Abstract
Oocyte maturation is a critical step in the completion of female gametogenesis in the ovary; thus, for subsequent fertilization and embryogenesis. Vitrification of embryo also has been shown to be closely associated with oocyte maturation. To improve the quality and developmental potential of bovine oocytes derived from in vitro maturation (IVM), Pre-IVM with C-type natriuretic peptide (CNP), melatonin (MT) and in combination, IGF1, FGF2, LIF (FLI) were supplemented in the IVM medium. In this current study, we cultured bovine oocytes in Pre-IVM with CNP for 6 h before transferring them to the IVM medium supplemented with MT and FLI. The developmental potential of bovine oocytes was then investigated by measuring the reactive oxygen species (ROS), the intracellular glutathione (GSH) and ATP levels, the transzonal projections (TZP), the mitochondrial membrane potential (ΔΨm), cacline-AM, and the expression of related genes (cumulus cells (CCs), oocytes, blastocysts). The results revealed that oocytes treated with a combination of CNP, MT, and FLI had dramatically improved the percentage of oocytes developed to blastocyst, ATP content, GSH levels, TZP intensity, the ΔΨm, cacline-AM fluorescence intensity, and considerably reduced ROS levels of oocytes. Furthermore, the survival rate and the hatched rate after vitrification of the CNP+MT+FLI group were significantly higher than those other groups. Thus, we speculated that CNP+MT+FLI increases the IVM of bovine oocytes. In conclusion, our findings deepen our understanding and provide new perspectives on targeting the combination of CNP, MT and FLI to enhance the quality and developmental potential of bovine oocytes.
1. Introduction
The breeding industry strongly supports the optimization of ovum pick up (OPU) and in vitro embryo production (IVP) as a way to improve genetics in dairy and beef cattle [1]. OPU combined with in vitro fertilization (IVF) is widely used for the genetic improvement of bovine embryos [2]. The embryo production efficiency of OPU-IVF has been reported to be higher than in vivo embryo production [3]. In order to maximize the breeding potential of elite cattle, OPU-IVF can hasten the breeding process by producing a large number of offspring from cattle with high genetic value; hence, increasing the selection intensity and decreasing the generation interval [4].
Even though there have been significant advancements in OPU technology, the production rate of in vitro blastocysts is still low [5]. The main reason for this reduced rate is that the developmental competence of OPU oocytes obtained through in vitro maturation (IVM) is lower compared to oocytes matured in vivo [6]. Synchronization of oocyte meiosis and cytoplasmic maturation is important for successful fertilization and for development before and after implantation [7], although it remains a challenge during IVM of oocytes. Oxidative stress is considered to be one of the factors of the impaired developmental capacity of oocytes and embryos [8]. Hence, enhancing the culture condition is crucial for boosting the quality and the developmental competence of oocytes derived from OPU.
Recent research have shown that C-type natriuretic peptide (CNP) can improve cytoplasmic maturation by maintaining sufficient cAMP levels to sustain the meiotic arrest of bovine oocytes for 6–8 h [9]. melatonin (MT) is a neurohormone secreted by the pineal gland in vertebrates and partly by other peripheral organs such as the retina, the intestine, immune-competent cells, and the gonads [10]. As a potent antioxidant, MT has been utilized to reduce intracellular oxidative stress and to prevent DNA damage in mouse oocytes [11], and decrease the generation of reactive oxygen species (ROS) during the maturation of mouse [12] and bovine [13] oocytes. Growth factors such as Insulin-like growth factor-1 (IGF1) can promote the rate and quality of oocyte maturation; subsequently, improving embryo development [14,15]. Similarly, fibroblast growth factor 2 (FGF2) can enhance nuclear maturation, cumulus cells (CCs)’ survival, and the extracellular matrix quality of bovine oocytes [16]. The leukemia inhibitory factor (LIF) promoted oocyte nuclear maturation and blastocyst development in porcine [17] and bovine [18] oocytes. Importantly, the supplementation of IGF1, FGF2, and LIF (FLI medium) improved porcine [19,20,21] and sheep [22] embryonic development in vitro. However, the mechanism through which the combination of CNP, MT, and FLI enhances the maturation and developmental capacity of oocytes in vitro has not been fully elucidated.
Therefore, the aim of this present study was to investigate how a Pre-IVM system with CNP, and supplementation with MT and FLI during IVM, affected the maturation quality and development potential of bovine oocytes. The findings of this study will contribute to establishing an efficient approach to improve the maturation and developmental capacity of bovine oocytes.
2. Materials and Methods
Animals were treated according to the recommendations of the Institutional Animal Care and Use Committee of the Chinese Academy of Agricultural Sciences.
2.1. Oocyte Collection
During the IVM experiment, an average of 5–8 oocytes were obtained per ovary, and the procedure was performed on 40 ovaries per session. Bovine ovaries were collected from the slaughterhouse and transported to the laboratory within 2 h at a temperature of 30–35 °C. Cumulus-oocyte complexes (COCs) were extracted from 2–8 mm follicles using a sterile 18-gauge needle. COCs were washed and those with at least 3 layers of CCs were chosen to be use for the experiment.
For the OPU experiment, an average of 20 oocytes were extracted per donor, and 4 donors were worked on per session. Oocytes were collected through an ultrasound-guided transvaginal procedure. Before OPU, donors were not given any hormone treatments. All collected COCs from a given donor and OPU session were treated together as one batch during the whole in vitro production procedure.
2.2. Oocytes Pre-IVM and IVM
The oocytes in the experiment were cultured in different IVM media as per the experiment design. The control group involved culturing the COCs in 4-well plates, with each well containing 500 µL of a basic IVM medium made up of TCM199 (Gibco BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone; Gibco BRL), 1 µg/mL estradiol, 10 µg/mL follicle-stimulating hormone (FSH), 50 ng/mL epidermal growth factor, and 10 µg/mL lutrinizing hormone (LH) for 22–24 h at a temperature of 38.5 °C under 5% CO2. In the first experiment, the COCs were first cultured in a pre-IVM medium containing TCM199 supplemented with 10% FBS and CNP (100 nM) in 4-well plates for 6 h at 38.5 °C under 5% CO2. They were then transferred to the basic IVM medium for an additional 22–24 h under the same conditions. For the second experiment, the COCs were cultured in a pre-IVM medium with CNP for 6 h at 38.5 °C under 5% CO2; then, transferred to the basic IVM medium with 10−9 M MT for 22–24 h under the same conditions. In the third experiment, the COCs were first cultured in a pre-IVM medium with CNP for 6 h at 38.5 °C under 5% CO2; then, transferred to the basic IVM medium with MT and FLI (IGF1 (20 ng/mL), FGF2 (40 ng/mL), LIF (20 ng/mL)) for 22–24 h under the same conditions.
2.3. IVF of Oocytes
The IVF procedures were carried out based on the techniques outlined by Brackett et al. [23] with some slight modifications. A straw of frozen semen was thawed in a water bath at 38 °C and mixed with 7 mL of washing medium in a 15 mL centrifuge tube. The mixture was then centrifuged twice at 1500 r/min for 5 min each time. The supernatant was discarded and the semen concentration was diluted to 1 × 107/mL; then, 10 μL of the sperm suspension was mixed with 90 μL of the fertilization medium, which contained 20–30 oocytes for insemination. After 16–18 h, zygotes were transferred to CR1aa medium for 48 h; then, the cleaved embryos were transferred to CR1aa medium containing 10% FBS for 5 days, and half of the medium was replaced every 48 h.
