Oxidative Stress and Antioxidants in Uterine Fibroids: Pathophysiology and Clinical Implications
Abstract
1. Introduction
2. Role of Reactive Oxygen Species in Female Reproductive Physiology and Disease
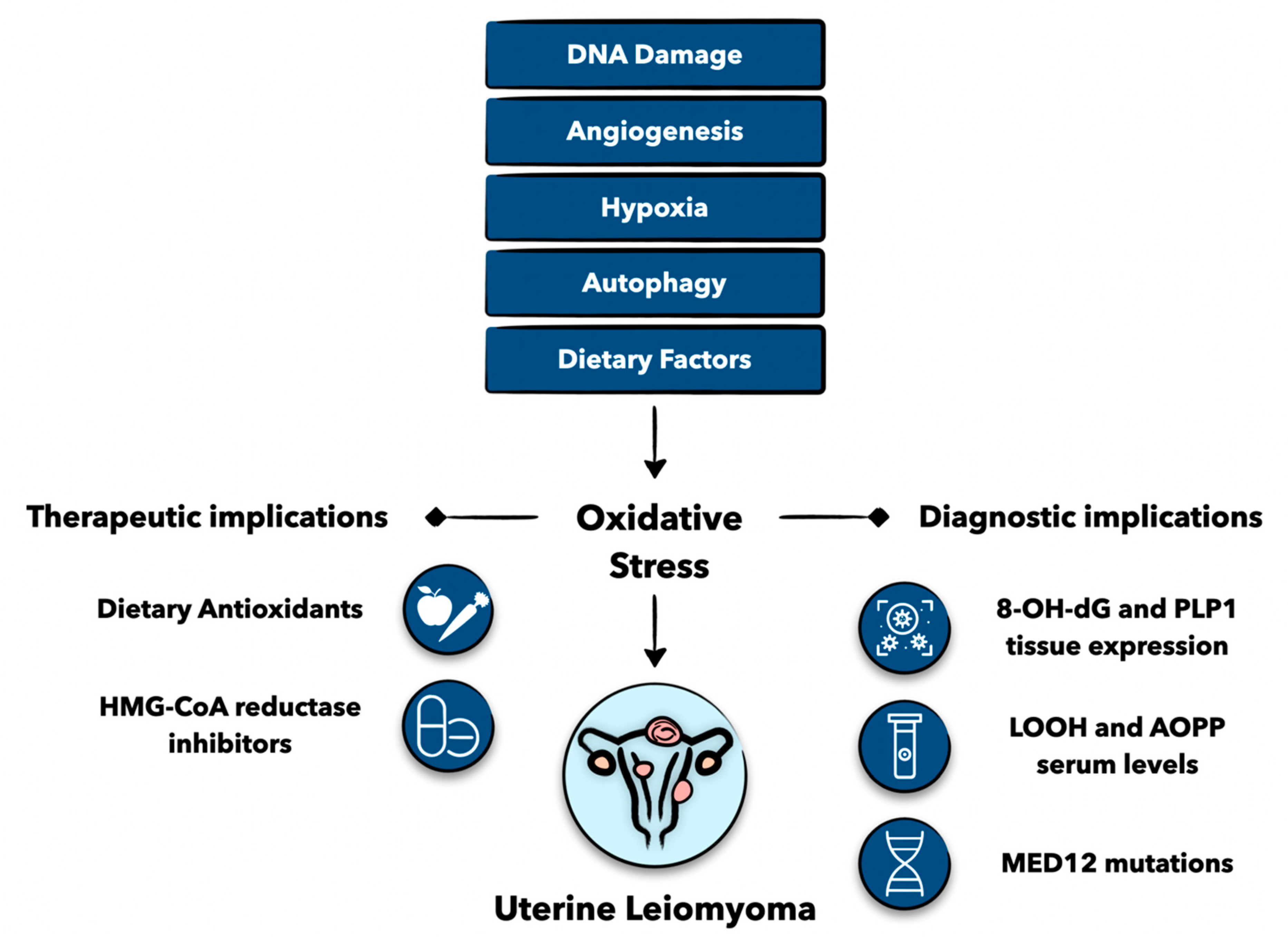
3. Pathophysiological Considerations
3.1. DNA Damage and Genetics
3.2. Hypoxia and Angiogenesis
3.3. Autophagy
3.4. Obesity and Oxidative Stress
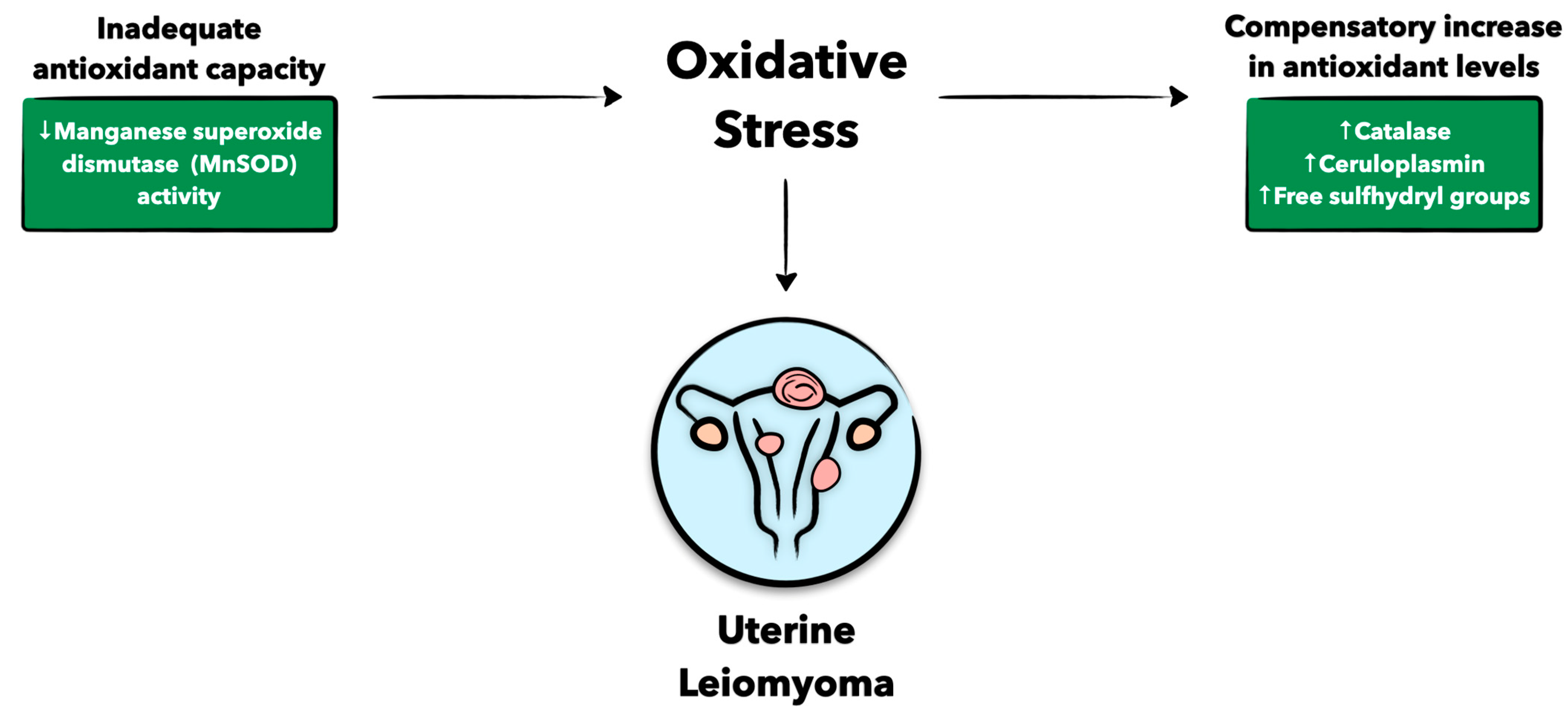
3.5. Antioxidant Defense
4. Clinical Implications
4.1. Biomarkers as Diagnostic Adjuncts
4.2. Dietary Antioxidants
4.3. Statins
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-OH-dG | 8-hydroxy-2′-deoxyguonosine |
| ADM | Adrenomedullin |
| AOPP | Advanced oxidation protein products |
| BHT | Butylated hydroxytoluene |
| BMI | Body mass index |
| BRCA1 | Breast cancer type 1 |
| CA-IX | Carbonic anhydrase |
| CAT | Catalase |
| Cp | Ceruloplasmin |
| DUOXI | Dual oxidase |
| EDC | Endocrine-disrupting chemicals |
| elF2a | Eukaryotic translation initiation factor 2a |
| ET1 | Endothelin 1 |
| FAP | Fibroblast activation protein |
| FH | Fumarate hydrase |
| GLUT-1 | Glucose transporter-1 |
| GnRHa | Gonadotropin-releasing hormone analogues |
| GPX | Glutathione peroxidase |
| H2O2 | Hydrogen peroxide |
| HIF1a | Hypoxia inducible factor 1α |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme A |
| iNOS | Nitric oxide synthase |
| LC3 | Microtubule-associated proteins 1A/1B light chain 3B |
| LDL | Low-density lipoprotein |
| LOOH | Lipid peroxidation products |
| MAD | Malondialdehyde |
| MED12 | Mediatory complex subunit 12 |
| MnSOD | Manganese superoxide dismutase |
| MPO | Myeloperoxidase |
| mTOR | Mammalian target of rapamycin |
| NOX4 | NADPH oxidase 4 |
| O2−• | Superoxide anion radical |
| OH• | Hydroxyl radical |
| PLP1 | Proteolipid protein 1 |
| RAD51 | RAD51 recombinase |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen Species |
| SH | Free sulfhydryl groups |
| Smad3 | SMAD family member 3 |
| SOD | Superoxide dismutase |
| TGF-b3 | Transforming growth factor-β3 |
| TIA1 | T-cell intracellular antigen 1 |
| TNFα | Tumor necrosis factor α |
| TSP1 | Thrombospondin |
| VEGF | Vascular endothelial growth factor |
References
- Yang, Q.; Ciebiera, M.; Bariani, M.V.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2022, 43, 678–719. [Google Scholar] [CrossRef] [PubMed]
- Alashqar, A.; El Ouweini, H.; Gornet, M.; Yenokyan, G.; Borahay, M.A. Cardiometabolic profile of women with uterine leiomyoma: A cross-sectional study. Minerva. Obs. Gynecol. 2022, 75, 27–38. [Google Scholar] [CrossRef] [PubMed]
- AlAshqar, A.; Reschke, L.; Kirschen, G.W.; Borahay, M.A. Role of inflammation in benign gynecologic disorders: From pathogenesis to novel therapiesdagger. Biol. Reprod. 2021, 105, 7–31. [Google Scholar] [CrossRef] [PubMed]
