Through Its Powerful Antioxidative Properties, L-Theanine Ameliorates Vincristine-Induced Neuropathy in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Experimental Protocol
2.4. Behavioral Assessment
2.4.1. Hot Plate Test
2.4.2. Acetone Drop Test
2.4.3. Paw Pressure Test
2.4.4. Von Frey Hair Test
2.5. Electrophysiological Studies
2.5.1. Motor Nerve Conduction Velocity Assessment
2.5.2. Sensory Nerve Conduction Velocity Determination
2.6. Biochemical Estimations
2.6.1. Total Protein Content
2.6.2. Measurement of Oxidative Stress
Nitrite Concentration
Malondialdehyde
Glutathione Concentration
Superoxide Dismutase Activity
Catalase Activity
2.6.3. Total Calcium
2.6.4. Measurement of Neuroinflammatory Markers
Interleukin-6, Interleukin-10, and Tumor Necrosis Factor-α
Myeloperoxidase
2.6.5. Measurement of the Apoptosis Marker
Caspase-3 Activity
2.7. Statistical Analysis
3. Results
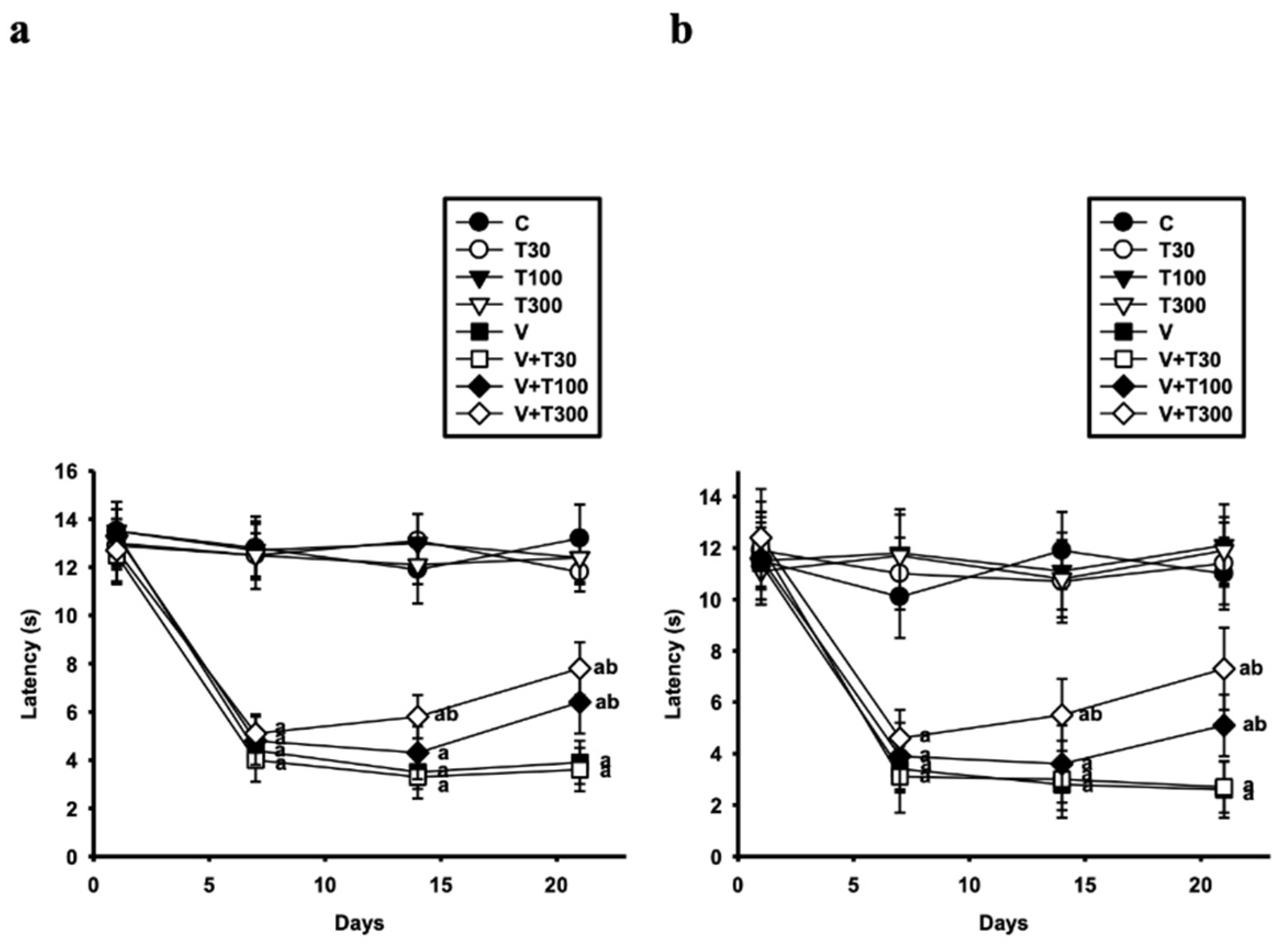
3.1. Effect of LT on the Development of Thermal Hyperalgesia and Allodynia Induced by VCR
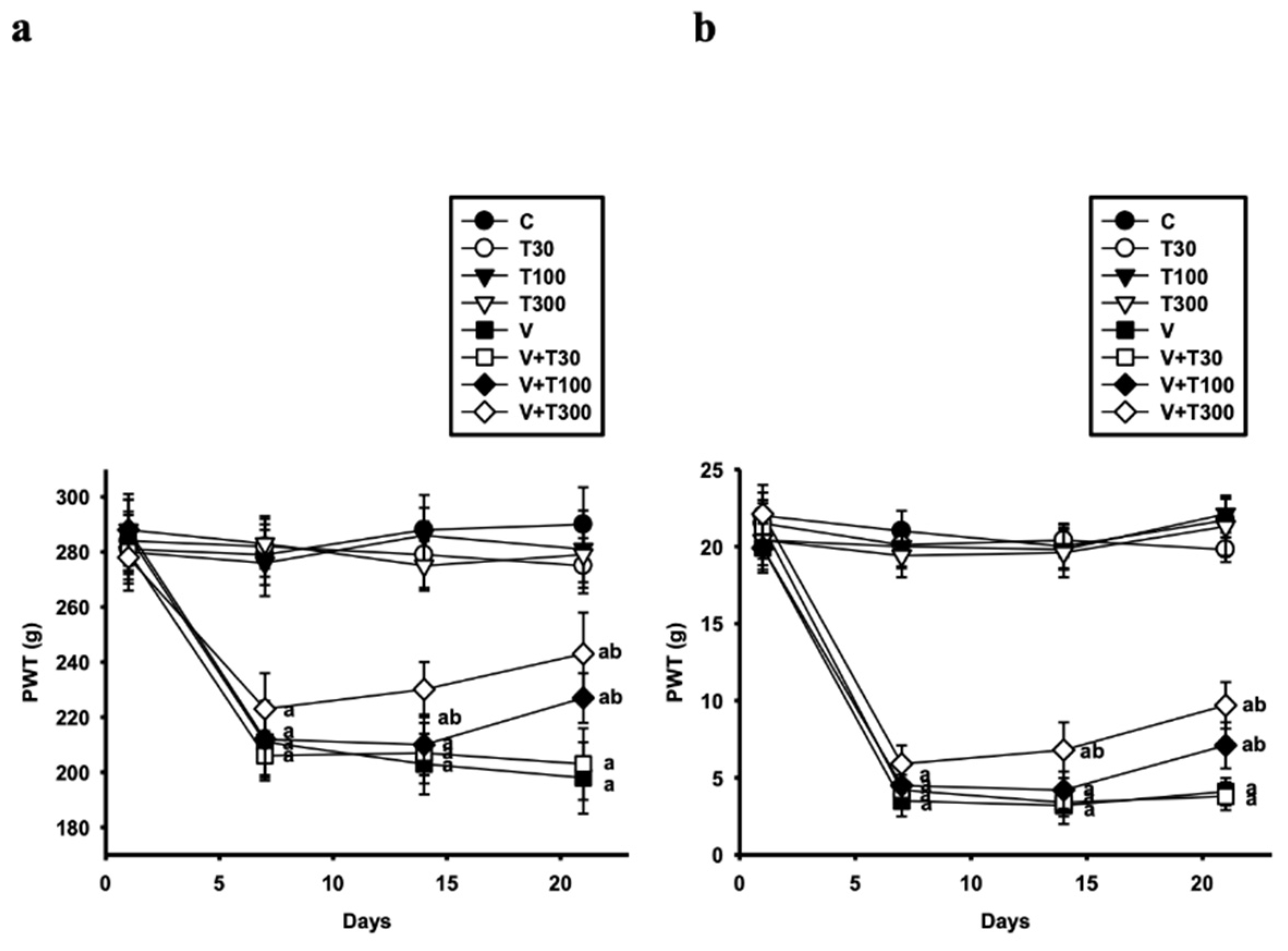
3.2. Effect of LT on the Development of Mechanical Hyperalgesia and Allodynia Induced by VCR
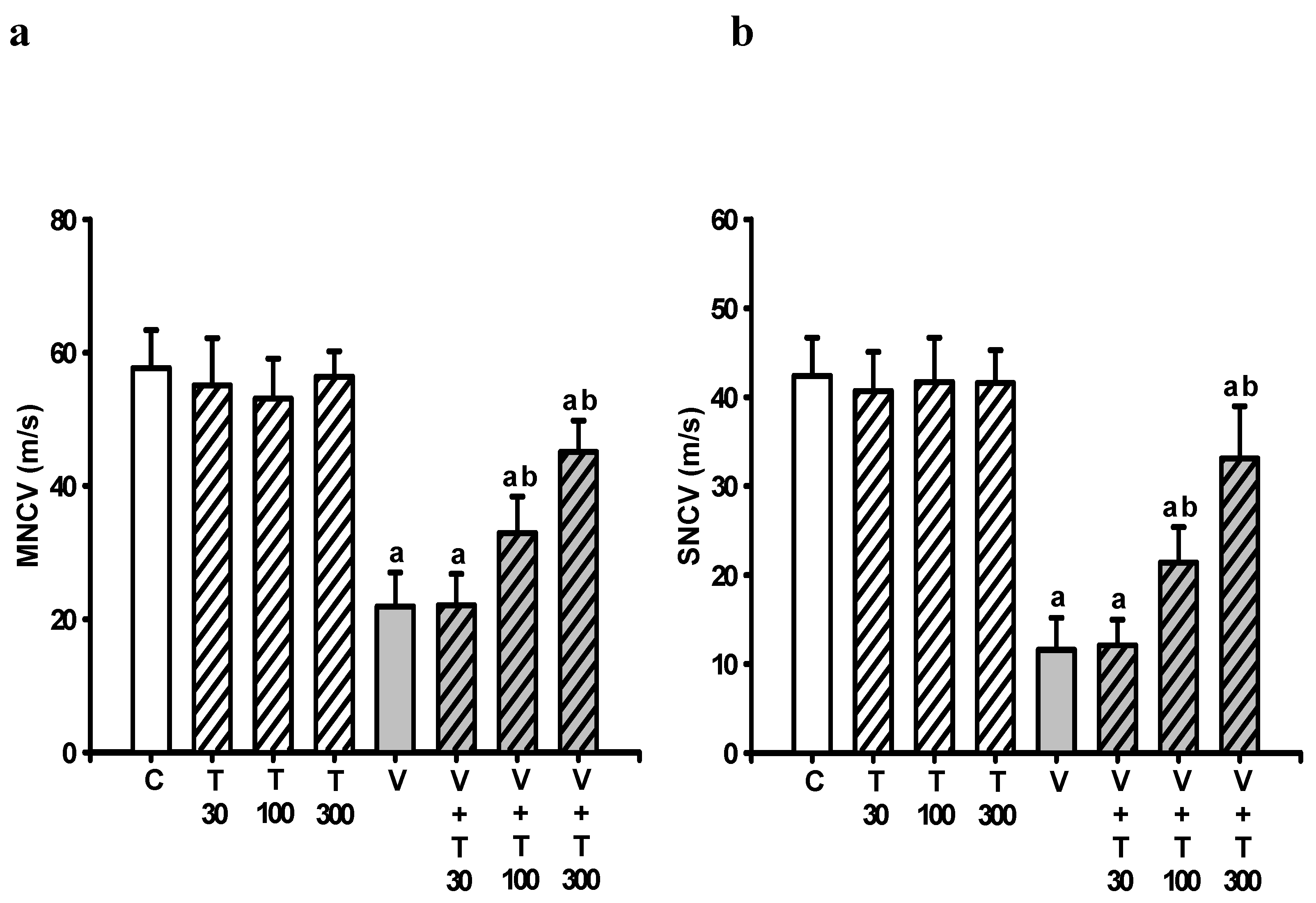
3.3. Effect of LT on the Loss of Neuronal Electrical Function Induced by VCR
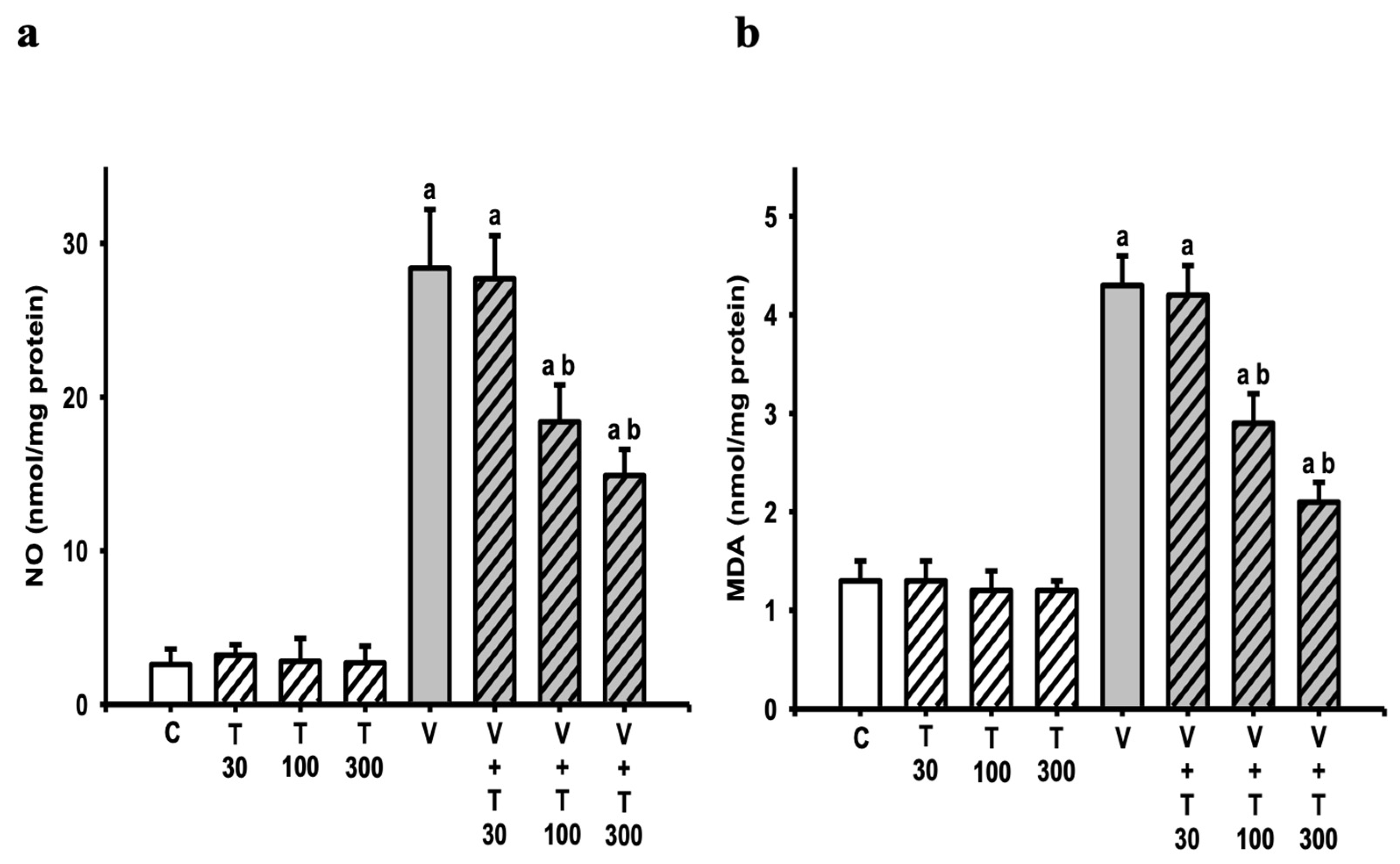
3.4. Effect of LT on the Increases in Sciatic Nitric Oxide and Lipid Peroxide Production Induced by VCR
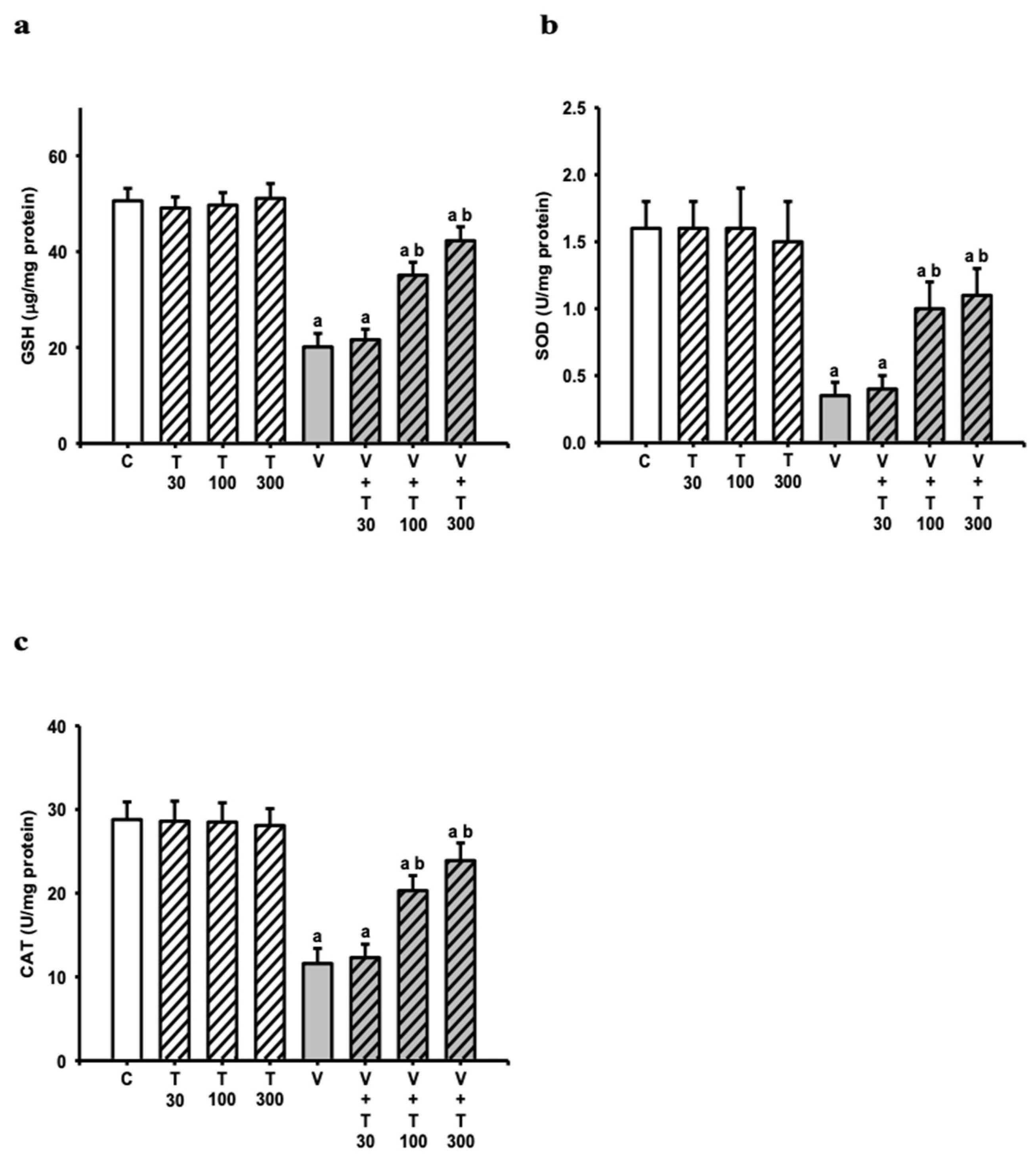
3.5. Effect of LT on the Decreases in Sciatic Antioxidation Power Induced by VCR
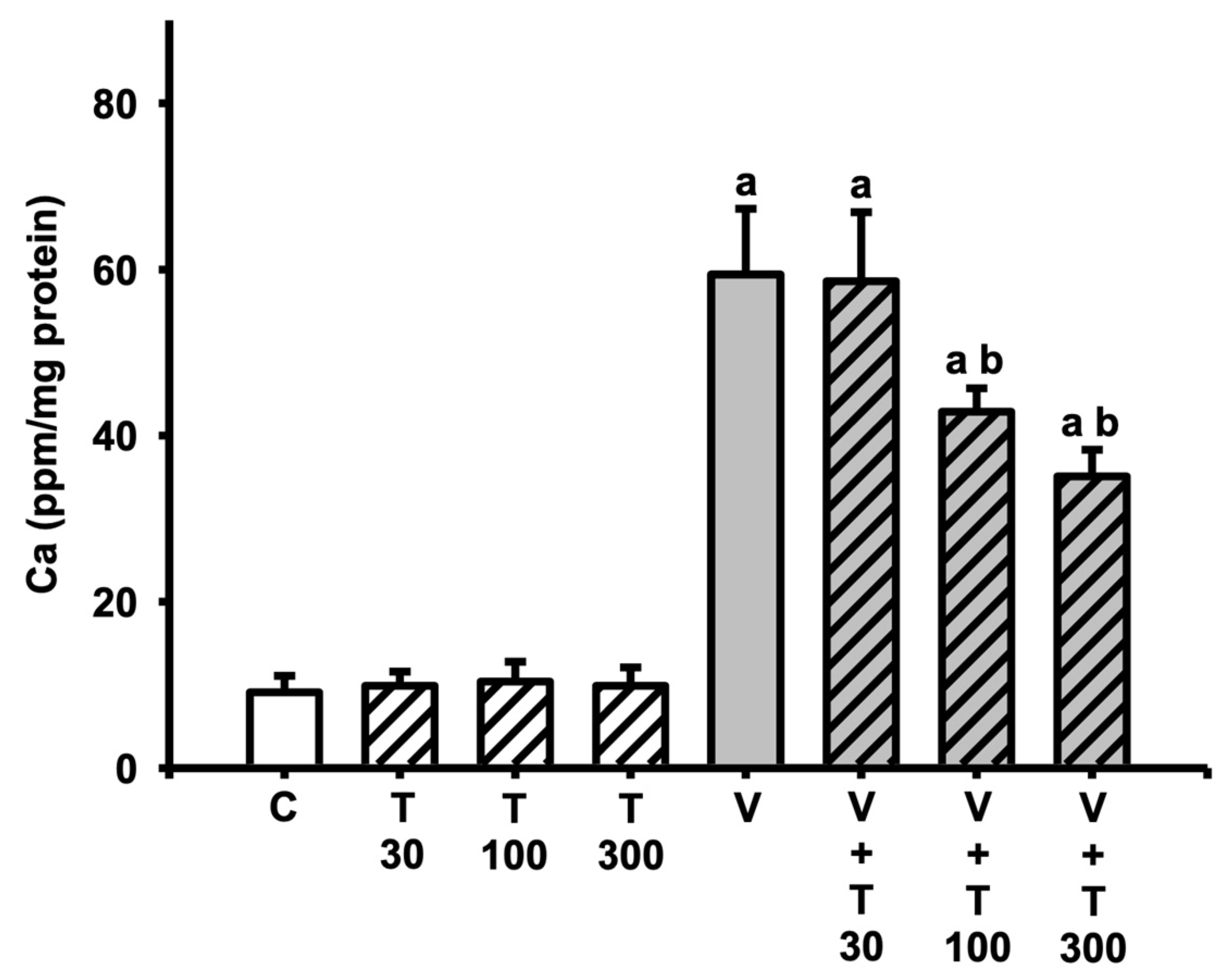
3.6. Effect of LT on the Increases in Sciatic Total Calcium Induced by VCR
3.7. Effect of LT on the Increases in Sciatic Neuroinflammatory and Apoptotic Markers Induced by VCR
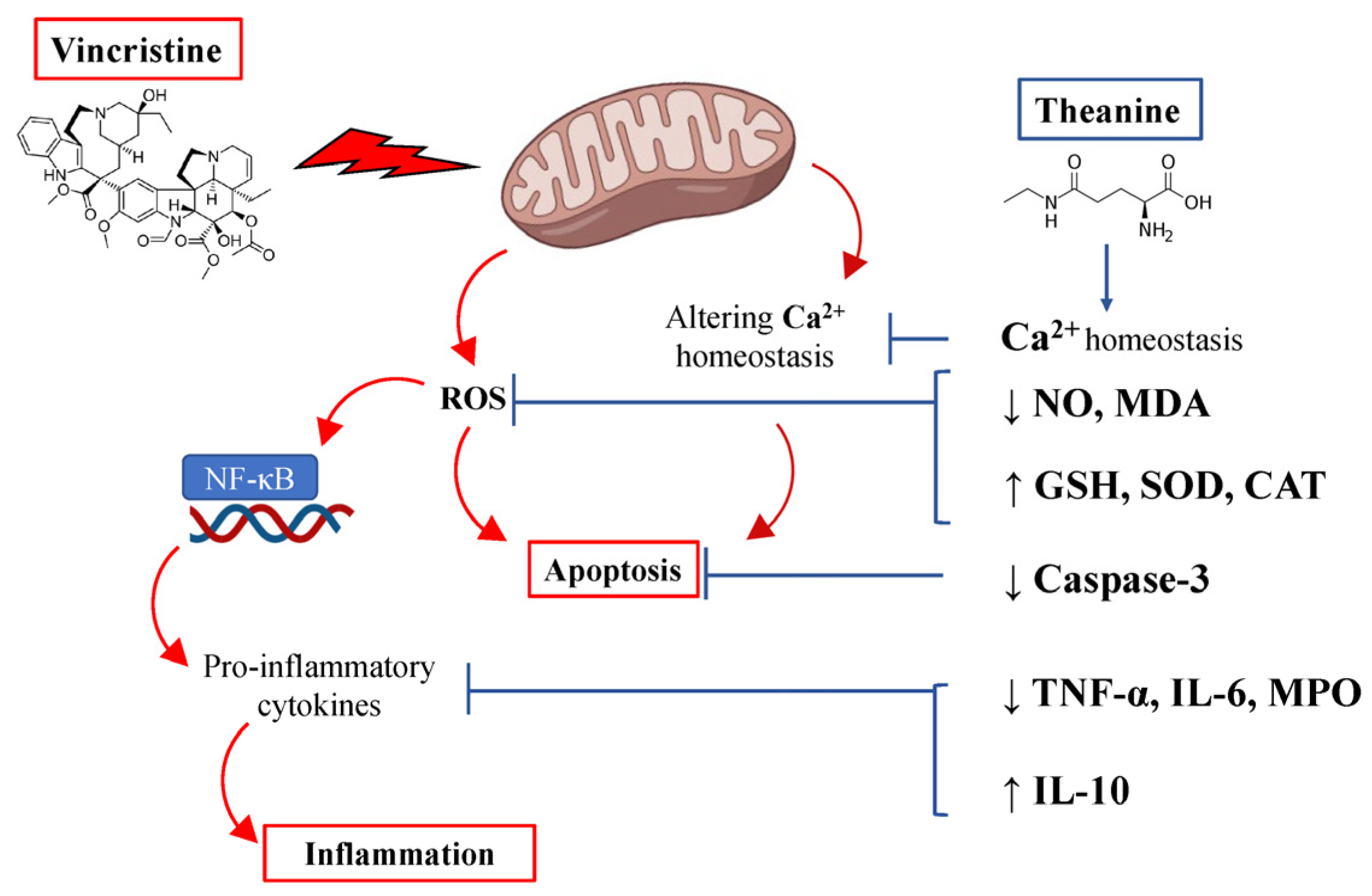