2.4. Vitrification and Thawing of Blastocyst
The methods of vitrification and thawing were changed slightly with reference to Zhao et al. [24]. For vitrification, blastocysts were exposed to a mixture of 10% EG and 10% DMSO for 30 s; then, placed in EDFSF40 for 25 s. The EDFSF40 consisted of FSF solution with 20% (v/v) EG and 20% (v/v) DMSO. The FSF solution contained 300 g/L Ficoll, 0.5 M sucrose, and 20% (v/v) FBS in the DPBS medium. Afterward, the blastocysts were immediately immersed in liquid nitrogen after being absorbed by an OPS. For thawing, the OPS was taken out of the liquid nitrogen and placed in 0.25 M sucrose solution, incubated for 1 min, followed by transfer to a 0.15 M sucrose solution at 38.5 °C for 5 min.
2.5. Analysis of ROS, ATP, and GSH in Oocytes
The ROS levels of the oocytes were evaluated according to Rahimi et al. [25] with some modifications. A group of 10 oocytes was washed 3 times with washing solution (0.1% polyvinyl alcohol (PVA)) and transferred to an M199 medium with fluorescent dye 2′,7-dichlorodihydrofluorescein diacetate (H2DCF-DA; Genmed Scientific Inc., Wilmington, DE, USA). Ten oocytes were incubated at 37 °C for 20 min; then, they were washed 3 times with washing solution and transferred into 96-well dishes. Luminescence was measured by a luminometer (Infinite M200; Tecan Group Ltd., Männedorf, Switzerland) and the abundance of ROS was expressed as photon counts per s (cps).
The ATP levels of a group of 10 oocytes were assessed using the ATP Bioluminescence Assay Kit (ATP Bioluminescence Assay Kit HS II, Roche diagnostics, GmbH Mannheim, Germany), as described in a study by Zhao et al. [26]. Ten oocytes were lysed using 20 μL of ATP-releasing agent. Samples and standard solutions were added to 96-well dishes plates, and each well contained 100 μL ATP detection solution. Luminescence was immediately measured by luminometer (InfiniteM200, Tecan Group Ltd) for 10 s. ATP levels of the samples were calculated based on a standard curve established using internal standards.
The glutathione (GSH) levels of oocytes were assessed using the procedure outlined by Ozawa et al. [27]. A group of 10 oocytes was washed 3 times in Dulbecco’s phosphate buffer solution (DPBS) containing 1 mg/mL polyvinylpyrrolidone (PVP); then, transferred into a microtube containing 50 µL of 6 mM DTNB, 350 µL of 0.33 mg/mL nicotinamide adenine dinucleotide phosphate (NADPH), and 90 µL of distilled water. To start the reaction, 5 μL of 250 U/mL GSH reductase was added. Absorbance was monitored at 412 nm with a spectrophotometer for 3 min, with readings recorded every 30 s. The total GSH content in the oocytes was calculated according to the constructed standard curve.
2.6. Analysis of TZPs in Oocytes
The transzonal projections (TZP) immunofluorescence staining in the oocytes was assessed using the procedure outlined by Yuan et al. [19]. The oocytes were washed 3 times in PBS containing 0.1% PVA, fixed with paraformaldehyde at 37 °C for 30 min, treated with 0.1% Triton X-100 for 5 min, and blocked with a 1 mg/mL BSA-PVA-PBS solution for 1 h. They were then incubated in Rhodamine Phalloidin (ab235138, 1:1000 dilution; Abcam, Cambridge, UK) for 2 h at 37 °C in the dark; then, imaged with confocal microscopy (TCS SP8; Lecia). The fluorescent intensity of the TZP staining was analyzed with ImageJ-Pro-Plus 6.0 software (Version1.40; National Institutes of Health). The fluorescent pixel values of the oocytes were measured from 10 different zona pellucida regions, and each region had the same area; then, the background fluorescence values were subtracted from the final values, and the statistically significant differences among the groups were analyzed.
2.7. qRT-PCR of Candidate Genes in CCs, Oocytes and Blastocysts
COCs were denuded of CCs by treatment with 0.01% hyaluronidase. The total RNA from CCs and oocytes was extracted using TRIzol reagent (Invitrogen, Waltham, MA, USA) as per the manufacturer’s instructions. The total RNA was reversely transcribed to synthesize the first-strand cDNA using random hexamers. The gene’s expression levels of blastocysts was measured using the Single Cell-to-CT quantitative real-time PCR kit (Life Technologies, Carlsbad, CA, USA), following the manufacturer’s protocol. qRT-PCR of mRNA was performed using an ABI 7500 SDS instrument (Applied Biosystems, Foster City, CA, USA) using the following parameters: a 2 min preheat at 95 °C, followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C. The gene expression fold change was analyzed with the 2−△△Ct method, with β-actin used as the reference gene and the results expressed as a ratio to β-actin levels. The PCR primers used in this study are shown in Table 1.

Table 1.
Primers used for qRT-PCR of candidate genes in CCs, oocytes, and blastocysts.
2.8. ΔΨm Examination of Oocytes
The mitochondrial membrane potential (ΔΨm) of oocytes was evaluated by utilizing JC-1 staining (Molecular Probes, Sigma-Aldrich, Missouri, MO, USA). In brief, the oocytes were gathered and incubated with 10 μg/mL JC-1 in 5% CO2 at 38.5 °C for 30 min. Afterward, they were rinsed 3 times with DPBS and subjected to analysis using a laser-scanning confocal microscope (TE2000-U; Nikon, Tokyo, Japan). The examination was conducted using a 488 nm wavelength, with the emission wavelength set at 530 nm. The fluorescent pixel values of the oocytes were measured from 10 different cytoplasmic regions and 10 different cortical regions, and each region had the same area; then, the cytoplasmic mean was subtracted from the cortical mean. The red and green fluorescence ratio was used to analyze ΔΨm.
2.9. Calcein-AM Staining of Oocytes
After IVM, the oocytes were mechanically denuded and transferred to 1 μM calcein-AM labeled for 25 min. The oocytes were washed 3 times in DPBS and then immediately examined by the fluorescence microscopy (Nikon, Tokyo, Japan). The fluorescence intensity of calcein-AM is in pixels [28]. The fluorescent pixel values of the oocytes were measured from 10 different cytoplasmic regions, and each region had the same area; then, the background fluorescence values were subtracted from the final values, and the statistically significant differences among the groups were analyzed.
2.10. Statistical Analysis
The data were presented as mean ± standard error. The analysis was performed using a one-way analysis of variance (anova) with Duncan’s test through SAS software (SAS Institute, Cary, NC, USA), and p < 0.05 was deemed statistically significant. All the experiments were repeated at least 3 times.
3. Results
3.1. Effect of the Combination Treatment of CNP, MT, and FLI on the Maturation of Bovine Oocytes Collected Post Mortem or Intra Vitam by OPU Method
As shown in Table 2, the nuclear maturation rate of the bovine oocytes collected post mortem of the CNP+MT+FLI group (92.95 ± 2.48%) was significantly higher than that of the CNP group (83.69 ± 4.85%), the CNP+MT group (85.33 ± 3.57%), and the control group (75.61 ± 5.02%; p < 0.05). Furthermore, the nuclear maturation rate of the oocytes collected Intra Vitam by OPU of the CNP+MT+FLI group (90.48 ± 3.14%) was significantly higher than that of the control group (81.66 ± 2.63; p < 0.05).

