Improving Blueberry Fruit Nutritional Quality through Physiological and Genetic Interventions: A Review of Current Research and Future Directions
Abstract
1. Introduction
2. Blueberry Species and Characteristics
3. Major Phytochemicals in Blueberry Fruits and Leaves
| Nutritional Composition | (mg/100 g) | References | ||
|---|---|---|---|---|
| Vitamins | Vitamin C | 3.4–9.5 | [25] | |
| Vitamin B complex | Vitamin B1 | 19.6–26.7 | [25] | |
| Vitamin B2 | 38.0–70.2 | [25] | ||
| Vitamin B3 | 1.0–1.7 | [25] | ||
| Vitamin B6 | 0.052 | [52] | ||
| Vitamin E | 0.57 | [52] | ||
| Vitamin K | 56.1–79.9 | [25] | ||
| Vitamin A | 5.0–83.1 | [25] | ||
| Macro-elements | Nitrogen | 74.4–103.1 | [28] | |
| Calcium | 6.6–15.2 | [28] | ||
| Magnesium | 4.5–10.1 | [28] | ||
| Potassium | 66.2–98.0 | [28] | ||
| Phosphorus | 6.8–20.3 | [28] | ||
| Sulphur | 10.1–25.4 | [28] | ||
| Micro-elements | Iron | 0.15–0.57 | [25] | |
| Manganese | 0.14–1.52 | [28] | ||
| Copper | 0.01–0.09 | [28] | ||
| Boron | 0.08–0.14 | [25] | ||
| Molybdenum | 0.003–0.012 | [28] | ||
| Zinc | 0.06–0.13 | [25] | ||
| Total Phenolic Content | 393 ± 52 | [52] | ||
| Total Flavonoids | 2.5–387.48 | [53] | ||
| Anthocyanidins (mg/kg FW) | 134 | [54] | ||
| Anthocyanins | 233 ± 34 | [52] | ||
| Malvidins | 22–33% | [53] | ||
| Delphinidins | 27–40% | [53] | ||
| Petunidins | 19–26% | [55] | ||
| Cyanidins | 5.7–14% | [55] | ||
| Peonidins | 1.4–4.5% | [55] | ||
| Flavonols (mg/kg FW) | 38–46 | [52,54] | ||
| Quercetin | 24 | [56] | ||
| Myricetin | 26 | [56] | ||
| Flavanols (mg/kg FW) | 1.1 | [54] | ||
| Carotenoids | Lutein | 1.53 | [26] | |
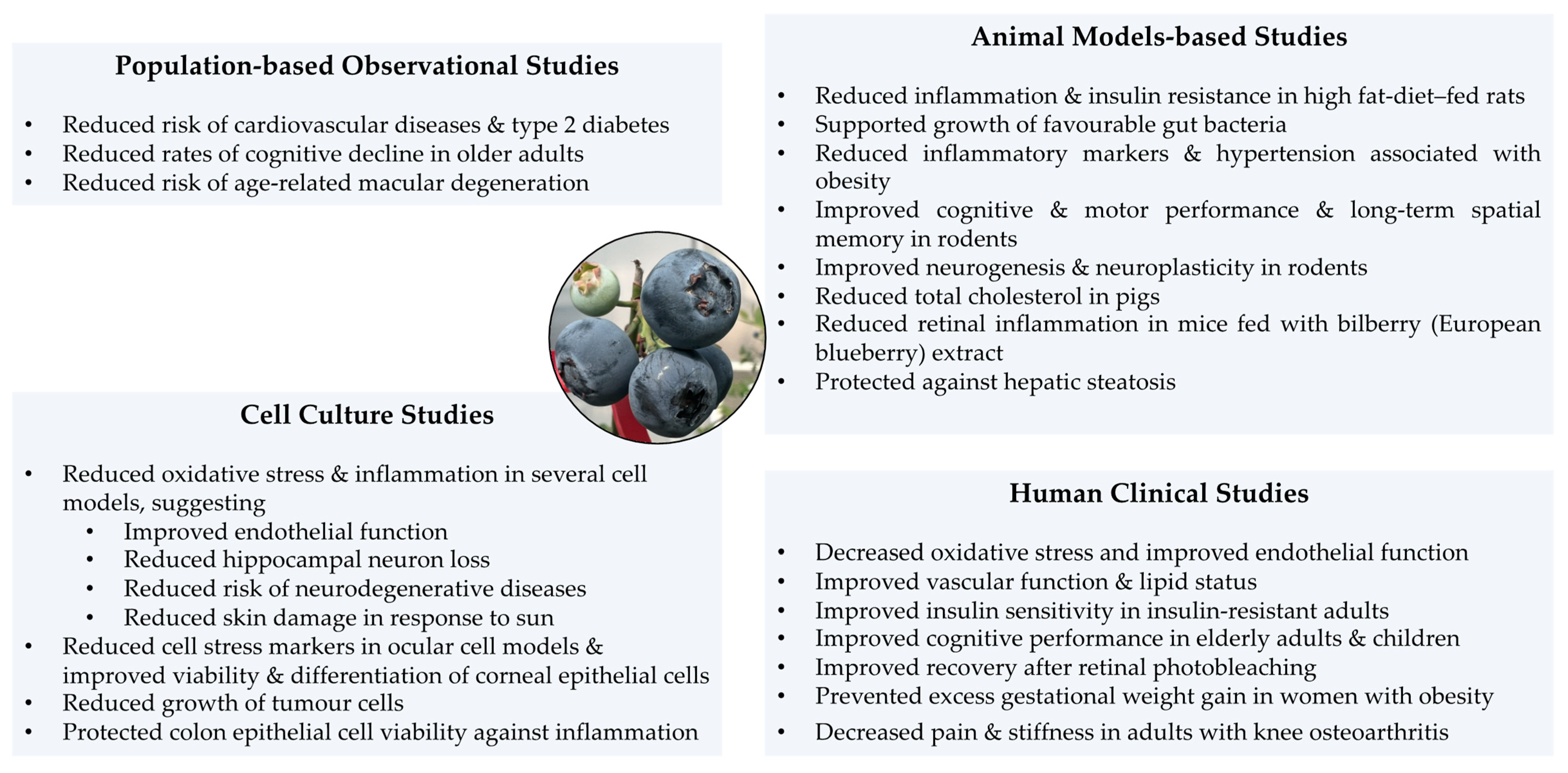
4. Health Benefits of Blueberry Consumption
5. Blueberry Production Systems
6. Light as a Regulator of Phytochemical Content with Antioxidant Activity
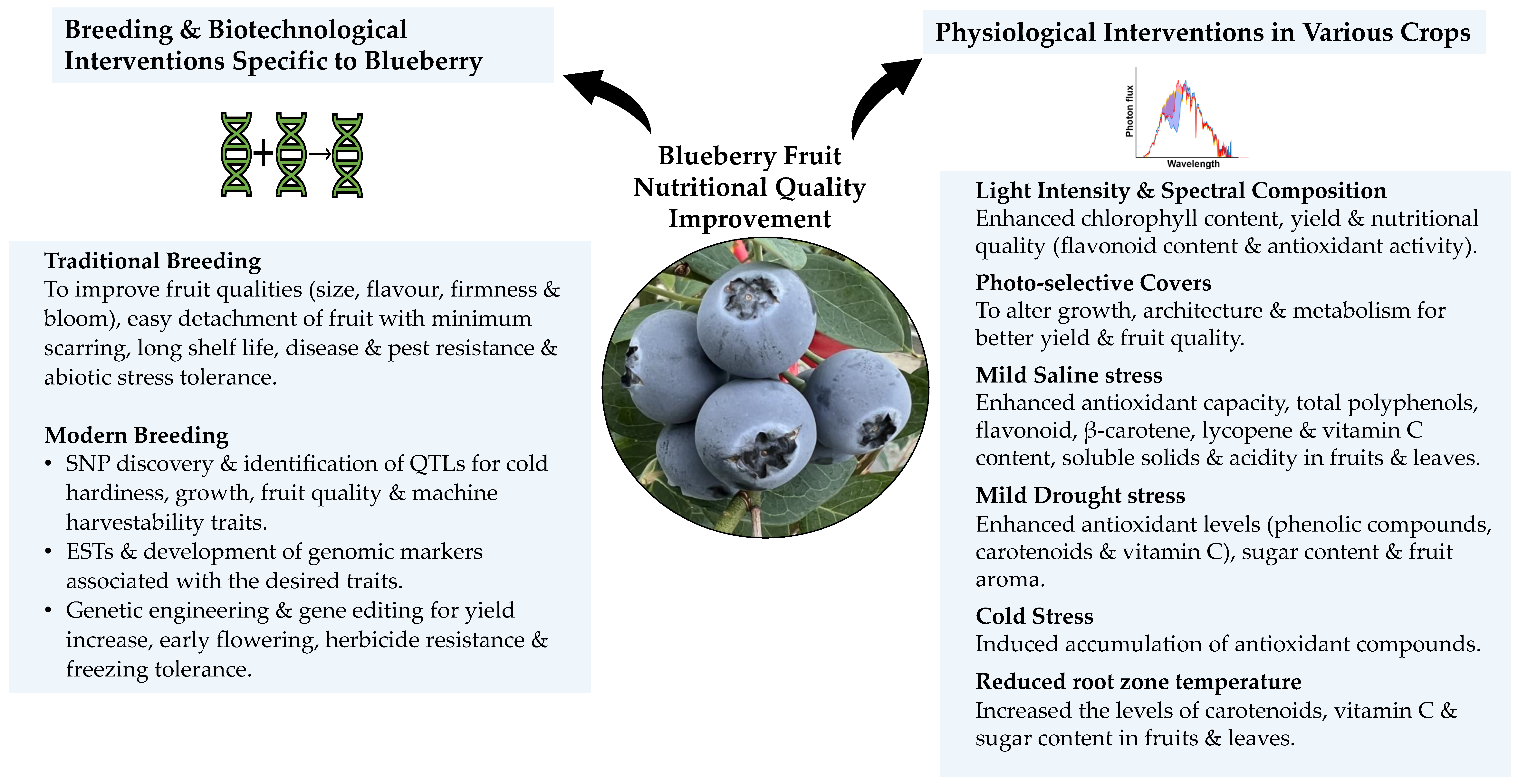
