New Directions to Approach Oxidative Stress Related to Physical Activity and Nutraceuticals in Normal Aging and Neurodegenerative Aging
Abstract
1. Introduction
2. Methods of the Literature Review
3. Oxidative Stress and Aging Process Pathophysiology
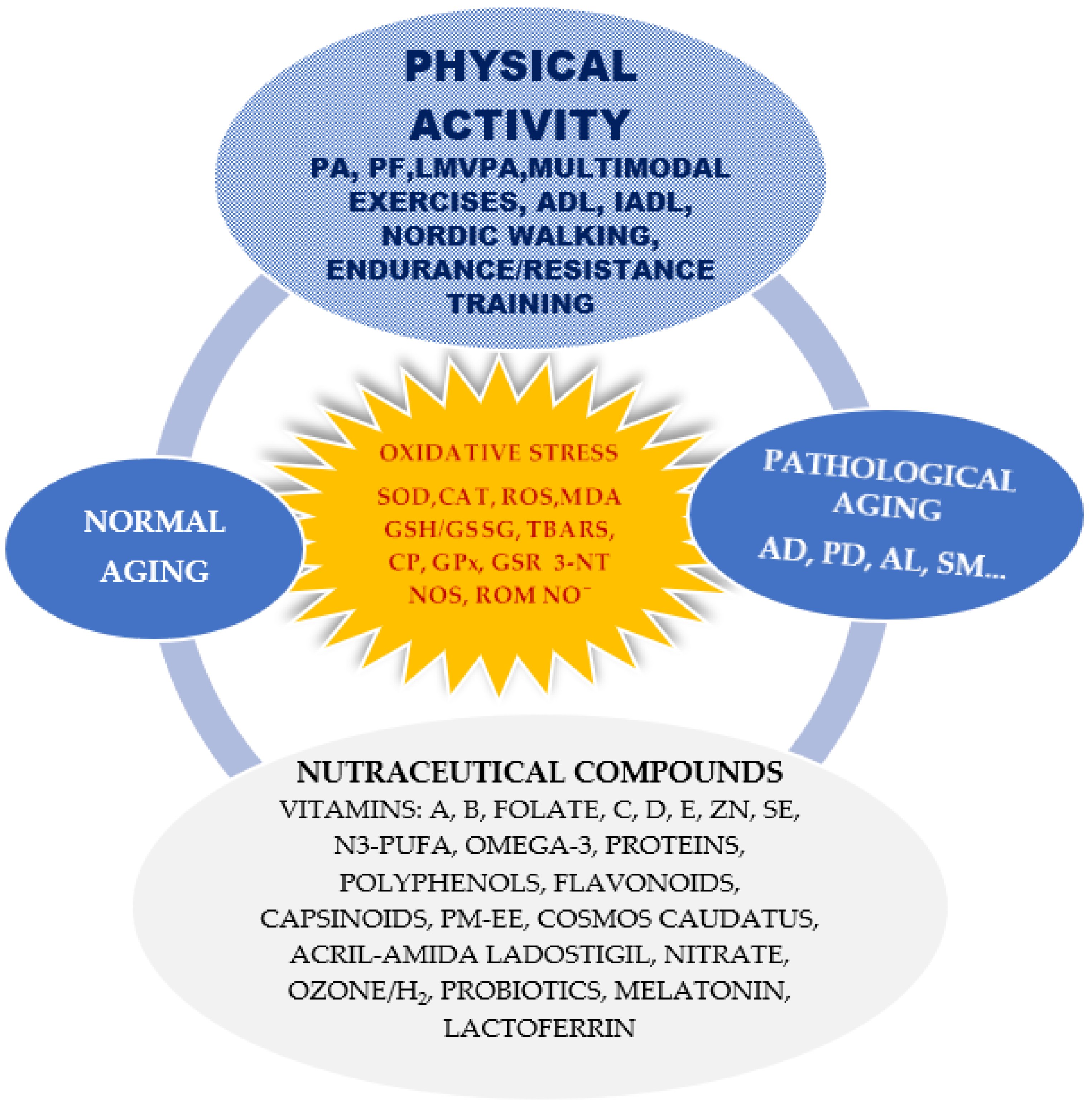
3.1. Instruments/Tools—Determinants of Oxidative Stress, Inflammation Status, Nutraceutical Compounds and Scales of Assessment of Motor and Cognitive Functions on Normal Aging and Aging Pathology
3.1.1. Oxidative Stress Biomarkers/Tools
3.1.2. Inflammatory Markers/Tools
3.1.3. Nutraceutical Compounds/Tools
3.1.4. Motor and Cognitive Scales/Tools
3.2. The Impact of Interventions through Physical Activity and Nutraceutical Compounds on Oxidative Stress for Healthy Aging
3.3. The Influence of Physical Activity on Oxidative Stress for Healthy Older Adults
3.4. The Impact of Recovery Using Physical Activity and Nutraceutical Compounds on Oxidative Stress for Neurodegenerative Diseases
3.5. The Effects of Recovery Using Nutraceutical (Antioxidant) Biomolecules on Oxidative Stress for Neurodegenerative Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Correa-Muñoz, E.; Arronte-Rosales, A.; Mendoza-Núñez, V.M. Oxidative stress risk is increased with a sedentary lifestyle during aging in Mexican women. Oxid. Med. Cell. Longev. 2021, 2021, 9971765. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Tanisawa, K.; Kawakami, R.; Usui, C.; Ito, T.; Tabata, H.; Nakamura, N.; Kurosawa, S.; Choi, W.; Ma, S.; et al. Determinants of Resting Oxidative Stress in Middle-Aged and Elderly Men and Women: WASEDA’S Health Study. Oxid. Med. Cell. Longev. 2021, 2021, 5566880. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Idelchik, M.D.P.S.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- To, T.; Terebessy, E.; Zhu, J.; Zhang, K.; Lakey, P.S.; Shiraiwa, M.; Hatzopoulou, M.; Minet, L.; Weichenthal, S.; Dell, S.; et al. Does early life exposure to exogenous sources of reactive oxygen species (ROS) increase the risk of respiratory and allergic diseases in children? A longitudinal cohort study. Environ. Health 2022, 21, 90. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Visioli, F.; Artaria, C. Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017, 8, 39–63. [Google Scholar] [CrossRef]
- Cuadrado, A.; Manda, G.; Hassan, A.; Alcaraz, M.J.; Barbas, C.; Daiber, A.; Ghezzi, P.; León, R.; López, M.G.; Oliva, B.; et al. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Mesquita, P.H.C.; Lamb, D.A.; Godwin, J.S.; Osburn, S.C.; Ruple, B.A.; Moore, J.H.; Vann, C.G.; Huggins, K.W.; Fruge, A.D.; Young, K.C.; et al. Effects of resistance training on the redox status of skeletal muscle in older adults. Antioxidants 2021, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz, M.; Rowiński, R.; Kornatowski, M.; Dąbrowski, A.; Kędziora-Kornatowska, K.; Strachecka, A. Relation of moderate physical activity to blood markers of oxidative stress and antioxidant defense in the elderly. Oxid. Med. Cell. Longev. 2019, 2019, 5123628. [Google Scholar] [CrossRef]
- Giolo, J.S.; Costa, J.G.; da Cunha-Junior, J.P.; Pajuaba, A.C.; Taketomi, E.A.; de Souza, A.V.; Caixeta, D.C.; Peixoto, L.G.; de Oliveira, E.P.; Everman, S.; et al. The effect of isoflavone supplementation plus combined exercise on lipids levels and inflammatory and oxidative stress markers in postmenopausal women. Nutrients 2018, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Wiecek, M.; Szygula, Z.; Gradek, J.; Kusmierczyk, J.; Szymura, J. Whole body cryotherapy increases the activity of nitric oxide synthase in older men. Biomolecules 2021, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.; Salama, R.M.; Schaalan, M.F. A pilot study on the effect of lactoferrin on Alzheimer’disease pathological sequelae: Impact of the p-Akt/PTEN pathway. Biomed. Pharmacother. 2019, 111, 714–723. [Google Scholar] [CrossRef]
- Morucci, G.; Ryskalin, L.; Pratesi, S.; Branca, J.J.V.; Modesti, A.; Modesti, P.A.; Gulisano, M.; Gesi, M. Effects of a 24-week exercise program on functional fitness, oxidative stress, and salivary cortisol levels in elderly subjects. Medicina 2022, 58, 1341. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Gabr, S.A.; Answer, S.; Li, H. Associations between vitamin E, oxidative stress markers, total homocysteine levels, and physical activity or cognitive capacity in older adult. Sci. Rep. 2021, 11, 12867. [Google Scholar] [CrossRef]
- Veronese, N.; Dominguez, L.J.; Ragusa, S.; Solimando, L.; Smith, L.; Bolzetta, F.; Maggi, S.; Barbagallo, M. Dietary acrylamide and physical performance tests: A cross-sectional analysis. PLoS ONE 2021, 16, e025932. [Google Scholar] [CrossRef]
- Ku, B.J.; Ko, K.; Shin, K.O.; Bae, J.Y. Effect of regular Taekwondo self-defense training on oxidative stress and inflammation markers in postmenopausal women. Healthcare 2021, 9, 985. [Google Scholar] [CrossRef]
- Jiménez-Delgado, A.; Ortiz, G.G.; Delgado-Lara, D.L.; González-Usigli, H.A.; González-Ortiz, L.J.; Cid-Hernández, M.; Cruz-Serrano, J.A.; Pacheco-Moisés, F.P. Effect of melatonin administration on mitochondrial activity and oxidative stress markers in patients with Parkinson’s disease. Oxid. Med. Cell. Longev. 2021, 2021, 5577541. [Google Scholar] [CrossRef]
- Balmes, J.R.; Arjomandi, M.; Bromberg, P.A.; Costantini, M.G.; Dagincourt, N.; Hazucha, M.J.; Hollenbeck-Pringle, D.; Rich, D.Q.; Stark, P.; Frampton, M.W. Ozone effects on blood markers of systemic inflammation, oxidative stress, endothelial function, and thrombosis: The multicenter ozone study in older subjects. PLoS ONE 2019, 14, e0222601. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortés, C.; Capó, X.; Bibiloni, M.D.M.; Martorell, M.; Ferrer, M.D.; Argelich, E.; Bouzas, C.; Carreres, S.; Tur, J.A.; Pons, A.; et al. Peripheral blood mononuclear cells antioxidant adaptations to regular physical activity in elderly people. Nutrients 2018, 10, 1555. [Google Scholar] [CrossRef]