- Kirschen, G.W.; AlAshqar, A.; Miyashita-Ishiwata, M.; Reschke, L.; El Sabeh, M.; Borahay, M.A. Vascular biology of uterine fibroids: Connecting fibroids and vascular disorders. Reproduction 2021, 162, R1–R18. [Google Scholar] [CrossRef]
- Fletcher, N.M.; Abusamaan, M.S.; Memaj, I.; Saed, M.G.; Al-Hendy, A.; Diamond, M.P.; Saed, G.M. Oxidative stress: A key regulator of leiomyoma cell survival. Fertil Steril 2017, 107, 1387–1394.e1381. [Google Scholar] [CrossRef]
- Santulli, P.; Borghese, B.; Lemarechal, H.; Leconte, M.; Millischer, A.E.; Batteux, F.; Chapron, C.; Borderie, D. Increased serum oxidative stress markers in women with uterine leiomyoma. PLoS ONE 2013, 8, e72069. [Google Scholar] [CrossRef]
- Fletcher, N.M.; Saed, M.G.; Abu-Soud, H.M.; Al-Hendy, A.; Diamond, M.P.; Saed, G.M. Uterine fibroids are characterized by an impaired antioxidant cellular system: Potential role of hypoxia in the pathophysiology of uterine fibroids. J. Assist. Reprod. Genet. 2013, 30, 969–974. [Google Scholar] [CrossRef]
- Miyashita-Ishiwata, M.; El Sabeh, M.; Reschke, L.D.; Afrin, S.; Borahay, M.A. Hypoxia induces proliferation via NOX4-Mediated oxidative stress and TGF-beta3 signaling in uterine leiomyoma cells. Free Radic Res. 2022, 56, 163–172. [Google Scholar] [CrossRef]
- Nasiadek, M.; Krawczyk, T.; Sapota, A. Tissue levels of cadmium and trace elements in patients with myoma and uterine cancer. Hum. Exp. Toxicol. 2005, 24, 623–630. [Google Scholar] [CrossRef]
- Pavone, D.; Clemenza, S.; Sorbi, F.; Fambrini, M.; Petraglia, F. Epidemiology and Risk Factors of Uterine Fibroids. Best Pr. Res. Clin. Obs. Gynaecol. 2018, 46, 3–11. [Google Scholar] [CrossRef]
- Maghraby, N.; El Noweihi, A.M.; El-Melegy, N.T.; Mostafa, N.A.M.; Abbas, A.M.; El-Deek, H.E.M.; Radwan, E. Increased Expression of Fibroblast Activation Protein is Associated with Autophagy Dysregulation and Oxidative Stress in Obese Women with Uterine Fibroids. Reprod. Sci. 2022, 29, 448–459. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Asif, H.; Feng, Y.; Kohrn, B.F.; Kennedy, S.R.; Kim, J.J.; Wei, J.J. Myometrial oxidative stress drives MED12 mutations in leiomyoma. Cell Biosci. 2022, 12, 111. [Google Scholar] [CrossRef]
- Xu, X.; Kim, J.J.; Li, Y.; Xie, J.; Shao, C.; Wei, J.J. Oxidative stress-induced miRNAs modulate AKT signaling and promote cellular senescence in uterine leiomyoma. J. Mol. Med. 2018, 96, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss. Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; de Groot, H. Role of reactive oxygen species in cell toxicity. Toxicol. Lett. 1992, 64–65, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Sugino, N.; Nakamura, Y.; Okuno, N.; Ishimatu, M.; Teyama, T.; Kato, H. Effects of ovarian ischemia-reperfusion on luteal function in pregnant rats. Biol. Reprod. 