Nymphoides peltata Root Extracts Improve Atopic Dermatitis by Regulating Skin Inflammatory and Anti-Oxidative Enzymes in 2,4-Dinitrochlorobenzene (DNCB)-Induced SKH-1 Hairless Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Plant Material and Extraction
2.3. Cell Culture and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.4. Animal Studies
2.5. Evaluation of Ear Swelling in the Oxazolone-Induced BALB/c Mice
2.6. Evaluation of AD Lesion Severity in the 2,4-Dinitrochlorobenzene (DNCB)-Induced SKH-1 Hairless Mice
2.7. Histological Examination
2.8. Immunohistochemistry
2.9. Measurement of Serum IL-4 and IgE by ELISA
2.10. Immunoblotting Analysis
2.11. Isolation and HPLC Analysis of NPR Extract
2.12. Statistical Analysis
3. Results
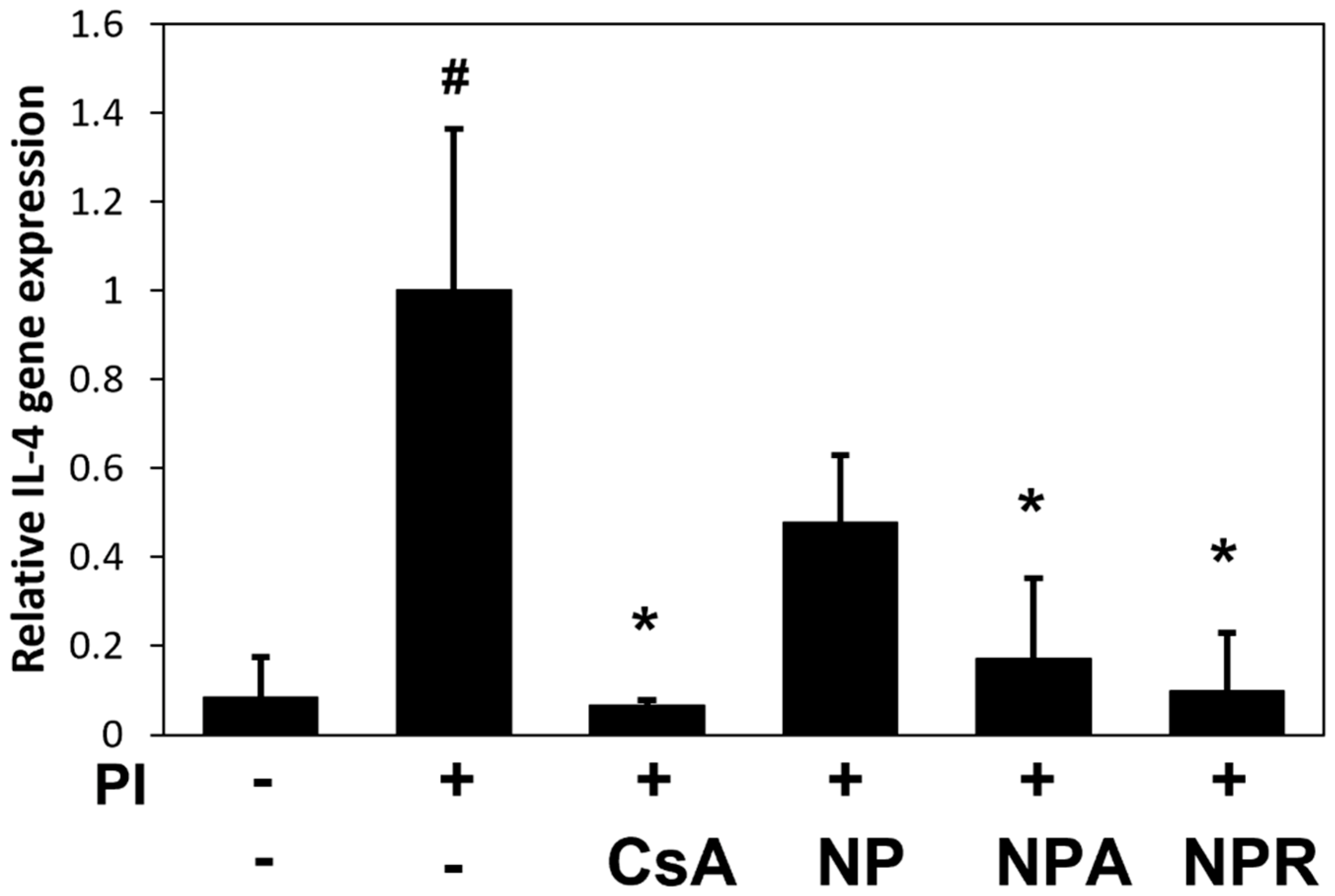
3.1. Inhibitory Effects of N. peltata Extracts (NP, NPA, and NPR) on IL-4 in RBL-2H3 Cells
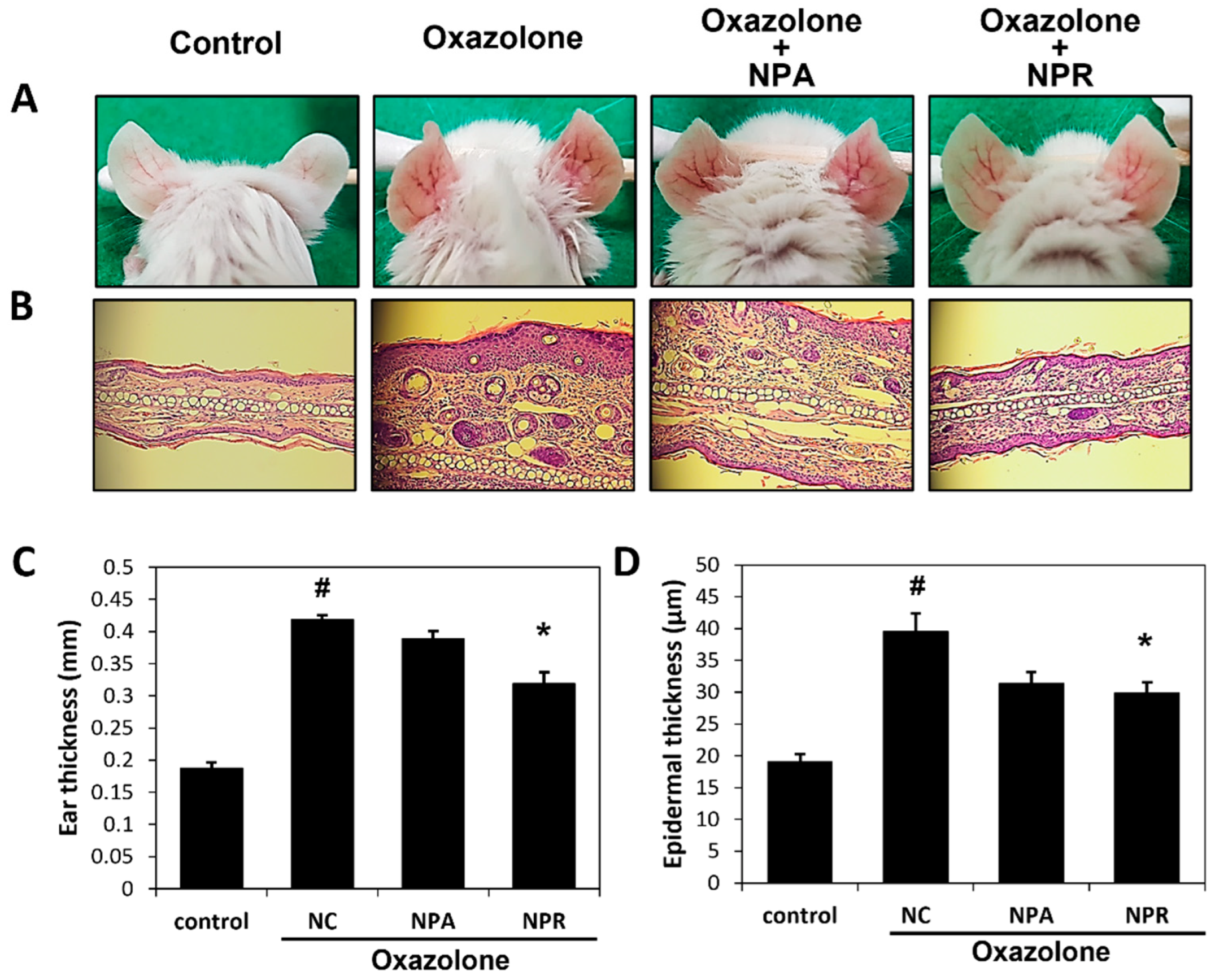
3.2. Inhibitory Effects of N. peltata Extracts (NP, NPA, and NPR) on Oxazolone-Induced Histological Changes BALB/c Mice
3.3. Effects of NPR Extract on Histological Changes in DNCB-SKH-1 Hairless Mice
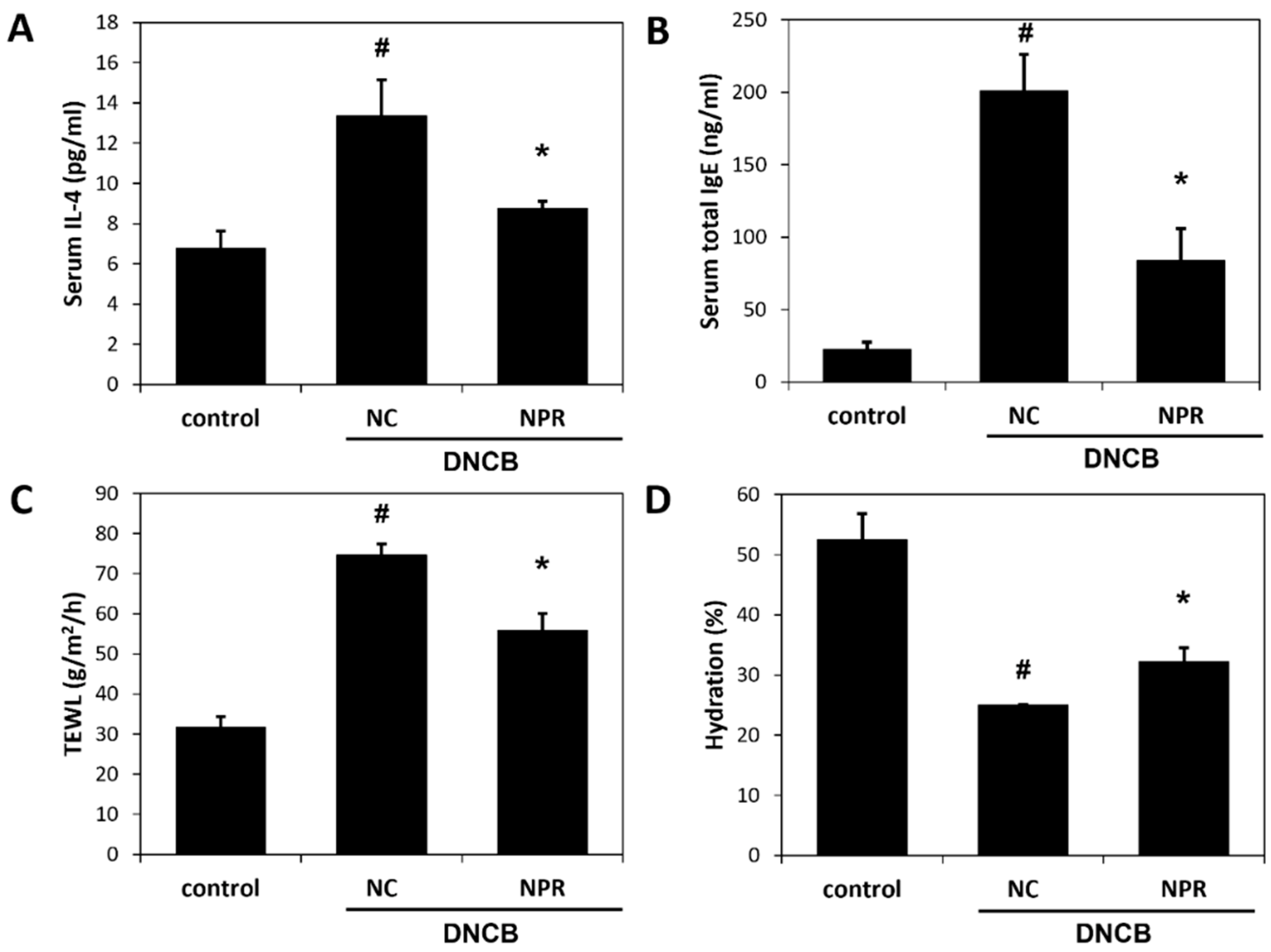
3.4. Effects of NPR Extract on Serum Concentrations of IL-4 and IgE and on Skin Barrier Function in DNCB-SKH-1 Hairless Mice
3.5. Effect of NPR Extract on Skin Barrier Function Associated Proteins in DNCB-SKH-1 Hairless Mice
3.6. Effect of NPR Extract on Antioxidant Enzyme Expressions DNCB-SKH-1 Hairless Mice
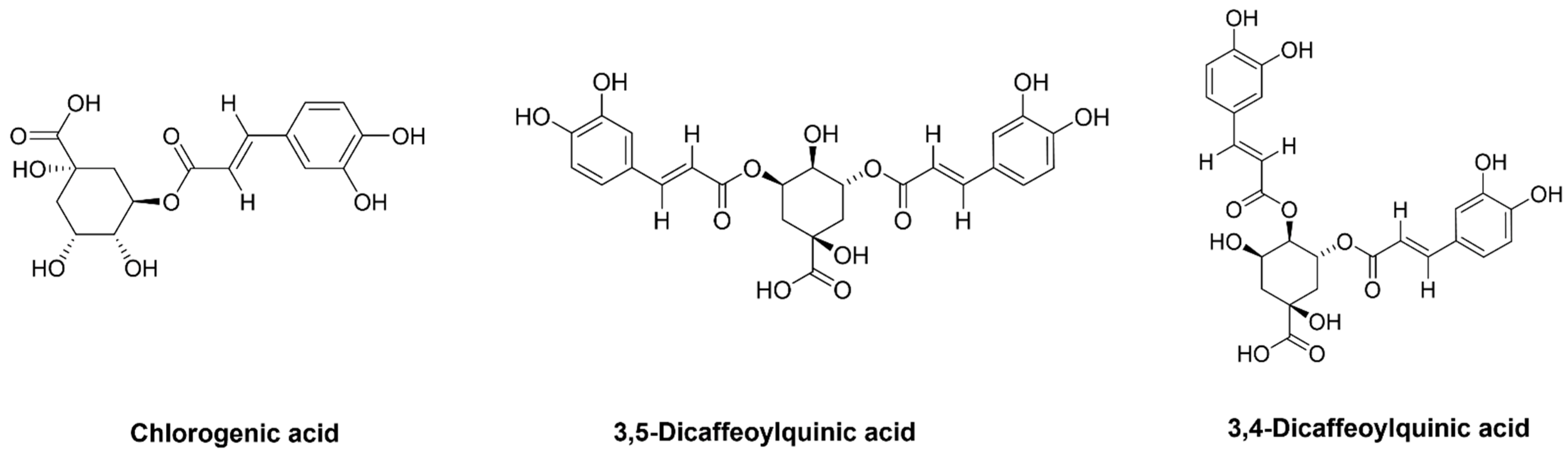
3.7. Isolation of Compounds from NPR Extract
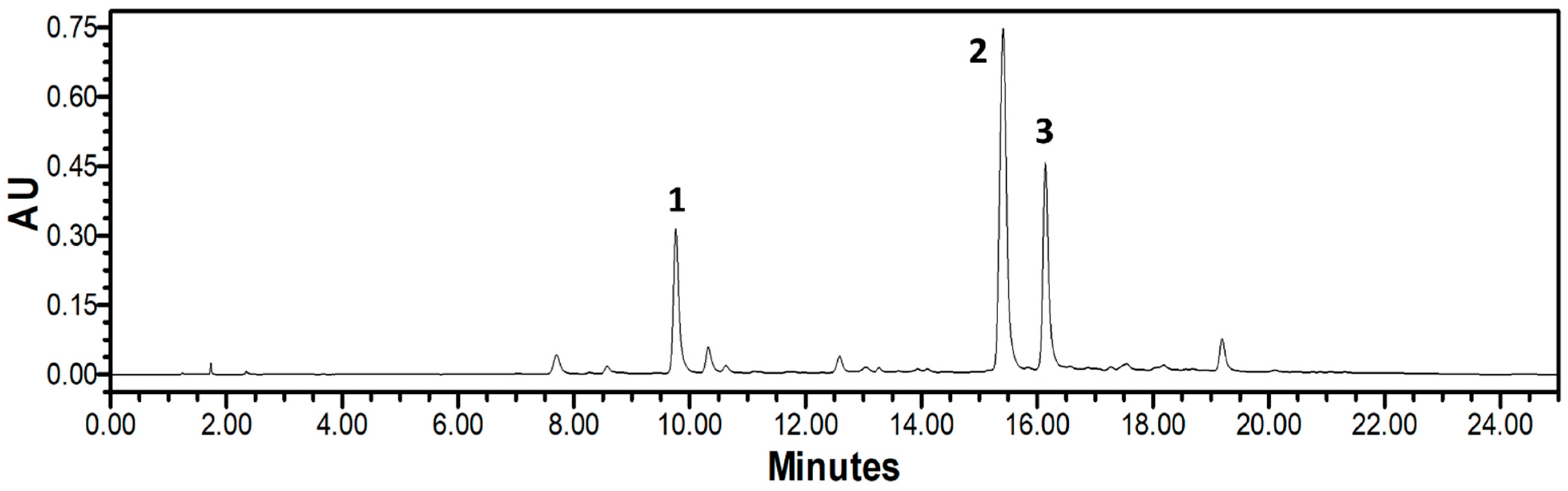
3.8. HPLC-PDA Analysis of NPR Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thestrup-Pedersen, K. Clinical aspects of atopic dermatitis. Clin. Exp. Dermatol. 2008, 25, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Paller, A.S. Atopic dermatitis and the atopic march. J. Allergy Clin. Immunol. 