Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress
Abstract
1. Introduction
2. Revolutionary Implications of mNPs in Pharmaceutical Research
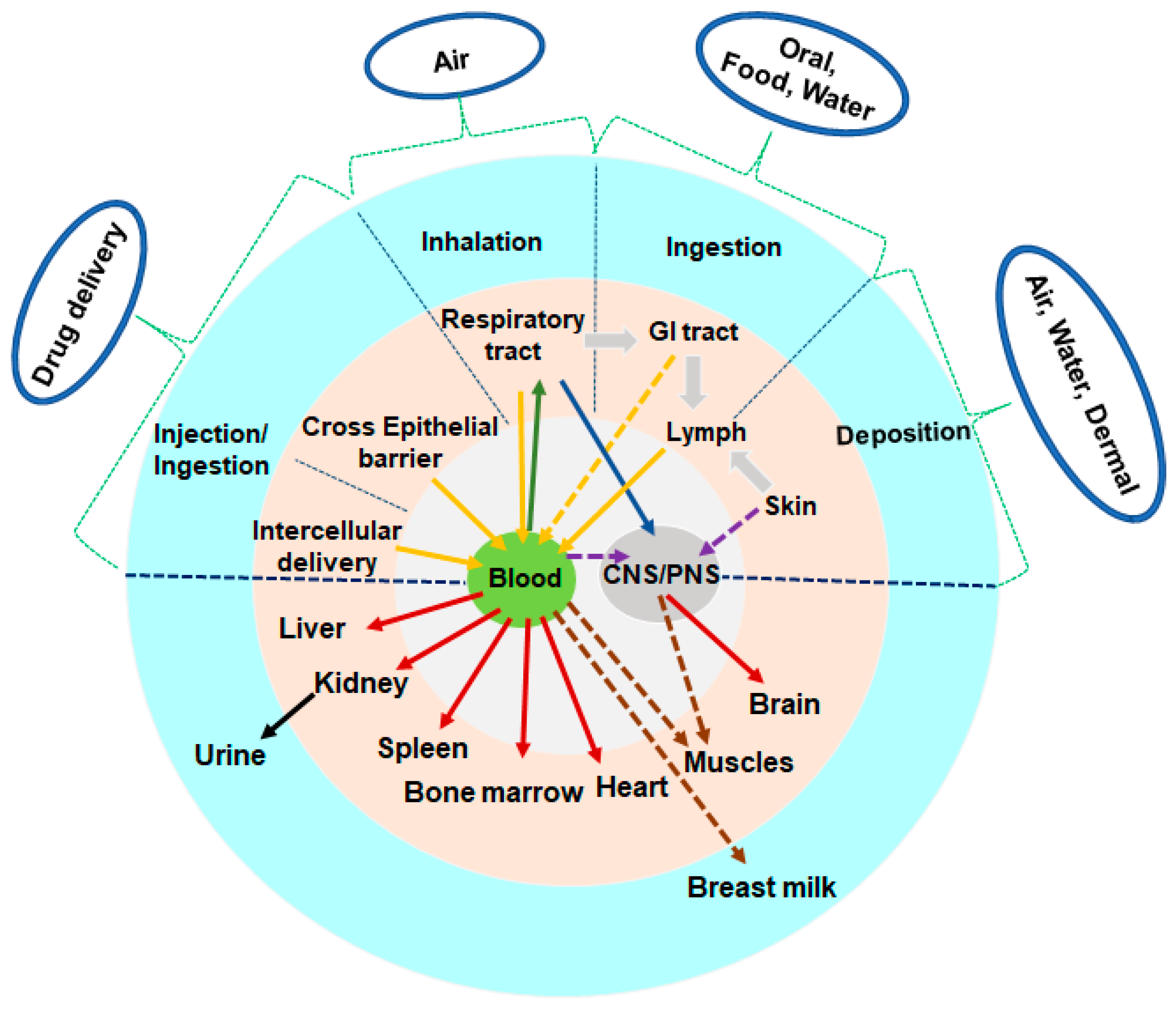
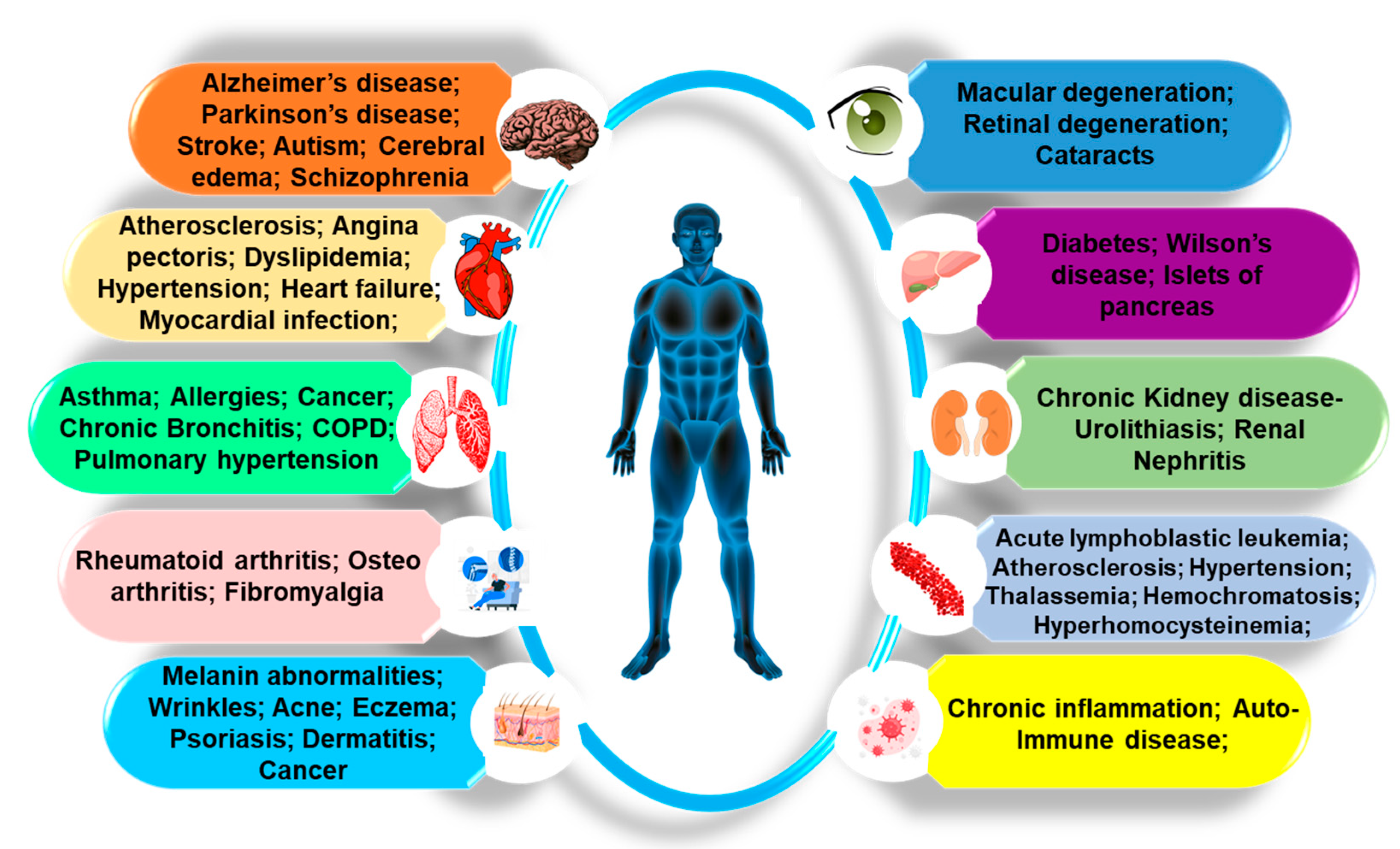
3. Physio-Pathological Implications of mNPs
The Adverse Impacts of Various mNPs on the Organs
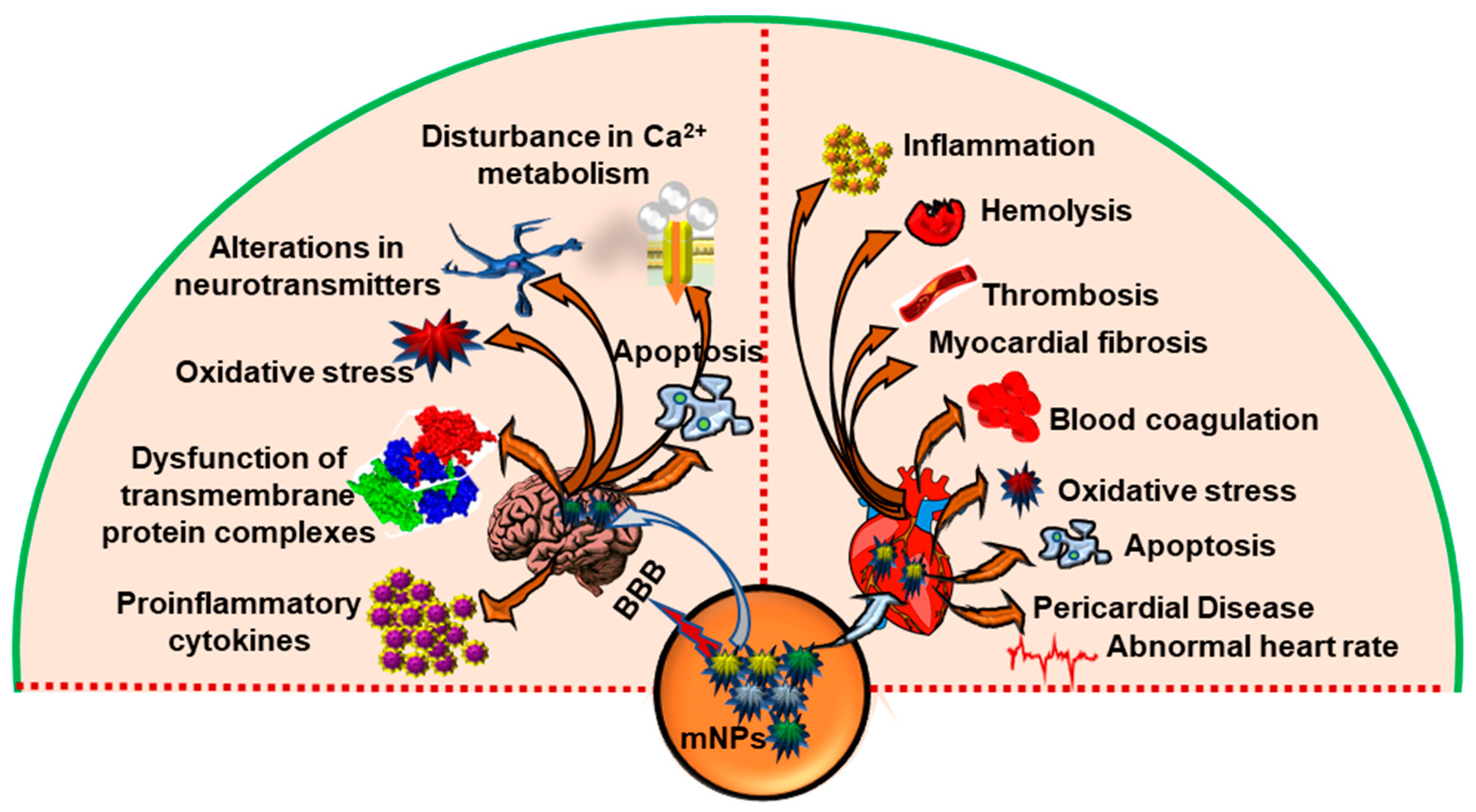
4. Effects of mNPs on Cytotoxicity and Cellular Damage
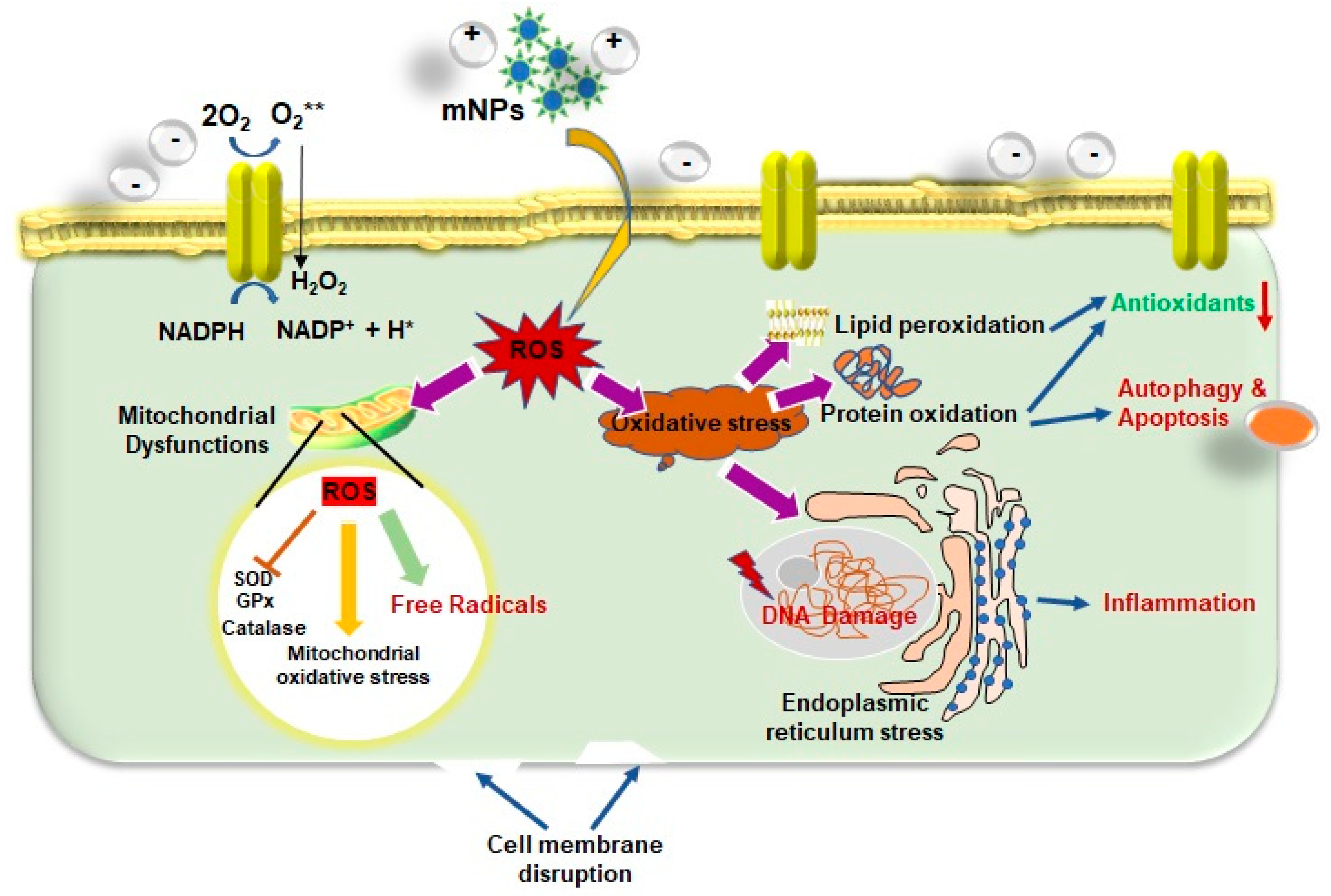
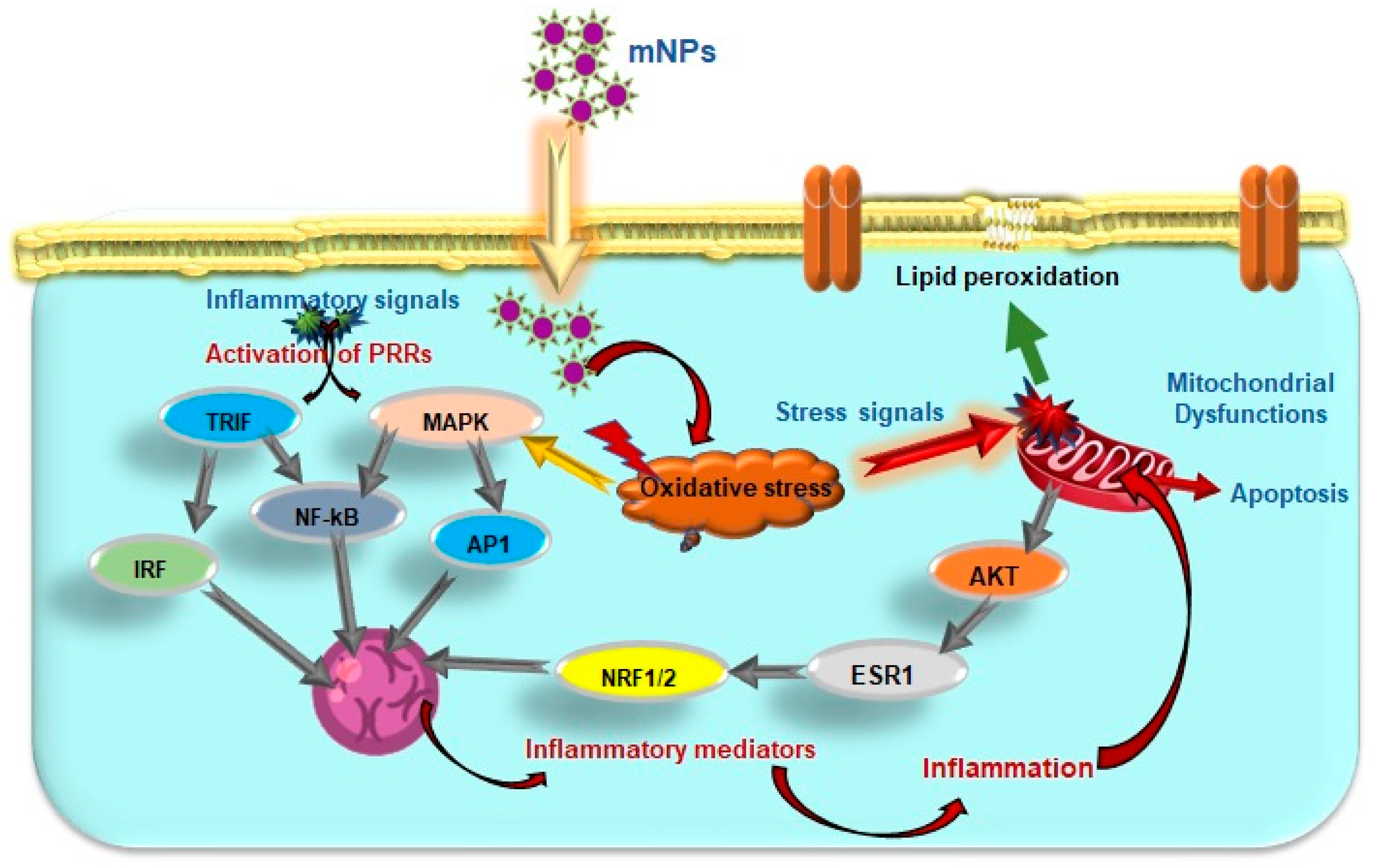
4.1. Influence of mNPs on Excessive ROS Generation
4.2. Impact of mNPs-Induced Oxidative Stress
4.3. Effects of mNPs on Cellular Signaling and Immune Response
5. Conclusions and Further Prospective
Author Contributions
Funding
Conflicts of Interest
References
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef]
- Chusuei, C.C.; Wu, C.-H.; Mallavarapu, S.; Hou, F.Y.S.; Hsu, C.-M.; Winiarz, J.G.; Aronstam, R.S.; Huang, Y.-W. Cytotoxicity in the age of nano: The role of fourth period transition metal oxide nanoparticle physicochemical properties. Chem.-Biol. Interact. 2013, 206, 319–326. [Google Scholar] [CrossRef]
- Kreuter, J.; Gelperina, S. Use of Nanoparticles for Cerebral Cancer. Tumori J. 2008, 94, 271–277. [Google Scholar] [CrossRef]
- Aitken, R.J.; Chaudhry, M.; Boxall, A.; Hull, M. Manufacture and use of nanomaterials: Current status in the UK and global trends. Occup. Med. 2006, 56, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Pietroiusti, A.; Fadeel, B.; Kagan, V.E. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharm. 2012, 261, 121–133. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, V. Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef]
- Casals, E.; Vázquez-Campos, S.; Bastús, N.G.; Puntes, V. Distribution and potential toxicity of engineered inorganic nanoparticles and carbon nanostructures in biological systems. TrAC Trends Anal. Chem. 2008, 27, 672–683. [Google Scholar] [CrossRef]
- Wang, S.-H.; Lee, C.-W.; Chiou, A.; Wei, P.-K. Size-dependent endocytosis of gold nanoparticles studied by three-dimensional mapping of plasmonic scattering images. J. Nanobiotechnol. 2010, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Dizaj, A. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.B.; Jiang, S.; Zhang, Y. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials 2007, 28, 1565–1571. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J.; Liu, L.; Huang, Y.; Mason, R.P. Exploring feasibility of multicolored CdTe quantum dots for in vitro and in vivo fluorescent imaging. J. Nanosci. Nanotechnol. 2008, 8, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Hardman, S.a.; Cope, A.; Swann, A.; Bell, P.; Naylor, A.; Hayes, P. An in vitro model to compare the antimicrobial activity of silver-coated versus rifampicin-soaked vascular grafts. Ann. Vasc. Surg. 2004, 18, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Biofunctional Text. Ski. 2006, 33, 17–34. [Google Scholar]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef]
- Raffi, M.; Hussain, F.; Bhatti, T.; Akhter, J.; Hameed, A.; Hasan, M. Antibacterial characterization of silver nanoparticles against E. coli ATCC-15224. J. Mater. Sci. Technol. 2008, 24, 192–196. [Google Scholar]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.; Dille, J.; Godet, S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Chaloupka, K.; Malam, Y.; Seifalian, A.M. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010, 28, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M.; Samadian, H.; Barabadi, H.; Azarnezhad, A.; Ahmadi, A. Redox interactions and genotoxicity of metal-based nanoparticles: A comprehensive review. Chem.-Biol. Interact. 2019, 312, 108814. [Google Scholar] [CrossRef]
- Duan, D.; Fan, K.; Zhang, D.; Tan, S.; Liang, M.; Liu, Y.; Zhang, J.; Zhang, P.; Liu, W.; Qiu, X.; et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 2015, 74, 134–141. [Google Scholar] [CrossRef]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, H. The synergistic effect and mechanism of doxorubicin-ZnO nanocomplexes as a multimodal agent integrating diverse anticancer therapeutics. Int. J. Nanomed. 2013, 8, 1835. [Google Scholar]