- Liu, S.Z.; Valencia, A.P.; VanDoren, M.P.; Shankland, E.G.; Roshanravan, B.; Conley, K.E.; Marcinek, D. Astaxanthin supplementation enhances metabolic adaptation with aerobic training in the elderly. J. Physiol. Rep. 2021, 9, e14887. [Google Scholar] [CrossRef] [PubMed]
- Valado, A.; Fortes, S.; Morais, M.; Barreira, R.; Figueiredo, J.P.; Caseiro, A. Impact of hydrotherapy an antioxidant enzyme activity in an elderly population. Geriatrics 2022, 7, 64. [Google Scholar] [CrossRef]
- Żychowska, M.; Grzybkowska, A.; Zasada, M.; Piotrowska, A.; Dworakowska, D.; Czerwińska-Ledwig, O.; Pilch, W.; Antosiewicz, J. Effect of six weeks 1000 mg/day vitamin C supplementation and healthy training in elderly women on genes expression associated with the immune response-a randomized controlled trial. J. Int. Soc. Sports Nutr. 2021, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Oggioni, C.; Jakovljevic, D.G.; Klonizakis, M.; Ashor, A.W.; Ruddock, A.; Ranchordas, M.; Williams, E.; Siervo, M. Dietary nitrate does not modify blood pressure and cardiac output at rest during exercise in older adults: A randomized cross-over study. Int. J. Food Sci. Nutr. 2018, 69, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Amirato, G.R.; Borges, J.O.; Marques, D.L.; Santos, J.M.B.; Santos, C.A.F.; Andrade, M.S.; Furtado, G.E.; Rossi, M.; Luis, L.N.; Zambonatto, R.F.; et al. L-glutamine supplementation enhances strength and power of knee muscles and improves glycemia control and plasma redox balance in exercising elderly women. Nutrients 2021, 13, 1025. [Google Scholar] [CrossRef]
- Ton, A.M.M.; Campagnaro, B.P.; Alves, G.A.; Aires, R.; Côco, L.Z.; Arpini, C.M.; Guerra, E.; Oliveira, T.; Campos-Toimil, M.; Meyrelles, S.S.; et al. Oxidative stress and dementia in Alzheimer’s patients: Effects of symbiotic supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 2638703. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of dietary supplementation of asthaxanthin and sesamin on daily fatigue: A randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef]
- Ten Haaf, D.S.M.; Bongers, C.C.W.G.; Hulshof, H.G.; Eijsvogels, T.M.H.; Hopman, M.T.E. The impact of protein supplementation on exercise-induced muscle damage, soreness and fatigue following prolonged walking exercise in vital older adults: A randomized double-blind placebo-controlled trial. Nutrients 2020, 12, 1806. [Google Scholar] [CrossRef]
- Aparicio-Ugarriza, R.; Díaz, Á.E.; Palacios, G.; Bibiloni, M.D.M.; Julibert, A.; Tur, J.A.; González-Gross, M. Association between blood marker analyses regarding physical fitness levels in Spanish older adults: A cross-sectional study from the PHYSMED project. PLoS ONE 2018, 13, e0206307. [Google Scholar] [CrossRef]
- Yokoyama, K.; Yamada, Y.; Akamatsu, Y.; Yoshinaka, Y.; Yamamoto, A.; Koizumi, T.; Ohyama, K.; Suzuki, K.; Hashimoto, M.; Sato, H.; et al. Effects of capsinoids on daily physical activity, body composition and cold hypersensitivity in middle-age and older adults: A randomized study. Nutrients 2020, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Munguia, L.; Rubio-Gayosso, I.; Ramirez-Sanchez, I.; Ortiz, A.; Hidalgo, I.; Gonzalez, C.; Meaney, E.; Villarreal, F.; Najera, N.; Ceballos, G. High flavonoid cocoa supplement ameliorates plasma oxidative stress and inflammation levels while improving mobility and quality of life in older subjects: A double-blind randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.O.; Xu, H.; Moser, L.; Adeoye, P.; Lin, A.W.; Tangney, C.C.; Risacher, S.L.; Saykin, A.J.; Considine, R.V.; Unverzagt, F.W. Mind food and speed of processing training in older adults with low education, the MINDSpeed Alzheimer’s disease prevention pilor trial. Contemp. Clin. Trials 2019, 84, 105814. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Michie, K.L.; Gries, K.J.; Zhang, X.; Ryan, Z.C.; Lanza, I.R. A randomized trial of the effects of dietary n3-PUFAs on skeletal muscle function and acute exercise response in healthy older adults. Nutrients 2022, 14, 3537. [Google Scholar] [CrossRef]
- Gaitán, J.M.; Moon, H.Y.; Stremlau, M.; Dubal, D.B.; Cook, D.B.; Okonkwo, O.C.; van Praag, H. Effects of aerobic exercise training on systemic biomarkers and cognition in late middle-age adults at risk for Alzheimer’s disease. Front. Endocrinol. 2021, 12, 660181. [Google Scholar] [CrossRef]
- Vyas, C.M.; Ogata, S.; Reynolds, C.F., 3rd; Mischoulon, D.; Chang, G.; Cook, N.R.; Manson, J.E.; Crous-Bou, M.; De Vivo, I.; Okereke, O.I. Lifestyle and behavioral factors and mithocondrial DNA copy number in a diverse cohort of mid-life and older adults. PLoS ONE 2020, 15, e0237235. [Google Scholar] [CrossRef]
- Batista, R.A.B.; de Branco, F.M.S.; Nehme, R.; de Oliveira, E.P.; Pena, G.D.G. Association between Plasma Omega-3 and Handgrip Strength According to Glycohemoglobin Levels in Older Adults: Results from NHANES 2011–2012. Nutrients 2022, 14, 4060. [Google Scholar] [CrossRef]
- An, Y.; Feng, L.; Zhang, X.; Wang, Y.; Wang, Y.; Tao, L.; Qin, Z.; Xiao, R. Dietary intakes and biomarker patterns of folate, vitamin B6 and vitamin B12 can be associated with cognitive impairment by hypermethylation of redox-related genes NUDT15 and TXNRD1. Clin. Epigenetics 2019, 11, 139. [Google Scholar] [CrossRef]