1993, 49, 354–358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sugino, N.; Karube-Harada, A.; Taketani, T.; Sakata, A.; Nakamura, Y. Withdrawal of ovarian steroids stimulates prostaglandin F2alpha production through nuclear factor-kappaB activation via oxygen radicals in human endometrial stromal cells: Potential relevance to menstruation. J. Reprod. Dev. 2004, 50, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Paszkowski, T.; Clarke, R.N.; Hornstein, M.D. Smoking induces oxidative stress inside the Graafian follicle. Hum. Reprod. 2002, 17, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Guerin, P.; El Mouatassim, S.; Menezo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Sugino, N.; Shimamura, K.; Takiguchi, S.; Tamura, H.; Ono, M.; Nakata, M.; Nakamura, Y.; Ogino, K.; Uda, T.; Kato, H. Changes in activity of superoxide dismutase in the human endometrium throughout the menstrual cycle and in early pregnancy. Hum. Reprod. 1996, 11, 1073–1078. [Google Scholar] [CrossRef]
- Agarwal, A.; Allamaneni, S.S. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod. Biomed. Online 2004, 9, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Toprani, S.M.; Kelkar Mane, V. Role of DNA damage and repair mechanisms in uterine fibroid/leiomyomas: A review. Biol. Reprod. 2021, 104, 58–70. [Google Scholar] [CrossRef]
- Chiaffarino, F.; Ricci, E.; Cipriani, S.; Chiantera, V.; Parazzini, F. Cigarette smoking and risk of uterine myoma: Systematic review and meta-analysis. Eur. J. Obs. Gynecol. Reprod. Biol. 2016, 197, 63–71. [Google Scholar] [CrossRef]
- Prusinski Fernung, L.E.; Al-Hendy, A.; Yang, Q. A Preliminary Study: Human Fibroid Stro-1(+)/CD44(+) Stem Cells Isolated From Uterine Fibroids Demonstrate Decreased DNA Repair and Genomic Integrity Compared to Adjacent Myometrial Stro-1(+)/CD44(+) Cells. Reprod. Sci. 2019, 26, 619–638. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Makinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.W.; Kang, H.J.; Bae, I. BRCA1 and Oxidative Stress. Cancers 2014, 6, 771–795. [Google Scholar] [CrossRef]
- Xu, L.; Wu, T.; Lu, S.; Hao, X.; Qin, J.; Wang, J.; Zhang, X.; Liu, Q.; Kong, B.; Gong, Y.; et al. Mitochondrial superoxide contributes to oxidative stress exacerbated by DNA damage response in RAD51-depleted ovarian cancer cells. Redox. Biol. 2020, 36, 101604. [Google Scholar] [CrossRef]
- Yang, Q.; Laknaur, A.; Elam, L.; Ismail, N.; Gavrilova-Jordan, L.; Lue, J.; Diamond, M.P.; Al-Hendy, A. Identification of Polycomb Group Protein EZH2-Mediated DNA Mismatch Repair Gene MSH2 in Human Uterine Fibroids. Reprod. Sci. 2016, 23, 1314–1325. [Google Scholar] [CrossRef][Green Version]
- Kurjak, A.; Kupesic-Urek, S.; Miric, D. The assessment of benign uterine tumor vascularization by transvaginal color Doppler. Ultrasound Med. Biol. 1992, 18, 645–649. [Google Scholar] [CrossRef]