7. Light Interventions to Enhance Antioxidant Capacity in Fruits
8. Mild Abiotic Stress Interventions to Enhance Antioxidant Capacity in Fruits
9. Blueberry Breeding and Genomics
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R. Fruits, Vegetables, and Health: A Comprehensive Narrative, Umbrella Review of the Science and Recommendations for Enhanced Public Policy to Improve Intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red Fruits Composition and Their Health Benefits-A Review. Foods 2022, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ríos, A.; Laso, J.; Aldaco, R.; Margallo, M. Superfoods: A Super Impact on Health and the Environment? Curr. Opin. Environ. Sci. Health 2023, 31, 100410. [Google Scholar] [CrossRef]
- Shahbandeh, M. Value of the Superfoods Market Worldwide from 2021 to 2027. 2022. Available online: https://www.statista.com/statistics/1078437/superfoods-market-value-worldwide/ (accessed on 20 October 2022).
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health Promoting Properties of Blueberries: A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Tobar-Bolaños, G.; Casas-Forero, N.; Orellana-Palma, P.; Petzold, G. Blueberry juice: Bioactive compounds, health impact, and concentration technologies-A review. J. Food Sci. 2021, 86, 5062–5077. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef]
- FAOSTAT. Production: Crops: Blueberry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021; Available online: https://www.fao.org/faostat/en/#home (accessed on 20 October 2022).
- International Blueberry Organization 2021. Available online: https://www.internationalblueberry.org/2021-report/ (accessed on 20 October 2022).
- Zhang, J. IBO Reports on Status of Global Blueberry Industry. 2022. Available online: https://www.producereport.com/article/ibo-reports-status-global-blueberry-industry (accessed on 20 October 2022).