2003, 112, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.M.; Boguniewicz, M.; Howell, M.D.; Nomura, I.; Hamid, Q.A. New insights into atopic dermatitis. J. Clin. Investig. 2004, 113, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Novak, N. Pathogenesis of atopic dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Grewe, M.; Bruijnzeel-Koomen, C.A.; Schöpf, E.; Thepen, T.; Langeveld-Wildschut, A.G.; Ruzicka, T.; Krutmann, J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol. Today 1998, 19, 359–361. [Google Scholar] [CrossRef]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cell in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef]
- O’Regan, G.M.; Irvine, A.D. The role of filaggrin in the atopic diathesis. Clin. Exp. Allergy 2010, 40, 965–972. [Google Scholar] [CrossRef]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic dermatitis: Immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef]
- Van Smeden, J.; Janssens, M.; Kaye, E.C.J.; Caspers, P.J.; Lavrijsen, A.P.; Vreeken, R.J.; Bouwstra, J.A. The importance of free fatty acid chain length for the skin barrier function in atopic eczema patients. Exp. Dermatol. 2014, 23, 45–52. [Google Scholar] [CrossRef]
- Lodén, M. Role of topical emollients and moisturizers in the treatment of dry skin barrier disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef]
- Spergel, J.M. Immunology and Treatment of Atopic Dermatitis. Am. J. Clin. Dermatol. 2008, 9, 233–244. [Google Scholar] [CrossRef]
- Tomi, N.S.; Luger, T.A. The treatment of atopic dermatitis with topical immunomodulators. Clin. Dermatol. 2003, 21, 215–224. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.-K. Oxidative Stress in Atopic Dermatitis. Oxid. Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef]
