The Impact of a Very Short Abstinence Period on Assisted Reproductive Technique Outcomes: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
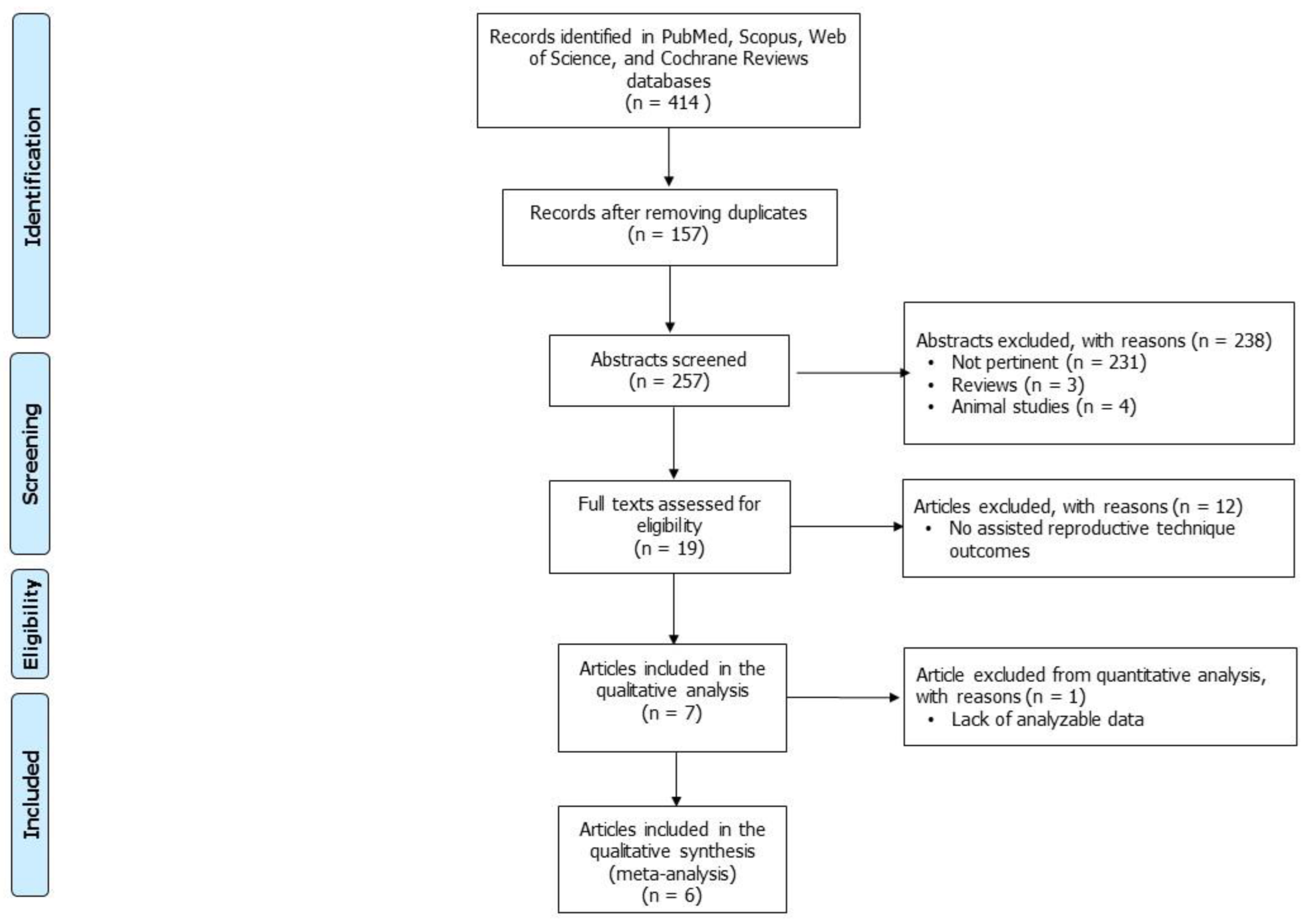
2. Materials and Methods
2.1. Sources
2.2. Study Selection
2.3. Data Extraction
2.4. Quality of Evidence Evaluation
2.5. Statistical Analysis
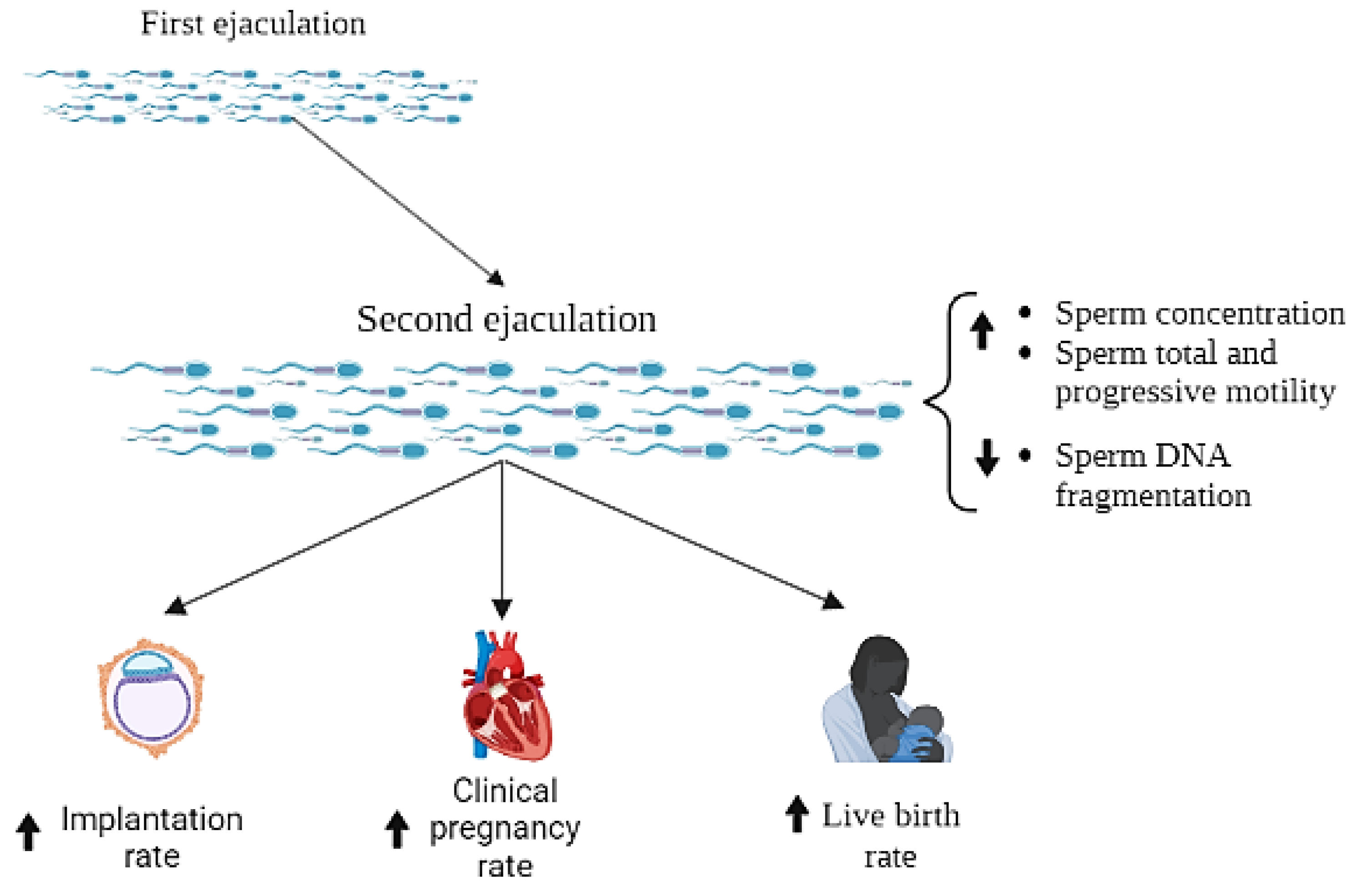
3. Results
3.1. Effects of a Very Short Period of Abstinence on ART Outcomes: Qualitative Analysis
3.2. Effects of a Short Period of Abstinence on ART Outcome: Quantitative Analysis
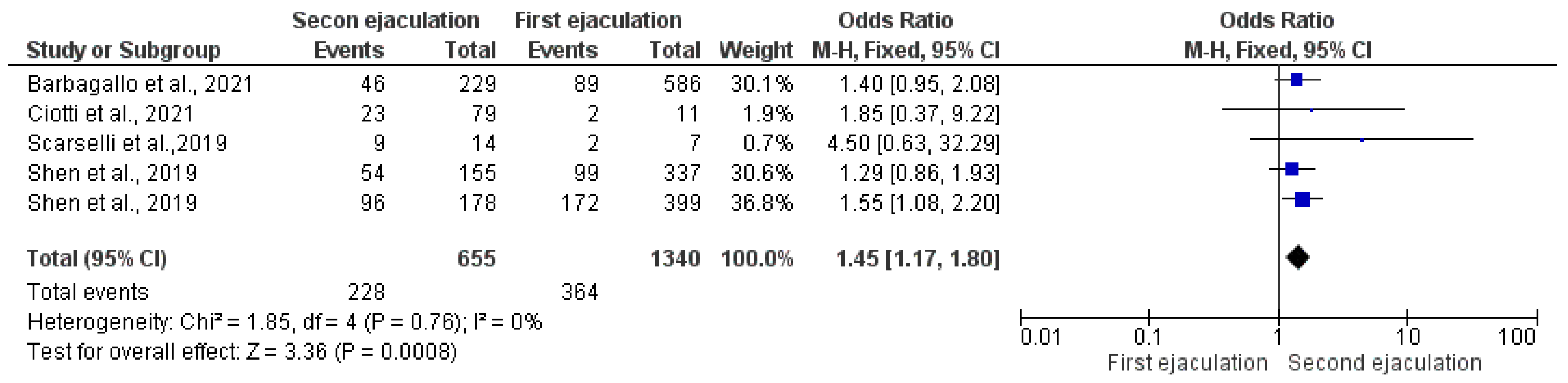
3.2.1. Fertilization Rate
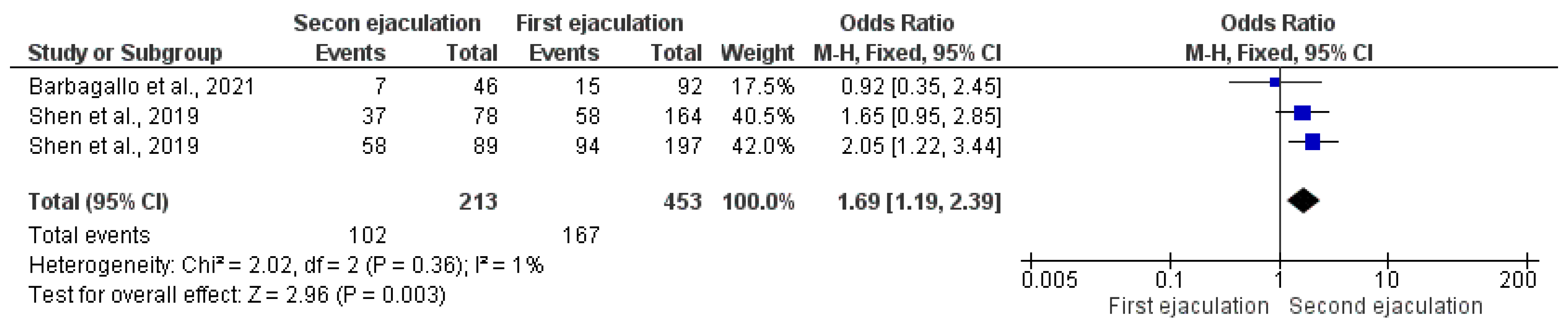
3.2.2. Implantation Rate
3.2.3. Clinical Pregnancy Rate
3.2.4. Live Birth Rate
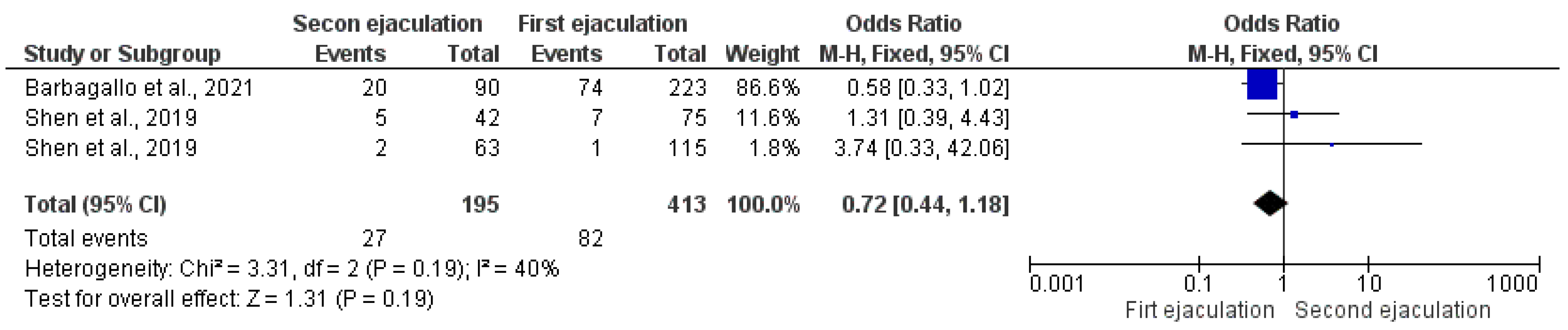
3.2.5. Miscarriage Rate
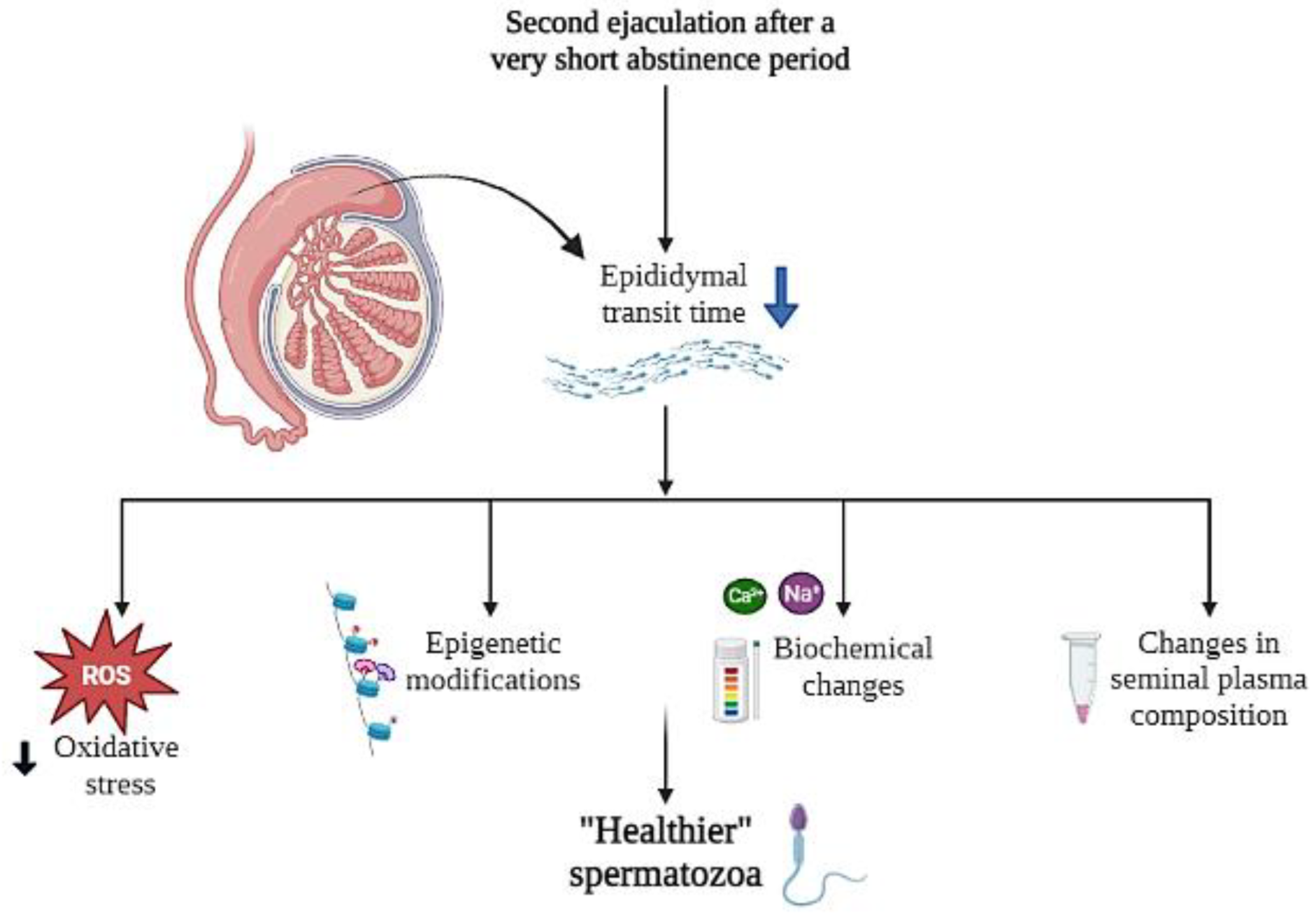
4. Discussion
5. Conclusions
6. Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sauer, M.V. In vitro fertilization (IVF): A review of 3 decades of clinical innovation and technological advancement. Ther. Clin. Risk Manag. 2006, 2, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Crafa, A.; Calogero, A.E.; Cannarella, R.; Mongioi’, L.M.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. The Burden of Hormonal Disorders: A Worldwide Overview With a Particular Look in Italy. Front. Endocrinol. 2021, 12, 694325. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.M.; Millar, K.; Jones, C.; Fatum, M.; Coward, K. Deleterious effects of obesity upon the hormonal and molecular mechanisms controlling spermatogenesis and male fertility. Hum. Fertil. 2015, 18, 184–193. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Gusmano, C.; Barone, N.; Burrello, N.; Aversa, A.; Calogero, A.E.; La Vignera, S. Temporal Trend of Conventional Sperm Parameters in a Sicilian Population in the Decade 2011–2020. J. Clin. Med. 2021, 10, 993. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Giacone, F.; Iacoviello, L.; Vicari, E.; Mongioi’, L.; Calogero, A.E. In vitro effects of nicotine on sperm motility and bio-functional flow cytometry sperm parameters. Int. J. Immunopathol. Pharmacol. 2013, 26, 739–746. [Google Scholar] [CrossRef]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Cimino, L.; Magagnini, M.C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Molecular Mechanisms Underlying the Relationship between Obesity and Male Infertility. Metabolites 2021, 11, 840. [Google Scholar] [CrossRef]
- Leisegang, K.; Dutta, S. Do lifestyle practices impede male fertility? Andrologia 2021, 53, e13595. [Google Scholar] [CrossRef]