- Lee, S.; Yun, H.-S.; Kim, S.-H. The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials 2011, 32, 9434–9443. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar] [CrossRef]
- Hua, X.; Yang, Q.; Dong, Z.; Zhang, J.; Zhang, W.; Wang, Q.; Tan, S.; Smyth, H.D. Magnetically triggered drug release from nanoparticles and its applications in anti-tumor treatment. Drug Deliv. 2017, 24, 511–518. [Google Scholar] [CrossRef]
- Corot, C.; Robert, P.; Idée, J.-M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Canaparo, R.; Foglietta, F.; Limongi, T.; Serpe, L. Biomedical Applications of Reactive Oxygen Species Generation by Metal Nanoparticles. Materials 2021, 14, 53. [Google Scholar] [CrossRef]
- Ivanova, P.; Dzięgielewski, K.; Drozd, M.; Skorupska, S.; Grabowska-Jadach, I.; Pietrzak, M. Nanoparticles of chosen noble metals as reactive oxygen species scavengers. Nanotechnology 2021, 32, 055704. [Google Scholar] [CrossRef] [PubMed]
- Alaraby, M.; Hernández, A.; Marcos, R. Systematic in vivo study of NiO nanowires and nanospheres: Biodegradation, uptake and biological impacts. Nanotoxicology 2018, 12, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Chanda, N.; Kan, P.; Watkinson, L.D.; Shukla, R.; Zambre, A.; Carmack, T.L.; Engelbrecht, H.; Lever, J.R.; Katti, K.; Fent, G.M. Radioactive gold nanoparticles in cancer therapy: Therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. In Nanomedicine in Cancer; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2017; pp. 753–774. [Google Scholar]
- Xie, H.; Wang, Z.J.; Bao, A.; Goins, B.; Phillips, W.T. In vivo PET imaging and biodistribution of radiolabeled gold nanoshells in rats with tumor xenografts. Int. J. Pharm. 2010, 395, 324–330. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Env. Health Perspect 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.J.; Nelson, B.C. Mechanisms and measurements of nanomaterial-induced oxidative damage to DNA. Anal. Bioanal. Chem. 2010, 398, 613–650. [Google Scholar] [CrossRef]
- Dhawan, A.; Sharma, V. Toxicity assessment of nanomaterials: Methods and challenges. Anal. Bioanal. Chem. 2010, 398, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, P.; Karimi, H.; Khazaei, M. Review on Metal-Based Nanoparticles: Role of Reactive Oxygen Species in Renal Toxicity. Chem. Res. Toxicol. 2020, 33, 2503–2514. [Google Scholar] [CrossRef]
- AshRrani, P.; Low Kah Mun, G.; Hande, M.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Cheng, F.-Y.; Chiu, H.-W.; Tsai, J.-C.; Fang, C.-Y.; Chen, C.-W.; Wang, Y.-J. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials 2014, 35, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Knaapen, A.M.; Borm, P.J.; Albrecht, C.; Schins, R.P. Inhaled particles and lung cancer. Part A: Mechanisms. Int. J. Cancer 2004, 109, 799–809. [Google Scholar] [CrossRef]
- Kovacic, P.; Somanathan, R. Biomechanisms of nanoparticles (toxicants, antioxidants and therapeutics): Electron transfer and reactive oxygen species. J. Nanosci. Nanotechnol. 2010, 10, 7919–7930. [Google Scholar] [CrossRef]
- Li, J.J.; Muralikrishnan, S.; Ng, C.T.; Yung, L.Y.; Bay, B.H. Nanoparticle-induced pulmonary toxicity. Exp. Biol. Med. 2010, 235, 1025–1033. [Google Scholar] [CrossRef]
- Gojova, A.; Guo, B.; Kota, R.S.; Rutledge, J.C.; Kennedy, I.M.; Barakat, A.I. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: Effect of particle composition. Environ. Health Perspect. 2007, 115, 403–409. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.; Sethu, S.; Lim, H.K.; Balaji, G.; Valiyaveettil, S.; Hande, M.P. Differential regulation of intracellular factors mediating cell cycle, DNA repair and inflammation following exposure to silver nanoparticles in human cells. Genome Integr. 2012, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Xu, K.; Gu, J.; Huang, L.; Zhang, L.; Liu, N.; Kong, J.; Xing, M.; Zhang, L. Characterization of superparamagnetic iron oxide nanoparticle-induced apoptosis in PC12 cells and mouse hippocampus and striatum. Toxicol. Lett. 2018, 292, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, B.; Toborek, M. Autophagy is involved in nanoalumina-induced cerebrovascular toxicity. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 212–221. [Google Scholar] [CrossRef]