Table 2.
The combination treatment of CNP, MT, and FLI on the maturation ability of oocytes.
3.2. Effect of the Combination Treatment of CNP, MT, and FLI on the Developmental Ability of Bovine Oocytes and IVF-Derived
The cleavage rate and the blastocysts development rate of the CNP+MT+FLI group (91.61 ± 5.17%, 51.41 ± 4.73%) were significantly higher than those of the CNP group (79.58 ± 3.14%, 35.40 ± 1.90%), the CNP+MT group (85.03 ± 4.84%, 42.40 ± 3.94%), and the control group (73.51 ± 2.47%, 30.15 ± 2.15%; p < 0.05). The survival rate and the hatched rate after vitrification of the CNP+MT+FLI group (97.37 ± 5.35%, 94.59 ± 8.23%) were significantly higher than those of the CNP group (90.00 ± 8.43%, 85.19 ± 7.26%), the CNP+MT group (91.43 ± 7.19%, 87.50 ± 6.48%), and the control group (90.32 ± 8.17%, 80.77 ± 6.75%; p < 0.05). For the OPU oocytes, the cleavage rate and the blastocysts development rate of the CNP+MT+FLI group (89.36 ± 4.25%, 41.67 ± 2.17%) were significantly higher than those of the control group (75.22 ± 4.38%, 30.49 ± 1.87%; p < 0.05), as presented in Table 3.

Table 3.
The combination treatment of CNP, MT, and FLI on the developmental ability of bovine oocytes and IVF-derived.
3.3. Effect of the Combination Treatment of CNP, MT, and FLI on Gene Expression in Bovine IVF Blastocysts
Meanwhile, as shown in Figure 1, the mRNA expression levels of mitochondrial genes (NDUFS8, TFAM, PINK1) in the blastocysts of the CNP+MT+FLI group were significantly higher compared to the other groups. The mRNA expression of the anti-apoptosis gene BCL2 in the CNP+MT+FLI group was significantly higher compared to the other groups, which was in contrast to the expression of the pro-apoptosis gene BAX (p < 0.05).
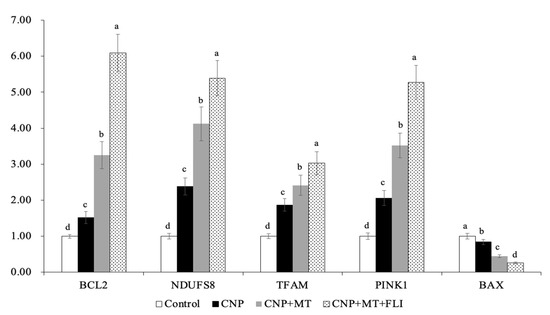
Figure 1.
Effect of the combination treatment of CNP, MT, and FLI on the gene expression in blastocysts. a, b, c, d Values with different superscripts indicate significant differences between groups (p < 0.05).
3.4. Effect of the Combination Treatment of CNP, MT, and FLI on ROS, ATP, and GSH Levels in Bovine Oocytes
The ROS levels in the oocytes of the CNP group, the CNP+MT group, and the CNP+MT+FLI group were significantly lower compared to the control group, and the ROS level of the CNP+MT+FLI group was significantly lower compared to the other groups (Figure 2A). The ATP and GSH levels in the oocytes of the CNP group (1.43 ± 0.03 pmol, 9.67 ± 0.04 pmol), the CNP+MT group (1.76 ± 0.02 pmol, 13.99 ± 0.27 pmol), and the CNP+MT+FLI group (2.23 ± 0.06 pmol, 18.40 ± 0.17 pmol) were significantly higher (p < 0.05) compared to the control group (1.05 ± 0.02 pmol, 8.09 ± 0.87 pmol), and the ATP and GSH levels of the CNP+MT+FLI group were significantly higher (p < 0.05) compared to the other groups (Figure 2B,C).
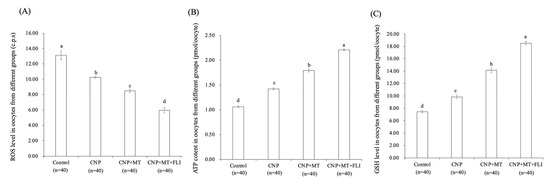
Figure 2.
Effect of the combination treatment CNP, MT, and FLI on the ROS, ATP, and GSH levels in bovine oocytes. (A) Effect of CNP, MT, and FLI on ROS level in bovine oocytes. (B) Effect of CNP, MT, and FLI on ATP content in bovine oocytes. (C) Effect of CNP, MT, and FLI on GSH level in bovine oocytes. a, b, c, d Values with different superscripts indicate significant differences between groups (p < 0.05).
3.5. Effect of the Combination Treatment of CNP, MT, and FLI on TZPs in Bovine Oocytes
As shown in Figure 3, the fluorescence intensity of the CNP group, the CNP+MT group, and the CNP+MT+FLI group was significantly higher compared to the control group, and the fluorescence intensity of the CNP+MT+FLI group was significantly lower compared to the other groups (p < 0.05).
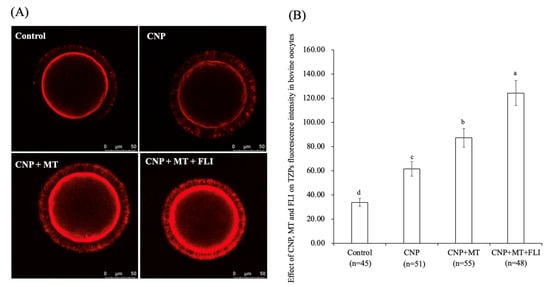
Figure 3.
Effect of the combination treatment CNP, MT, and FLI on TZPs fluorescence intensity in bovine oocytes. (A) Representative image of TZPs staining in bovine oocytes. (B) Effect of the combination treatment of CNP + MT + FLI on TZPs in bovine oocytes. Bar = 50 μm. a, b, c, d Values with different superscripts indicate significant differences between groups (p < 0.05).
3.6. Effect of the Combination Treatment of CNP, MT, and FLI on the ΔΨm in Bovine Oocytes
As shown in Figure 4, the ΔΨm of the CNP group, the CNP+MT group, and the CNP+MT+FLI group was significantly higher compared to the control group, and the ΔΨm of the CNP+MT+FLI group was significantly higher compared to the other groups (p < 0.05).
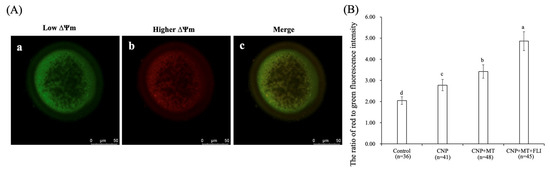
Figure 4.
Effect of the combination treatment CNP, MT, and FLI on the ΔΨm in bovine oocytes. (A) Representative image staining of JC-1 staining in bovine oocytes. a: Green: JC-1 monomeric form (low ΔΨm); b: Red: JC-1-aggregated form (higher ΔΨm); c: merging of images with green and red fluorescence. Bar = 20 μm. (B) Effect of CNP+MT+FLI on the ratio of red to green fluorescence intensity in bovine oocytes. a, b, c, d Values with different superscripts indicate significant differences between groups (p < 0.05).