- Song, G.-Q.; Hancock, J.F. Recent Advances in Blueberry Transformation. Int. J. Fruit Sci. 2012, 12, 316–332. [Google Scholar] [CrossRef]
- Yue, C.; Wang, J. Consumer Preferences for Fresh Blueberry Attributes. Acta Hortic. 2017, 1180, 1–8. [Google Scholar] [CrossRef]
- Gallardo, R.K.; Zhang, Q.; Dossett, M.; Polashock, J.J.; Rodriguez-Saona, C.; Vorsa, N.; Edger, P.P.; Ashrafi, H.; Babiker, E.; Finn, C.E.; et al. Breeding Trait Priorities of the Blueberry Industry in the United States and Canada. HortScience 2018, 53, 1021–1028. [Google Scholar] [CrossRef]
- Mezzetti, B. The Sustainable Improvement of European Berry Production, Quality and Nutritional Value in a Changing Environment: Strawberries, Currants, Blackberries, Blueberries and Raspberries—The EUBerry Project. Acta Hortic. 2016, 1117, 309–314. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Li, Y.; Sun, X.; Wang, D.; Wang, W. Response of Bioactive Phytochemicals in Vegetables and Fruits to Environmental Factors. Eur. J. Nutr. Food Saf. 2019, 9, 233–247. [Google Scholar] [CrossRef]
- Pinto-Morales, F.; Retamal-Salgado, J.; Lopéz, M.D.; Zapata, N.; Vergara-Retamales, R.; Pinto-Poblete, A. The Use of Compost Increases Bioactive Compounds and Fruit Yield in Calafate Grown in the Central South of Chile. Agriculture 2022, 12, 98. [Google Scholar] [CrossRef]
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; Crop Production Science in Horticulture Series; CABI: Boston, MA, USA, 2018. [Google Scholar]
- Lobos, G.A.; Hancock, J.F. Breeding Blueberries for a Changing Global Environment: A Review. Front. Plant Sci. 2015, 6, 782. [Google Scholar] [CrossRef] [PubMed]
- Sakhanokho, H.F.; Rinehart, T.A.; Stringer, S.J.; Islam-Faridi, M.N.; Pounders, C.T. Variation in Nuclear DNA Content and Chromosome Numbers in Blueberry. Sci. Hortic. 2018, 233, 108–113. [Google Scholar] [CrossRef]
- Sargent, S.A.; Takeda, F.; Williamson, J.G.; Berry, A.D. Harvest of Southern Highbush Blueberry with a Modfied, Over-the-Row Mechanical Harvester: Use of Soft-Catch Surfaces to Minimize Impact Bruising. Agronomy 2021, 11, 1412. [Google Scholar] [CrossRef]
- Olmstead, J.W.; Finn, C.E. Breeding Highbush Blueberry Cultivars Adapted to Machine Harvest for the Fresh Market. Horttechnology 2014, 24, 290–294. [Google Scholar] [CrossRef]
- Chu, W.; Gao, H.; Chen, H.; Fang, X.; Zheng, Y. Effects of Cuticular Wax on the Postharvest Quality of Blueberry Fruit. Food Chem. 2018, 239, 68–74. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.; Li, J.; Zhang, H.; Li, Y.; Farooq, S.; Bacha, S.A.S.; Wang, J. Evaluation of Sugar and Organic Acid Composition and Their Levels in Highbush Blueberries from Two Regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Bushway, R.J.; Gann, D.M.; Cook, W.P.; Bushway, A.A. Mineral and Vitamin Content of Lowbush Blueberries (Vaccinium angustifolium Ait.). J. Food Sci. 1983, 48, 1878. [Google Scholar] [CrossRef]
- Nadulski, R.; Masłowski, A.; Mazurek, A.; Sobczak, P.; Szmigielski, M.; Żukiewicz-Sobczak, W.; Niedziółka, I.; Mazur, J. Vitamin C and Lutein Content of Northern Highbush Blueberry (Vaccinium corymbosum L.) Juice Processed Using Freezing and Thawing. J. Food Meas. Charact. 2019, 13, 2521–2528. [Google Scholar] [CrossRef]
- Dróżdż, P.; Šėžienė, V.; Pyrzynska, K. Mineral Composition of Wild and Cultivated Blueberries. Biol. Trace Elem. Res. 2018, 181, 173–177. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A.; Čekstere, G.; Pormale, J. Research on the Mineral Composition of Cultivated and Wild Blueberries and Cranberries. Agron. Res. 2018, 16, 454–463. [Google Scholar] [CrossRef]
- Morita, M.; Naito, Y.; Yoshikawa, T.; Niki, E. Antioxidant Capacity of Blueberry Extracts: Peroxyl Radical Scavenging and Inhibition of Plasma Lipid Oxidation Induced by Multiple Oxidants. J. Berry Res. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An Alternative Use of Horticultural Crops: Stressed Plants as Biofactories of Bioactive Phenolic Compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef]
- Pandhair, V.; Sekhon, B.S. Reactive Oxygen Species and Antioxidants in Plants: An Overview. J. Plant Biochem. Biotechnol. 2006, 15, 71–78. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising Natural Compounds against Viral Infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly) Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Yu, R.; Wu, C.; Fan, G.; Li, T. Effects of Postharvest Application of Methyl Jasmonate on Physicochemical Characteristics and Antioxidant System of the Blueberry Fruit. Sci. Hortic. 2019, 258, 108785. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, Anthocyanins, and Flavonoids Contents and the Antioxidant Capacity of Various Cultivars of Highbush and Half-High Blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Kalt, W.; Ryan, D.A.; Duy, J.C.; Prior, R.L.; Ehlenfeldt, M.K.; Vander Kloet, S.P. Interspecific Variation in Anthocyanins, Phenolics, and Antioxidant Capacity among Genotypes of Highbush and Lowbush Blueberries (Vaccinium section Cyanococcus spp.). J. Agric. Food Chem. 2001, 49, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Ferlemi, A.-V.; Lamari, F. Berry Leaves: An Alternative Source of Bioactive Natural Products of Nutritional and Medicinal Value. Antioxidants 2016, 5, 17. [Google Scholar] [CrossRef]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-Specific Distribution of Phenolic Compounds in Bilberry (Vaccinium myrtillus) and ‘Northblue’Blueberry (Vaccinium corymbosum × V. angustifolium). Food Chem. 2008, 110, 156–160. [Google Scholar] [CrossRef]