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyette-Davis, J.A.; Hou, S.; Abdi, S.; Dougherty, P.M. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 2018, 8, 363–375. [Google Scholar] [CrossRef]
- Zhang, S.M. Chemotherapy-induced peripheral neuropathy and rehabilitation: A review. Semin. Oncol. 2021, 48, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Hu, Y.H.; Li, D.Y.; Zhang, Y.; Guo, H.L.; Li, Y.M.; Chen, F.; Xu, J. Vincristine-induced peripheral neuropathy: A mini-review. Neurotoxicology 2020, 81, 161–171. [Google Scholar] [CrossRef]
- Alenezi, S.K. The Ameliorative Effect of Thymoquinone on Vincristine-Induced Peripheral Neuropathy in Mice by Modulating Cellular Oxidative Stress and Cytokine. Life 2023, 13, 101. [Google Scholar] [CrossRef]
- Gautam, M.; Ramanathan, M. Ameliorative potential of flavonoids of Aegle marmelos in vincristine-induced neuropathic pain and associated excitotoxicity. Nutr. Neurosci. 2021, 24, 296–306. [Google Scholar] [CrossRef]
- Khalilzadeh, M.; Panahi, G.; Rashidian, A.; Hadian, M.R.; Abdollahi, A.; Afshari, K.; Shakiba, S.; Norouzi-Javidan, A.; Rahimi, N.; Momeny, M.; et al. The protective effects of sumatriptan on vincristine—induced peripheral neuropathy in a rat model. Neurotoxicology 2018, 67, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Jibira, Y.; Boakye-Gyasi, E.; Kofi Mensah Abotsi, W.; Amponsah, I.K.; Adongo, D.W.; Woode, E. Hydroethanolic Stem Bark Extract of Burkea africana Attenuates Vincristine-Induced Peripheral Neuropathy in Rats. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 7232579. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.S.; Li, Y.X.; Zhang, M.T.; Du, J.; Ma, P.S.; Yao, W.X.; Zhou, R.; Niu, Y.; Sun, T.; Yu, J.Q. Neuroprotective Effect of Matrine in Mouse Model of Vincristine-Induced Neuropathic Pain. Neurochem. Res. 2016, 41, 3147–3159. [Google Scholar] [CrossRef]