- Calogero, A.E.; Fiore, M.; Giacone, F.; Altomare, M.; Asero, P.; Ledda, C.; Romeo, G.; Mongioì, L.M.; Copat, C.; Giuffrida, M.; et al. Exposure to multiple metals/metalloids and human semen quality: A cross-sectional study. Ecotoxicol. Environ. Saf. 2021, 215, 112165. [Google Scholar] [CrossRef]
- Perrone, P.; Lettieri, G.; Marinaro, C.; Longo, V.; Capone, S.; Forleo, A.; Pappalardo, S.; Montano, L.; Piscopo, M. Molecular Alterations and Severe Abnormalities in Spermatozoa of Young Men Living in the “Valley of Sacco River” (Latium, Italy): A Preliminary Study. Int. J. Environ. Res. Public Health 2022, 19, 11023. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Effects of Bisphenols on Testicular Steroidogenesis. Front. Endocrinol. 2020, 11, 373. [Google Scholar] [CrossRef]
- Ješeta, M.; Navrátilová, J.; Franzová, K.; Fialková, S.; Kempisty, B.; Ventruba, P.; Žáková, J.; Crha, I. Overview of the Mechanisms of Action of Selected Bisphenols and Perfluoroalkyl Chemicals on the Male Reproductive Axes. Front. Genet. 2021, 12, 692897. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Gül, M.; Rambhatla, A.; Agarwal, A. Temporal decline of sperm concentration: Role of endocrine disruptors. Endocrine 2023, 79, 1–16. [Google Scholar] [CrossRef]
- Loutradi, K.E.; Tarlatzis, B.C.; Goulis, D.G.; Zepiridis, L.; Pagou, T.; Chatziioannou, E.; Grimbizis, G.F.; Papadimas, I.; Bontis, I. The effects of sperm quality on embryo development after intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2006, 23, 69–74. [Google Scholar] [CrossRef]
- Puscheck, E.E.; Jeyendran, R.S. The impact of male factor on recurrent pregnancy loss. Curr. Opin. Obstet. Gynecol. 2007, 19, 222–228. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240030787 (accessed on 3 December 2021).