3.7. Effect of the Combination Treatment of CNP, MT, and FLI on the Calcein-AM Fluorescence Intensity of Bovine Oocytes
As shown in Figure 5, the calcein-AM fluorescence intensity of the CNP group, the CNP+MT group, and the CNP+MT+FLI group was significantly higher compared to the control group, and the calcein-AM fluorescence intensity of the CNP+MT+FLI group was significantly higher compared to the other groups (p < 0.05).
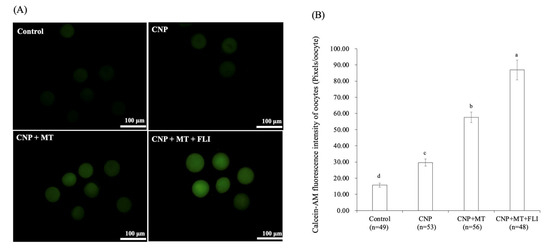
Figure 5.
Effect of the combination treatment CNP, MT, and FLI on calcein-AM fluorescence intensity of bovine oocytes. (A) Representative image staining of calcein-AM in bovine oocytes. Bar = 100 μm. (B) Effect of CNP+MT+FLI on the calcein-AM fluorescence intensity of bovine oocytes. a, b, c, d Values with different superscripts indicate significant differences between groups (p < 0.05).
3.8. Effect of the Combination Treatment of CNP, MT, and FLI on Gene Expression in Bovine Oocytes and CCs
Meanwhile, as shown in Figure 6, the mRNA expression levels of the spindle gene (MPS1), the mitotic arrest deficient genes (Mad1, Mad2), and the TZP-related genes (Myol0, FScn1, Daam1) in the oocytes of the CNP+MT+FLI group were significantly higher compared to the other groups (p < 0.05). The mRNA expression levels of the cumulus expansion-related genes (HAS2, TNFAIP6, PTGS2) in CCs of the CNP+MT+FLI group were significantly higher compared to the other groups, and the stress-related genes (CYP11A1, BAD, TP53) in CCs of the CNP+MT+FLI group were significantly lower compared to the other groups (p < 0.05), as shown in Figure 7.
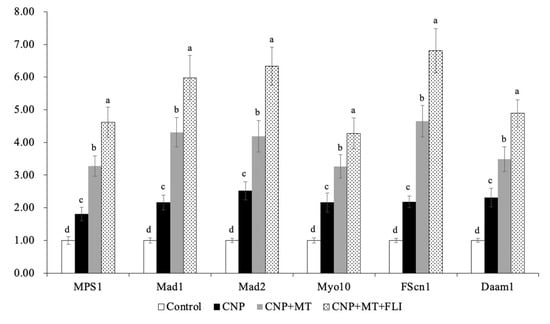
Figure 6.
Effect of the combination treatment CNP, MT, and FLI on the gene expression in bovine oocytes. a, b, c, d Values with different superscripts indicate significant differences between groups (p < 0.05).
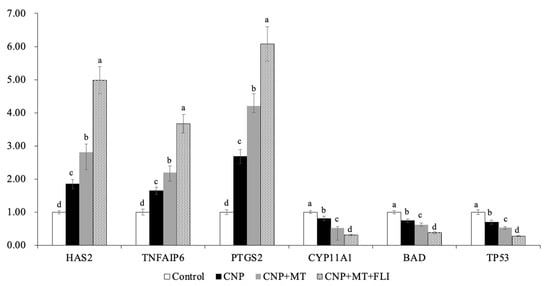
Figure 7.
Effect of the combination treatment of CNP, MT, and FLI on the gene expression in CCs. a, b, c, d Values with different superscripts indicate significant differences between groups (p < 0.05).
4. Discussion
Recent studies revealed that pre-IVM with CNP enhanced the developmental competence of the bovine oocytes [29,30,31]. Our study also demonstrated that the use of a novel IVM system based on CNP pretreatment considerably enhanced the developmental competence of the bovine oocytes (Table 2), which was due to CNP increased oocytes cGMP levels [32]. Evidence from numerous studies on humans [33], bovine [26,34,35], and pigs [36,37] has shown that MT supplementation was beneficial for the IVM of oocytes and their development; and, reducing ROS levels depicts why the combination treatment of CNP and MT significantly enhanced the developmental competence of the bovine oocytes. The oocytes treated with CNP, MT, and FLI showed the highest development potential because FLI promoted porcine oocyte IVM by activating MAPK1/3 [19,38].
Earlier studies have found that CNP may raise cGMP levels, which would boost the maturation rate and the development of vitrified mouse oocytes [39], explaining the survival and hatching rate of the CNP group following vitrification. MT improves the development ability of the vitrified oocytes by reducing ROS [24], and the addition of FLI to the maturation medium of the bovine oocytes can increase cytoskeleton integrity and decrease post-thaw cell apoptosis, and improve the developmental ability of the vitrified bovine oocytes [40]. Our finding explained that the survival and hatching rates of the CNP+MT+FLI group after vitrification were significantly greater than those of the control group, as indicated in Table 3.
TFAM is an autosomal mitochondrial gene that is essential for the transcription and replication of mitochondrial DNA, and silencing TFAM induces mitochondrial dysfunction [41]. In addition, PINK1 is stabilized on the outer membrane of depolarized mitochondria to initiate mitophagy [42]. NDUFS8 is a subunit of the NADH dehydrogenase (ubiquitin) complex; it is located in the inner mitochondrial membrane and is involved in the electron transport chain [43]. The results of our research revealed that the expression levels of TFAM, PINK1, and NDUFA8 were significantly increased due to CNP+MT+FLI, indicating an improvement in mitochondrial function (Figure 1). Moreover, BAX is a pro-apoptotic protein that causes cell death, while BCL-2 is an anti-apoptotic protein that promotes cell survival [44]. Our results demonstrated that the apoptosis of blastocyst was suppressed by the combination treatment of the CNP+MT+FLI because the expression level of the BCL-2 gene was significantly elevated, and the expression level of the BAX gene was decreased (Figure 1).
Foregoing research have found that pre-IVM CNP treatment can significantly reduce ROS levels by inducing SIRT1 abundance and the regulation of genes involved in antioxidant defense [31], promoting ATP production by stimulating mitochondrial development in oocytes [45,46]. GSH was transferred from bovine CCs to oocytes via gap junctions and extended communication functions, which contributed to the accumulation of GSH in the oocytes [30]. These shreds of evidence help to explain the increased ATP and GSH levels, and the decreased ROS levels in the CNP group (Figure 2). In oocytes, MT promotes the antioxidant defense system (NADPH and GSH) and the capacity to scavenge ROS by increasing G6PDH activity [47]. Meanwhile, MT also increases mitochondrial energy production by activating the SIRT1/PGC-1α pathway [48]. In bovine and porcine oocytes, MT supplementation has been shown to decrease intra-oocyte H2O2 and boost intra-oocyte GSH and ATP levels [26,49]. Previous experiments showed that the treatment with IGF1 significantly increased the GSH and decreased the ROS level in oocytes [50]. In addition, IGF1 may act by modulating the expression of the cytochrome c oxidase subunit 1 gene, which, in turn, increases ATP levels by acting indirectly on the respiratory chain activity of oocytes [51]. Hence, we found the highest level of GSH and ATP, and the lowest level of ROS in the CNP+MT+FLI group.