- Emmert, H.; Fonfara, M.; Rodriguez, E.; Weidinger, S. NADPH oxidase inhibition rescues keratinocytes from elevated oxidative stress in a 2D atopic dermatitis and psoriasis model. Exp. Dermatol. 2020, 29, 749–758. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Larson, D. Reproduction strategies in introduced Nymphoides peltata populations revealed by genetic markers. Aquat. Bot. 2007, 86, 402–406. [Google Scholar] [CrossRef]
- Du, Y.; Wang, R.; Zhang, H.; Liu, J. Anti-tumor constituents of the wetland plant Nymphoides peltata: A case study for the potential utilization of constructed wetland plant resources. Nat. Prod. Commun. 2015, 10, 233–236. [Google Scholar]
- Khan, Z.R.; Chowdhury, N.S.; Sharmin, S.; Sohrab, M.H. Medicinal values of aquatic plant genus Nymphoides grown in Asia: A review. Asian Pac. J. Trop. Biomed. 2018, 8, 113–119. [Google Scholar] [CrossRef]
- Ravishankar, K.; Rao, S.N.; Sastry, V.G. Analgesic and Anti-inflammatory activities of ethanolic, ethyl acetate, and hexane extracts of Nymphoides hydrophylla in experimental animals. Int. J. Green Pharm. 2020, 14, 393. [Google Scholar]
- Nocchi, N.; Duarte, H.M.; Pereira, R.C.; Konno, T.U.P.; Soares, A.R. Effects of UV-B radiation on secondary metabolite production, antioxidant activity, photosynthesis and herbivory interactions in Nymphoides humboldtiana (Menyanthaceae). J. Photochem. Photobiol. B Biol. 2020, 212, 112021. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, V.; Arora, S.; Murali, A.; Yoganarasimhan, S.N. Anti-Convulsant Activity of Aqueous and Alcohol Extracts of Roots and Rhizomes of Nymphoides indica (L.) Kuntze in Swiss Albino Mice. J. Nat. Remedies 2009, 9, 68–73. [Google Scholar] [CrossRef]
- Adnan Amin, A.; Tuenter, E.; Exarchou, V.; Upadhyay, A.; Cos, P.; Maes, L.; Apers, S.; Pieters, L. Phytochemical and Pharmacological Investigations on Nymphoides indica Leaf Extracts. Phytother. Res. 2016, 10, 1624–1633. [Google Scholar] [CrossRef]