- Kvist, U.; Björndahl, L. Manual on Basic Semen Analysis; Oxford University Press: Oxford, UK, 2002; Published in association with ESHRE. [Google Scholar]
- Levitas, E.; Lunenfeld, E.; Weiss, N.; Friger, M.; Har-Vardi, I.; Koifman, A.; Potashnik, G. Relationship between the duration of sexual abstinence and semen quality: Analysis of 9,489 semen samples. Fertil. Steril. 2005, 83, 1680–1686. [Google Scholar] [CrossRef]
- Henkel, R. Sperm preparation: State-of-the-art–physiological aspects and application of advanced sperm preparation methods. Asian J. Androl. 2012, 14, 260–269. [Google Scholar] [CrossRef]
- Barbagallo, F.; Cannarella, R.; Crafa, A.; Manna, C.; La Vignera, S.; Condorelli, R.A.; Calogero, A.E. The Impact of a Very Short Abstinence Period on Conventional Sperm Parameters and Sperm DNA Fragmentation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7303. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-Analysis of Observational Studies in Epidemiology. A Proposal for Reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R. The PICO strategy for the research question construction and evidence search. Rev. Lat.-Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.; Farrington, D.P.; Eisner, M.P. Drawing conclusions about causes from systematic reviews of risk factors: The Cambridge Quality Checklists. J. Exp. Criminol. 2009, 5, 1–23. [Google Scholar] [CrossRef]
- Barash, A.; Lurie, S.; Weissman, A.; Insler, V. Comparison of sperm parameters, in vitro fertilization results, and subsequent pregnancy rates using sequential ejaculates, collected two hours apart, from oligoasthenozoospermic men. Fertil. Steril. 1995, 64, 1008–1011. [Google Scholar] [CrossRef]
- Sugiyam, R.; Al-Salem, J.A.; Nishi, Y.; Sugiyama, R.; Shirai, A.; Inoue, M.; Irahara, M. Improvement of sperm motility by short interval sequential ejaculation in oligoasthenozoospermic patients. Arch. Med. Sci. 2008, 4, 438–442. [Google Scholar]
- Shen, Z.Q.; Shi, B.; Wang, T.R.; Jiao, J.; Shang, X.J.; Wu, Q.J.; Zhou, Y.M.; Cao, T.F.; Du, Q.; Wang, X.X.; et al. Characterization of the Sperm Proteome and Reproductive Outcomes with in Vitro, Fertilization after a Reduction in Male Ejaculatory Abstinence Period. Mol. Cell. Proteom. 2019, 8 (Suppl. S1), S109–S117. [Google Scholar] [CrossRef]
- Scarselli, F.; Cursio, E.; Muzzì, S.; Casciani, V.; Ruberti, A.; Gatti, S.; Greco, P.; Varricchio, M.T.; Minasi, M.G.; Greco, E. How 1 h of abstinence improves sperm quality and increases embryo euploidy rate after PGT-A: A study on 106 sibling biopsied blastocysts. J. Assist. Reprod. Genet. 2019, 36, 1591–1597. [Google Scholar] [CrossRef]
- Ciotti, P.M.; Calza, N.; Zuffa, S.; Notarangelo, L.; Nardi, E.; Damiano, G.; Cipriani, L.; Porcu, E. Two subsequent seminal productions: A good strategy to treat very severe oligoasthenoteratozoospermic infertile couples. Andrology 2021, 9, 1185–1191. [Google Scholar] [CrossRef]
- Barbagallo, F.; Calogero, A.E.; Condorelli, R.A.; Farrag, A.; Jannini, E.A.; La Vignera, S.; Manna, C. Does a Very Short Length of Abstinence Improve Assisted Reproductive Technique Outcomes in Infertile Patients with Severe Oligo-Asthenozoospermia? J. Clin. Med. 2021, 10, 4399. [Google Scholar] [CrossRef]
- Patel, D.V.; Patel, T.; Maheshwari, N.; Soni, S.; Patel, R.G. Retrospective Analysis of the First Collection versus the Second Collection in Severe Oligo-asthenoteratozoospermia Cases in Self-Intracytoplasmic Sperm Injection Patients. J. Hum. Reprod. Sci. 2022, 15, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, Q.; Li, X.; Guo, J.; Zhang, L.; Quan, Y.; Ma, M.; Yang, Y. The Effect of Male Sexual Abstinence Periods on the Clinical Outcomes of Fresh Embryo Transfer Cycles Following Assisted Reproductive Technology: A Meta-Analysis. Am. J. Mens Health 2020, 14, 1557988320933758. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F.; et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, G.; Almossawi, O.; Zeirideen Zaid, R.; Ilahibuccus, A.; Al-Habib, A.; Muneer, A.; Okolo, S. Semen characteristics in consecutive ejaculates with short abstinence in subfertile males. Reprod. Biomed. Online 2016, 32, 323–328. [Google Scholar] [CrossRef]
