composition of meat and estimated indices of lipid metabolism in different poultry genotypes reared under organic system. Poult. Sci. 2012, 91, 2039–2045. [Google Scholar] [CrossRef]
- Rey, A.I.; López-Bote, C.J.; Kerry, J.P.; Lynch, P.B.; Buckley, D.J.; Morrissey, P.A. Modification of lipid composition and oxidation in porcine muscle and muscle microsomes as affected by dietary supplementation of n−3 with either n−9 or n−6 fatty acids and α-tocopheryl acetate. Anim. Feed Sci. Technol. 2004, 113, 223–238. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Klobasa, F.; Werhahn, E.; Butler, J.E. Composition of sow milk during lactation. J. Anim. Sci. 1987, 64, 1458–1466. [Google Scholar] [CrossRef]
- Rosales, C.A.; Meza-Herrera, C.A.; Morón, F.J.; Flores, M.J.; Gámez, H.G.; Cuevas, V.; Liu, S.M. Effects of vitamin E supply during late gestation and early lactation upon colostrum composition, milk production and quality in nutritional restricted ewes. Small Rum. Res. 2015, 133, 77–81. [Google Scholar] [CrossRef]
- Chen, J.; Han, J.H.; Guan, W.T.; Chen, F.; Wang, C.X.; Zhang, Y.Z.; Lv, Y.T.; Lin, G. Selenium and vitamin E in sow diets: I. Effect on antioxidant status and reproductive performance in multiparous sows. Anim. Feed Sci. Technol. 2016, 221, 111–123. [Google Scholar] [CrossRef]
- Mahan, D.C.; Kim, Y.Y.; Stuart, R.I. Effect of vitamin E sources (RRR- or all-rac-α-tocopheryl acetate) and levels on sow reproductive performance, serum, tissue, and milk α-tocopherol contents over a fiveparity period, and the effects on the progeny. J. Anim. Sci. 2000, 78, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, C.; Danielsen, V. Lactational dietary fat levels and sources influence milk composition and performance of sows and their progeny. Livest. Prod. Sci. 2004, 91, 95–105. [Google Scholar] [CrossRef]
- Pinelli-Saavedraa, A.; Scaife, J.R. Pre- and postnatal transfer of vitamins E and C to piglets in sows supplemented with vitamin E and vitamin C. Livest. Prod. Sci. 2005, 97, 231–240. [Google Scholar] [CrossRef]
- Mahan, D.C. Effects of dietary vitamin E on sow reproductive performance over a five-parity period. J. Anim. Sci. 1994, 72, 2870–2879. [Google Scholar] [CrossRef] [PubMed]