- Li, M.Y.; Liu, H.Y.; Wu, D.T.; Kenaan, A.; Geng, F.; Li, H.B.; Gunaratne, A.; Li, H.; Gan, R.Y. L-Theanine: A Unique Functional Amino Acid in Tea (Camellia sinensis L.) With Multiple Health Benefits and Food Applications. Front. Nutr. 2022, 9, 853846. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Khan, M.S.; Kamboh, A.A.; Alagawany, M.; Khafaga, A.F.; Noreldin, A.E.; Qumar, M.; Safdar, M.; Hussain, M.; Abd El-Hack, M.E.; et al. L-theanine: An astounding sui generis amino acid in poultry nutrition. Poult. Sci. 2020, 99, 5625–5636. [Google Scholar] [CrossRef]
- Wang, D.X.; Gao, Q.; Wang, T.T.; Qian, F.; Wang, Y.J. Theanine: The unique amino acid in the tea plant as an oral hepatoprotective agent. Asia Pac. J. Clin. Nutr. 2017, 26, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.; Gupta, G.D.; Singh, S. l-Theanine ameliorates motor deficit, mitochondrial dysfunction, and neurodegeneration against chronic tramadol induced rats model of Parkinson’s disease. Drug Chem. Toxicol. 2022, 45, 2097–2108. [Google Scholar] [CrossRef]
- Unno, K.; Muguruma, Y.; Inoue, K.; Konishi, T.; Taguchi, K.; Hasegawa-Ishii, S.; Shimada, A.; Nakamura, Y. Theanine, Antistress Amino Acid in Tea Leaves, Causes Hippocampal Metabolic Changes and Antidepressant Effects in Stress-Loaded Mice. Int. J. Mol. Sci. 2020, 22, 193. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Wang, M.H.; Soung, H.S.; Tseng, H.C.; Fang, C.H.; Lin, Y.W.; Yang, C.C.; Tsai, C.C. Neuroprotective effect of L-theanine in a rat model of chronic constriction injury of sciatic nerve-induced neuropathic pain. J. Formos. Med. Assoc. 