- Alipour, H.; Duus, R.K.; Wimmer, R.; Dardmeh, F.; Du Plessis, S.S.; Jørgensen, N.; Christiansen, O.B.; Hnida, C.; Nielsen, H.I.; Van Der Horst, G. Seminal plasma metabolomics profiles following long (4–7 days) and short (2 h) sexual abstinence periods. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 178–183. [Google Scholar] [CrossRef]
- Agarwal, A.; Parekh, N.; Panner Selvam, M.K.; Henkel, R.; Shah, R.; Homa, S.T.; Ramasamy, R.; Ko, E.; Tremellen, K.; Esteves, S.; et al. Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens Health 2019, 37, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Varner, D.D. Effect of daily spermatozoan production but not age on transit time of spermatozoa through the human epididymis. Biol. Reprod. 1988, 39, 812–817. [Google Scholar] [CrossRef]
- Mayorga-Torres, B.J.; Camargo, M.; Agarwal, A.; du Plessis, S.S.; Cadavid, Á.P.; Cardona Maya, W.D. Influence of ejaculation frequency on seminal parameters. Reprod. Biol. Endocrinol. 2015, 13, 47. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Mens Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The effect of sperm DNA fragmentation on live birth rate after IVF or ICSI: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 30, 120–127. [Google Scholar] [CrossRef]
- Borges, E., Jr.; Zanetti, B.F.; Setti, A.S.; Braga, D.P.A.F.; Provenza, R.R.; Iaconelli, A., Jr. Sperm DNA fragmentation is correlated with poor embryo development, lower implantation rate, and higher miscarriage rate in reproductive cycles of non-male factor infertility. Fertil. Steril. 2019, 112, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.S. Function of sperm chromatin structural elements in fertilization and development. Mol. Hum. Reprod. 2010, 16, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.S.; Daneshmand, S.T.; Garner, F.C.; Aguirre, M.; Hudson, C.; Thomas, S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil. Steril. 2011, 96, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Roque, M.; Haahr, T.; Geber, S.; Esteves, S.C.; Humaidan, P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: A systematic review and meta-analysis of reproductive outcomes. Hum. Reprod. Update 2019, 25, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Díez, C.; González-Rojo, S.; Lombó, M.; Herráez, M.P. Impact of sperm DNA damage and oocyte-repairing capacity on trout development. Reproduction 2016, 152, 57–67. [Google Scholar] [CrossRef]
- Vendrell, X.; Ferrer, M.; García-Mengual, E.; Muñoz, P.; Triviño, J.C.; Calatayud, C.; Rawe, V.Y.; Ruiz-Jorro, M. Correlation between aneuploidy, apoptotic markers and DNA fragmentation in spermatozoa from normozoospermic patients. Reprod. Biomed. Online 2014, 28, 492–502. [Google Scholar] [CrossRef] [PubMed]


| Inclusion | Exclusion | |
|---|---|---|
| Population | Human male in reproductive age | / |
| Intervention | Short second ejaculation (within 4 h) | Second ejaculation > 4 h |
| Comparison | Ejaculation after a period of sexual abstinence between 2–7 days | / |
| Outcome | ART outcome: FR, IR, CPR, LBR, MR | / |
| Study type | Observational, cohort, cross-sectional, case-control, RCTs | Case reports; comments; letters to the editor; systematic or narrative reviews; in vitro studies; studies on animals |