During the development of follicles and the growth of oocytes, TZPs emerge from CCs and penetrate the zona pellucida. Some TZPs reach the oolemma to establish a direct link between the oocytes and granulosa cells [52]. Communication through TZPs and gap junctions is crucial for the regulation of meiosis in oocytes and the maturation of the oocyte [53,54]. CNP increases cAMP levels by regulating cGMP, and cAMP is mediated by TZPs from CCs to the oocyte [55]. It has been proven that CNP-treated oocytes showed a higher density of TZP and functionally open gap junctions [55,56]. Similarly, our experiments also showed that the TZP level in the oocytes of the CNP group was significantly higher than that of the control group. Meanwhile, supplementation of MT increased the number and density of TZPs by improving the gap junction intercellular communication (GJIC) activity of bovine oocytes [28]. It was shown that FLI increased oocyte TZPs through activating MAPK activation [19]. All of these studies supported the highest TZP level in the CNP+MT+FLI group. Oocyte maturation and development are supported by CCs transferring metabolites and signaling molecules to the oocytes [57], indicating that the development ability of the CNP+MT+FLI group was improved.
Mitochondria are the major source of ATP, and play a vital role in cellular metabolism; ΔΨm activity is closely related to both the developmental potential of oocytes and embryos [58]. It is reported that treatment with CNP before IVM increases the relative abundance of PGC-1α, NRF1, NRF2, and TFAM mRNA transcripts in bovine oocytes, leading to enhanced ΔΨm levels and mitochondrial function [31], which explains the higher ΔΨm level in the oocytes from the CNP group. MT also blocks MPTP to preserve the ΔΨm level of oocytes [59,60]. Furthermore, IGF-I may play a regulatory role in the increase of ΔΨm in oocytes by regulating the expression of the cytochrome c oxidase subunit 1 gene [51]. This evidence led to elucidating the rationale for the highest ΔΨm in the CNP+MT+FLI group (Figure 4B).
GJIC between the CCs and the oocytes was assessed using the fluorescent dye calcein after IVM. It was previously established that the increased level of calcein-AM fluorescence intensity in the oocytes of the CNP group was due to the increased gene expression of connexin alpha 1 and 4 (GJA1 and GJA4) in CCs and the ability of COCs in follicles to take up calcein through gap junctions [61]. Similarly, MT supplementation resulted in increased GJIC activity of bovine oocytes by expanding the GJA4 expression [28]. LIF stimulated the expression of GJA4 for GJIC during follicle development [62]. Our experiments showed that the CNP+MT+FLI group significantly improved the level of calcein-AM fluorescence intensity in oocytes, which explained the increased development ability of bovine oocytes (Figure 5).
Myo10, FScn1 and Daam1 are genes related to the key structural components of TZP in oocytes [47]; Myo10 promotes the formation of actin-TZPs [63], FScn1 mediates cell interactions and migration, and cytoplasmic microfilaments that contribute to cell structure and intracellular movements [64], and Daam1 belongs to the formin family and regulates the nucleation of unbranched actin filaments [65]. A previous study revealed that MT significantly increased the mRNA levels of the Myo10, Fscn1, and Daam1 in mice [47]. Our findings also showed that CNP+MT+FLI significantly increased the expression levels of Myo10, FScn1, and Daam1, which proved that the TZP in oocytes was enhanced (Figure 6). MPS1 is a bispecific kinase which is the core protein of SAC and regulates the mitotic recruitment of other SAC proteins [66]. SAC-related genes and proteins are believed to have a significant regulatory effect on the normal assembly of spindles and segregation of chromosomes during meiosis [67]. In particular, Mad1, Mad2, and Mps1 are crucial in spindle assembly [68] and their proper expression is necessary for chromosome segregation and attachment to dynamic microtubules [67]. Notably, when MT was added to the IVM medium, there was a significant increase in the relative expression of Mps1 and Mad1 in developing mature oocytes [69]. Our study showed that the mRNA expression of MPS1, Mad1, and Mad2 was significantly enhanced by the combination treatment of CNP, MT, and FLI, and this was because MT and growth factors can promote the development of oocytes (Figure 6).
HAS2 is the main enzyme for the synthesis of hyaluronic acid, which is necessary for the expansion of the CCs and signaling during ovulation [70]. PTGS2 is the key enzyme in prostaglandin biosynthesis [71]. TNFAIP6 gene coding hyaluronan binding proteins is necessary for cumulus expansion [72]. The earlier study showed that HAS2 and PGR expressions in the CCs of the mice receiving MT increased compared to the control groups [73], and Tian et al. [74] found that the MT has efficient ovine cumulus expansion. Furthermore, it has been demonstrated that FGF2 in CCs plays a crucial role in promoting cumulus expansion and increasing the expression of genes associated with expansion (HAS2 and PTGS2) [75,76]. Yuan et al. [19] have found that stress-related genes (CYP11A1, BAD, and TP53) were down-regulated (p < 0.05) in CCs from COCs cultured in an FLI medium. Our experiments showed that the mRNA expression of HAS2, TNFAIP6, and PTGS2 was significantly improved by the combination treatment of CNP, MT, and FLI, indicating that the CCs expansion factors were increased (Figure 7). On the contrary, the mRNA expression of CYP11A1, BAD, and TP53 was significantly decreased, indicating that the stress of CCs was inhibited by the combined treatment of CNP+MT+FLI.
5. Conclusions
The combination of CNP, MT, and FLI during IVM significantly improved the quality and development ability of bovine oocytes. Likewise, a combination of CNP, MT, and FLI also increased the ATP, GSH, TZP, ΔΨm, cacline-AM fluorescence intensity of oocytes, and decreased the ROS of oocytes. Our findings inferred that combining CNP, MT, and FLI in the maturation medium resulted in a profound improvement in oocyte competence and in vitro embryo development.
Elucidating the importance of CNP, MT, and FLI for cytobiochemical and molecular balance of intracellular anti- and pro-oxidative processes in IVM-derived heifer/cow oocytes might be helpful for elaborating a variety of biotechnological and embryological parameters. The combination of these parameters might result in the efficient generation of bovine and other mammalian high-quality embryos not only by standard IVF [77,78] but also by intracytoplasmic sperm injection (ICSI) [79,80] or by somatic cell nuclear transfer (SCNT) [81,82].