- Johansson, S.; Göransson, U.; Luijendijk, T.; Backlund, A.; Claeson, P.; Bohlin, L. A Neutrophil Multitarget Functional Bioassay to Detect Anti-inflammatory Natural Products. J. Nat. Prod. 2002, 65, 32–41. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, D.H.; Yu, J.M.; Park, C.B.; Park, T.S.; Park, B.J. Anti-wrinkle effects of extracts and solvent fractions from Nymphoides peltata on CCD-986sk. J. Appl. Biol. Chem. 2017, 60, 357–362. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, J.C.; Chung, H.Y.; Choi, J.S. Antioxidant Flavonoids and Chlorogenic Acid from the Leaves of Eriobotrya japonica. Arch. Pharm. Res. 1999, 22, 213–218. [Google Scholar] [CrossRef]
- D’Amelio, N.; Papamokos, G.; Dreyer, J.; Carloni, P.; Navarini, L. NMR Studies of Hetero-Association of Caffeine with di-O-Caffeoylquinic Acid Isomers in Aqueous Solution. Food Biophys. 2015, 10, 235–243. [Google Scholar] [CrossRef]
- Shi, S.; Huang, K.; Zhang, Y.; Zhao, Y.; Du, Q. Purification and identification of antiviral components from Laggera pterodonta by high-speed counter-current chromatography. J. Chromatogr. B 2007, 859, 119–124. [Google Scholar] [CrossRef]
- Siddique, N.A.; Mujeeb, M.; Najmi, A.K.; Akram, M. Evaluation of antioxidant activity, quantitative estimation of phenols and flavonoids in different parts of Aegle marmelos. S. Afr. J. Bot. 2010, 4, 001–005. [Google Scholar] [CrossRef]
- Mohd-Esa, N.; Hern, F.S.; Ismail, A.; Yee, C.L. Antioxidant activity in different parts of roselle (Hibiscus sabdariffa L.) extracts and potential exploitation of the seeds. Food Chem. 2010, 122, 1055–1060. [Google Scholar] [CrossRef]
- Kaplan, M.; Mutlu, E.A.; Benson, M.; Fields, J.Z.; Banan, A.; Keshavarzian, A. Use of herbal preparations in the treatment of oxidant-mediated inflammatory disorders. Complement. Ther. Med. 2007, 15, 207–216. [Google Scholar] [CrossRef]