- Wang, L.-J.; Wu, J.; Wang, H.-X.; Li, S.-S.; Zheng, X.-C.; Du, H.; Xu, Y.-J.; Wang, L.-S. Composition of Phenolic Compounds and Antioxidant Activity in the Leaves of Blueberry Cultivars. J. Funct. Foods 2015, 16, 295–304. [Google Scholar] [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. The Chemical and Biological Profiles of Leaves from Commercial Blueberry Varieties. Plants 2020, 9, 1193. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Variation of Phenolic Profile and Antioxidant Activity of North American Highbush Blueberry Leaves with Variation of Time of Harvest and Cultivar. Ind. Crops Prod. 2014, 62, 147–155. [Google Scholar] [CrossRef]
- Fujii, K.; Ota, Y.; Nishiyama, K.; Kunitake, H.; Yamasaki, Y.; Tari, H.; Araki, K.; Arakawa, T.; Yamasaki, M. Blueberry Leaf Polyphenols Prevent Body Fat Accumulation in Mice Fed High-Fat, High-Sucrose Diet. J. Oleo Sci. 2019, 68, 471–479. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Li, Y.; Chen, H.; Wang, C.; Wong, V.K.W.; Jiang, Z.; Zhang, W. Phytotherapy Using Blueberry Leaf Polyphenols to Alleviate Non-Alcoholic Fatty Liver Disease through Improving Mitochondrial Function and Oxidative Defense. Phytomedicine 2020, 69, 153209. [Google Scholar] [CrossRef]
- Kelly, E.; Vyas, P.; Weber, J. Biochemical Properties and Neuroprotective Effects of Compounds in Various Species of Berries. Molecules 2017, 23, 26. [Google Scholar] [CrossRef]
- Vyas, P.; Kalidindi, S.; Chibrikova, L.; Igamberdiev, A.U.; Weber, J.T. Chemical Analysis and Effect of Blueberry and Lingonberry Fruits and Leaves against Glutamate-Mediated Excitotoxicity. J. Agric. Food Chem. 2013, 61, 7769–7776. [Google Scholar] [CrossRef] [PubMed]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and Leaves from Wild Blueberry Plants Contain Diverse Polyphenols and Decrease Neuroinflammatory Responses in Microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Harris, C.S.; Burt, A.J.; Saleem, A.; Le, P.M.; Martineau, L.C.; Haddad, P.S.; Bennett, S.A.L.; Arnason, J.T. A Single HPLC-PAD-APCI/MS Method for the Quantitative Comparison of Phenolic Compounds Found in Leaf, Stem, Root and Fruit Extracts of Vaccinium angustifolium. Phytochem. Anal. 2007, 18, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G. Compositional and Functional Properties of Saskatoon Berry and Blueberry. Int. J. Fruit Sci. 2005, 5, 101–120. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a Source of Bioactive Compounds for the Treatment of Obesity, Type 2 Diabetes and Chronic Inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid Glycosides and Antioxidant Capacity of Various Blackberry, Blueberry and Red Grape Genotypes Determined by High-Performance Liquid Chromatoraphy/ Mass Spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the Flavonols Quercetin, Myricetin, and Kaempferol in 25 Edible Berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef]
- Riso, P.; Klimis-Zacas, D.; Del Bo’, C.; Martini, D.; Campolo, J.; Vendrame, S.; Møller, P.; Loft, S.; De Maria, R.; Porrini, M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur. J. Nutr. 2013, 52, 949–961. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.S.; Pellenz, F.M.; de Souza, B.M.; Crispim, D. Blueberry Consumption and Changes in Obesity and Dia-betes Mellitus Outcomes: A Systematic Review. Metabolites 2022, 13, 19. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.F.; Lucca, A.B.A.; Ribeiro, E.; Silva, V.R.; Macedo, L.R.; Silva, M. Blueberry intervention improves metabolic syndrome risk factors: Systematic review and meta-analysis. Nutr. Res. 2021, 91, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef]
- Driscoll, K.; Deshpande, A.; Datta, R.; Ramakrishna, W. Anti-inflammatory Effects of Northern Highbush Blueberry Extract on an In Vitro Inflammatory Bowel Disease Model. Nutr. Cancer 2020, 72, 1178–1190. [Google Scholar] [CrossRef]
- Felgus-Lavefve, L.; Howard, L.; Adams, S.H.; Baum, J.I. The Effects of Blueberry Phytochemicals on Cell Models of Inflammation and Oxidative Stress. Adv. Nutr. 2022, 13, 1279–1309. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Stamps, R.H.; Giglia, F.F. Environmental Modification Inside Photoselective Shadehouses. HortScience 2013, 48, 975–979. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Bastías, R.M.; Wilckens, R.; Paulino, L. Influence of Microclimatic Conditions under High Tunnels on the Physiological and Productive Responses in Blueberry’O’Neal’. Chil. J. Agric. Res. 2015, 75, 291–297. [Google Scholar] [CrossRef]
- Retamal-Salgado, J.; Vásquez, R.; Fischer, S.; Hirzel, J.; Zapata, N. Decrease in Artificial Radiation with Netting Reduces Stress and Improves Rabbit-Eye Blueberry (Vaccinium virgatum Aiton)‘Ochlockonee’Productivity. Chil. J. Agric. Res. 2017, 77, 226–233. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, Q.; Zheng, K.; Zhao, S.; Wang, P.; Cheng, J.; Zhang, X.; Chen, X. Effects of Diffuse Light on Microclimate of Solar Greenhouse, and Photosynthesis and Yield of Greenhouse-Grown Tomatoes. HortScience 2020, 55, 1605–1613. [Google Scholar] [CrossRef]
- Demchak, K. Small Fruit Production in High Tunnels. HortTechnology 2009, 19, 44–49. [Google Scholar] [CrossRef]
- Holcman, E.; Sentelhas, P.C.; da C. Mello, S. Microclimatic Changes Caused by Different Plastic Coverings in Greenhouses Cultivated with Cherry Tomato in Southern Brazil. Rev. Bras. Meteorol. 2015, 30, 125–133. [Google Scholar] [CrossRef]
- Sandri, M.A.; Andriolo, J.L.; Witter, M.; Dal Ross, T. Effect of Shading on Tomato Plants Grow under Greenhouse. Hortic. Bras. 2003, 21, 642–645. [Google Scholar] [CrossRef]