2022, 121, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Dutta, A.; Phukan, B.C.; Manivasagam, T.; Thenmozhi, A.J.; Bhattacharya, P.; Paul, R.; Borah, A. Neuroprotective attributes of L-theanine, a bioactive amino acid of tea, and its potential role in Parkinson’s disease therapeutics. Neurochem. Int. 2019, 129, 104478. [Google Scholar] [CrossRef]
- Eddy, N.B.; Touchberry, C.F.; Lieberman, J.E. Synthetic Analgesics; Methadone Isomers and Derivatives. J. Pharmacol. Exp. Ther. 1950, 98, 121–137. [Google Scholar] [PubMed]
- Choi, Y.; Yoon, Y.W.; Na, H.S.; Kim, S.H.; Chung, J.M. Behavioral Signs of Ongoing Pain and Cold Allodynia in a Rat Model of Neuropathic Pain. Pain 1994, 59, 369–376. [Google Scholar]
- Randall, L.O.; Selitto, J.J. A Method for Measurement of Analgesic Activity on Inflamed Tissue. Arch. Int. Pharmacod. Ther. 1957, 111, 409–419. [Google Scholar]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative Assessment of Tactile Allodynia in the Rat Paw. J. Neurosci. Meth. 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Thomsen, K.; Rubin, I.; Lauritzen, M. NO- and non-NO-, non-prostanoid-dependent vasodilatation in rat sciatic nerve during maturation and developing experimental diabetic neuropathy. J. Physiol. 2002, 543, 977–993. [Google Scholar] [CrossRef]
- Saini, A.K.; Kumar, A.; Sharma, S.S. Preventive and curative effect of edaravone on nerve functions and oxidative stress in experimental diabetic neuropathy. Eur. J. Pharmacol. 2007, 568, 164–172. [Google Scholar] [CrossRef]
- Kurokawa, K.; de Almeida, D.F.; Zhang, Y.; Hebert, C.D.; Page, J.G.; Schweikart, K.M.; Oh, S.J. Sensory nerve conduction of the plantar nerve compared with other nerve conduction tests in rats. Clin. Neurophysiol. 