- Malek Rivan, N.F.; Shahar, S.; Rajab, N.F.; Singh, D.K.A.; Din, N.C.; Hazlina, M.; Hamid, T.A. Cognitive frailty among Malaysian older adults: Baseline findings from the LRGS TUA cohort study. Clin. Interv. Aging 2019, 14, 1343–1352. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Lee, W.J.; Shin, Y.W.; Kim, D.E.; Kweon, M.H.; Kim, M. Effect of desalted Salicornia europaeaL. Ethanol extract (PM-EE) on the subjects complaining memory dysfunction without dementia: A 12 week, randomized, double-blind, placebo-controlled clinical trial. Sci. Rep. 2020, 10, 19914. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jeon, B.H. The effects of fermented Laminaria japonica on short-term working memory and physical fitness in the elderly. Evid. Based Complement. Alternat Med. 2018, 2018, 8109621. [Google Scholar]
- Hong, C.T.; Hu, C.J.; Lin, H.Y.; Wu, D. Effects of concomitant use of hydrogen water and photo biomodulation on Parkinson disease. Medicine 2021, 100, e24191. [Google Scholar] [CrossRef] [PubMed]
- Coles, L.D.; Tuite, P.J.; Öz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Cloyd, J.C.; Terpstra, M. Repeated-dose oral N-acetylcysteine in Parkinson disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol. 2018, 58, 158–167. [Google Scholar] [CrossRef]
- Gibson, G.E.; Luchsinger, J.A.; Cirio, R.; Chen, H.; Franchino-Elder, J.; Hirsch, J.A.; Bettendorff, L.; Chen, Z.; Flowers, S.A.; Gerber, L.M.; et al. Benfotiamine and cognitive decline in Alzheimer’s disease: Results of a randomized placebo-controlled phase IIa clinical trial. J. Alzheimers. Dis. 2020, 78, 989–1010. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Geffen, Y.; Rabinowitz, J.; Thomas, R.G.; Schmidt, R.; Ropele, S.; Weinstock, M. Low-dose ladostigil for mild cognitive impairment. Ladostigil Study Group. Neurology 2019, 93, 1474–1484. [Google Scholar] [CrossRef]
- Al Ali, M.; Alqubaisy, M.; Aljaafari, M.N.; Al Ali, A.O.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.S.; Lim, S.H.E. Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef]
- Daimiel, L.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Schröder, H.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; Lopez-Miranda, J.; et al. Physical fitness and physical activity association with cognitive function and quality of life: Baseline cross-sectional analysis of the PREDIMED-Plus trial. Sci. Rep. 2020, 10, 3472. [Google Scholar] [CrossRef]
- Netz, Y.; Ben-Zaken, S.; Zeev, A.; Dunsky, A. Correlates of Early-Stage Frailty-Sleep, Fitness, Oxidative Stress, and BMI. Front. Med. 2021, 7, 594710. [Google Scholar] [CrossRef]
- Silva, L.A.D.; Tortelli, L.; Motta, J.; Menguer, L.; Mariano, S.; Tasca, G.; Silveira, G.B.; Pinho, R.A.; Silveira, P.C.L. Effects of aquatic exercise on mental health, functional autonomy and oxidative stress in depressed elderly individuals: A randomized clinical trial. Clinics 2019, 74, e322. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Lim, P.J.; Tsubosaka, M.; Kim, H.K.; Miyashita, M.; Suzuki, K.; Tan, E.L.; Shibata, S. Effects of increased daily physical activity on mental health biomarkers in postmenopausal women. J. Phys. Ther. Sci. 2019, 31, 408–413. [Google Scholar] [CrossRef]
- You, Y.X.; Shahar, S.; Rajab, N.F.; Haron, H.; Yahya, H.M.; Mohamad, M.; Din, N.C.; Maskat, M.Y. Effects of 12 weeks Cosmos caudatus supplement among older adults mild cognitive impairment: A randomized double-blind and placebo-controlled trial. Nutrients 2021, 13, 434. [Google Scholar] [CrossRef]
- Yoritaka, A.; Kobayashi, Y.; Hayashi, T.; Saiki, S.; Hattori, N. Randomized double-blind placebo-controlled trial of hydrogen in halation for Parkinson’s disease: A pilot study. Neurol. Sci. 2021, 42, 4767–4770. [Google Scholar] [CrossRef]
- Zaenker, P.; Favret, F.; Lonsdorfer, E.; Muff, G.; de Seze, J.; Isner-Horobeti, M.E. High-intensity interval training combined with resistance training improves physiological capacities, strength and quality of life in multiple sclerosis patients: A pilot study. Eur. J. Phys. Rehabil. Med. 2018, 54, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Isoda, H.; Okura, T. Evaluation of beneficial effect of a dual-task exercise based on Japanese traditional games in older adults: A pilot study. Aging 2020, 12, 18957–18969. [Google Scholar] [CrossRef] [PubMed]
| Oxidative Stress Tools | Inflammatory Tools | Nutraceutical Compounds Tools | Motor and Cognitive Scales |
|---|---|---|---|
| SOD | Lipid profile | AX/AX + Sesamin | IPAQ |
| TBARS | IL1/IL1b/IL6 /IL-8/IL-10 IL-12 | Beetroot juice (Nitrate dietary) NO3− | MET |
| FRAP | I CAM-3 | Vitamin C | HOMA-IR |
| ROS/iNOS ROM | E/P-selectin Adhesion molecules | VAS/NPRS | |
| TOS/TOC TAS/TAC/TACV | Thrombomodulin Endothelin-1 | Capsinoids | 1RM |
| 3-NT | TUBB | Isoflavone | POMS2/TMD |
| CP | CRP | Cryotherapy | GDS/S-GDS |
| MDA | TNFα | N3 PUFA | BDHQ |
| GPx SOD/GPx ratio | HCY | European/Spanish/ Majorcan/ dietary intake | PASE |
| PCOOH | BNDF | Vitamins A/C/D Tocopherol | PHQ-8 |
| CAT | 8-iso PGF2α | Beta-carotene | PSQI/OSA-MA |
| GSR | IL-12/IL-10 ratio /IL-12-p70/ IL-8/IL-10 ratio | Acrylamide | MMSE/ MoCA |
| HSPs | Ferritin | Cocoa beverage powder | DS/DSF /DSB |
| H2O2 | Fibrinogen | L-glutamine | TMT/ATMT/CDT |
| BAP | Ozone | SF-36-HRQOL | |
| TRX | Vitamin E/Zn/Se | RAPA/GPAQ | |
| GRd | Protein intake | EQ-5D | |
| AOPP | Niacin | COWAT | |
| GSH/GSSG | FST | UPDRS/H&Y /PDQ | |
| NO | PM-EE | LOTCA | |
| 8-OHdG | Melatonin | D-KEFS–CWI | |
| H2/Photo-modulation | RAVLT/ CVLT/AVLT /LMT/SDMT | ||
| Benfotiamine | IADL/ADL | ||
| Vitamin B | WHODAS/DHQ/FFQ Er-Med | ||
| NAC | FFQ | ||
| Ladostigil | BDI/BAI | ||
| Polyphenols/ Mind foods | BBS | ||
| Kefir Probiotics | EDSS /SEP-59/MSQOL/ | ||
| LF | ADAS–cog/ ADCS–ADL/DAD/ CERAD-K/CDR | ||
| CC | NPI/RBANS/NTB |