- Uluer, E.T.; Inan, S.; Ozbilgin, K.; Karaca, F.; Dicle, N.; Sanci, M. The role of hypoxia related angiogenesis in uterine smooth muscle tumors. Biotech. Histochem. 2015, 90, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Hockel, M.; Wree, A.; Leo, C.; Horn, L.C.; Vaupel, P. Lack of hypoxic response in uterine leiomyomas despite severe tissue hypoxia. Cancer Res. 2008, 68, 4719–4726. [Google Scholar] [CrossRef] [PubMed]
- Miyashita-Ishiwata, M.; El Sabeh, M.; Reschke, L.D.; Afrin, S.; Borahay, M.A. Differential response to hypoxia in leiomyoma and myometrial cells. Life Sci. 2022, 290, 120238. [Google Scholar] [CrossRef] [PubMed]
- Pejic, S.; Todorovic, A.; Stojiljkovic, V.; Cvetkovic, D.; Lucic, N.; Radojicic, R.M.; Saicic, Z.S.; Pajovic, S.B. Superoxide dismutase and lipid hydroperoxides in blood and endometrial tissue of patients with benign, hyperplastic and malignant endometrium. Acad. Bras. Cienc. 2008, 80, 515–522. [Google Scholar] [CrossRef]
- Fletcher, N.M.; Saed, M.G.; Abuanzeh, S.; Abu-Soud, H.M.; Al-Hendy, A.; Diamond, M.P.; Saed, G.M. Nicotinamide adenine dinucleotide phosphate oxidase is differentially regulated in normal myometrium versus leiomyoma. Reprod. Sci. 2014, 21, 1145–1152. [Google Scholar] [CrossRef]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Aissani, B.; Wiener, H.; Zhang, K. Multiple hits for the association of uterine fibroids on human chromosome 1q43. PLoS ONE 2013, 8, e58399. [Google Scholar] [CrossRef]
- Li, P.; Wu, Y.; Wu, H.; Xiong, Q.; Zhao, N.; Chen, G.; Wu, C.; Xiao, H. Functional Characterization of FH Mutation c.557G>A Underlies Uterine Leiomyomas. Int. J. Mol. Sci. 2022, 23, 1452. [Google Scholar] [CrossRef]
- AlAshqar, A.; Patzkowsky, K.; Afrin, S.; Wild, R.; Taylor, H.S.; Borahay, M.A. Cardiometabolic Risk Factors and Benign Gynecologic Disorders. Obs. Gynecol. Surv. 2019, 74, 661–673. [Google Scholar] [CrossRef]
- Gallagher, C.S.; Makinen, N.; Harris, H.R.; Rahmioglu, N.; Uimari, O.; Cook, J.P.; Shigesi, N.; Ferreira, T.; Velez-Edwards, D.R.; Edwards, T.L.; et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat. Commun. 2019, 10, 4857. [Google Scholar] [CrossRef]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Lashen, H.; Fear, K.; Sturdee, D.W. Obesity is associated with increased risk of first trimester and recurrent miscarriage: Matched case-control study. Hum. Reprod. 2004, 19, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Schmidt, M.D.; Dwyer, T.; Norman, R.J.; Venn, A.J. Obesity and menstrual irregularity: Associations with SHBG, testosterone, and insulin. Obesity 2009, 17, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Lin, Z.; Vasquez, E.; Luan, X.; Guo, F.; Xu, L. Association between obesity and the risk of uterine fibroids: A systematic review and meta-analysis. J. Epidemiol. Community Health 2021, 75, 197–204. [Google Scholar] [CrossRef]