2004, 115, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Taskiran, D.; Kutay, F.Z.; Sozmen, E.; Pogun, S. Sex differences in nitrite/nitrate levels and antioxidant defense in rat brain. Neuroreport 1997, 8, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Cheeseman, K.H. Determination of Aldehydic Lipid-Peroxidation Products—Malonaldehyde and 4-Hydroxynonenal. Method Enzymol. 1990, 186, 407–421. [Google Scholar]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A Simple Method for Clinical Assay of Superoxide-Dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Berker, K.I.; Olgun, F.A.O.; Ozyurt, D.; Demirata, B.; Apak, R. Modified Folin-Ciocalteu Antioxidant Capacity Assay for Measuring Lipophilic Antioxidants. J. Agric. Food Chem. 2013, 61, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Severinghaus, J.W.; Ferrebee, J.W. Calcium Determination by Flame Photometry—Methods for Serum, Urine, and Other Fluids. J. Biol. Chem. 1950, 187, 621–630. [Google Scholar] [CrossRef]
- Martucci, C.; Trovato, A.E.; Costa, B.; Borsani, E.; Franchi, S.; Magnaghi, V.; Panerai, A.E.; Rodella, L.F.; Valsecchi, A.E.; Sacerdote, P.; et al. The purinergic antagonist PPADS reduces pain related behaviours and interleukin-1 beta, interleukin-6, iNOS and nNOS overproduction in central and peripheral nervous system after peripheral neuropathy in mice. Pain 2008, 137, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative Assay for Acute Intestinal Inflammation Based on Myeloperoxidase Activity—Assessment of Inflammation in Rat and Hamster Models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.L.; Yarnitsky, D. Mechanical Hyperalgesias in Neuropathic Pain Patients—Dynamic and Static Subtypes. Ann. Neurol. 