- Lemonakis, N.; Poudyal, H.; Halabalaki, M.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.; Gikas, E. The LC–MS-based metabolomics of hydroxytyrosol administration in rats reveals amelioration of the metabolic syndrome. J. Chromatogr. B 2017, 1041, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Bars-Cortina, D.; Lopez de las Hazas, M.C.; Benavent-Vallés, A.; Motilva, M.J. Impact of dietary supplementation with olive and thyme phenols on alpha-tocopherol concentration in the muscle and liver of adult Wistar rats. Food Funct. 2018, 9, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Aguinaga, M.A.; Haro, A.; Lara, L.; Gómez-Carballar, F.; Nieto, R.; Aguilera, J.F. Utilization of milk fatty acids by the suck ling Iberian piglets. Animal 2016, 10, 1786–1795. [Google Scholar] [CrossRef]
- Okayasu, T.; Kameda, K.; Ono, T.; Imai, Y. Effect of dietary vitamin B2 and vitamin E on the D-9-desaturase and catalase activities in rat liver microsomes. Biochim. Biophys. Acta 1977, 489, 389–402. [Google Scholar]
- Fuhrmann, H.; Sallman, H.P. Phospholipid fatty acid of brain and liver are modified by -tocopherol and dietary fat in growing chicks. Br. J. Nutr. 1996, 76, 109–122. [Google Scholar] [CrossRef]
- Lebold, K.M.; Jump, D.B.; Miller, G.W.; Wright, C.L.; Labut, E.M.; Barton, C.L.; Tanguay, R.L.; Traber, M.G. Vitamin E Deficiency Decreases Long-Chain PUFA in Zebrafish (Danio rerio). J. Nutr. 2011, 141, 2113–2118. [Google Scholar] [CrossRef]
- Raclot, T.; Groscolas, R. Differential mobilization of white adipose tissue fatty acids according to chain length, unsaturation, and positional isomerism. J. Lipid Res. 1993, 34, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Ann. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef] [PubMed]
- Tosi, F.; Sartori, F.; Guarini, P.; Olivieri, O.; Martinelli, N. Delta-5 and Delta-6 desaturases: Crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. Adv. Exp. Med. Biol. 2014, 824, 61–81. [Google Scholar] [PubMed]
- Hu, P.; Yang, H.; Bo, L.; Zhao, D.; Wang, J.; Zhu, W. Dynamic changes of fatty acids and minerals in sow milk during lactation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 603–611. [Google Scholar] [CrossRef] [PubMed]
| Control | VE | HXT | VE+HXT | RMSE 1 | p VE 2 | p HXT | p VE × HXT | |
|---|---|---|---|---|---|---|---|---|
| Colostrum | ||||||||
| Dry matter, % | 21.60 | 20.64 | 22.40 | 22.39 | 3.981 | 0.760 | 0.466 | 0.793 |
| Lactose, % | 3.64 | 5.04 | 3.91 | 4.32 | 1.437 | 0.090 | 0.728 | 0.399 |
| Fat, % | 5.55 | 5.39 | 5.98 | 6.97 | 2.504 | 0.938 | 0.498 | 0.810 |
| Tocopherol, µg/mL | 13.44 b | 15.65 ab | 12.90 b | 25.62 a | 5.972 | 0.006 | 0.093 | 0.058 |