Author Contributions
Conceptualization, B.Y. and X.X.; Methodology, H.Z. (Hang Zhang) and X.F.; formal analysis, H.H., W.D. and H.Z. (Huabin Zhu); data curation: S.L. and W.Y.; visualization: X.Z.; writing original draft preparation, P.Z.; writing—review and editing, A.K., S.U. and X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the earmarked fund for the National Key R&D Program of China (2022YFE0100200), the National Natural Science Foundation of China (32161143032; 31972568), the Natural Science Foundation of Beijing (6202027), the marked fund for CARS (CARS36), the National Germplasm Center of Domestic Animal Resources and the Agricultural Science and Technology Innovation Program (ASTIP-2016-IAS-06), the Transformation Fund Program for Agricultural Science and Technology Achievements of Hebei Province (21626604D), the Key Research and Development Program of Hebei Province (21326608D), and the Development and demonstration of Holstein Breeding and ovum pick-up and in vitro Fertilization (OPU-IVF) technology (G2022003015L).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (protocol code: IAS2023-4, date of approval: 8 February 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Merton, J.S.; Knijn, H.M.; Flapper, H.; Dotinga, F.; Roelen, B.A.; Vos, P.L.; Mullaart, E. Cysteamine supplementation during in vitro maturation of slaughterhouse- and opu-derived bovine oocytes improves embryonic development without affecting cryotolerance, pregnancy rate, and calf characteristics. Theriogenology 2013, 80, 365–371. [Google Scholar] [CrossRef]
- Balboula, A.Z.; Aboelenain, M.; Sakatani, M.; Yamanaka, K.I.; Bai, H.; Shirozu, T.; Kawahara, M.; Hegab, A.E.O.; Zaabel, S.M.; Takahashi, M. Effect of E-64 Supplementation during in vitro Maturation on the Developmental Competence of Bovine OPU-Derived Oocytes. Genes 2022, 13, 324. [Google Scholar] [CrossRef]
- Merton, J.S.; de Roos, A.P.; Koenen, E.P.; Roelen, B.A.; Vos, P.L.; Mullaart, E.; Knijn, H.M. Bovine OPU-derived oocytes can be matured in vitro for 16-28 h with similar developmental capacity. Reprod. Domest. Anim. 2012, 47, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Fukumoto, Y.; Yamamoto, S.; Ogata, Y.; Horiuchi, T. Variations in bovine embryo production between individual donors for OPU-IVF are closely related to glutathione concentrations in oocytes during in vitro maturation. Theriogenology 2018, 113, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Deb, G.K.; Jin, J.I.; Kwon, T.H.; Choi, B.H.; Bang, J.I.; Dey, S.R.; Cho, I.R.; Kong, I.K. Improved blastocyst development of single cow OPU-derived presumptive zygotes by group culture with agarose-embedded helper embryos. Reprod. Biol. Endocrinol. 2011, 9, 121. [Google Scholar] [CrossRef]
- Nagano, M. Acquisition of developmental competence and in vitro growth culture of bovine oocytes. J. Reprod. Dev. 2019, 65, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.S.; Marques, K.N.G.; Bragança, L.G.M.; Lodde, V.; Luciano, A.M.; Buratini, J. Synchronization of germinal vesicle maturity improves efficacy of in vitro embryo production in Holstein cows. Theriogenology 2020, 154, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, I.; Zare-Shahneh, A.; Goodarzi, A.; Baghshahi, H.; Fouladi-Nashta, A.A. The effect of Tempo and MitoTEMPO on oocyte maturation and subsequent embryo development in bovine model. Theriogenology 2021, 176, 128–136. [Google Scholar]
- Soto-Heras, S.; Paramio, M.T.; Thompson, J.G. Effect of pre-maturation with C-type natriuretic peptide and 3-isobutyl-1-methylxanthine on cumulus-oocyte com-munication and oocyte developmental competence in cattle. Anim. Reprod. Sci. 2019, 202, 49–57. [Google Scholar]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Hu, L.L.; Li, H.G.; Li, X.M.; Xu, Y.; Pang, Y.Q.; Wang, B.; Wang, J.L.; Sun, S.C. Nonylphenol exposure-induced oocyte quality deterioration could be reversed by melatonin supplementation in mice. Environ. Pollut. 2022, 305, 119317. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, S.; Salehi, M.; Farifteh-Nobijari, F.; Hosseini, T.; Hosseini, S.; Ghazifard, A.; Novin, M.; Fallah-Omrani, V.; Nourozian, M.; Hosseini, A. Melatonin modifies histone acetylation during in vitro maturation of mouse oocytes. Cell J. 2018, 20, 244–249. [Google Scholar]
- Yang, M.; Tao, J.; Chai, M.; Wu, H.; Wang, J.; Li, G.; He, C.; Xie, L.; Ji, P.; Dai, Y.; et al. Melatonin improves the quality of inferior bovine oocytes and promoted their subsequent IVF embryo development: Mechanisms and results. Molecules 2017, 22, 2059. [Google Scholar] [CrossRef]
- Kaya, A.; Sağirkaya, H.; Misirlioğlu, M.; Gümen, A.; Parrish, J.J.; Erdoğan Memili, E. Leptin and IGF-I improve bovine embryo quality in vitro. Anim. Reprod. 2017, 14, 1151–1160. [Google Scholar] [CrossRef]
- Sato, A.; Sarentonglaga, B.; Ogata, K.; Yamaguchi, M.; Hara, A.; Atchalalt, K.; Sugane, N.; Fukumori, R.; Nagao, Y. Effects of insulin-like growth factor-1 on the in vitro maturation of canine oocytes. J. Reprod. Dev. 2018, 64, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Barros, R.G.; Lima, P.F.; Soares, A.C.S.; Sanches, L.; Price, C.A.; Buratini, J. Fibroblast growth factor 2 regulates cumulus differentiation under the control of the oocyte. J. Assist. Reprod. Genet. 2019, 36, 905–913. [Google Scholar] [CrossRef]
- Dang-Nguyen, T.Q.; Haraguchi, S.; Kikuchi, K.; Somfai, T.; Bodó, S.; Nagai, T. Leukemia inhibitory factor promotes porcine oocyte maturation and is accompanied by activation of signal transducer and activator of transcription 3. Mol. Reprod. Dev. 2014, 81, 230–239. [Google Scholar] [CrossRef]
- Mo, X.; Wu, G.; Yuan, D.; Jia, B.; Liu, C.; Zhu, S.; Hou, Y. Leukemia inhibitory factor enhances bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2014, 81, 608–618. [Google Scholar] [CrossRef]
- Yuan, Y.; Spate, L.D.; Redel, B.K.; Tian, Y.; Zhou, J.; Prather, R.S.; Roberts, R.M. Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation. Proc. Natl. Acad. Sci. USA 2017, 114, E5796–E5804. [Google Scholar] [CrossRef]
- Serrano Albal, M.; Silvestri, G.; Kiazim, L.G.; Vining, L.M.; Zak, L.J.; Walling, G.A.; Haigh, A.M.; Harvey, S.C.; Harvey, K.E.; Griffin, D.K. Supplementation of porcine in vitromaturation medium with FGF2, LIF, and IGF1 enhances cytoplasmic maturation in prepubertal gilts oocytes and improves embryo quality. Zygote 2022, 30, 801–808. [Google Scholar] [CrossRef]
- Currin, L.; Glanzner, W.G.; Gutierrez, K.; de Macedo, M.P.; Guay, V.; Baldassarre, H.; Bordignon, V. Optimizing swine in vitro embryo production with growth factor and antioxidant supplementation during oocyte maturation. Theriogenology 2022, 194, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Qi, Q.; Yan, F.; Wang, C.; Hou, F.; Ren, W.; Zhang, L.; Hou, J. Enhancing the developmental competence of prepubertal lamb oocytes by supplementing the in vitro maturation medium with sericin and the fibroblast growth factor 2—Leukemia inhibitory factor–Insulin-like growth factor 1 combination. Theriogenology 2021, 159, 13–19. [Google Scholar] [CrossRef]