- Sun, K.; Xie, Y.; Zhao, N.; Li, Z. A case-control study of the relationship between visceral fat and development of uterine fibroids. Exp. Ther. Med. 2019, 18, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; El Sabah, M.; Manzoor, A.; Miyashita-Ishiwata, M.; Reschke, L.; Borahay, M.A. Adipocyte coculture induces a pro-inflammatory, fibrotic, angiogenic, and proliferative microenvironment in uterine leiomyoma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166564. [Google Scholar] [CrossRef]
- Maggio, M.; Lauretani, F.; Basaria, S.; Ceda, G.P.; Bandinelli, S.; Metter, E.J.; Bos, A.J.; Ruggiero, C.; Ceresini, G.; Paolisso, G.; et al. Sex hormone binding globulin levels across the adult lifespan in women--the role of body mass index and fasting insulin. J. Endocrinol. Investig. 2008, 31, 597–601. [Google Scholar] [CrossRef]
- Reschke, L.; Afrin, S.; El Sabah, M.; Charewycz, N.; Miyashita-Ishiwata, M.; Borahay, M.A. Leptin induces leiomyoma cell proliferation and extracellular matrix deposition via JAK2/STAT3 and MAPK/ERK pathways. F S Sci. 2022, 3, 383–391. [Google Scholar] [CrossRef]
- Azziz, R. Reproductive endocrinologic alterations in female asymptomatic obesity. Fertil. Steril. 1989, 52, 703–725. [Google Scholar]
- Vignini, A.; Sabbatinelli, J.; Clemente, N.; Delli Carpini, G.; Tassetti, M.; Zagaglia, G.; Ciavattini, A. Preperitoneal Fat Thicknesses, Lipid Profile, and Oxidative Status in Women With Uterine Fibroids. Reprod. Sci. 2017, 24, 1419–1425. [Google Scholar] [CrossRef]
- Vural, M.; Camuzcuoglu, H.; Toy, H.; Camuzcuoglu, A.; Aksoy, N. Oxidative stress and prolidase activity in women with uterine fibroids. J. Obs. Gynaecol. 2012, 32, 68–72. [Google Scholar] [CrossRef]
- Markowska, A.; Mardas, M.; Gajdzik, E.; Zagrodzki, P.; Markowska, J. Oxidative stress markers in uterine fibroids tissue in pre- and postmenopausal women. Clin. Exp. Obs. Gynecol. 2015, 42, 725–729. [Google Scholar] [CrossRef]
- Asare, G.A.; Akuffo, G.; Doku, D.; Asiedu, B.; Santa, S. Dynamics of urinary oxidative stress biomarkers: 8-hydroxy-2′-deoxyguanosine and 8-isoprostane in uterine leiomyomas. J. Midlife Health 2016, 7, 8–14. [Google Scholar] [PubMed]
- Shahbazi, S.; Zarei, S.; Torfeh, M.; Fatahi, N. Q192R variant in paraoxonase 1 gene confers susceptibility to leiomyoma. J. Cancer Res. Ther. 2020, 16, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Vidimar, V.; Gius, D.; Chakravarti, D.; Bulun, S.E.; Wei, J.J.; Kim, J.J. Dysfunctional MnSOD leads to redox dysregulation and activation of prosurvival AKT signaling in uterine leiomyomas. Sci. Adv. 2016, 2, e1601132. [Google Scholar] [CrossRef]
- Lethaby, A.; Puscasiu, L.; Vollenhoven, B. Preoperative medical therapy before surgery for uterine fibroids. Cochrane Database Syst. Rev. 2017, 11, CD000547. [Google Scholar] [CrossRef]
- Bradley, L.D.; Gueye, N.A. The medical management of abnormal uterine bleeding in reproductive-aged women. Am. J. Obs. Gynecol. 2016, 214, 31–44. [Google Scholar] [CrossRef]