- Bastías, R.M.; Corelli-Grappadelli, L. Light Quality Management in Fruit Orchards: Physiological and Technological Aspects. Chil. J. Agric. Res. 2012, 72, 574. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Cobo, N.; del Pozo, A. Spectral Irradiance, Gas Exchange Characteristics and Leaf Traits of Vaccinium corymbosum L.‘Elliott’Grown under Photo-Selective Nets. Environ. Exp. Bot. 2012, 75, 142–149. [Google Scholar] [CrossRef]
- Cardeñosa, V.; Girones-Vilaplana, A.; Muriel, J.L.; Moreno, D.A.; Moreno-Rojas, J.M. Influence of Genotype, Cultivation System and Irrigation Regime on Antioxidant Capacity and Selected Phenolics of Blueberries (Vaccinium corymbosum L.). Food Chem. 2016, 202, 276–283. [Google Scholar] [CrossRef]
- Katsoulas, N.; Bari, A.; Papaioannou, C. Plant Responses to UV Blocking Greenhouse Covering Materials: A Review. Agronomy 2020, 10, 1021. [Google Scholar] [CrossRef]
- Zoratti, L.; Jaakola, L.; Häggman, H.; Giongo, L. Modification of Sunlight Radiation through Colored Photo-Selective Nets Affects Anthocyanin Profile in Vaccinium Spp. Berries. PLoS ONE 2015, 10, e0135935. [Google Scholar] [CrossRef]
- Kendrick, R.E. (Ed.) Photomorphogenesis in Plants, 2nd ed.; Kluwer Academic Publ.: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Hong, G.-J.; Hu, W.-L.; Li, J.-X.; Chen, X.-Y.; Wang, L.-J. Increased Accumulation of Artemisinin and Anthocyanins in Artemisia annua Expressing the Arabidopsis Blue Light Receptor CRY1. Plant Mol. Biol. Rep. 2009, 27, 334–341. [Google Scholar] [CrossRef]
- Mawphlang, O.I.L.; Kharshiing, E.V. Photoreceptor Mediated Plant Growth Responses: Implications for Photoreceptor Engineering toward Improved Performance in Crops. Front. Plant Sci. 2017, 8, 1181. [Google Scholar] [CrossRef] [PubMed]
- McCree, K.J. Photosynthetically Active Radiation. In Physiological Plant Ecology I; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 41–55. [Google Scholar] [CrossRef]
- Palma, C.F.F.; Castro-Alves, V.; Morales, L.O.; Rosenqvist, E.; Ottosen, C.-O.; Strid, Å. Spectral Composition of Light Affects Sensitivity to UV-B and Photoinhibition in Cucumber. Front. Plant Sci. 2021, 11, 610011. [Google Scholar] [CrossRef] [PubMed]
- Legendre, R.; van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Franklin, K.A.; Quail, P.H. Phytochrome Functions in Arabidopsis Development. J. Exp. Bot. 2010, 61, 11–24. [Google Scholar] [CrossRef] [PubMed]
- González, C.V.; Fanzone, M.L.; Cortés, L.E.; Bottini, R.; Lijavetzky, D.C.; Ballaré, C.L.; Boccalandro, H.E. Fruit-Localized Photoreceptors Increase Phenolic Compounds in Berry Skins of Field-Grown Vitis vinifera L. cv. Malbec. Phytochemistry 2015, 110, 46–57. [Google Scholar] [CrossRef]
- McMahon, M.J.; Kelly, J.W. Influence of Spectral Filters on Height, Leaf Chlorophyll, and Flowering of Rosa × Hybrida ‘Meirutral’. J. Environ. Hortic. 1990, 8, 209–211. [Google Scholar] [CrossRef]
- Zhou, Y.; Singh, B.R. Red light stimulates flowering and anthocyanin biosynthesis in American cranberry. Plant Growth Regul. 2002, 38, 165–171. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Gam, D.T.; Khoi, P.H.; Ngoc, P.B.; Linh, L.K.; Hung, N.K.; Anh, P.T.L.; Thu, N.T.; Hien, N.T.T.; Khanh, T.D.; Ha, C.H. LED Lights Promote Growth and Flavonoid Accumulation of Anoectochilus roxburghii and are Linked to the Enhanced Expression of Several Related Genes. Plants 2020, 9, 1344. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Moreira-Rodríguez, M.; Benavides, J. UVA and UVB Radiation as Innovative Tools to Biofortify Horticultural Crops with Nutraceuticals. Horticulturae 2022, 8, 387. [Google Scholar] [CrossRef]
- Jenkins, G.I. The UV-B Photoreceptor UVR8: From Structure to Physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of Light Quality on the Accumulation of Phytochemicals in Vegetables Produced in Controlled Environments: A Review: Effects of Light on Vegetable Phytochemicals. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Du, W.-X.; Avena-Bustillos, R.J.; Breksa, A.P.; McHugh, T.H. UV-B Light as a Factor Affecting Total Soluble Phenolic Contents of Various Whole and Fresh-Cut Specialty Crops. Postharvest Biol. Technol. 2014, 93, 72–82. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Du, W.-X.; Woods, R.; Olson, D.; Breksa, A.P.; McHugh, T.H. Ultraviolet-B Light Treatment Increases Antioxidant Capacity of Carrot Products: UV-B Treatment Increases Phenolics in Carrots. J. Sci. Food Agric. 2012, 92, 2341–2348. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Howard, L. Blueberry Fruit Response to Postharvest Application of Ultraviolet Radiation. Postharvest Biol. Technol. 2008, 47, 280–285. [Google Scholar] [CrossRef]
- Maya-Cano, D.A.; Arango-Varela, S.; Santa-Gonzalez, G.A. Phenolic Compounds of Blueberries (Vaccinium spp.) as a Protective Strategy against Skin Cell Damage Induced by ROS: A Review of Antioxidant Potential and Antiproliferative Capacity. Heliyon 2021, 7, e06297. [Google Scholar] [CrossRef] [PubMed]
- Escobar Bravo, R.; Chen, G.; Grosser, K.; Van Dam, N.M.; Leiss, K.A.; Klinkhamer, P.G.L. Ultraviolet Radiation Enhances Salicylic Acid-Mediated Defense Signaling and Resistance to Pseudomonas Syringae DC3000 in a Jasmonic Acid-Deficient Tomato Mutant. Plant Signal. Behav. 2019, 14, e1581560. [Google Scholar] [CrossRef]
- Yavaş, İ.; Ünay, A.; Ali, S.; Abbas, Z. UV-B Radiations and Secondary Metabolites. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 147. [Google Scholar] [CrossRef]