| Authors | Design | Dietary Intake | Age/ Gender | Physical Activity | Primary Outcomes | Secondary Outcomes | Tools/ Instruments | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Liu S.Z. et al. (2021) [23] | 42 subjects: 23 females 17 males | Nutraceutical dietary (AX) AX group PL (placebo) group | 65–82 years | 12 weeks endurance training for 3 sessions/week | FATox (g/min) CHO (g/min) RER Exercise efficiency (%) = work (kcal/min)/energy expenditure (kcal/min) × 10 | GXT, TA, blood chemistry test: lipid profile (total cholesterol, HDL, LDL, triglycerides insulin level) | Treadmill, custom-built exercise device | Improved exercise efficiency for males, enhanced FA tox for both sexes and groups, increased fat oxidation under the same exercise intensity, improved exercise efficiency, decreased carbohydrate oxidation during lower-intensity training in older males. |
| Oggioni C. et al. (2018) [26] | 20 participants 10 females 10 males | Nitrate dietary (NO3−) beetroot juice: 70 mL × 2/day for seven days | 60–70 years | Cycling with 10-M, pedalling cadence of 50 rpm, vigorous/moderate-intensity activity, walking, sitting | IPAQ, MET, haemodynamic parameters: BP, CO, CI, SV, HR, AIx | Glucose, insulin, HOMA-IR, IL-6, cGMP, E-selectin, P-selectin, thrombomodulin ICAM-3, 3-NT | Bicycle ergometer, micromanometer | The nitrate nutraceutical diet did not favourably influence the haemodynamic and cardiac parameters at rest or during physical activity of various degrees, nor the oxidative stress. |
| Żychowska M. et al. (2021) [17] | 24 women SUP (supported group), CON (control) group | 1000 mg vitamin C/day for 6 weeks | ≥65 years | 6-week training program three times/week 60 min, either session, gyrokinesis–Nordic walking, stabilisation training | VO2 max, muscle mass, fat mass, BMI, TOS/TOC, TAS/TAC vitamin C prooxidative/antioxidative ratio | TUBB, IL-1, IL-6, IL-10, CCL2, CRP | Cyclo-ergometer Ergoline (60 rpm cadence) | Decreased BMI and increasing muscle mass after regular exercise training-related CON group, insignificant changes for both groups after 6 weeks of exercise training supported/not supported with vitamin C, non-influencing oxidative/antioxidative balance after support using vitamin C and health training, decreased IL-6 and increasing IL-10 mRNA in supported group, small increase in IL-1 after aerobic training and nutraceutical vitamin C supplementation. |
| Yokoyama K. et al. (2020) [32] | 69 subjects 52 females 17 males CP group PL group | 9 mg capsinoids (Capsicum anuum L., CH-19 Sweet) daily for 12 weeks | 52–87 years | VPA (≥6 METs), LMPA (1.5–5.9 METs), physical strength test, 10 m walking time | FAS METs, energy expenditure (Kcal/day = METs × time × body weight (kg) × 1.05 | Mood profile: POMS2, TMD, LBM, BMI, % body fat, visceral fat index, muscle mass %, FFM (kg), VAS | Accelerometer with 3 axes, stadiometer, Scala Yamato with weight, activity meter | In terms of body composition, ingestion of capsinoids decreased waist circumference, body fat percentage and visceral fat index, increasing energy expenditure in sedentary participants. Homeostatic capacity regulation with decreased chilly sensation in adults 80 years or older. Enhanced energy expenditure, oxidative phosphorylation in muscles, insulin resistance and lipids’ use under conditions of increased amount and time devoted to LMPA. |
| Jéssica S. Giolo et al. (2018) [13] | 32 females ISO group = 17 PL group = 15 | 100 mg/day isoflavone nutraceutical for 10 weeks intervention | 50–70 years | Aerobic training, resistance training (7 exercises, 30 sessions, 10 weeks 50 min/session) | BMI, VE/O2 ratio, VE/CO2 ratio, 1RM | Total cholesterol, triglycerides, HDL, LDL, uric acid, HBA1c, IL-8, IL-1β, TNF, IL-10, SOD, TBARS, FRAP | Stadiometer Sanny, treadmill (5.5 km/hour) | Lipid profile and markers of oxidative stress were not influenced by isoflavone nutraceuticals and the association with aerobic or resistance exercise, enhanced IL-8 levels and reduced total cholesterol from aerobic and resistance training promotion. |
| Wiecek M. et al. (2021) [14] | RUN = 10 UTR = 10 | WBC—24 exposures to −130° for 3 min in cryochamber (whole-body cryotherapy), 3 times/week, 7 days | 53–56 years | 3–5 times/week, 55–150 km at 2 marathons/year 10/6 METs—very hard/hard PA for RUN, 4 METs—moderate-intensity exercise for UTR | BMI, Height, Fat mass, % body fat, Haemoglobin (g/dL), Haematocrit (%), ESR (mm/h), Leucocytes (103/µL), Platelets (103/µL), Fasting glucose, HBA1c, Total cholesterol, Triglycerides, HDL, LDL, Total protein, Fibrinogen (g/L), AIP | iNOS, ADMA, 3-NTR, CRP, IL-6, IL-1β, IL-10, HCY | Bamet KN-1 cryogenic chamber, Stopwatch | Increased i-NOS levels in older adults after 24 WBC treatments, no change in the level of 3-NTR, which is an indicator of nitro-oxidative stress, stimulated lipid oxidation using IL-6 metabolic effect, decreased IL-6 levels in RUN group compared with UTR group. |
| Kunz H.E. et al. (2022) [35] | 63 elderly n3-PUFA = 30, PL = 33 Male = 29 Female = 34 | n3-PUFA diet for 6 months | 71.5 ± 4.8 years | Free-living physical activity (MVPA), cardiorespiratory fitness (VO2 max), 1RM, leg endurance test | BMI, SMI, body fat (%) RBC-EPA RBC-DHA CRP, ESR, lean mass (kg), leg lean mass (kg) | ROS, jO2, jATP, ACR, Phe, Tyr, FSR | Waist-worn accelerometer, treadmill | Enhanced anabolic response after supplementation with n3-PUFA related to acute and resistance physical activity, physical mobility maintenance and strengthened skeletal muscles, increased resistance training and strength gain, improved ATP level (contraction energy) after n3-PUFA during physical training with decreased protein synthesis rate, involving anti-inflammatory status after n3-PUFA addition. |