- Marciniak, A.; Szydlowska, I.; Brodowska, A.; Wisniewska, B.; Nawrocka-Rutkowska, J.; Starczewski, A. New methods of uterine fibroids treatment. Pol. Merkur Lek. 2016, 41, 303–305. [Google Scholar]
- Fruehauf, J.H.; Back, W.; Eiermann, A.; Lang, M.C.; Pessel, M.; Marlinghaus, E.; Melchert, F.; Volz-Koster, S.; Volz, J. High-intensity focused ultrasound for the targeted destruction of uterine tissues: Experiences from a pilot study using a mobile HIFU unit. Arch. Gynecol. Obs. 2008, 277, 143–150. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Li, C.; Li, B.; Ouyang, L. Minimally invasive surgery for uterine fibroids. Ginekol. Pol. 2020, 91, 149–157. [Google Scholar] [CrossRef]
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Prim. 2016, 2, 16043. [Google Scholar] [CrossRef] [PubMed]
- Foksinski, M.; Kotzbach, R.; Szymanski, W.; Olinski, R. The level of typical biomarker of oxidative stress 8-hydroxy-2′-deoxyguanosine is higher in uterine myomas than in control tissues and correlates with the size of the tumor. Free Radic Biol. Med. 2000, 29, 597–601. [Google Scholar] [CrossRef]
- de Carvalho, L.F.; Abrao, M.S.; Biscotti, C.; Sharma, R.; Agarwal, A.; Falcone, T. Mapping histological levels of 8-hydroxy-2′-deoxyguanosine in female reproductive organs. J. Mol. Histol. 2013, 44, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Liao, Z.; Li, S.; Wu, R.; Li, J.; Ren, F.; Zhang, H. PLP1 may serve as a potential diagnostic biomarker of uterine fibroids. Front. Genet. 2022, 13, 1045395. [Google Scholar] [CrossRef] [PubMed]
- Pejic, S.; Kasapovic, J.; Todorovic, A.; Stojiljkovic, V.; Pajovic, S.B. Lipid peroxidation and antioxidant status in blood of patients with uterine myoma, endometrial polypus, hyperplastic and malignant endometrium. Biol. Res. 2006, 39, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Lemarechal, H.; Allanore, Y.; Chenevier-Gobeaux, C.; Kahan, A.; Ekindjian, O.G.; Borderie, D. Serum protein oxidation in patients with rheumatoid arthritis and effects of infliximab therapy. Clin. Chim. Acta 2006, 372, 147–153. [Google Scholar] [CrossRef]
- Allanore, Y.; Borderie, D.; Lemarechal, H.; Ekindjian, O.G.; Kahan, A. Acute and sustained effects of dihydropyridine-type calcium channel antagonists on oxidative stress in systemic sclerosis. Am. J. Med. 2004, 116, 595–600. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 22–38. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Chen, H.Y.; Ali, M.; Shieh, T.M.; Huang, Y.J.; Wang, K.L.; Chang, H.Y.; Huang, T.C.; Hong, Y.H.; Hsia, S.M. The Role of Cell Proliferation and Extracellular Matrix Accumulation Induced by Food Additive Butylated Hydroxytoluene in Uterine Leiomyoma. Nutrients 2021, 13, 74. [Google Scholar] [CrossRef]