- Hussain, Z.; Thu, H.E.; Shuid, A.N.; Kesharwani, P.; Khan, S.; Hussain, F. Phytotherapeutic potential of natural herbal medicines for the treatment of mild-to-severe atopic dermatitis: A review of human clinical studies. Biomed. Pharmacother. 2017, 93, 596–608. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Howell, M.D.; Fairchild, H.R.; Kim, B.E.; Bin, L.; Boguniewicz, M.; Redzic, J.S.; Hansen, K.C.; Leung, D.Y.M. Th2 Cytokines Act on S100/A11 to Downregulate Keratinocyte Differentiation. J. Investig. Dermatol. 2008, 128, 2248–2258. [Google Scholar] [CrossRef]
- Udkoff, J.; Silverberg, J.I. Validation of Scratching Severity as an Objective Assessment for Itch. J. Investig. Dermatol. 2018, 138, 1062–1068. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Bissonnette, R.; Ungar, B.; Suárez-Fariñas, M.; Ardeleanu, M.; Esaki, H.; Suprun, M.; Estrada, Y.; Xu, H.; Peng, X.; et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2019, 143, 155–172. [Google Scholar] [CrossRef]
- Pickard, C.; Smith, A.M.; Cooper, H.; Strickland, I.; Jackson, J.; Healy, E.; Friedmann, P.S. Investigation of Mechanisms Underlying the T-Cell Response to the Hapten 2,4-Dinitrochlorobenzene. J. Investig. Dermatol. 2007, 127, 630–637. [Google Scholar] [CrossRef]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal Models of Atopic Dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Elias, P.M.; Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr. Allergy Asthma Rep. 2019, 9, 265–272. [Google Scholar] [CrossRef]
- Montero-Vilchez, T.; Segura-Fernández-Nogueras, M.-V.; Pérez-Rodríguez, I.; Soler-Gongora, M.; Martinez-Lopez, A.; Fernández-González, A.; Molina-Leyva, A.; Arias-Santiago, S. Skin Barrier Function in Psoriasis and Atopic Dermatitis: Transepidermal Water Loss and Temperature as Useful Tools to Assess Disease Severity. J. Clin. Med. 2021, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Armengot-Carbo, M.; Hernández-Martín, Á.; Torrelo, A. The Role of Filaggrin in the Skin Barrier and Disease Development Filagrina: Papelen la barrera cutánea y eneldesarrollo de patología. Actas Dermosifiliogr. 