1993, 33, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Djouhri, L.; Lawson, S.N. A beta-fiber nociceptive primary afferent neurons: A review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res. Rev. 2004, 46, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Prasanth, K.G.; Balaji, B. Effect of curcumin in mice model of vincristine-induced neuropathy. Pharm. Biol. 2015, 53, 838–848. [Google Scholar] [CrossRef]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef]
- Kiguchi, N.; Maeda, T.; Kobayashi, Y.; Saika, F.; Kishioka, S. Involvement of Inflammatory Mediators in Neuropathic Pain Caused by Vincristine. Int. Rev. Neurobiol. 2009, 85, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.D.; Culver, D.G.; Levey, A.I.; Nash, N.R. Very early activation of m-calpain in peripheral nerve during Wallerian degeneration. J. Neurol. Sci. 2002, 196, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, A.; Flatters, S.J.L. Mitochondrial dysfunction in the pathogenesis of chemotherapy-induced peripheral neuropathy. Mitochondrial Dysfunct. Neurodegener. Peripher. Neuropathies 2019, 145, 83–126. [Google Scholar] [CrossRef]
- Nagasawa, K.; Aoki, H.; Yasuda, E.; Nagai, K.; Shimohama, S.; Fujimoto, S. Possible involvement of group I mGluRs in neuroprotective effect of theanine. Biochem. Biophys. Res. Commun. 2004, 320, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, V.R.K.; Shanmugam, P.; Krishnan, U.M.; Muthuraman, A. Ameliorative effect of Vernonia cinerea in vincristine-induced painful neuropathy in rats. Toxicol. Ind. Health 2014, 30, 794–805. [Google Scholar] [CrossRef]
- Vashistha, B.; Sharma, A.; Jain, V. Ameliorative potential of ferulic acid in vincristine-induced painful neuropathy in rats: An evidence of behavioral and biochemical examination. Nutr. Neurosci. 2017, 20, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jeong, W.; Jun, I.G.; Park, J.Y. Antiallodynic and anti-inflammatory effects of intrathecal R-PIA in a rat model of vincristine-induced peripheral neuropathy. Korean J. Anesthesiol. 2020, 73, 434–444. [Google Scholar] [CrossRef]