- Takenaka, S.; Karg, E.; Roth, C.; Schulz, H.; Ziesenis, A.; Heinzmann, U.; Schramel, P.; Heyder, J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ. Health Perspect. 2001, 109, 547–551. [Google Scholar] [PubMed]
- Kakkar, V.; Kaur, I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011, 49, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, L.E.; Patchin, E.S.; Chiu, P.-L.; Brandenberger, C.; Smiley-Jewell, S.; Pinkerton, K.E. Nose-to-brain transport of aerosolised quantum dots following acute exposure. Nanotoxicology 2014, 8, 885–893. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Liu, Y.; Li, B.; Chen, C.; Wu, G. Oxidative stress and acute changes in murine brain tissues after nasal instillation of copper particles with different sizes. J. Nanosci. Nanotechnol. 2014, 14, 4534–4540. [Google Scholar] [CrossRef]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Yin, J.; Li, W.; Kang, C.; Huang, Q.; Li, Q. Systematic influence induced by 3 nm titanium dioxide following intratracheal instillation of mice. J. Nanosci. Nanotechnol. 2010, 10, 8544–8549. [Google Scholar] [CrossRef]
- Li, X.-b.; Zheng, H.; Zhang, Z.-r.; Li, M.; Huang, Z.-y.; Schluesener, H.J.; Li, Y.-y.; Xu, S.-q. Glia activation induced by peripheral administration of aluminum oxide nanoparticles in rat brains. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Takács, S.; Szabó, A.; Oszlánczi, G.; Pusztai, P.; Sápi, A.; Kónya, Z.; Papp, A. Repeated simultaneous cortical electrophysiological and behavioral recording in rats exposed to manganese-containing nanoparticles. Acta Biol. Hung. 2012, 63, 426–440. [Google Scholar] [CrossRef]
- Oszlánczi, G.; Papp, A.; Szabó, A.; Nagymajtényi, L.; Sápi, A.; Kónya, Z.; Paulik, E.; Vezér, T. Nervous system effects in rats on subacute exposure by lead-containing nanoparticles via the airways. Inhal. Toxicol. 2011, 23, 173–181. [Google Scholar] [CrossRef]
- De Simone, U.; Roccio, M.; Gribaldo, L.; Spinillo, A.; Caloni, F.; Coccini, T. Human 3D cultures as models for evaluating magnetic nanoparticle CNS cytotoxicity after short-and repeated long-term exposure. Int. J. Mol. Sci. 2018, 19, 1993. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol. Brain 2013, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.; Linemann, T.; Pondman, K.M.; Lichota, J.; Kim, K.S.; Pieters, R.J.; Visser, G.M.; Moos, T. Uptake and transport of superparamagnetic iron oxide nanoparticles through human brain capillary endothelial cells. ACS Chem. Neurosci. 2013, 4, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, A.; Zhang, Y.; Wang, J.; Shao, L.; Wei, L. Central nervous system toxicity of metallic nanoparticles. Int. J. Nanomed. 2015, 10, 4321–4340. [Google Scholar] [CrossRef]
- Shimizu, M.; Tainaka, H.; Oba, T.; Mizuo, K.; Umezawa, M.; Takeda, K. Maternal exposure to nanoparticulate titanium dioxide during the prenatal period alters gene expression related to brain development in the mouse. Part. Fibre Toxicol. 2009, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Suzuki, K.-i.; Ishihara, A.; Kubo-Irie, M.; Fujimoto, R.; Tabata, M.; Oshio, S.; Nihei, Y.; Ihara, T.; Sugamata, M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J. Health Sci. 2009, 55, 95–102. [Google Scholar] [CrossRef]
- Hougaard, K.S.; Jackson, P.; Jensen, K.A.; Sloth, J.J.; Löschner, K.; Larsen, E.H.; Birkedal, R.K.; Vibenholt, A.; Boisen, A.-M.Z.; Wallin, H.; et al. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part. Fibre Toxicol. 2010, 7, 16. [Google Scholar] [CrossRef]
- Kan, H.; Pan, D.; Castranova, V. Engineered nanoparticle exposure and cardiovascular effects: The role of a neuronal-regulated pathway. Inhal. Toxicol. 2018, 30, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, Y.; Ni, Y.; Ning, F.; Shang, L.; Ma, A. Size dependent effects of Gold Nanoparticles in ISO-induced Hyperthyroid Rats. Sci. Rep. 2018, 8, 10960. [Google Scholar] [CrossRef]