- Bariani, M.V.; Rangaswamy, R.; Siblini, H.; Yang, Q.; Al-Hendy, A.; Zota, A.R. The role of endocrine-disrupting chemicals in uterine fibroid pathogenesis. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 380–387. [Google Scholar] [CrossRef]
- Galvez-Ontiveros, Y.; Paez, S.; Monteagudo, C.; Rivas, A. Endocrine Disruptors in Food: Impact on Gut Microbiota and Metabolic Diseases. Nutrients 2020, 12, 1158. [Google Scholar] [CrossRef] [PubMed]
- Szydlowska, I.; Nawrocka-Rutkowska, J.; Brodowska, A.; Marciniak, A.; Starczewski, A.; Szczuko, M. Dietary Natural Compounds and Vitamins as Potential Cofactors in Uterine Fibroids Growth and Development. Nutrients 2022, 14, 734. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Akhtar, M.M.; Ciavattini, A.; Giannubilo, S.R.; Protic, O.; Janjusevic, M.; Procopio, A.D.; Segars, J.H.; Castellucci, M.; Ciarmela, P. Use of dietary phytochemicals to target inflammation, fibrosis, proliferation, and angiogenesis in uterine tissues: Promising options for prevention and treatment of uterine fibroids? Mol. Nutr. Food Res. 2014, 58, 1667–1684. [Google Scholar] [CrossRef]
- Wise, L.A.; Radin, R.G.; Palmer, J.R.; Kumanyika, S.K.; Boggs, D.A.; Rosenberg, L. Intake of fruit, vegetables, and carotenoids in relation to risk of uterine leiomyomata. Am. J. Clin. Nutr. 2011, 94, 1620–1631. [Google Scholar] [CrossRef]
- Ciebiera, M.; Ali, M.; Zgliczynska, M.; Skrzypczak, M.; Al-Hendy, A. Vitamins and Uterine Fibroids: Current Data on Pathophysiology and Possible Clinical Relevance. Int. J. Mol. Sci. 2020, 21, 5528. [Google Scholar] [CrossRef]
- Roshdy, E.; Rajaratnam, V.; Maitra, S.; Sabry, M.; Allah, A.S.; Al-Hendy, A. Treatment of symptomatic uterine fibroids with green tea extract: A pilot randomized controlled clinical study. Int. J. Womens Health 2013, 5, 477–486. [Google Scholar] [PubMed]
- Grandi, G.; Del Savio, M.C.; Melotti, C.; Feliciello, L.; Facchinetti, F. Vitamin D and green tea extracts for the treatment of uterine fibroids in late reproductive life: A pilot, prospective, daily-diary based study. Gynecol. Endocrinol. 2022, 38, 63–67. [Google Scholar] [CrossRef]
- Heinonen, H.R.; Mehine, M.; Makinen, N.; Pasanen, A.; Pitkanen, E.; Karhu, A.; Sarvilinna, N.S.; Sjoberg, J.; Heikinheimo, O.; Butzow, R.; et al. Global metabolomic profiling of uterine leiomyomas. Br. J. Cancer 2017, 117, 1855–1864. [Google Scholar] [CrossRef]
- Arauz, J.; Rivera-Espinoza, Y.; Shibayama, M.; Favari, L.; Flores-Beltran, R.E.; Muriel, P. Nicotinic acid prevents experimental liver fibrosis by attenuating the prooxidant process. Int. Immunopharmacol. 2015, 28, 244–251. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kostantinou, A.; Kougias, M.; Kazazis, C. Statins and cancer. Anticancer Agents Med. Chem. 2014, 14, 706–712. [Google Scholar] [CrossRef]
- Borahay, M.A.; Kilic, G.S.; Yallampalli, C.; Snyder, R.R.; Hankins, G.D.; Al-Hendy, A.; Boehning, D. Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells. J. Biol. Chem. 2014, 289, 35075–35086. [Google Scholar] [CrossRef] [PubMed]