| Aparicio-Ugarriza R. et al. (2018) [31] | 429 older adults Females = 57% Males = 43% | Low fitness group (low PF), Medium fitness group (medium PF), High fitness group (high PF), PHYSMED project, dietary intake (European, Spanish or Majorcan food) | 55–85 years | Chair stand test, aerobic endurance, dynamic balance test (8-foot up-and-go), 6-minute walk test, handgrip strength | BMI, TDW, FFM, | 25(OH)D, total cholesterol, vitamin B12, folate, triglycerides, HDL-c/LDL-c, HCY, creatinine, urea, uric acid, glucose, total protein albumin, haemoglobin, haematocrit, RBC folate, Fe/FER | Handgrip dynamometer | Decreased vitamin B12 and triglycerides in blood concentration and increased HCY, TC, HDL, LDL and creatinine levels, especially in the low PF group. |
| Kawamura T. et al. (2021) [2] | 873 participants Females—296 Males—577 | Dietary intake with vitamin A, C, tocopherol, beta-carotene, WASEDA’S health study | 50–65 years | PF: leg extension power, CRF (mL/kg/min) | BDHQ, BMI, LBM, body fat %, HR, blood pressure, VO2 max | Fasting glucose, HBA1c, TC, HDL-c, LDL-c, TG, TBARS, PC, F2-IsoP, vitamin A, vitamin C α-tocopherol, β-carotene | - | Decrease in oxidative stress by about 10% after physical fitness and nutraceutical intake (with vitamin A, C, tocopherol and beta-carotene) for elderly females and males. |
| Veronese N. et al. (2021) [18] | 4436 participants Females—2578 Males—1858 | Dietary acrylamide intake | 61.3 years | 20 m repeated 2 times, 400 m walking, chair stands repeated 5 times | FFQ, BMI | PASE | - | Poor physical performance was associated with higher acrylamide dietary intake. |
| Imai A. et al. (2018) [29] | 22 volunteers 12—AS group 10—Placebo group Females—11 Males—11 | AS food antioxidants (3 mg astaxanthin and 5 mg sesamin), visual display terminal task, ergometer task | About 60 years | Mental tasks—4 h/day, four sets of 30 min and four sets of 30 min using advanced TMT, physical tasks— 4 h/day with cycle ergometer, 4 weeks | VAS, CFQ, BMI, POMS2, OSA-MA | PCOOH | Bicycle ergometer, plethysmograph | Increased antifatigue effect after AS supplementation, PCOOH level augmentation during physical and mental tasks was reduced in AS. |
| Munguia L. et al. (2019) [33] | 60 subjects, Flavonoid (F) group, nonflavonoid (NF) group but highly alkalinised, placebo group | Cocoa beverage powder once/day | 55–70 years | 12 weeks 6 min walking test, 30 min/day training | TUG, sit-up test, 2 min step-in-place test, glycaemia, TG, HDL-c, LDL-c, TG/HDL index | SMI, EQ-5D | Handgrip strength | Reduced oxidative stress through dietary intake intervention, improved functional and cognitive profile with physical activity and nutraceuticals from the flavonoid group. |
| Amirato G.R. et al. (2021) [27] | 44 participants, exercising group (PE)—21, non-exercising group (NPE)—23 | L-glutamine (Gln) nutraceutical (maltodextrin) 30 days | 60–80 years | Endurance training, strength training, 60–75 min/session 3 times/week | FFT, TUG, 5XST, IPAQ, BMI | D-fructoso amine, insulin, GSH, GSSG, iron, uric acid, TBARs, average power of extensor and flexor knee muscles | Cycle ergometer | Gln administration stimulated gluconeogenesis and controlled glycaemia levels for older adults, increasing redox potential and metabolic immunological, cardiovascular, respiratory and neuromotor adaptations using endurance and strength training for elderly women. |
| Vyas C.M. et al. (2020) [37] | 391 participants, non-Hispanic white—183, Black—110, Hispanic—98 | Behavioural factors (cigarette smoking, alcohol consumption, depression) Nutraceutical dietary supplement with vitamin D and omega-3 | Mean 67 years | Low, intermediate and vigorous physical activity (LMVPA) training (<5.5–29.9 > 30 MET hours/week) | METs PHQ-8, BMI | mtDNAcn | Bicycle | Lowered mtDNA copy number for smoking participants and negative impact, especially on Black population, not related to other lifestyle and behavioural factors for lower mtDNA copy. |
| Balmes J.R. et al. (2019) [21] | 87 participants 65%–females 35%–males | Ozone-controlled chambers with different concentrations (0 ppb, 70 ppb, 120 ppb) | 55–70 years | Moderate exercise training | BMI, CT, LDL-c, CRP, IL-6, 8-isoprostane, P-selectin, monocyte-platelets conjugated | Endothelin-1, 3-NT, GSTM1, fibrinogen | - | Decreased 3-NT levels and increased CRP, 8-isoprostane, ET-1 and IL-6 proinflammatory markers after ozone exposure to elderly. However, changes in serum levels of proinflammatory factors had no significant effect. |
| Busquets-Cortés C. et al. (2018) [22] | 127 participants Female—66 Male—61 Inactive group—40 Intermediate group—41 Active group—46 | European food, Spanish food, dietary intake with vitamin C, vitamin E, Zn, Se | 55–80 years | Intermediate and active exercise training | BMI, body fat % | CAT, SOD, GRd, GPx, MDA, 3-NT, TrxR1, PBMCs | - | Increased antioxidative activity and attenuated inflammatory status-related aging due to an active lifestyle and regular physical activity promotion, improved oxidative metabolism capacity in PBMCs and enhanced antioxidant defences. |
| Ten Haaf D.S.M, et al. (2020) [30] | 104 subjects 81%—male 19%—female protein group—50 placebo group—54 | Protein dietary intake for 12 weeks with 2 nutraceutical proteins/day | 67–73 years | 30/40/50 km/day walking training (endurance exercises), 3 days, consecutive | BMI, NPRS | CK, CRP, IL-6, IL-10 | - | Protein nutraceutical administered to healthy older adults did not affect muscle damage, soreness and fatigue after prolonged moderate-intensity walking training. |