| Retinol, µg/mL | 0.83 | 0.80 | 0.68 | 1.46 | 0.589 | 0.200 | 0.439 | 0.169 |
| Milk day 7 | ||||||||
| Dry matter, % | 18.06 | 16.05 | 17.33 | 17.16 | 3.957 | 0.445 | 0.893 | 0.518 |
| Lactose, % | 5.36 ab | 5.23 b | 6.51 ab | 6.48 a | 1.696 | 0.896 | 0.056 | 0.942 |
| Fat, % | 7.37 | 6.40 | 7.49 | 9.08 | 2.359 | 0.721 | 0.113 | 0.144 |
| Protein, % | 5.59 a | 4.89 b | 5.00 ab | 4.91 b | 0.632 | 0.091 | 0.226 | 0.196 |
| Tocopherol, µg/mL | 3.49 b | 3.95 ab | 3.50b | 4.25 a | 0.888 | 0.062 | 0.641 | 0.641 |
| Retinol, µg/mL | 0.25 b | 0.33 ab | 0.46 ab | 0.52 a | 0.202 | 0.346 | 0.010 | 0.911 |
| Milk day 20 | ||||||||
| Dry matter, % | 17.37 | 16.91 | 17.94 | 17.10 | 1.879 | 0.418 | 0.637 | 0.811 |
| Lactose, % | 6.05 | 5.21 | 7.99 | 6.06 | 2.106 | 0.105 | 0.101 | 0.515 |
| Fat, % | 7.47 | 7.57 | 7.97 | 7.55 | 2.257 | 0.859 | 0.784 | 0.773 |
| Protein, mg/mL | 5.35 ab | 5.11 b | 5.87 a | 5.30 ab | 0.563 | 0.081 | 0.125 | 0.449 |
| Tocopherol, µg/mL | 2.90 | 3.32 | 2.44 | 2.84 | 0.737 | 0.167 | 0.117 | 0.966 |
| Retinol, µg/mL | 0.39 | 0.48 | 0.48 | 0.55 | 0.122 | 0.415 | 0.421 | 0.891 |
| % Fatty Acids | Control | VE | HXT | VE+HXT | RMSE 12 | p VE 13 | p HXT | p VE × HXT |
|---|---|---|---|---|---|---|---|---|
| C14:0 | 1.25 | 1.15 | 1.27 | 1.22 | 0.139 | 0.180 | 0.418 | 0.678 |
| C14:1 | 0.04 | 0.03 | 0.04 | 0.02 | 0.021 | 0.146 | 0.240 | 0.796 |
| C16:0 | 22.01 | 22.27 | 22.12 | 23.37 | 1.449 | 0.328 | 0.470 | 0.601 |
| C16:1n−9 | 1.26 | 1.20 | 1.30 | 1.21 | 0.169 | 0.446 | 0.395 | 0.847 |
| C16:1n−7 | 3.70 | 3.89 | 3.48 | 3.11 | 0.873 | 0.916 | 0.275 | 0.636 |
| C17:0 | 1.10 | 1.07 | 1.04 | 0.96 | 0.306 | 0.652 | 0.495 | 0.851 |
| C17:1 | 0.46 | 0.47 | 0.49 | 0.50 | 0.068 | 0.982 | 0.433 | 0.807 |
| C18:0 | 6.27 | 6.43 | 6.06 | 6.75 | 0.972 | 0.580 | 0.676 | 0.889 |
| C18:1n−9 | 41.44 | 41.84 | 39.76 | 38.70 | 2.521 | 0.836 | 0.027 | 0.542 |
| C18:1n−7 | 3.05 | 3.58 | 3.14 | 3.15 | 0.608 | 0.046 | 0.829 | 0.848 |
| C18:2n−6 | 15.76 ab | 14.58 b | 17.52 a | 17.53 a | 1.643 | 0.225 | 0.002 | 0.529 |
| C18:3n−6 | 0.23 | 0.22 | 0.21 | 0.17 | 0.059 | 0.659 | 0.481 | 0.995 |
| C18:3n−3 | 0.94 ab | 0.82 b | 1.10 a | 1.09 a | 0.142 | 0.100 | 0.002 | 0.583 |
| C18:4n−3 | 0.20 | 0.18 | 0.18 | 0.17 | 0.063 | 0.671 | 0.830 | 0.732 |
| C20:0 | 0.09 | 0.08 | 0.07 | 0.05 | 0.041 | 0.540 | 0.494 | 0.790 |
| C20:1n−9 | 0.34 | 0.39 | 0.29 | 0.25 | 0.092 | 0.466 | 0.044 | 0.511 |