- Yousef, M.I.; Abuzreda, A.A.; Kamel, M.A. Cardiotoxicity and lung toxicity in male rats induced by long-term exposure to iron oxide and silver nanoparticles. Exp. Ther. Med. 2019, 18, 4329–4339. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, C.; Lee, Y.; Ryu, C.; Park, J.; Kim, Y. Acute Adverse Effects of Metallic Nanomaterials on Cardiac and Behavioral Changes in Daphnia magna. Environments 2022, 9, 26. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Xia, T. Understanding Nanomaterial-Liver Interactions to Facilitate the Development of Safer Nanoapplications. Adv. Mater. 2022, 34, e2106456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef]
- Thomson, A.W.; Knolle, P.A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 2010, 10, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Reed, K.L.; Warheit, D.B. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L.; Swain, K.A. Health effects related to nanoparticle exposures: Environmental, health and safety considerations for assessing hazards and risks. Pharmacol. Ther. 2008, 120, 35–42. [Google Scholar] [CrossRef]
- Jia, J.; Li, F.; Zhou, H.; Bai, Y.; Liu, S.; Jiang, Y.; Jiang, G.; Yan, B. Oral Exposure to Silver Nanoparticles or Silver Ions May Aggravate Fatty Liver Disease in Overweight Mice. Env. Sci. Technol. 2017, 51, 9334–9343. [Google Scholar] [CrossRef] [PubMed]
- Abdelhalim, M.A.; Jarrar, B.M. Gold nanoparticles administration induced prominent inflammatory, central vein intima disruption, fatty change and Kupffer cells hyperplasia. Lipids Health Dis. 2011, 10, 133. [Google Scholar] [CrossRef]
- Duan, J.; Liang, S.; Feng, L.; Yu, Y.; Sun, Z. Silica nanoparticles trigger hepatic lipid-metabolism disorder in vivo and in vitro. Int. J. Nanomed. 2018, 13, 7303–7318. [Google Scholar] [CrossRef]
- Yu, Y.; Duan, J.; Li, Y.; Li, Y.; Jing, L.; Yang, M.; Wang, J.; Sun, Z. Silica nanoparticles induce liver fibrosis via TGF-β(1)/Smad3 pathway in ICR mice. Int. J. Nanomed. 2017, 12, 6045–6057. [Google Scholar] [CrossRef] [PubMed]
- Parivar, K.; Fard, F.M.; Bayat, M.; Alavian, S.M.; Motavaf, M. Evaluation of iron oxide nanoparticles toxicity on liver cells of BALB/c rats. Iran. Red Crescent Med. J. 2016, 18, e28939. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xu, X.; Guo, M.; Wang, S.; Gao, S.; Zhu, S.; Rong, R. Synergistic toxicity of zno nanoparticles and dimethoate in mice: Enhancing their biodistribution by synergistic binding of serum albumin and dimethoate to zno nanoparticles. Environ. Toxicol. 2017, 32, 1202–1212. [Google Scholar] [CrossRef] [PubMed]
- Waters, K.M.; Masiello, L.M.; Zangar, R.C.; Tarasevich, B.J.; Karin, N.J.; Quesenberry, R.D.; Bandyopadhyay, S.; Teeguarden, J.G.; Pounds, J.G.; Thrall, B.D. Macrophage responses to silica nanoparticles are highly conserved across particle sizes. Toxicol. Sci. 2009, 107, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhouhua, W.; Jie, Z.; Xinlu, F.; Jinqiang, L.; Yuwen, Q.; Zhiying, H. Renal interstitial fibrosis induced by high-dose mesoporous silica nanoparticles via the NF-κB signaling pathway. Int. J. Nanomed. 2015, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Desouky, E.M.; Hozayen, W.G.; Bin-Jumah, M.; El-Nahass, E.-S.; Soliman, H.A.; Farghali, A.A. Mesoporous Silica Nanoparticles Trigger Liver and Kidney Injury and Fibrosis Via Altering TLR4/NF-κB, JAK2/STAT3 and Nrf2/HO-1 Signaling in Rats. Biomolecules 2019, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Wang, S.; Zhou, L.; Sun, L. The potential liver, brain, and embryo toxicity of titanium dioxide nanoparticles on mice. Nanoscale Res. Lett. 2017, 12, 478. [Google Scholar] [CrossRef]
- Chen, J.; Dong, X.; Zhao, J.; Tang, G. In vivo acute toxicity of titanium dioxide nanoparticles to mice after intraperitioneal injection. J. Appl. Toxicol. 2009, 29, 330–337. [Google Scholar] [CrossRef]