| Batista R.A.B. et al. (2022) [38] | 950 subjects 474—females 476—males | Glycohaemoglobin group (<5.7%), glycohaemoglobin group (>5.7%), dietary intake with omega-3 (2–4 weeks) | 50–85 years | Moderate or vigorous physical activity, strength training | BMI | Fasting glucose, HBA1c %, plasma omega-3, ALA, EPA, DHA | Handgrip strength- isokinetic dynamometer | Increased plasma omega-3 level improved muscle strength in elderly people with normal glycaemia, decreased inflammation and oxidative stress related to physical activity training of different degrees. |
| Authors | Participants | Characteristics | Age/Gender | Physical Exercises | Primary Outcomes | Secondary Outcomes | Tools | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Daimiel L. et al. (2020) [49] | 6874 participants 48.5% females, 51.5% males | PREDIMED-plus trial PF—physical fitness (PF quartiles) Chair stand test PA—physical activity (PA levels) Rapid assessment physical activity questionnaires | 60–70 years | Chair stand test (30 s), light physical activities, moderate physical activities (≤150 min/week), vigorous physical activities (≤75 min/week), high PA (>150 min/week) | BMI, Er-Med diet, MMSE, COWAT, DS, TMT, CDT | SF36-HRQL, RAPA, MET/min/week | Chair stand, Stopwatch | Enhanced scores for verbal and phonemic verbal fluency related to PF, decreased TMT time in PF, improved neurocognitive parameters related to PF, increased cognitive function and quality of life in activities correlated with PF and PA. |
| Alghadir A.H. et al. (2021) [17] | 106 individuals 44 females 62 males | Sedentary group (n = 29), moderately active group (n = 37), highly active group (n = 40) | 56–81 years | Active exercises LTPA | VO2 max, RER, HCY, Vitamin E, NO, TAC | GPAQ, METs, LOTCA, MMSE | Treadmill, Accelerometer | Decreased glycosylated haemoglobin and homocysteine levels related to moderate and high physical activity, lowered vitamin E levels related to degrees of cognitive capacity damage, increased serum levels of oxidative stress markers and α- and γ-tocopherol at higher-intensity physical activity, and better cognition capacity. |
| Netz Y. et al. (2021) [50] | 122 subjects | Younger elderly female (<74 years) elderly female (≥75 years) Males (n = 36) PA, PF | 65–82 years | PA: habitual physical activity, low-, moderate- or high-level exercises, PF | BMI, IPAQ-SF, MMSE, METs, PSQI, GDS | FI, VO2 max, H2O2 | Treadmill | The correlation of FI with oxidative stress markers, BMI, physical and fitness activities and sleep disorders suggested an improvement in motor function, mental and emotional status and sleep quality in elderly men and younger elderly women. |
| Ku B.J. et al. (2021) [19] | 16 women TSDG—8 Control G—8 | Postmenopausal status | >45 years | 12-week Taekwondo training course, 4 times/week, session—60 min | BMI, fat mass (kg), percent fat (%), LBM | MDA, SOD, IL-6, α-TNF | Wear M430 device | Decreased MDA and increased SOD after Taekwondo training course, reduced oxidative stress after physical activity, decreased IL-6 and TNF blood levels, improved agility and motor functions for menopausal women after intervention of Taekwondo training course. |
| Morucci G. et al. (2022) [16] | 18 participants 14—female 4—men | Functional fitness, reactive oxygen metabolites, biological antioxidant potential | 62–86 years | 24-week multimodal exercise program, 1 h/session, twice/week | BMI, body fat % FFTs (HST, CSST, BST, V-SRT, TUG, 6-MWT) | Salivary cortisol levels, ROM, EuroQol-5 dimension-3 level | Chairs, Elastic bands, Sticks, Electronic hand dynamometer | Increased flexibility for upper and lower limbs after multimodal exercise training application, fitness capacity maintenance from daily living activities, decreased body fat percentage improved muscle strength, aerobic endurance, dynamic balance and biomechanical and physiological parameters, cognitive function amelioration due to intense physical activity, which stabilised anti- and pro-oxidative balance status. |
| Takahashi M. et al. (2019) [52] | 38 participants | Active group (PA)—19 Control group—19 | 70.2 ± 3.9 years | 8 weeks MVPA, ADL | BMI, METs systolic blood pressure, diastolic blood pressure | GDS, step count (steps/day) BDNF, serotonin, ROMs, HEL, BAP, TRX | Uniaxial accelerometer | Increased step count, BDNF, serotonin levels after MVPA, depression prevention associated with decreased serotonin concentrations in older women due to PA, decreased oxidative stress, which promotes depression in older adults, using PA. |
| Mesquita P. et al. (2019) [11] | 13 Participants (males) | Muscle biopsies, HSPs | 64 ± 9 years | 6 weeks RT twice/week, 3 sets with 10–12 repetitions | mRNA analysis, FFM | CAT, GDS, SOD, GAPDH | Isokinetic dynamometer | Benefit effect promotion in redox status, especially for CAT and GDS in skeletal muscles after RT training. |
| Yoon J. et al. (2020) [56] | 24 participants, Synap group—15 Control group—9 | Synapsology (SYNAP), Dual-task exercise using traditional games | 65–77 years | 8 weeks, 2 times/week, 60 min/session | BMI, TUG, 5XST, 5 m habitual test, | TMPT, ROMs, BNDF, | Game-like dual-task | Improved motor and cognitive abilities for older adults who participated in intervention games such as dual-tasks. |
| Valado A. et al. (2022) [24] | 37 participants, experimental group—27, control group—10 | Therapeutic pool with aquatic exercise training | 60–89 years | hydrotherapy exercises, 15 sessions, 30 min/session | SOD | GPx, GR | - | Decreased bone damage risk due to aquatic exercise protocol application, increased enzyme-related antioxidant effects, hydrotherapy stimulated antioxidant defence for older adults. |