- Klem, K.; Ač, A.; Holub, P.; Kováč, D.; Špunda, V.; Robson, T.M.; Urban, O. Interactive Effects of PAR and UV Radiation on the Physiology, Morphology and Leaf Optical Properties of Two Barley Varieties. Environ. Exp. Bot. 2012, 75, 52–64. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of Light on Secondary Metabolites in Selected Leafy Greens: A Review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. A Review of Strawberry Photobiology and Fruit Flavonoids in Controlled Environments. Front. Plant Sci. 2021, 12, 611893. [Google Scholar] [CrossRef] [PubMed]
- Kadomura-Ishikawa, Y.; Miyawaki, K.; Noji, S.; Takahashi, A. Phototropin 2 Is Involved in Blue Light-Induced Anthocyanin Accumulation in Fragaria x Ananassa Fruits. J. Plant Res. 2013, 126, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 534. [Google Scholar] [CrossRef] [PubMed]
- Wimalasekera, R. Effect of Light Intensity on Photosynthesis. In Photosynthesis, Productivity and Environmental Stress; Ahmad, P., Abass Ahanger, M., Nasser Alyemeni, M., Alam, P., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 65–73. [Google Scholar] [CrossRef]
- Arakawa, O.; Hori, Y.; Ogata, R. Relative Effectiveness and Interaction of Ultraviolet-B, Red and Blue Light in Anthocyanin Synthesis of Apple Fruit. Physiol. Plant. 1985, 64, 323–327. [Google Scholar] [CrossRef]
- Panjai, L.; Noga, G.; Fiebig, A.; Hunsche, M. Effects of Continuous Red Light and Short Daily UV Exposure during Postharvest on Carotenoid Concentration and Antioxidant Capacity in Stored Tomatoes. Sci. Hortic. 2017, 226, 97–103. [Google Scholar] [CrossRef]
- Höll, J.; Lindner, S.; Walter, H.; Joshi, D.; Poschet, G.; Pfleger, S.; Ziegler, T.; Hell, R.; Bogs, J.; Rausch, T. Impact of Pulsed UV-B Stress Exposure on Plant Performance: How Recovery Periods Stimulate Secondary Metabolism While Reducing Adaptive Growth Attenuation. Plant Cell Environ. 2019, 42, 801–814. [Google Scholar] [CrossRef]
- Yang, J.; Shi, W.; Li, B.; Bai, Y.; Hou, Z. Preharvest and Postharvest UV Radiation Affected Flavonoid Metabolism and Antioxidant Capacity Differently in Developing Blueberries (Vaccinium corymbosum L.). Food Chem. 2019, 301, 125248. [Google Scholar] [CrossRef]
- Li, T.; Yamane, H.; Tao, R. Preharvest Long-Term Exposure to UV-B Radiation Promotes Fruit Ripening and Modifies Stage-Specific Anthocyanin Metabolism in Highbush Blueberry. Hortic. Res. 2021, 8, 67. [Google Scholar] [CrossRef]
- Luengo Escobar, A.; Alberdi, M.; Acevedo, P.; Machado, M.; Nunes-Nesi, A.; Inostroza-Blancheteau, C.; Reyes-Díaz, M. Distinct physiological and metabolic reprogramming by highbush blueberry (Vaccinium corymbosum) cultivars revealed during long-term UV-B radiation. Physiol. Plant. 2017, 160, 46–64. [Google Scholar] [CrossRef]
- Inostroza-Blancheteau, C.; Acevedo, P.; Loyola, R.; Arce-Johnson, P.; Alberdi, M.; Reyes-Díaz, M. Short-Term UV-B Radiation Affects Photosynthetic Performance and Antioxidant Gene Expression in Highbush Blueberry Leaves. Plant Physiol. Biochem. 2016, 107, 301–309. [Google Scholar] [CrossRef]
- Yemmireddy, V.; Adhikari, A.; Moreira, J. Effect of Ultraviolet Light Treatment on Microbiological Safety and Quality of Fresh Produce: An Overview. Front. Nutr. 2022, 9, 871243. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An Overview of LEDs’ Effects on the Production of Bioactive Compounds and Crop Quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Akimova, S.; Radzhabov, A.; Esaulko, A.; Samoshenkov, E.; Nechiporenko, I.; Kazakov, P.; Voskoboinikov, Y.; Matsneva, A.; Zubkov, A.; Aisanov, T. Improvement of Ex Vitro Growing Completion of Highbush Blueberry (Vaccinium corymbosum L.) in Containers. Forests 2022, 13, 1550. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.-H.; Kim, S.-K.; Lee, K.-H.; Park, J.-Y.; Nam, M.-W.; Choi, D.-H.; Lee, H.-I. LED Light for in vitro and ex vitro Efficient Growth of Economically Important Highbush Blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Ni, C.; Chen, J. Effect of LED Light on the Growth and Physiological Indices of Blueberry. Agron. J. 2022, 114, 2105–2112. [Google Scholar] [CrossRef]
- Figiel-Kroczyńska, M.; Ochmian, I.; Krupa-Małkiewiecz, M.; Lachowicz, S. Influence of Various Types of Light on Growth and Physicochemical Composition of Blueberry (Vaccinium corymbosum L.) Leaves. Acta Sci. Pol. Hortorum Cultus 2022, 21, 87–101. [Google Scholar] [CrossRef]
- Ordidge, M.; García-Macías, P.; Battey, N.H.; Gordon, M.H.; Hadley, P.; John, P.; Lovegrove, J.A.; Vysini, E.; Wagstaffe, A. Phenolic Contents of Lettuce, Strawberry, Raspberry, and Blueberry Crops Cultivated under Plastic Films Varying in Ultraviolet Transparency. Food Chem. 2010, 119, 1224–1227. [Google Scholar] [CrossRef]
- Shahak, Y. Photoselective Netting: An Overview of the Concept, Research and Development and Practical Implementation in Agriculture. Acta Hortic. 2014, 1015, 155–162. [Google Scholar] [CrossRef]
- Conde, A.; Chaves, M.M.; Geros, H. Membrane Transport, Sensing and Signaling in Plant Adaptation to Environmental Stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing Quality of Fresh Vegetables through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Flores, P.; Garrido, C.; Martinez, V. Changes in the Contents of Antioxidant Compounds in Pepper Fruits at Different Ripening Stages, as Affected by Salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, M.T.; Oba, S. Salinity Stress Accelerates Nutrients, Dietary Fiber, Minerals, Phytochemicals and Antioxidant Activity in Amaranthus tricolor Leaves. PLoS ONE 2018, 13, e0206388. [Google Scholar] [CrossRef] [PubMed]