2015, 106, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, A.; Nomura, T.; Rerknimitr, P.; Seidel, J.A.; Honda, T.; Kabashima, K. The interplay between genetic and environmental factors in the pathogenesis of atopic dermatitis. Immunol. Rev. 2017, 278, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Liddle, J.; Beneton, V.; Benson, M.; Bingham, R.; Bouillot, A.; Boullay, A.-B.; Brook, E.; Cryan, J.; Denis, A.; Edgar, E.; et al. A Potent and Selective Kallikrein-5 Inhibitor Delivers High Pharmacological Activity in Skin from Patients with Netherton Syndrome. J. Investig. Dermatol. 2021, 141, 2272–2279. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Motohashi, H.; Yamamoto, M. The Keap1-Nrf2 system in cancers: Stress response and anabolic metabolism. Front. Oncol. 2012, 2, 200. [Google Scholar] [CrossRef]
- Ogawa, T.; Ishitsuka, Y. The Role of KEAP1-NRF2 System in Atopic Dermatitis and Psoriasis. Antioxidants 2022, 11, 1397. [Google Scholar] [CrossRef]
- Beyer, T.A.; Auf dem Keller, U.; Braun, S.; Schäfer, M.; Werner, S. Roles and mechanisms of action of the Nrf2 transcription factor in skin morphogenesis, wound repair and skin cancer. Cell Death Differ. 2007, 14, 1250–1254. [Google Scholar] [CrossRef]
- Hennig, P.; Fenini, G.; Filippo, M.D.; Beer, H.-D. Electrophiles against (Skin) Diseases: More Than Nrf2. Biomolecules 2020, 10, 271. [Google Scholar] [CrossRef]
- Xian, Z.; Choi, Y.H.; Zheng, M.; Jiang, J.; Zhao, Y.; Wang, C.; Li, J.; Li, Y.; Li, L.; Piao, H.; et al. Imperatorin alleviates ROS-mediated airway remodeling by targeting the Nrf2/HO-1 signaling pathway. Biosci. Biotechnol. Biochem. 2020, 84, 898–910. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Hong, S.; Joo, T.; Jhoo, J.