| Sánchez-Rodríguez M.A. et al. (2021) [1] | 177 subjects (women) | Mexican community-dwelling women, behavioural factors | 46–69 years | PA (walking, aerobics exercises, swimming, yoga, running) > 30 min/day | BMI, glucose, cholesterol, triglycerides, HDL-c | MDA, GPx, SOD, uric acid, SOD/GPx ratios, SS (stress score) | - | Increased oxidative stress index associated with sedentary lifestyle, smoking and coffee and alcoholic beverage consumption. |
| Kozakiewicz M. et al. (2019) [12] | 327 participants Younger elderly inactive (YN)—112, younger elderly active (YA)—112, older inactive (ON)—128, older active (OA)—41 | Younger elderly—65–74 years, Older—90–99 years | 65–99 years | LTPA | BMI, METs, SDPAR, TC | MDA, SOD, CAT, GPx GR, SOD/GPx ratios, CP, isoprostanes | - | Increased GPx and CAT activity in both younger elderly and the oldest groups related to moderate physical activity, enhanced SOD/GPx ratios in younger elderly men compared with inactive older men of all groups, promoting moderate physical exercises caused decreased oxidative stress and had a beneficial effect on both younger elderly and the oldest groups. |
| Authors | Participants | Characteristics | Age/Gender | Physical Exercises | Primary Outcomes | Secondary Outcomes | Tools | Conclusions |
|---|---|---|---|---|---|---|---|---|
| Gaitán J.M. et al. (2021) [36] | 23 subjects: 50% female | Dietary nutraceuticals, UPA group, EPA group | 60–69 years | 26 weeks, aerobic exercise training, 150 min/week UPA, EPA, 3 sessions/week | VO2 peak CTSB, BDNF, Klotho protein, MMSE, CVLT | TC, TG, HDL-c, LDL-c, Non-HDL-c, lipid metabolites, nonlipid metabolism | Treadmill, accelerometer, | Increased CTSB associated with EPA, ameliorated cognitive function associated with aerobic training, decreased BDNF during physical activity, cardiorespiratory fitness associated with Klotho, enhanced phospholipids and PUFAs in the EPA group. |
| Malek Rivan N.F. et al. (2019) [40] | 815 participants: cognitively pre-frail group (37.4%), cognitively frail group (2.2%), | LRGS-TUA study, questionnaire–interview low niacin intake | 60 years | ADL, IADL, physical fitness, | BMI, MMSE, GDS, PASE, digit span, RAVLT, MoCA, MOS-SS, WHODAS, DHQ | SOD, MDA, DNA damage, vitamin D, BDNF, telomerase | Handgrip, chair | Decreased ADL associated with cognitive impairments and physical frailty, lowered niacin intake associated with enhanced risk of cognitive frailty in dementia, oxidative stress markers such as MDA and telomerase levels being suggestive of cognitive frailty in older adults. |
| Silva L.A.D. et al. (2019) [51] | Depression group—16 subjects, Non-depression group—14 subjects | Pool with depth of 1.20 m, 25 m × 12.5 m, water with temperature about 26 °C, dietary intake protein | 63.5 ± 8.8 years | 12 weeks of aquatic training, 2 times/week, 45 min/session | BMI, HR, Borg scale, BDI, BAI, TUG, BBS | GSH, SOD, nitric oxide, protein carbonylation | Stopwatch, ruler, chair | Aquatic training program contributed to decreasing anxiety, depression and other cognitive impairments, improved physical functions and SOD/GSH levels in the non-depression group, lowered oxidative stress after undergoing aquatic training. |
| Reid S.N.S. et al. (2019) [43] | 60 participants: FST group—32, control group—28 | Nutraceutical dietary intake with fermented Laminaria japonica (FST) 1.5 g/day for 6 weeks. | 67–81 years | Physical fitness | MMSE, numerical memory test, Raven’s test, Flanker test, iconic memory test, TMT, 6-MW, TUG | SOD, GSR, GPx, TBARS, 8-oxoDG, BDNF | Stopwatch, armchair | Increased cognitive functions after supplementation with FST, enhanced antioxidant activity for enzymes such as: SOD, GSR, GPx, decreased oxidative stress markers’ (TBARS and 8-oxoDG) levels after FST administration for 6 weeks, increased BDNF levels associated with neuromuscular integrity and physical functions after FST nutraceuticals. |
| Zaenker P. et al. (2018) [55] | 26 participants: female—19, male—7 | EDSS 0–5 Dietary intake | about 54 years | 12 weeks, high-intensity training—one session/week, resistance training—one session/week. | VO2 peak, HR, MTP | LET, QOL, SEP-59, MSQOL-54 | Cycle ergometer, dynamometer, weights, elastic bands | Improved functional capacity for resistance and endurance training in multiple sclerosis, enhanced quality of life, especially for men with multiple sclerosis. |
| Lee W.J. et al. (2020) [42] | 53 participants: PM-EE group—26, placebo group—27 | Nutraceutical with PM-EE times 12 weeks, 600 mg/day | 50–85 years | Regular exercises 2–3–4 times or every day | MMSE CERAD-K, K-CWST, S-GDS | ADAS–cog, memory, language, executive function | - | Improvement in K-CWST, ADAS–cog scales, enhanced cognitive task scores such as attention, perception, spoken language, working memory increased ADL abilities in AD patients. |
| Authors | Participants | Characteristics | Age/Gender | Primary Outcomes | Secondary Outcomes | Tools | Conclusions |
|---|---|---|---|---|---|---|---|
| Jiménez-Delgado A. et al. (2021) [20] | 26 participants with PD | Nutraceutical dietary melatonin—25 mg for 3 months, melatonin–placebo group, placebo–melatonin group | 60–69 years | Lipoperoxides, nitric oxide metabolites, carbonyl groups, CAT | Mitochondrial complex I activity, mitochondrial respiratory control ratio | Decreased oxidative stress markers after supplementation with melatonin, increased complex I activity, catalase and respiratory control ratio. | |
| Hong C.T. et al. (2021) [44] | 18 patients with PD | Combination of molecular hydrogen (H2) and Photo-biomodulation therapy for 2 weeks | 50–80 years | UPDRS part I, II and III H&Y (II/III) | Bun, creatinine, GOT, GPT, WBC, Hb, PLT | Light-emitting diode array | Decreased reactive oxidative species using H2 water intervention, stimulated mitochondrial functions, which increased ATP levels, alleviated cognitive function deterioration. |