- Brackett, B.G.; Oliphant, G. Capacitation of Rabbit Spermatozoa in vitro. Biol. Reprod. 1975, 12, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal. Res. 2016, 60, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, G.; Isachenko, E.; Sauer, H.; Isachenko, V.; Wartenberg, M.; Hescheler, J.; Mallmann, P.; Nawroth, F. Effect of different vitrification protocols for human ovarian tissue on reactive oxygen species and apoptosis. Reprod. Fertil. Dev. 2003, 15, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Wang, N.; Hao, H.S.; Li, C.Y.; Zhao, Y.H.; Yan, C.L.; Wang, H.Y.; Du, W.H.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal. Res. 2018, 64, 212445. [Google Scholar] [CrossRef]
- Ozawa, M.; Hirabayashi, M.; Kanai, Y. Developmental competence and oxidative state of mouse zygotes heat-stressed maternally or in vitro. Reproduction 2022, 124, 683–689. [Google Scholar] [CrossRef]
- Hao, T.; Xu, X.; Hao, H.; Du, W.; Pang, Y.; Zhao, S.; Zou, H.; Yang, S.; Zhu, H.; Yang, Y.; et al. Melatonin improves the maturation and developmental ability of bovine oocytes by up-regulating GJA4 to enhance gap junction intercellular communication. Reprod. Fertil. Dev. 2021, 33, 760–771. [Google Scholar] [CrossRef]
- Xi, G.; An, L.; Jia, Z.; Tan, K.; Zhang, J.; Wang, Z.; Zhang, C.; Miao, K.; Wu, Z.; Tian, J. Natriuretic peptide receptor 2 (NPR2) localized in bovine oocyte underlies a unique mechanism for C-type natriuretic peptide (CNP)-induced meiotic arrest. Theriogenology 2018, 106, 198–209. [Google Scholar] [CrossRef]
- Zhenwei, J.; Xianhua, Z. Pre-IVM treatment with C-type natriuretic peptide in the presence of cysteamine enhances bovine oocytes antioxidant defense ability and developmental competence in vitro. Iran. J. Vet. Res. 2019, 20, 173–179. [Google Scholar]
- Jia, Z.; Yang, X.; Liu, K. Treatment of cattle oocytes with C-type natriuretic peptide before in vitro maturation enhances oocyte mitochondrial function. Anim. Reprod. Sci. 2021, 225, 106685. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, C.; Xie, J.; Song, X.; Yin, B.; Liu, Q.; Hu, L.; Hao, H.; Geng, J.; Wang, P. Supplementation with low concentrations of melatonin improves nuclear maturation of human oocytes in vitro. J. Assist. Reprod. Genet. 2013, 30, 933–938. [Google Scholar] [CrossRef]
- Zhao, X.M.; Min, J.T.; Du, W.H.; Hao, H.S.; Liu, Y.; Qin, T.; Wang, D.; Zhu, H.B. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote 2015, 23, 525–536. [Google Scholar] [CrossRef]
- Yaacobi-Artzi, S.; Shimoni, C.; Kalo, D.; Hansen, P.J.; Roth, Z. Melatonin slightly alleviates the effect of heat shock on bovine oocytes and resulting blastocysts. Theriogenology 2020, 158, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, J.Y.; Kim, J.W.; Yang, S.G.; Jung, J.M.; Kim, M.J.; Kang, M.J.; Cho, Y.H.; Wee, G.; Yang, H.Y.; et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J. Pineal. Res. 2018, 64, e12458. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.A.; Cuello, C.; Parrilla, I.; Maside, C.; Ramis, G.; Cambra, J.M.; Vazquez, J.M.; Rodriguez-Martinez, H.; Gil, M.A.; Martinez, E.A. Exogenous Melatonin in the Culture Medium Does Not Affect the Development of In Vivo-Derived Pig Embryos but Substantially Improves the Quality of In Vitro-Produced Embryos. Antioxidants 2022, 11, 1177. [Google Scholar] [CrossRef]
- Procházka, R.; Bartková, A.; Němcová, L.; Murín, M.; Gad, A.; Marcollová, K.; Kinterová, V.; Lucas-Hahn, A.; Laurinčík, J. The Role of MAPK3/1 and AKT in the Acquisition of High Meiotic and Developmental Competence of Porcine Oocytes Cultured In Vitro in FLI Medium. Int. J. Mol. Sci. 2021, 22, 11148. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wei, Q.; Li, W.; Ge, J.; Zhao, X.; Ma, B. C-type natriuretic peptide improved vitrified-warmed mouse cumulus oocyte complexes developmental competence. Cryobiology 2016, 72, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Stoecklein Stoecklein, K.S.; Ortega, M.S.; Spate, L.D.; Murphy, C.N.; Prather, R.S. Improved cryopreservation of in vitro produced bovine embryos using FGF2, LIF, and IGF1. PLoS ONE 2021, 16, e0243727. [Google Scholar] [CrossRef]
- Koh, E.H.; Park, J.Y.; Park, H.S.; Jeon, M.J.; Ryu, J.W.; Kim, M.; Kim, S.Y.; Kim, M.S.; Kim, S.W.; Park, I.S.; et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 2007, 56, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef]
- Weiss, H.; Friedrich, T.; Hofhaus, G.; Preis, D. The respiratory-chain NADH dehydrogenase (Complex I) of mitochondria. Eur. J. Biochem. 1991, 197, 563–576. [Google Scholar] [CrossRef]
- Renault, T.T.; Dejean, L.M.; Manon, S. A brewing understanding of the regulation of Bax function by Bcl-xL and Bcl-2. Mech. Ageing Dev. 2017, 161, 201–210. [Google Scholar] [CrossRef]
- Zeng, H.T.; Ren, Z.; Guzman, L.; Wang, X.; Sutton-McDowall, M.L.; Ritter, L.J.; de Vos, M.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. Heparin and cAMP modulators interact during pre-in vitro maturation to affect mouse and human oocyte meiosis and developmental competence. Hum. Reprod. 2013, 28, 1536–1545. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, C.; Fan, X.; Li, R.; Zhang, J. Effect of c-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. Vitr. Cell Dev. Biol. Anim. 2017, 53, 199–206. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Wen, D.; Li, R.; Lu, S.; Xu, R.; Tang, Y.; Sun, Y.; Zhao, X.; Pan, M.; et al. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox. Biol. 2022, 49, 102215. [Google Scholar] [CrossRef]
- Niu, Y.J.; Zhou, W.; Nie, Z.W.; Shin, K.T.; Cui, X.S. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal. Res. 2020, 68, e12627. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal. Res. 2017, 62, e12388. [Google Scholar] [CrossRef]
- Yang, S.; Yang, Y.; Hao, H.; Du, W.; Pang, Y.; Zhao, S.; Zou, H.; Zhu, H.; Zhang, P.; Zhao, X. Supplementation of EGF, IGF-1, and Connexin 37 in IVM Medium Significantly Improved the Maturation of Bovine Oocytes and Vitrification of Their IVF Blastocysts. Genes 2022, 13, 805. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.R.; Ticianelli, J.S.; Ispada, J.; Risolia, P.H.B.; Rodrigues, T.A.; Paula-Lopes, F.F. The effect of insulin-like growth factor-I (IGF-I) on mitochondrial gene expression of bovine oocytes subjected to heat shock. Anim. Reprod. 2015, 12, 775. [Google Scholar]
- Baena, V.; Terasaki, M. Three-dimensional organization of transzonal projections and other cytoplasmic extensions in the mouse ovarian follicle. Sci. Rep. 2019, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Eppig, J. Mouse oocytes control metabolic co-operativity between oocytes and cumulus cells. Reprod. Fertil. Dev. 2005, 17, 1–2. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Luciano, A.M.; Richani, D.; Zeng, H.T.; Wang, X.; Vos, M.D.; Sugimura, S.; Smitz, J.; Richard, F.J.; Thompson, J.G. Oocyte maturation and quality: Role of cyclic nucleotides. Reproduction 2016, 152, R143–R157. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.; Xingping, G.; Haixia, C.; Zhulin, W.; Huaixiu, W. Assessment of cGMP level in medium during in vitro growth period of murine preantral follicles with and without supplementation of C-type natriuretic peptide. Zygote 2022, 30, 98–102. [Google Scholar] [CrossRef]