- Fanasca, S.; Colla, G.; Maiani, G.; Venneria, E.; Rouphael, Y.; Azzini, E.; Saccardo, F. Changes in Antioxidant Content of Tomato Fruits in Response to Cultivar and Nutrient Solution Composition. J. Agric. Food Chem. 2006, 54, 4319–4325. [Google Scholar] [CrossRef]
- Cardeñosa, V.; Medrano, E.; Lorenzo, P.; Sánchez-Guerrero, M.C.; Cuevas, F.; Pradas, I.; Moreno-Rojas, J.M. Effects of salinity and nitrogen supply on the quality and health-related compounds of strawberry fruits (Fragaria × ananassa cv. Primoris). J. Sci. Food Agric. 2015, 95, 2924–2930. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water Shortage and Quality of Fleshy Fruits—Making the Most of the Unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.A.; Chope, G.A.; Bordonaba, J.G. Effect of Water Deficit Irrigation and Inoculation with Botrytis cinerea on Strawberry (Fragaria × Ananassa) Fruit Quality. J. Agric. Food Chem. 2007, 55, 10812–10819. [Google Scholar] [CrossRef]
- Veit-Köhler, U.; Krumbein, A.; Kosegarten, H. Effect of Different Water Supply on Plant Growth and Fruit Quality of Lycopersicon esculentum. J. Plant Nutr. Soil Sci. 1999, 162, 583–588. [Google Scholar] [CrossRef]
- Modise, D.M.; Wright, C.J.; Atherton, J.G. Changes in Strawberry Aroma in Response to Water Stress. Bostwana J. Agric. Appl. Sci. 2006, 2, 50–60. [Google Scholar]
- Salgado Vargas, C.; Sánchez-García, P.; Volke-Haller, V.H.; Colinas León, H.T. Agronomic Response to Osmotic Stress of Blueberry (Vaccinium corymbosum L.). Agrociencia 2018, 2, 231–239. [Google Scholar]
- Janská, A.; Maršík, P.; Zelenková, S.; Ovesná, J. Cold Stress and Acclimation—What Is Important for Metabolic Adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Latorre-Moratalla, M.L.; Comas-Basté, O.; Toro-Funes, N.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Polyamines in Food. Front. Nutr. 2019, 6, 108. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to Cold and Heat Stress: Accumulation of Phenolic Compounds in Tomato and Watermelon Plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Seki, M.; Furusaki, S. Effect of Temperature and Its Shift on Growth and Anthocyanin Production in Suspension Cultures of Strawberry Cells. Plant Sci. 1997, 127, 207–214. [Google Scholar] [CrossRef]
- Yoon, Y.-E.; Kuppusamy, S.; Cho, K.M.; Kim, P.J.; Kwack, Y.-B.; Lee, Y.B. Influence of Cold Stress on Contents of Soluble Sugars, Vitamin C and Free Amino Acids Including Gamma-Aminobutyric Acid (GABA) in Spinach (Spinacia oleracea). Food Chem. 2017, 215, 185–192. [Google Scholar] [CrossRef]
- Medina, E.; Kim, S.H.; Yun, M.; Choi, W.G. Recapitulation of the Function and Role of ROS Generated in Response to Heat Stress in Plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, G.; Zhang, S.; Wang, Q.; Fu, Y.; Lu, H. Efficacy of Root Zone Temperature Increase in Root and Shoot Development and Hormone Changes in Different Maize Genotypes. Agriculture 2021, 11, 477. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Elevated Root-Zone Temperature Modulates Growth and Quality of Hydroponically Grown Carrots. Agric. Sci. 2015, 06, 749–757. [Google Scholar] [CrossRef]
- Tamura, A. Effect of Air Temperature on the Content of Sugar and Vitamin C of Spinach and Komatsuna. Hortic. Res. Jpn. 2004, 3, 187–190. [Google Scholar] [CrossRef]
- Chadirin, Y.; Hidaka, K.; Takahashi, T.; Sago, Y.; Wajima, T.; Kitano, M. Application of Temperature Stress to Roots of Spinach I. Effect of the Low Temperature Stress on Quality. Environ. Control Biol. 2011, 49, 133–139. [Google Scholar] [CrossRef]
- He, F.; Thiele, B.; Watt, M.; Kraska, T.; Ulbrich, A.; Kuhn, A.J. Effects of Root Cooling on Plant Growth and Fruit Quality of Cocktail Tomato during Two Consecutive Seasons. J. Food Qual. 2019, 2019, 3598172. [Google Scholar] [CrossRef]
- Hancock, J.F.; Olmstead, J.W.; Itle, R.A.; Callow, P.W.; Neils-Kraft, S.; Wheeler, E.J.; Mangandi, J.; Sooriyapathirana, S.S.; Rowland, L.J.; Mackey, T.A.; et al. Performance of an elite, hybrid family of a northern × southern highbush cross (‘Draper’ × ‘Jewel’). Euphytica 2018, 214. [Google Scholar] [CrossRef]
- Ochmian, I.; Błaszak, M.; Lachowicz, S.; Piwowarczyk, R. The Impact of Cultivation Systems on the Nutritional and Phytochemical Content, and Microbiological Contamination of Highbush Blueberry. Sci. Rep. 2020, 10, 16696. [Google Scholar] [CrossRef]
- Edger, P.P.; Iorizzo, M.; Bassil, N.V.; Benevenuto, J.; Ferrão, L.F.V.; Giongo, L.; Hummer, K.; Lawas, L.M.; Leisner, C.; Li, C.; et al. There and Back Again; Historical Perspective and Future Directions for Vaccinium Breeding and Research Studies. Hortic. Res. 2022, 9, uhac083. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Draper, A.D.; Clark, J.R. Performance of Southern Highbush Blueberry Cultivars Released by the U.S. Department of Agriculture and Cooperating State Agricultural Experiment Stations. HortTechnology 1995, 5, 127–130. [Google Scholar] [CrossRef]
- Vorsa, N.; Zalapa, J. Domestication, Genetics, and Genomics of the American Cranberry. In Plant Breeding Reviews; Goldman, I., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 279–315. [Google Scholar] [CrossRef]