| C20:2 | 0.48 | 0.50 | 0.46 | 0.45 | 0.079 | 0.388 | 0.558 | 0.938 |
| C20:3n−6 | 0.24 | 0.21 | 0.23 | 0.23 | 0.044 | 0.773 | 0.490 | 0.126 |
| C20:4n−6 | 1.13 | 1.09 | 1.22 | 1.06 | 0.198 | 0.611 | 0.239 | 0.979 |
| ∑SAT 1 | 30.72 | 30.99 | 30.57 | 32.36 | 2.276 | 0.479 | 0.819 | 0.691 |
| ∑MUFAs 2 | 50.30 | 51.41 | 48.51 | 46.94 | 3.410 | 0.828 | 0.057 | 0.535 |
| ∑PUFAs 3 | 18.99 ab | 17.60 b | 20.92 a | 20.70 a | 1.783 | 0.193 | 0.002 | 0.496 |
| ∑n−6 4 | 17.37 ab | 16.10 b | 19.18 a | 18.99 a | 1.649 | 0.198 | 0.002 | 0.502 |
| ∑n−3 5 | 1.14 ab | 1.00 b | 1.28 a | 1.25 a | 0.162 | 0.109 | 0.008 | 0.540 |
| Δ-9-desaturase 6 | 0.61 | 0.61 | 0.60 | 0.57 | 0.033 | 0.621 | 0.199 | 0.571 |
| Δ-5-desaturase 7 | 0.83 | 0.84 | 0.84 | 0.82 | 0.019 | 0.828 | 0.819 | 0.056 |
| Δ-6-desaturase 8 | 0.03 | 0.03 | 0.02 | 0.02 | 0.006 | 0.923 | 0.065 | 0.830 |
| Thioesterase (16-14) 9 | 0.95 | 0.95 | 0.95 | 0.95 | 0.006 | 0.067 | 0.759 | 0.943 |
| Elongase (18-16) 10 | 0.22 | 0.22 | 0.21 | 0.22 | 0.021 | 0.816 | 0.385 | 0.922 |
| Elongase (20-18) 11 | 0.01 | 0.01 | 0.01 | 0.01 | 0.007 | 0.529 | 0.757 | 0.765 |
| % Fatty Acids | Control | VE | HXT | VE+HXT | RMSE 12 | p VE 13 | p HXT | p VE × HXT |
|---|---|---|---|---|---|---|---|---|
| C14:0 | 3.08 | 2.77 | 2.96 | 2.90 | 0.544 | 0.370 | 0.981 | 0.534 |
| C14:1 | 0.19 | 0.15 | 0.19 | 0.17 | 0.058 | 0.168 | 0.777 | 0.763 |
| C16:0 | 29.81 | 28.29 | 28.07 | 28.55 | 3.049 | 0.639 | 0.507 | 0.372 |
| C16:1n−9 | 0.37 | 0.54 | 0.42 | 0.40 | 0.189 | 0.294 | 0.530 | 0.177 |
| C16:1n−7 | 7.82 | 7.01 | 7.57 | 7.13 | 1.653 | 0.305 | 0.916 | 0.756 |
| C17:0 | 0.71 | 0.90 | 0.78 | 0.72 | 0.195 | 0.358 | 0.417 | 0.076 |
| C17:1 | 0.41 b | 0.52 ab | 0.46 ab | 0.56 a | 0.096 | 0.008 | 0.205 | 0.896 |
| C18:0 | 4.62 | 4.92 | 4.45 | 5.35 | 0.877 | 0.071 | 0.694 | 0.351 |
| C18:1n−9 | 34.83 | 35.86 | 35.78 | 38.02 | 3.545 | 0.212 | 0.235 | 0.636 |
| C18:1n−7 | 1.85 | 2.44 | 2.42 | 2.39 | 0.515 | 0.149 | 0.170 | 0.111 |
| C18:2n−6 | 13.55 a | 13.35 a | 13.97 a | 11.19 b | 1.594 | 0.015 | 0.143 | 0.034 |
| C18:3n−6 | 0.10 b | 0.15 a | 0.13 b | 0.12 b | 0.028 | 0.094 | 0.700 | 0.018 |
| C18:3n−3 | 0.97 a | 0.94 a | 0.99 a | 0.78 b | 0.120 | 0.011 | 0.135 | 0.054 |
| C18:4n−3 | 0.18 | 0.22 | 0.19 | 0.18 | 0.042 | 0.390 | 0.343 | 0.149 |
| C20:0 | 0.10 | 0.12 | 0.10 | 0.09 | 0.044 | 0.573 | 0.285 | 0.302 |
| C20:1n−9 | 0.30 | 0.38 | 0.34 | 0.37 | 0.108 | 0.160 | 0.762 | 0.576 |