| Coles L.D. et al. (2018) [41] | 5 participants (3 female, 2 male) with PD | Nutraceutical with NAC 6000 mg/day for 28 days | 54–73 years | UPDRS (I-III), H&Y, | NAC, Cys, GSH, GSH/GSSG, CAT, MDA, 4-HNE | MRS-Siemens Magnetom | Decreased oxidative stress markers after NAC administration, increased cysteine levels and implicit antioxidant capacity, such as GSH/GSSG and catalase. |
| Yoritaka A. et al. (2021) [54] | 15 patients PD | Molecular hydrogen (H2) inhalation, 2 L/min, 2 times/day, 1 hour, for 16 weeks. | 50–70 years | MDS-UPDRS, PDQ-39 | N1, N8-dyacetylspermidin, 8-hydroxy-2-deoxiguanosine | MHG-2000α-H2 producing machine | H2 gas supplementation is sure and safe but did not beneficially influence PD patients. |
| Gibson G.E. et al. (2020) [46] | 70 patients with AD, benfotiamine group—34, placebo group—36 | Nutraceutical with oral benfotiamine for 12 months, 300 mg, twice/day | 60 years | ADAS–cog, MMSE, METS, SRT, NPI, ADCS–ADL | CDR, FDG, thiamine (Th), AGE | PET (brain positron emission tomography) | Ameliorated cognitive and functional status in AD patients after administration of nutraceuticals with benfotiamine. |
| An Y. et al. (2019) [39] | MCI group—102, control group—68 | Dietary nutraceutical intake with vitamin B, EMCOA study | 50–70 years | MMSE, MoCA, SDMT, AVLT (IR, SR, LR) LMT, DSF, DSB, FFQ (33 items) | Vitamin B6, folate, vitamin B12, Hcy, ROS, MDA, 8-OHdG, 8-isoPGF2α | MALDI-TOF mass spectrometry | Decreased cognitive decline associated with vitamin B12 intake deficiency, adequate diet with folate and vitamin B6 was associated with better cognitive reserves, enhanced Hcy levels and oxidative stress markers in MCI patients with vitamin B deficiency. |
| You Y.X. et al. (2021) [53] | 48 participants | Supplemented nutraceutical with CC 250 mg twice daily for 12 weeks | 60–70 years | MMSE, digit span, RAVLT, VR, POMS | BNDF, COX-2, GSH, MDA, iNOS, SOD | Improved global cognitive function after CC supplementation for 12 weeks, ameliorated mood status after CC, decreased lipid peroxidation and implicit oxidative stress markers. | |
| Schneider L.S. et al. (2019) [47] | 210 participants, ladostigil group—103, placebo group—107 | Addition ladostigil 10 mg/day for 3 years | 55–85 years | CDR (score-0.5), MMSE (>24), WMS-RC (≤18), | NTB, DAD, RAVLT, GDS (>5), medial temporal lobe atrophy scale (>1) | Ameliorated neurodegeneration with ladostigil in mild cognitive impairments after administration at a low dose. | |
| Clark D.O. et al. (2019) [34] | 180 participants, MINDspeed study | MindFoods dietary intake (high-polyphenol food) and speed training (playing control games) for 12 weeks | 60–69 years | FFQ, MMSE, Trail-Making Test, GDS, RBANS | Proinflammatory cytokines, anti-inflammatory cytokines, TBARS, mRNA | iPad, BrainHQ program | Decreased proinflammatory cytokines and oxidative stress markers after intervention with dietary intake of MindFoods and MINDspeed (cognitive training), enhanced anti-inflammatory cytokine levels after administration of nutraceuticals for patients with AD. |
| Ton A.M.M. et al. (2020) [28] | 13 participants: women—11 men—2 | Probiotic kefir nutraceutical dietary intake (2 mL/kg/day) for 90 days | 78 ± 7 years | MMSE, immediate memory test, delayed memory test, Cookie Theft Picture Test, similarity test, Boston Naming Test, TMT-A, Clock-Drawing Test | IL-6, IL-1b, TNF-α, IL-8, IL12p70, IL-10, IL-8/IL-10 ratio, IL-12/IL-10 ratio, ROS, AOPP, NO, PARP-1 | Recall board, picture | Improved cognitive functions, such as memory, visual–spatial function, language abstraction and conceptualisation skills, decreased oxidative stress markers associated with ROS, Increased NO levels and implicit mitochondrial activity enhancement due to lowered membrane potential and improved antioxidant capacity. |
| Mohamed W.A. et al. (2019) [15] | 50 participants: female—22, male—28, LF group, placebo group | Supplemented with lactoferrin (LF) 250 mg/day for 3 months | 60–85 years | MMSE, CDR, ADAS–cog 11 | PI3K, p-Akt, Ach, 5-HT, MDA, NO, GSH, TAC, IL-6, IL-10, p-tau, Aβ42, caspase-3, HSPs, cholesterol | Decreased A beta 42, cholesterol, proinflammatory and oxidative stress markers after LF supplementation for 3 months, lowered heat-shock protein, caspase-3, and p-tau upon intake, ameliorated cognitive functions for patients with AD due to decreased inflammation and oxidative status. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacanoiu, M.V.; Danoiu, M.; Rusu, L.; Marin, M.I. New Directions to Approach Oxidative Stress Related to Physical Activity and Nutraceuticals in Normal Aging and Neurodegenerative Aging. Antioxidants 2023, 12, 1008. https://doi.org/10.3390/antiox12051008
Bacanoiu MV, Danoiu M, Rusu L, Marin MI. New Directions to Approach Oxidative Stress Related to Physical Activity and Nutraceuticals in Normal Aging and Neurodegenerative Aging. Antioxidants. 2023; 12(5):1008. https://doi.org/10.3390/antiox12051008
Chicago/Turabian StyleBacanoiu, Manuela Violeta, Mircea Danoiu, Ligia Rusu, and Mihnea Ion Marin. 2023. "New Directions to Approach Oxidative Stress Related to Physical Activity and Nutraceuticals in Normal Aging and Neurodegenerative Aging" Antioxidants 12, no. 5: 1008. https://doi.org/10.3390/antiox12051008
APA StyleBacanoiu, M. V., Danoiu, M., Rusu, L., & Marin, M. I. (2023). New Directions to Approach Oxidative Stress Related to Physical Activity and Nutraceuticals in Normal Aging and Neurodegenerative Aging. Antioxidants, 12(5), 1008. https://doi.org/10.3390/antiox12051008