- Hancock, J.F. (Ed.) Temperate Fruit Crop Breeding: Germsplasm to Genomics; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Broman, K.W.; Sen, S. A Guide to QTL Mapping with R/Qtl; Statistics for Biology and Health; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Rowland, L.J.; Ogden, E.L.; Bassil, N.; Buck, E.J.; McCallum, S.; Graham, J.; Brown, A.; Wiedow, C.; Campbell, A.M.; Haynes, K.G.; et al. Construction of a Genetic Linkage Map of an Interspecific Diploid Blueberry Population and Identification of QTL for Chilling Requirement and Cold Hardiness. Mol. Breed. 2014, 34, 2033–2048. [Google Scholar] [CrossRef]
- Rowland, L.J.; Ogden, E.L.; Vinyard, B.T. Phenotypic Evaluation of a Hybrid Diploid Blueberry Population for Plant Development and Fruit Quality Traits. Agronomy 2020, 10, 1067. [Google Scholar] [CrossRef]
- Cappai, F.; Amadeu, R.R.; Benevenuto, J.; Cullen, R.; Garcia, A.; Grossman, A.; Ferrão, L.F.V.; Munoz, P. High-Resolution Linkage Map and QTL Analyses of Fruit Firmness in Autotetraploid Blueberry. Front. Plant Sci. 2020, 11, 562171. [Google Scholar] [CrossRef]
- Qi, X.; Ogden, E.L.; Bostan, H.; Sargent, D.J.; Ward, J.; Gilbert, J.; Iorizzo, M.; Rowland, L.J. High-Density Linkage Map Construction and QTL Identification in a Diploid Blueberry Mapping Population. Front. Plant Sci. 2021, 12, 692628. [Google Scholar] [CrossRef] [PubMed]
- Colle, M.; Leisner, C.P.; Wai, C.M.; Ou, S.; Bird, K.A.; Wang, J.; Wisecaver, J.H.; Yocca, A.E.; Alger, E.I.; Tang, H.; et al. Haplotype-Phased Genome and Evolution of Phytonutrient Pathways of Tetraploid Blueberry. GigaScience 2019, 8, giz012. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Kumar, P.; Singh, A.; Aggarwal, K.; Roy, J.; Sharma, V.; Rawat, S. Development of Polymorphic EST-SSR Markers and Their Applicability in Genetic Diversity Evaluation in Rhododendron Arboreum. Mol. Biol. Rep. 2020, 47, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Rowen, L.; Lasky, S.; Hood, L. Deciphering Genomes through Automated Large-Scale Sequencing. Methods Microbiol. 1999, 28, 155–192. [Google Scholar]
- Die, J.V.; Rowland, L.J. Advent of Genomics in Blueberry. Mol. Breed. 2013, 32, 493–504. [Google Scholar] [CrossRef]
- Rowland, L.J.; Alkharouf, N.; Darwish, O.; Ogden, E.L.; Polashock, J.J.; Bassil, N.V.; Main, D. Generation and Analysis of Blueberry Transcriptome Sequences from Leaves, Developing Fruit, and Flower Buds from Cold Acclimation through Deacclimation. BMC Plant Biol. 2012, 12, 46. [Google Scholar] [CrossRef]
- Li, X.; Sun, H.; Pei, J.; Dong, Y.; Wang, F.; Chen, H.; Sun, Y.; Wang, N.; Li, H.; Li, Y. De Novo Sequencing and Comparative Analysis of the Blueberry Transcriptome to Discover Putative Genes Related to Antioxidants. Gene 2012, 511, 54–61. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Liu, Z.; Cui, X.; Zhang, T.; Li, Y.; Zhang, L. Comparative Transcriptome Sequencing and de Novo Analysis of Vaccinium Corymbosum during Fruit and Color Development. BMC Plant Biol. 2016, 16, 223. [Google Scholar] [CrossRef]
- Qi, X.; Ogden, E.L.; Die, J.V.; Ehlenfeldt, M.K.; Polashock, J.J.; Darwish, O.; Alkharouf, N.; Rowland, L.J. Transcriptome Analysis Identifies Genes Related to the Waxy Coating on Blueberry Fruit in Two Northern-Adapted Rabbiteye Breeding Populations. BMC Plant Biol. 2019, 19, 460. [Google Scholar] [CrossRef]
- Bhatt, D.S.; Debnath, S.C. Genetic Diversity of Blueberry Genotypes Estimated by Antioxidant Properties and Molecular Markers. Antioxidants 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Genome Database for Vaccinium. Available online: https://www.vaccinium.org/ (accessed on 20 October 2022).
- Darwish, O.; Rowland, L.J.; Alkharouf, N.W. BBGD454: A Database for Transcriptome Analysis of Blueberry Using 454 Sequences. Bioinformation 2013, 9, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Gao, X. Transcriptomic Changes Reveal Gene Networks Responding to the Overexpression of a Blueberry DWARF AND DELAYED FLOWERING 1 Gene in Transgenic Blueberry Plants. BMC Plant Biol. 2017, 17, 106. [Google Scholar] [CrossRef]
- Song, G.-Q.; Walworth, A.E.; Hancock, J.F. Stability of Transgenes in Blueberry. Int. J. Fruit Sci. 2012, 12, 333–341. [Google Scholar] [CrossRef]
- Walworth, A.; Song, G. The Cold-Regulated Genes of Blueberry and Their Response to Overexpression of VcDDF1 in Several Tissues. Int. J. Mol. Sci. 2018, 19, 1553. [Google Scholar] [CrossRef] [PubMed]
- Omori, M.; Yamane, H.; Osakabe, K.; Osakabe, Y.; Tao, R. Targeted Mutagenesis of CENTRORADIALIS Using CRISPR/Cas9 System through the Improvement of Genetic Transformation Efficiency of Tetraploid Highbush Blueberry. J. Hortic. Sci. Biotechnol. 2021, 96, 153–161. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishna, P.; Pandey, G.; Thomas, R.; Parks, S. Improving Blueberry Fruit Nutritional Quality through Physiological and Genetic Interventions: A Review of Current Research and Future Directions. Antioxidants 2023, 12, 810. https://doi.org/10.3390/antiox12040810
Krishna P, Pandey G, Thomas R, Parks S. Improving Blueberry Fruit Nutritional Quality through Physiological and Genetic Interventions: A Review of Current Research and Future Directions. Antioxidants. 2023; 12(4):810. https://doi.org/10.3390/antiox12040810
Chicago/Turabian StyleKrishna, Priti, Gareema Pandey, Richard Thomas, and Sophie Parks. 2023. "Improving Blueberry Fruit Nutritional Quality through Physiological and Genetic Interventions: A Review of Current Research and Future Directions" Antioxidants 12, no. 4: 810. https://doi.org/10.3390/antiox12040810
APA StyleKrishna, P., Pandey, G., Thomas, R., & Parks, S. (2023). Improving Blueberry Fruit Nutritional Quality through Physiological and Genetic Interventions: A Review of Current Research and Future Directions. Antioxidants, 12(4), 810. https://doi.org/10.3390/antiox12040810