-W. Antioxidant and Anti-inflammatory Activities of 3,5-Dicaffeoylquinic Acid Isolated from Ligulariafischeri Leaves. Food Sci. Biotechnol. 2015, 24, 257–263. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Amelioration of Oxidative Stress in Caco-2 Cells Treated with Pro-inflammatory Proteins by Chlorogenic Acid Isomers via Activation of the Nrf2–Keap1–ARE-Signaling Pathway. J. Agric. Food Chem. 2018, 66, 11008–11017. [Google Scholar] [CrossRef]



| Position | Compound 1 | Compound 2 | Compound 3 | |||
|---|---|---|---|---|---|---|
| δH (Multi, J in Hz) | δc | δH (Multi, J in Hz) | δc | δH (Multi, J in Hz) | δc | |
| 1 | 76.30 | 73.31 | 74.32 | |||
| 2ax | 1.98–1.85 (m) | 1.90–1.84 (m) | 1.99 (d, 12.4) | 41.12 | ||
| 2eq | 1.75–1.65 (m) | 2.10 (dd, 13.7, 3.9) | 2.14 (d, 10.5) | 41.12 | ||
| 3 | 3.99 (d, 7.1) | 71.57 | 5.13 (dd, 7.3, 3.7) | 71.75 | 5.38 (td, 8.5, 4.4) | 68.38 |
| 4 | 71.76 | 71.51 | 4.94 (dd, 8.3, 3.0) | 74.32 | ||
| 5 | 5.06 (d, 6.7), | 74.71 | 5.13 (dd, 7.3, 3.7) | 71.51 | 4.16 (s) | 68.31 |
| 6eq | 1.98–1.85 (m) | 37.88 | 1.90–1.84 (m) | 35.54 | 1.91–1.81 (m) | 38.10 |
| 6ax | 1.75–1.65 (m) | 37.88 | 2.02 (dd, 13.5, 8.0) | 35.54 | 2.14 (d, 10.5) | 38.10 |
| 7 | 176.01 | 176.41 | 175.42 | |||
| 1′ | 126.10 | 126.18 | 125.94 | |||
| 2′ | 7.00 (d, 2.1) | 115.27 | 7.01 (dd, 5.6, 2.1) | 116.35 | 7.01 (dd, 3.5, 2.1) | 116.31 |
| 3′ | 146.11 | 145.61 | 146.04 | |||
| 4′ | 148.89 | 148.91 | 149.00 | |||
| 5′ | 6.72 (d, 8.1), | 116.29 | 6.73 (dd, 8.2, 2.1) | 115.34 | 6.73 (dd, 8.2, 2.1) | 115.38 |
| 6′ | 6.94 (dd, 8.1, 2.0) | 121.87 | 6.94 (dd, 7.2, 2.0) | 122.04 | 6.96 (dd, 8.8, 2.1) | 121.99 |
| 7′ | 7.39 (d, 15.9) | 145.40 | 7.42 (dd, 15.9, 12.2), | 146.11 | 7.45 (dd, 15.9, 12.2) | 146.11 |
| 8′ | 6.14 (d, 15.9), | 114.91 | 6.21 (d, 15.9) | 115.04 | 6.21 (d, 15.9) | 114.37 |
| 9′ | 166.48 | 166.82 | 166.57 | |||