- Milić, M.; Leitinger, G.; Pavičić, I.; Zebić Avdičević, M.; Dobrović, S.; Goessler, W.; Vinković Vrček, I. Cellular uptake and toxicity effects of silver nanoparticles in mammalian kidney cells. J. Appl. Toxicol. 2015, 35, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Axson, J.L.; Stark, D.I.; Bondy, A.L.; Capracotta, S.S.; Maynard, A.D.; Philbert, M.A.; Bergin, I.L.; Ault, A.P. Rapid kinetics of size and pH-dependent dissolution and aggregation of silver nanoparticles in simulated gastric fluid. J. Phys. Chem. C 2015, 119, 20632–20641. [Google Scholar] [CrossRef]
- Nosrati, H.; Hamzepoor, M.; Sohrabi, M.; Saidijam, M.; Assari, M.J.; Shabab, N.; Gholami Mahmoudian, Z.; Alizadeh, Z. The potential renal toxicity of silver nanoparticles after repeated oral exposure and its underlying mechanisms. BMC Nephrol. 2021, 22, 228. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. Int. J. 2008, 27, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Boudreau, M.D.; Yin, J.-J. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef] [PubMed]
- Özel, R.E.; Alkasir, R.S.; Ray, K.; Wallace, K.N.; Andreescu, S. Comparative evaluation of intestinal nitric oxide in embryonic zebrafish exposed to metal oxide nanoparticles. Small 2013, 9, 4250–4261. [Google Scholar] [CrossRef]
- Wang, Y.; Aker, W.G.; Hwang, H.-m.; Yedjou, C.G.; Yu, H.; Tchounwou, P.B. A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci. Total Environ. 2011, 409, 4753–4762. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Zhao, J.; Xing, B. Toxicity and internalization of CuO nanoparticles to prokaryotic alga Microcystis aeruginosa as affected by dissolved organic matter. Environ. Sci. Technol. 2011, 45, 6032–6040. [Google Scholar] [CrossRef] [PubMed]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Albrecht, R.M. Gastrointestinal persorption and tissue distribution of differently sized colloidal gold nanoparticles. J. Pharm. Sci. 2001, 90, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in Understanding Size and Shape Dependent Toxicity of Gold Nanomaterials in Human Skin Keratinocytes. Chem. Phys. Lett. 2008, 463, 145–149. [Google Scholar] [CrossRef]
- Goodman, C.M.; McCusker, C.D.; Yilmaz, T.; Rotello, V.M. Toxicity of Gold Nanoparticles Functionalized with Cationic and Anionic Side Chains. Bioconj. Chem. 2004, 15, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.; Fang, X.; Sun, Y.; Bakhtina, A.; Ramakrishnan, A.; Sokolov, J.; Ulman, A.; Rafailovich, M. Adverse effects of citrate/gold nanoparticles on human dermal fibroblasts. Small 2006, 2, 766–773. [Google Scholar] [CrossRef]
- Yen, H.J.; Hsu, S.H.; Tsai, C.L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Fukami, G.; Hashimoto, K.; Koike, K.; Okamura, N.; Shimizu, E.; Iyo, M. Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004, 1016, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Blair, D.; Dufort, F.J.; Gumina, M.R.; Huang, Z.; Hong, G.; Wagner, D.; Canahan, D.; Kempa, K.; Ren, Z.F.; et al. Interaction between carbon nanotubes and mammalian cells: Characterization by flow cytometry and application. Nanotechnology 2008, 19, 345102. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Mirsattari, S.; Hammond, R.; Sharpe, M.; Leung, F.; Young, G. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology 2004, 62, 1408–1410. [Google Scholar] [CrossRef]
- Cioffi, N.; Torsi, L.; Ditaranto, N.; Tantillo, G.; Ghibelli, L.; Sabbatini, L.; Bleve-Zacheo, T.; D’Alessio, M.; Zambonin, P.G.; Traversa, E. Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties. Chem. Mater. 2005, 17, 5255–5262. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Toyooka, T.; Ibuki, Y. Simple and easy method to evaluate uptake potential of nanoparticles in mammalian cells using a flow cytometric light scatter analysis. Environ. Sci. Technol. 2007, 41, 3018–3024. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, X.; Guo, J.; Gu, A.Z.; Li, D.; Chen, J. Toxicity Assessment of Nano-ZnO Exposure on the Human Intestinal Microbiome, Metabolic Functions, and Resistome Using an In Vitro Colon Simulator. Environ. Sci. Technol. 2021, 55, 6884–6896. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chai, X.; Li, X.; Ren, G.; Yang, X.; Yang, Z. Nano-CuO causes cell damage through activation of dose-dependent autophagy and mitochondrial lncCyt b-AS/ND5-AS/ND6-AS in SH-SY5Y cells. Toxicol. Mech. Methods 2022, 32, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Ma, L.; X, Z.-g.; Zhang, H.; Lin, B. A comparative study of lung toxicity in rats induced by three types of nanomaterials. Nanoscale Res. Lett. 2013, 8, 521. [Google Scholar] [CrossRef]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Jeng, H.A.; Swanson, J. Toxicity of metal oxide nanoparticles in mammalian cells. J. Environ. Sci. Health Part A 2006, 41, 2699–2711. [Google Scholar] [CrossRef]
- Grassian, V.H.; O’Shaughnessy, P.T.; Adamcakova-Dodd, A.; Pettibone, J.M.; Thorne, P.S. Inhalation exposure study of titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environ. Health Perspect. 2007, 115, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating nanoscale titania structure with toxicity: A cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Gurr, J.R.; Wang, A.S.; Chen, C.H.; Jan, K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, T.J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Env. Toxicol. Pharm. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Lademann, J.; Weigmann, H.; Rickmeyer, C.; Barthelmes, H.; Schaefer, H.; Mueller, G.; Sterry, W. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Ski. Pharm. Appl. Ski. Physiol. 1999, 12, 247–256. [Google Scholar] [CrossRef]
- Zhou, Y.; Yokel, R.A. The chemical species of aluminum influences its paracellular flux across and uptake into Caco-2 cells, a model of gastrointestinal absorption. Toxicol. Sci. 2005, 87, 15–26. [Google Scholar] [CrossRef]
- Hoet, P.H.; Brüske-Hohlfeld, I.; Salata, O.V. Nanoparticles–known and unknown health risks. J. Nanobiotechnol. 2004, 2, 12. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.S.; Sharma, A. Nanoparticles aggravate heat stress induced cognitive deficits, blood-brain barrier disruption, edema formation and brain pathology. Prog. Brain Res. 2007, 162, 245–273. [Google Scholar] [CrossRef]
- Chen, D.; Xi, T.; Bai, J. Biological effects induced by nanosilver particles: In vivo study. Biomed. Mater. 2007, 2, S126–S128. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- Kashiwada, S. Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environ. Health Perspect. 2006, 114, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Kumar, S.; Paulraj, R. Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. J. Nanopart. Res. 2015, 17, 49. [Google Scholar] [CrossRef]
- Hu, R.; Zheng, L.; Zhang, T.; Gao, G.; Cui, Y.; Cheng, Z.; Cheng, J.; Hong, M.; Tang, M.; Hong, F. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. J. Hazard Mater. 2011, 191, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The chemistry of oxygen radicals and other derived species. Free Radic. Biol. Med. 1989, 22–85. [Google Scholar]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Tee, J.K.; Ong, C.N.; Bay, B.H.; Ho, H.K.; Leong, D.T. Oxidative stress by inorganic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 414–438. [Google Scholar] [CrossRef]
- Wissing, D.; Mouritzen, H.; Jäättelä, M. TNF-induced mitochondrial changes and activation of apoptotic proteases are inhibited by A20. Free Radic. Biol. Med. 1998, 25, 57–65. [Google Scholar] [CrossRef]
- Singh, I.; Pahan, K.; Khan, M.; Singh, A.K. Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J. Biol. Chem. 1998, 273, 20354–20362. [Google Scholar] [CrossRef] [PubMed]
- Sidoti-de Fraisse, C.; Rincheval, V.; Risler, Y.; Mignotte, B.; Vayssière, J.L. TNF-alpha activates at least two apoptotic signaling cascades. Oncogene 1998, 17, 1639–1651. [Google Scholar] [CrossRef]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [CrossRef]
- Sorce, S.; Krause, K.H. NOX enzymes in the central nervous system: From signaling to disease. Antioxid. Redox Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Chartrand, K.; Reynolds, A.; Vitvitsky, V.; Banerjee, R.; Gendelman, H.E. Ion channel blockade attenuates aggregated alpha synuclein induction of microglial reactive oxygen species: Relevance for the pathogenesis of Parkinson’s disease. J. Neurochem. 2007, 100, 503–519. [Google Scholar] [CrossRef]