| C20:2 | 0.35 | 0.44 | 0.40 | 0.40 | 0.091 | 0.239 | 0.916 | 0.192 |
| C20:3n−6 | 0.14 b | 0.22 a | 0.16 b | 0.13 b | 0.052 | 0.285 | 0.071 | 0.012 |
| C20:4n−6 | 0.60 b | 0.80 a | 0.64 ab | 0.56 b | 0.166 | 0.288 | 0.101 | 0.029 |
| ∑SAT 1 | 38.31 | 37.01 | 36.35 | 37.60 | 3.300 | 0.980 | 0.570 | 0.293 |
| ∑MUFAs 2 | 45.78 | 46.89 | 47.17 | 49.04 | 3.128 | 0.199 | 0.128 | 0.740 |
| ∑PUFAs 3 | 15.91 a | 16.11 a | 16.48 a | 13.36 b | 1.731 | 0.028 | 0.093 | 0.013 |
| ∑n−6 4 | 14.40 a | 14.52 a | 14.90 a | 12.00 b | 1.650 | 0.027 | 0.102 | 0.018 |
| ∑n−3 5 | 1.15 a | 1.16 a | 1.18 a | 0.96 b | 0.116 | 0.018 | 0.062 | 0.014 |
| Δ-9-desaturase 6 | 0.53 | 0.55 | 0.55 | 0.55 | 0.035 | 0.625 | 0.326 | 0.683 |
| Δ-5-desaturase 7 | 0.80 | 0.79 | 0.80 | 0.81 | 0.048 | 0.945 | 0.675 | 0.498 |
| Δ-6-desaturase 8 | 0.02 b | 0.03 a | 0.02 ab | 0.02 ab | 0.005 | 0.016 | 0.883 | 0.427 |
| Δ-5+Δ-6-desaturase | 0.05 b | 0.07 a | 0.05 b | 0.05 ab | 0.013 | 0.032 | 0.279 | 0.135 |
| Thioesterase (16-14) 9 | 0.91 | 0.91 | 0.90 | 0.91 | 0.011 | 0.255 | 0.597 | 0.959 |
| Elongase (18-16) 10 | 0.14 | 0.15 | 0.14 | 0.16 | 0.031 | 0.096 | 0.579 | 0.662 |
| Elongase (20-18) 11 | 0.02 | 0.02 | 0.02 | 0.02 | 0.008 | 0.917 | 0.173 | 0.157 |
| Control | VE | HXT | VE+HXT | RMSE 1 | p VE 2 | p HXT | p VE × HXT | |
|---|---|---|---|---|---|---|---|---|
| Milk day 7, nmols MDA/mg protein | ||||||||
| MDA 0 min | 0.15 a,b | 0.14 b | 0.17 a | 0.15 ab | 0.024 | 0.134 | 0.075 | 0.263 |
| MDA 30 min | 0.14 | 0.10 | 0.14 | 0.12 | 0.063 | 0.183 | 0.713 | 0.664 |
| MDA 90 min | 0.61 a | 0.28 b | 0.42 ab | 0.25 b | 0.188 | 0.001 | 0.126 | 0.240 |
| MDA 120 min | 0.30 a | 0.11 b | 0.31 | 0.16 ab | 0.164 | 0.010 | 0.592 | 0.770 |
| Total ∑MDA 3 | 1.20 a | 0.56 c | 1.05 ab | 0.69 bc | 0.368 | 0.001 | 0.932 | 0.298 |
| Milk day 20, nmols MDA/mg protein | ||||||||
| MDA 0 min | 0.15 | 0.12 | 0.14 | 0.14 | 0.037 | 0.317 | 0.760 | 0.253 |
| MDA 30 min | 0.11 b | 0.10 b | 0.24 a | 0.13 b | 0.091 | 0.097 | 0.039 | 0.182 |
| MDA 90 min | 0.22 b | 0.15 b | 0.48 a | 0.23 b | 0.128 | 0.005 | 0.003 | 0.079 |
| MDA 120 min | 0.24 | 0.10 | 0.23 | 0.21 | 0.167 | 0.259 | 0.456 | 0.394 |
| Total ∑MDA | 0.72 ab | 0.48 b | 1.09 a | 0.72 ab | 0.328 | 0.026 | 0.028 | 0.604 |
| Intercept | ±s.d. 1 | Slope | ±s.d. | Variable x | r | R2 | p (Linear) 2 | |
|---|---|---|---|---|---|---|---|---|
| Milk on day 7 | ||||||||
| Total MDA3 | −0.424 ± 0.62 | 0.107 ± 0.05 | % C18:2 n−6 in milk | 0.39 | 0.16 | 0.034 | ||