| 1″ | 126.10 | 125.94 | ||||
| 2″ | 7.01 (dd, 5.6, 2.1) | 116.29 | 7.01 (dd, 3.5, 2.1), | 116.25 | ||
| 3″ | 145.27 | 146.04 | ||||
| 4″ | 148.78 | 149.00 | ||||
| 5″ | 6.73 (dd, 8.2, 2.1) | 115.17 | 6.73 (dd, 8.2, 2.1) | 115.38 | ||
| 6″ | 6.94 (dd, 7.2, 2.0) | 121.84 | 6.96 (dd, 8.8, 2.1) | 121.90 | ||
| 7″ | 7.42 (dd, 15.9, 12.2), | 146.11 | 7.45 (dd, 15.9, 12.2) | 146.08 | ||
| 8″ | 6.15 (d, 15.9) | 114.76 | 6.15 (d, 15.9) | 114.16 | ||
| 9″ | 166.36 | 166.18 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-Y.; Park, N.-J.; Jegal, H.; Paik, J.-H.; Choi, S.; Kim, S.-N.; Yang, M.H. Nymphoides peltata Root Extracts Improve Atopic Dermatitis by Regulating Skin Inflammatory and Anti-Oxidative Enzymes in 2,4-Dinitrochlorobenzene (DNCB)-Induced SKH-1 Hairless Mice. Antioxidants 2023, 12, 873. https://doi.org/10.3390/antiox12040873
Kim T-Y, Park N-J, Jegal H, Paik J-H, Choi S, Kim S-N, Yang MH. Nymphoides peltata Root Extracts Improve Atopic Dermatitis by Regulating Skin Inflammatory and Anti-Oxidative Enzymes in 2,4-Dinitrochlorobenzene (DNCB)-Induced SKH-1 Hairless Mice. Antioxidants. 2023; 12(4):873. https://doi.org/10.3390/antiox12040873
Chicago/Turabian StyleKim, Tae-Young, No-June Park, Hyun Jegal, Jin-Hyub Paik, Sangho Choi, Su-Nam Kim, and Min Hye Yang. 2023. "Nymphoides peltata Root Extracts Improve Atopic Dermatitis by Regulating Skin Inflammatory and Anti-Oxidative Enzymes in 2,4-Dinitrochlorobenzene (DNCB)-Induced SKH-1 Hairless Mice" Antioxidants 12, no. 4: 873. https://doi.org/10.3390/antiox12040873
APA StyleKim, T.-Y., Park, N.-J., Jegal, H., Paik, J.-H., Choi, S., Kim, S.-N., & Yang, M. H. (2023). Nymphoides peltata Root Extracts Improve Atopic Dermatitis by Regulating Skin Inflammatory and Anti-Oxidative Enzymes in 2,4-Dinitrochlorobenzene (DNCB)-Induced SKH-1 Hairless Mice. Antioxidants, 12(4), 873. https://doi.org/10.3390/antiox12040873