- Schäppi, M.G.; Jaquet, V.; Belli, D.C.; Krause, K.-H. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin. Immunopathol. 2008, 30, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Vallyathan, V.; Shi, X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ. Health Perspect. 1997, 105, 165–177. [Google Scholar]
- Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 592, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Song, B.; Zhang, Y.; Liu, J.; Feng, X.; Zhou, T.; Shao, L. Is Neurotoxicity of Metallic Nanoparticles the Cascades of Oxidative Stress? Nanoscale Res. Lett. 2016, 11, 291. [Google Scholar] [CrossRef]
- Zheng, F.; Gonçalves, F.M.; Abiko, Y.; Li, H.; Kumagai, Y.; Aschner, M. Redox toxicology of environmental chemicals causing oxidative stress. Redox Biol. 2020, 34, 101475. [Google Scholar] [CrossRef]
- Khalili Fard, J.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef]
- Fahmy, B.; Cormier, S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. Vitr. 2009, 23, 1365–1371. [Google Scholar] [CrossRef]
- Chan, H.W.; Liu, T.; Verdile, G.; Bishop, G.; Haasl, R.J.; Smith, M.A.; Perry, G.; Martins, R.N.; Atwood, C.S. Copper Induces Apoptosis of Neuroblastoma Cells Via Post-translational Regulation of the Expression of Bcl-2-family Proteins and the tx Mouse is a Better Model of Hepatic than Brain Cu Toxicity. Int. J. Clin. Exp. Med. 2008, 1, 76–88. [Google Scholar]
- Zou, L.; Cheng, G.; Xu, C.; Liu, H.; Wang, Y.; Li, N.; Fan, X.; Zhu, C.; Xia, W. Copper Nanoparticles Induce Oxidative Stress via the Heme Oxygenase 1 Signaling Pathway in vitro Studies. Int. J. Nanomed. 2021, 16, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lai, W.; Liu, X.; Yang, H.; Fang, Y.; Tian, L.; Li, K.; Nie, H.; Zhang, W.; Shi, Y.; et al. Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J. Hazard. Mater. 2021, 401, 123349. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.A.; Ali, D.; Alarafi, S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef]
- Podila, R.; Brown, J.M. Toxicity of engineered nanomaterials: A physicochemical perspective. J. Biochem. Mol. Toxicol. 2013, 27, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, M.; Sil, P.C. Nanotoxicity: Oxidative stress mediated toxicity of metal and metal oxide nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Howden, P.J.; Faux, S.P. Fibre-induced lipid peroxidation leads to DNA adduct formation in Salmonella typhimurium TA104 and rat lung fibroblasts. Carcinogenesis 1996, 17, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Onuma, K.; Sato, Y.; Ogawara, S.; Shirasawa, N.; Kobayashi, M.; Yoshitake, J.; Yoshimura, T.; Iigo, M.; Fujii, J.; Okada, F. Nano-scaled particles of titanium dioxide convert benign mouse fibrosarcoma cells into aggressive tumor cells. Am. J. Pathol. 2009, 175, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Zou, L.; Hartono, D.; Ong, C.N.; Bay, B.H.; Lanry Yung, L.Y. Gold nanoparticles induce oxidative damage in lung fibroblasts in vitro. Adv. Mater. 2008, 20, 138–142. [Google Scholar] [CrossRef]
- Ruiz, P.; Katsumiti, A.; Nieto, J.A.; Bori, J.; Jimeno-Romero, A.; Reip, P.; Arostegui, I.; Orbea, A.; Cajaraville, M.P. Short-term effects on antioxidant enzymes and long-term genotoxic and carcinogenic potential of CuO nanoparticles compared to bulk CuO and ionic copper in mussels Mytilus galloprovincialis. Mar. Environ. Res. 2015, 111, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Sheng, J.; Hosseinian, F.; Willmore, W.G. Nanoparticle Effects on Stress Response Pathways and Nanoparticle-Protein Interactions. Int. J. Mol. Sci. 2022, 23, 7962. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Reed, W.; Wu, W.; Bromberg, P.A.; Graves, L.M.; Samet, J.M. Zn2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2006, 290, L1028–L1035. [Google Scholar] [CrossRef] [PubMed]