| Total MDA | −0.486 ± 0.59 | 1.593 ± 0.65 | % C18:3 n−3 in milk | 0.42 | 0.18 | 0.021 | ||
| Total MDA | −0.391 ± 0.68 | 0.088 ± 0.04 | ∑PUFAs in milk 4 | 0.36 | 0.13 | 0.050 | ||
| Total MDA | 0.525 ± 0.13 | 9.133 ± 2.44 | MDA in piglets after weaning | 0.72 | 0.52 | 0.003 | ||
| Milk on day 20 | ||||||||
| Total MDA | −1.298 ± 0.51 | 0.189 ± 0.05 | % C18:2 n−6 in milk | 0.65 | 0.43 | 0.001 | ||
| Total MDA | −1.510 ± 0.66 | 0.173 ± 0.05 | ∑PUFAs in milk | 0.59 | 0.35 | 0.002 | ||
| Total MDA | 2.683 ± 0.73 | −0.097 ±0.04 | Sow’s plasma catalase on day 20 | –0.58 | 0.34 | 0.017 | ||
| Total MDA | 1.063 ± 0.13 | −0.183 ± 0.06 | Sow’s plasma α-tocopherol (µg/mL) on day 20 | –0.67 | 0.45 | 0.006 | ||
| Total MDA | 0.344 ± 0.12 | 7.238 ± 2.06 | MDA in piglets after weaning | 0.73 | 0.53 | 0.005 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laviano, H.D.; Gómez, G.; Muñoz, M.; García-Casco, J.M.; Nuñez, Y.; Escudero, R.; Molina, A.H.; González-Bulnes, A.; Óvilo, C.; López-Bote, C.; et al. Dietary Vitamin E and/or Hydroxytyrosol Supplementation to Sows during Late Pregnancy and Lactation Modifies the Lipid Composition of Colostrum and Milk. Antioxidants 2023, 12, 1039. https://doi.org/10.3390/antiox12051039
Laviano HD, Gómez G, Muñoz M, García-Casco JM, Nuñez Y, Escudero R, Molina AH, González-Bulnes A, Óvilo C, López-Bote C, et al. Dietary Vitamin E and/or Hydroxytyrosol Supplementation to Sows during Late Pregnancy and Lactation Modifies the Lipid Composition of Colostrum and Milk. Antioxidants. 2023; 12(5):1039. https://doi.org/10.3390/antiox12051039
Chicago/Turabian StyleLaviano, Hernan D., Gerardo Gómez, María Muñoz, Juan M. García-Casco, Yolanda Nuñez, Rosa Escudero, Ana Heras Molina, Antonio González-Bulnes, Cristina Óvilo, Clemente López-Bote, and et al. 2023. "Dietary Vitamin E and/or Hydroxytyrosol Supplementation to Sows during Late Pregnancy and Lactation Modifies the Lipid Composition of Colostrum and Milk" Antioxidants 12, no. 5: 1039. https://doi.org/10.3390/antiox12051039
APA StyleLaviano, H. D., Gómez, G., Muñoz, M., García-Casco, J. M., Nuñez, Y., Escudero, R., Molina, A. H., González-Bulnes, A., Óvilo, C., López-Bote, C., & Rey, A. I. (2023). Dietary Vitamin E and/or Hydroxytyrosol Supplementation to Sows during Late Pregnancy and Lactation Modifies the Lipid Composition of Colostrum and Milk. Antioxidants, 12(5), 1039. https://doi.org/10.3390/antiox12051039