- Vallejo, R.; Tilley, D.M.; Vogel, L.; Benyamin, R. The Role of Glia and the Immune System in the Development and Maintenance of Neuropathic Pain. Pain Pract. 2010, 10, 167–184. [Google Scholar] [CrossRef]
- Sommer, C.; Leinders, M.; Uceyler, N. Inflammation in the pathophysiology of neuropathic pain. Pain 2018, 159, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P. Mechanisms for Reducing Neuropathic Pain. Mol. Neurobiol. 2020, 57, 67–87. [Google Scholar] [CrossRef]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- McKelvey, R.; Berta, T.; Old, E.; Ji, R.R.; Fitzgerald, M. Neuropathic Pain Is Constitutively Suppressed in Early Life by Anti-Inflammatory Neuroimmune Regulation. J. Neurosci. 2015, 35, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Ding, Q.; Gao, C.J.; Sun, X.D. Resveratrol attenuates neuropathic pain through balancing pro-inflammatory and anti-inflammatory cytokines release in mice. Int. Immunopharmacol. 2016, 34, 165–172. [Google Scholar] [CrossRef]
- Soung, H.S.; Wang, M.H.; Chang, K.C.; Chen, C.N.; Chang, Y.; Yang, C.C.; Tseng, H.C. (L)-Theanine Decreases Orofacial Dyskinesia Induced by Reserpine in Rats. Neurotox. Res. 2018, 34, 375–387. [Google Scholar] [CrossRef]
- Chen, C.N.; Wang, M.H.; Soung, H.S.; Chen, S.M.; Fang, C.H.; Lin, Y.W.; Tseng, H.C. (L)-Theanine Ameliorated Rotenone-Induced Parkinsonism-like Symptoms in Rats. Neurotox. Res. 2022, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Kawashiri, T.; Kobayashi, D.; Egashira, N.; Tsuchiya, T.; Shimazoe, T. Oral administration of Cystine and Theanine ameliorates oxaliplatin-induced chronic peripheral neuropathy in rodents. Sci. Rep. 2020, 10, 12665. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Sato, R.; Komura, T.; Ichikawa, H.; Hirashima, T.; Otake, S.; Akazawa, N.; Yazawa, T.; Abe, T.; Okada, T.; et al. Protective effect of the oral administration of cystine and theanine on oxaliplatin-induced peripheral neuropathy: A pilot randomized trial. Int. J. Clin. Oncol. 2020, 25, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.N.; Chang, K.C.; Wang, M.H.; Tseng, H.C.; Soung, H.S. Protective Effect of L-Theanine on Haloperidol-Induced Orofacial. Chin. J. Physiol. 2018, 61, 35–41. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-C.; Wang, M.-H.; Soung, H.-S.; Tseng, H.-C.; Lin, F.-H.; Chang, K.-C.; Tsai, C.-C. Through Its Powerful Antioxidative Properties, L-Theanine Ameliorates Vincristine-Induced Neuropathy in Rats. Antioxidants 2023, 12, 803. https://doi.org/10.3390/antiox12040803
Yang C-C, Wang M-H, Soung H-S, Tseng H-C, Lin F-H, Chang K-C, Tsai C-C. Through Its Powerful Antioxidative Properties, L-Theanine Ameliorates Vincristine-Induced Neuropathy in Rats. Antioxidants. 2023; 12(4):803. https://doi.org/10.3390/antiox12040803
Chicago/Turabian StyleYang, Chih-Chuan, Mao-Hsien Wang, Hung-Sheng Soung, Hsiang-Chien Tseng, Feng-Huei Lin, Kuo-Chi Chang, and Cheng-Chia Tsai. 2023. "Through Its Powerful Antioxidative Properties, L-Theanine Ameliorates Vincristine-Induced Neuropathy in Rats" Antioxidants 12, no. 4: 803. https://doi.org/10.3390/antiox12040803
APA StyleYang, C.-C., Wang, M.-H., Soung, H.-S., Tseng, H.-C., Lin, F.-H., Chang, K.-C., & Tsai, C.-C. (2023). Through Its Powerful Antioxidative Properties, L-Theanine Ameliorates Vincristine-Induced Neuropathy in Rats. Antioxidants, 12(4), 803. https://doi.org/10.3390/antiox12040803