- Tal, T.; Graves, L.; Silbajoris, R.; Bromberg, P.; Wu, W.; Samet, J. Inhibition of protein tyrosine phosphatase activity mediates epidermal growth factor receptor signaling in human airway epithelial cells exposed to Zn2+. Toxicol. Appl. Pharmacol. 2006, 214, 16–23. [Google Scholar] [CrossRef]
- Bellomo, E.; Abro, A.; Hogstrand, C.; Maret, W.; Domene, C. Role of Zinc and Magnesium Ions in the Modulation of Phosphoryl Transfer in Protein Tyrosine Phosphatase 1B. J. Am. Chem. Soc. 2018, 140, 4446–4454. [Google Scholar] [CrossRef]
- Khan, H.A.; Abdelhalim, M.A.K.; Alhomida, A.S.; Al-Ayed, M.S. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. BioMed Res. Int. 2013, 2013, 590730. [Google Scholar] [CrossRef]
- Senapati, V.A.; Gupta, G.S.; Pandey, A.K.; Shanker, R.; Dhawan, A.; Kumar, A. Zinc oxide nanoparticle induced age dependent immunotoxicity in BALB/c mice. Toxicol. Res. 2017, 6, 342–352. [Google Scholar] [CrossRef]
- Lim, D.; Roh, J.y.; Eom, H.j.; Choi, J.Y.; Hyun, J.; Choi, J. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 2012, 31, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Liu, Y.; Gorospe, M.; Xu, Q.; Holbrook, N.J. Activation of Mitogen-activated Protein Kinase by H2O2: ROLE IN CELL SURVIVAL FOLLOWING OXIDANT INJURY (∗). J. Biol. Chem. 1996, 271, 4138–4142. [Google Scholar] [CrossRef]
- Tournier, C.; Thomas, G.; Pierre, J.; Jacquemin, C.; Pierre, M.; Saunier, B. Mediation by arachidonic acid metabolites of the H2O2-induced stimulation of mitogen-activated protein kinases (extracellular-signal-regulated kinase and c-Jun NH2-terminal kinase). Eur. J. Biochem. 1997, 244, 587–595. [Google Scholar] [CrossRef]
- Pujalté, I.; Passagne, I.; Brouillaud, B.; Tréguer, M.; Durand, E.; Ohayon-Courtès, C.; L’Azou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 2011, 8, 10. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.R.; Tkach, A.V.; Yanamala, N.; Mercer, R.; Young, S.-H.; Fadeel, B.; Kagan, V.E.; Shvedova, A.A. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part. Fibre Toxicol. 2012, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, A.K.; Timblin, C.R.; Shukla, A.; Rincón, M.; Mossman, B.T. Activation of NF-κB-dependent gene expression by silica in lungs of luciferase reporter mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2002, 282, L968–L975. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.; Tresini, M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Zhang, X.; Young, H.A.; Mao, Y.; Shi, X. Chromium (VI)-induced nuclear factor-κB activation in intact cells via free radical reactions. Carcinogenesis 1995, 16, 2401–2405. [Google Scholar] [CrossRef]
- Gorshkova, Y.; Barbinta-Patrascu, M.-E.; Bokuchava, G.; Badea, N.; Ungureanu, C.; Lazea-Stoyanova, A.; Răileanu, M.; Bacalum, M.; Turchenko, V.; Zhigunov, A.; et al. Biological Performances of Plasmonic Biohybrids Based on Phyto-Silver/Silver Chloride Nanoparticles. Nanomaterials 2021, 11, 1811. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.-E.; Gorshkova, Y.; Ungureanu, C.; Badea, N.; Bokuchava, G.; Lazea-Stoyanova, A.; Bacalum, M.; Zhigunov, A.; Petrovic, S. Characterization and Antitumoral Activity of Biohybrids Based on Turmeric and Silver/Silver Chloride Nanoparticles. Materials 2021, 14, 4726. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, Y.; Suminda, G.G.D.; Heo, Y.; Kim, M.; Ghosh, M.; Son, Y.-O. Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants 2023, 12, 703. https://doi.org/10.3390/antiox12030703
Min Y, Suminda GGD, Heo Y, Kim M, Ghosh M, Son Y-O. Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants. 2023; 12(3):703. https://doi.org/10.3390/antiox12030703
Chicago/Turabian StyleMin, Yunhui, Godagama Gamaarachchige Dinesh Suminda, Yunji Heo, Mangeun Kim, Mrinmoy Ghosh, and Young-Ok Son. 2023. "Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress" Antioxidants 12, no. 3: 703. https://doi.org/10.3390/antiox12030703
APA StyleMin, Y., Suminda, G. G. D., Heo, Y., Kim, M., Ghosh, M., & Son, Y.-O. (2023). Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants, 12(3), 703. https://doi.org/10.3390/antiox12030703