| Authors and Year of Publication | Checklist for Correlates | Checklist for Risk Factors | Checklist for Casual Risk Factors | Total |
|---|---|---|---|---|
| Barash et al., 1995 [27] | 1 | 3 | 6 | 10/15 |
| Sugiyam et al., 2008 [28] | 1 | 3 | 6 | 10/15 |
| Shen et al., 2019 [29] | 2 | 3 | 6 | 11/15 |
| Scarselli et al., 2019 [30] | 2 | 3 | 6 | 11/15 |
| Ciotti et al., 2021 [31] | 1 | 2 | 6 | 9/15 |
| Barbagallo et al., 2021 [32] | 2 | 2 | 6 | 10/15 |
| Patel et al., 2022 [33] | 1 | 2 | 6 | 9/15 |
| Authors & Years | Study Design | Couples Enrolled | Semen Characterstics of Male Partners | Type of ART | Abstinence Period of First Ejaculate | Abstinence Period of Second Ejaculate | Method Used for Semen Analysis | ET | ART Outcome Assessed | Main Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Barash et al., 1995 [27] | Prospective study | n = 39 | OA | IVF | 3 days | 2h | NA | Fresh | FR No. of Embryo CPR per ET | ↑ FR ↑ no. of Embryo ↑ CPR |
| Sugiyam et al., 2008 [28] | Prospective study | n = 22 | OA | IUI IVF | 3–5 days | 30–60 min | NA | Fresh | FR Embryo quality CPR per ET | ↑ FR - Embryo quality - CPR per ET |
| Shen et al., 2019 [29] | Prospective study | n = 167 (experimental couples) | N | IVF | 3–7 days | 1–3 h | WHO, 2010 | Fresh | IR CPR Early miscarriage LBR | - IR - CPR - Early miscarriage - LBR |
| n = 361 (control couples) | N | IVF | 3–7 days | / | Frozen | IR CPR Early miscarriage LBR | ↑ IR ↑ CPR - Early miscarriage ↑ LBR | |||
| Scarselli et al., 2019 [30] | Prospective study | n = 22 | OAT | ICSI | 2–5 days | 1 h | WHO, 2010 | Frozen | FR Euploid blastocyst CPR IR | - FR ↑ Euploid blastocyst - CPR - IR |
| Ciotti et al., 2021 [31] | Retrospective study | n = 116 | Study group 1 = severe OAT (n = 75 cycles) | ICSI | 2–3 days | 2 h | NA | Fresh | FR IR CPR LBR MR Embryo quality | - FR - IR - CPR - LBR - MR - Embryo quality |
| Control Group 0 = N or mild OAT (n = 52 cycles) | 2–3 days | / | ||||||||
| Barbagallo et al., 2021 [32] | Retrospective study | n = 313 | Group 1 = N or mild OA (n = 223) | ICSI | 2–7 days | / | WHO, 2010 | Fresh | FR IR CPR LBR MR Embryo quality | - FR - IR ↑ CPR - LBR - MR ↑ Embryo quality |
| Group 2 = severe OA (n = 90) | 2–7 days | 1 h | ||||||||
| Patel et al., 2022 [33] | Retrospective study | n = 97 | Group A = OAT (n = 56) | ICSI | 2–7 days | / | WHO, 2010 | / | FR Embryo 1 grade | - FR ↓ Embryo 1 grade |
| Group B = OAT (n = 41) | 2–7 days | 1 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbagallo, F.; Cannarella, R.; Crafa, A.; La Vignera, S.; Condorelli, R.A.; Manna, C.; Calogero, A.E. The Impact of a Very Short Abstinence Period on Assisted Reproductive Technique Outcomes: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 752. https://doi.org/10.3390/antiox12030752
Barbagallo F, Cannarella R, Crafa A, La Vignera S, Condorelli RA, Manna C, Calogero AE. The Impact of a Very Short Abstinence Period on Assisted Reproductive Technique Outcomes: A Systematic Review and Meta-Analysis. Antioxidants. 2023; 12(3):752. https://doi.org/10.3390/antiox12030752
Chicago/Turabian StyleBarbagallo, Federica, Rossella Cannarella, Andrea Crafa, Sandro La Vignera, Rosita A. Condorelli, Claudio Manna, and Aldo E. Calogero. 2023. "The Impact of a Very Short Abstinence Period on Assisted Reproductive Technique Outcomes: A Systematic Review and Meta-Analysis" Antioxidants 12, no. 3: 752. https://doi.org/10.3390/antiox12030752
APA StyleBarbagallo, F., Cannarella, R., Crafa, A., La Vignera, S., Condorelli, R. A., Manna, C., & Calogero, A. E. (2023). The Impact of a Very Short Abstinence Period on Assisted Reproductive Technique Outcomes: A Systematic Review and Meta-Analysis. Antioxidants, 12(3), 752. https://doi.org/10.3390/antiox12030752