- Santiquet, N.; Papillon-Dion, E.; Djender, N.; Guillemette, C.; Richard, F.J. New elements in the C-type natriuretic peptide signaling pathway inhibiting swine in vitro oocyte meiotic resumption. Biol. Reprod. 2014, 91, 16. [Google Scholar] [CrossRef]
- Soares, A.C.S.; Lodde, V.; Barros, R.G.; Price, C.A.; Luciano, A.M.; Buratini, J. Steroid hormones interact with natriuretic peptide C to delay nuclear maturation, to maintain oocyte-cumulus communication and to improve the quality of in vitro-produced embryos in cattle. Reprod. Fertil. Dev. 2017, 29, 2217–2224. [Google Scholar] [CrossRef]
- May-Panloup, P.; Chretien, M.F.; Malthiery, Y.; Reynier, P. Mitochondrial DNA in the oocyte and the developing embryo. Curr. Top. Dev. Biol. 2007, 77, 51–83. [Google Scholar]
- Tan, D.X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- El-Raey, M.; Geshi, M.; Somfai, T.; Kaneda, M.; Hirako, M.; Abdel-Ghaffar, A.E.; Nagai, T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 2011, 78, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Rouhollahi, V.S.; Hajian, M.; Jafarpour, F.; Ghazvini, Z.F.; Nasr-Esfahani, M.H. Granulosa secreted factors improve the developmental competence of cumulus oocyte complexes from small antral follicles in sheep. PLoS ONE 2020, 15, e0229043. [Google Scholar]
- Cadoret, V.; Jarrier-Gaillard, P.; Papillier, P.; Monniaux, D.; Guérif, F.; Dalbies-Tran, R. Leukaemia inhibitory factor modulates the differentiation of granulosa cells during sheep in vitro preantral to antral follicle development and improves oocyte meiotic competence. Mol. Hum. Reprod. 2021, 27, gaab051. [Google Scholar] [CrossRef] [PubMed]
- Granados-Aparici, S.; Volodarsky-Perel, A.; Yang, Q.; Anam, S.; Tulandi, T.; Buckett, W.; Son, W.Y.; Younes, G.; Chung, J.T.; Jin, S.; et al. MYO10 promotes transzonal projection-dependent germ line-somatic contact during mammalian folliculogenesis†. Biol. Reprod. 2022, 107, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.C. Roles of fascin in cell adhesion and motility. Curr. Opin. Cell Biol. 2004, 16, 590–596. [Google Scholar] [CrossRef]
- Venditti, M.; Fasano, C.; Santillo, A.; Aniello, F.; Minucci, S. First evidence of DAAM1 localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. C R Biol. 2018, 341, 228–234. [Google Scholar] [CrossRef]
- Marquardt, J.R.; Fisk, H.A. ARHGEF17 sets the timer for retention of Mps1 at kinetochores. J. Cell Biol. 2016, 212, 615–616. [Google Scholar] [CrossRef]
- Hached, K.; Xie, S.Z.; Buffin, E.; Cladiere, D.; Rachez, C.; Sacras, M.; Sorger, P.K.; Wassmann, K. Mps1 at kinetochores is essential for female mouse meiosis I. Development 2011, 138, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C.; Kim, N.H. Spindle assembly checkpoint and its regulators in meiosis. Hum. Reprod. Update. 2012, 18, 60–72. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, B.; Qazi, I.H.; Yang, H.; Guo, S.; Yang, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Han, H.; et al. Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicel Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes. Cells 2019, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, T.; Segers, I.; Wathlet, S.; Smitz, J. The cumulus cell gene expression profile of oocytes with different nuclear maturity and potential for blastocyst formation. J. Assist. Rerod. Genet. 2011, 28, 31–40. [Google Scholar] [CrossRef]
- Marei, W.F.; Abayasekara, D.R.; Wathes, D.C.; Fouladi-Nashta, A.A. Role of PTGS2-generated PGE2 during gonadotrophin-induced bovine oocyte maturation and cumulus cell expansion. Reprod. Biomed. Online. 2014, 28, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Calder, M.D.; Caveney, A.N.; Westhusin, M.E.; Watson, A.J. Cyclooxygenase-2 and prostaglandin E(2) (PGE(2)) receptor messenger RNAs are affected by bovine oocyte maturation time and cumulus-oocyte complex quality, and PGE(2) induces moderate expansion of the bovine cumulus in vitro. Biol. Reprod. 2001, 65, 135–140. [Google Scholar] [CrossRef]
- Ezzati, M.; Roshangar, L.; Soleimani Rad, J.; Karimian, N. Evaluating the Effect of Melatonin on HAS2, and PGR expression, as well as Cumulus Expansion, and Fertility Potential in Mice. Cell J. 2018, 20, 108–112. [Google Scholar]
- Tian, X.; Wang, F.; Zhang, L.; He, C.; Ji, P.; Wang, J.; Zhang, Z.; Dongying, L.; Ab-ulizi, W.; Wang, X.; et al. Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. Int. J. Mol. Sci. 2017, 18, 4834. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Davis, J.S.; Chen, C.; Li, Z.; Cao, Y.; Sun, H.; Shao, B.S.; Lin, Y.X.; Wang, Y.S.; Yang, L.G.; et al. FGF2/FGFR signaling promotes cumulus-oocyte complex maturation in vitro. Reproduction 2021, 161, 205–214. [Google Scholar] [CrossRef]
- Dhali, A.; Javvaji, P.K.; Kolte, A.P.; Francis, J.R.; Roy, S.C.; Sejian, V. Temporal expression of cumulus cell marker genes during in vitro maturation and oocyte developmental competence. J. Assist. Reprod. Genet. 2017, 34, 1493–1500. [Google Scholar] [CrossRef]
- Timlin, C.L.; Lynn, A.; Wooldridge, L.K.; Uh, K.; Ealy, A.D.; White, R.R.; Lee, K.; Mercadante, V.R.G. Physical parameters of bovine activated oocytes and zygotes as predictors of development success. Zygote 2021, 29, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, G.; Wartalski, K.; Romek, M.; Samiec, M.; Duda, M. The Molecular Quality and Mitochondrial Activity of Porcine Cumulus-Oocyte Complexes Are Affected by Their Exposure to Three Endocrine-Active Compounds under 3D In Vitro Maturation Conditions. Int. J. Mol. Sci. 2022, 23, 4572. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Muñoz, E.; Contreras, M.J.; Arias, M.E.; Felmer, R. Bovine ICSI: Limiting factors, strategies to improve its efficiency and alternative approaches. Zygote 2022, 30, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Briski, O.; Salamone, D.F. Past, present and future of ICSI in livestock species. Anim. Reprod. Sci. 2022, 246, 106925. [Google Scholar] [CrossRef]
- Skrzyszowska, M.; Samiec, M. Generating Cloned Goats by Somatic Cell Nuclear Transfer—Molecular Determinants and Application to Transgenics and Biomedicine. Int. J. Mol. Sci. 2021, 22, 7490. [Google Scholar] [CrossRef] [PubMed]
- Samiec, M.; Skrzyszowska, M.; Lipiński, D. Pseudophysiological transcomplementary activation of reconstructed oocytes as a highly efficient method used for producing nuclear-transferred pig embryos originating from transgenic foetal fibroblast cells. Pol. J. Vet. Sci. 2012, 15, 509–516. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).