- Borahay, M.A.; Fang, X.; Baillargeon, J.G.; Kilic, G.S.; Boehning, D.F.; Kuo, Y.F. Statin use and uterine fibroid risk in hyperlipidemia patients: A nested case-control study. Am. J. Obs. Gynecol. 2016, 215, 750.e1–750.e8. [Google Scholar] [CrossRef] [PubMed]
- Borahay, M.A.; Vincent, K.; Motamedi, M.; Sbrana, E.; Kilic, G.S.; Al-Hendy, A.; Boehning, D. Novel effects of simvastatin on uterine fibroid tumors: In vitro and patient-derived xenograft mouse model study. Am. J. Obs. Gynecol. 2015, 213, e191–e198. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Islam, M.S.; Patzkowsky, K.; Malik, M.; Catherino, W.H.; Segars, J.H.; Borahay, M.A. Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells. Am. J. Obs. Gynecol. 2020, 223, 733.e1–733.e14. [Google Scholar] [CrossRef]
- Afrin, S.; El Sabeh, M.; Islam, M.S.; Miyashita-Ishiwata, M.; Malik, M.; Catherino, W.H.; Akimzhanov, A.M.; Boehning, D.; Yang, Q.; Al-Hendy, A.; et al. Simvastatin modulates estrogen signaling in uterine leiomyoma via regulating receptor palmitoylation, trafficking and degradation. Pharm. Res. 2021, 172, 105856. [Google Scholar] [CrossRef]

| Biomarker | Diagnostic Usage |
|---|---|
| 8-OH-dG | Protein found in fibroid tissue; levels correlated with tumor size; can be found in urine |
| PLP1 | mRNA and protein found in fibroid tissues |
| LOOH | Elevated in the sera of women with hyperplastic and neoplastic endometrial cells |
| Carbonyl groups and AOPPs | Increased in sera of women with fibroids |
| Thiols | Decreased in sera of women with fibroids |
| MED12 | Somatic mutation present in 70% of fibroids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlAshqar, A.; Lulseged, B.; Mason-Otey, A.; Liang, J.; Begum, U.A.M.; Afrin, S.; Borahay, M.A. Oxidative Stress and Antioxidants in Uterine Fibroids: Pathophysiology and Clinical Implications. Antioxidants 2023, 12, 807. https://doi.org/10.3390/antiox12040807
AlAshqar A, Lulseged B, Mason-Otey A, Liang J, Begum UAM, Afrin S, Borahay MA. Oxidative Stress and Antioxidants in Uterine Fibroids: Pathophysiology and Clinical Implications. Antioxidants. 2023; 12(4):807. https://doi.org/10.3390/antiox12040807
Chicago/Turabian StyleAlAshqar, Abdelrahman, Bethlehem Lulseged, Akailah Mason-Otey, Jinxiao Liang, Umme Aoufa Mafruha Begum, Sadia Afrin, and Mostafa A. Borahay. 2023. "Oxidative Stress and Antioxidants in Uterine Fibroids: Pathophysiology and Clinical Implications" Antioxidants 12, no. 4: 807. https://doi.org/10.3390/antiox12040807
APA StyleAlAshqar, A., Lulseged, B., Mason-Otey, A., Liang, J., Begum, U. A. M., Afrin, S., & Borahay, M. A. (2023). Oxidative Stress and Antioxidants in Uterine Fibroids: Pathophysiology and Clinical Implications. Antioxidants, 12(4), 807. https://doi.org/10.3390/antiox12040807







