Antibacterial Activity of ZnSe, ZnSe-TiO2 and TiO2 Particles Tailored by Lysozyme Loading and Visible Light Irradiation
Abstract
1. Introduction
- (a)
- Through formation of a hybrid complex by lysozyme loading (Lys/ZnSe, Lys/ZnSe-TiO2 and Lys/TiO2);
- (b)
- By exposing the light sensitive samples (ZnSe, ZnSe-TiO2 and TiO2) and their hybrid complexes (Lys/ZnSe, Lys/ZnSe-TiO2 and Lys/TiO2) to visible light irradiation before the antibacterial assay on Staphylococcus aureus and Micrococcus lysodeikticus.
2. Materials and Methods
2.1. Synthesis of Inorganic Matrices (ZnSe, ZnSe-TiO2, TiO2)
2.2. Lysozyme Adsorption on ZnSe, ZnSe-TiO2 and TiO2 Samples
2.3. Characterization Methods
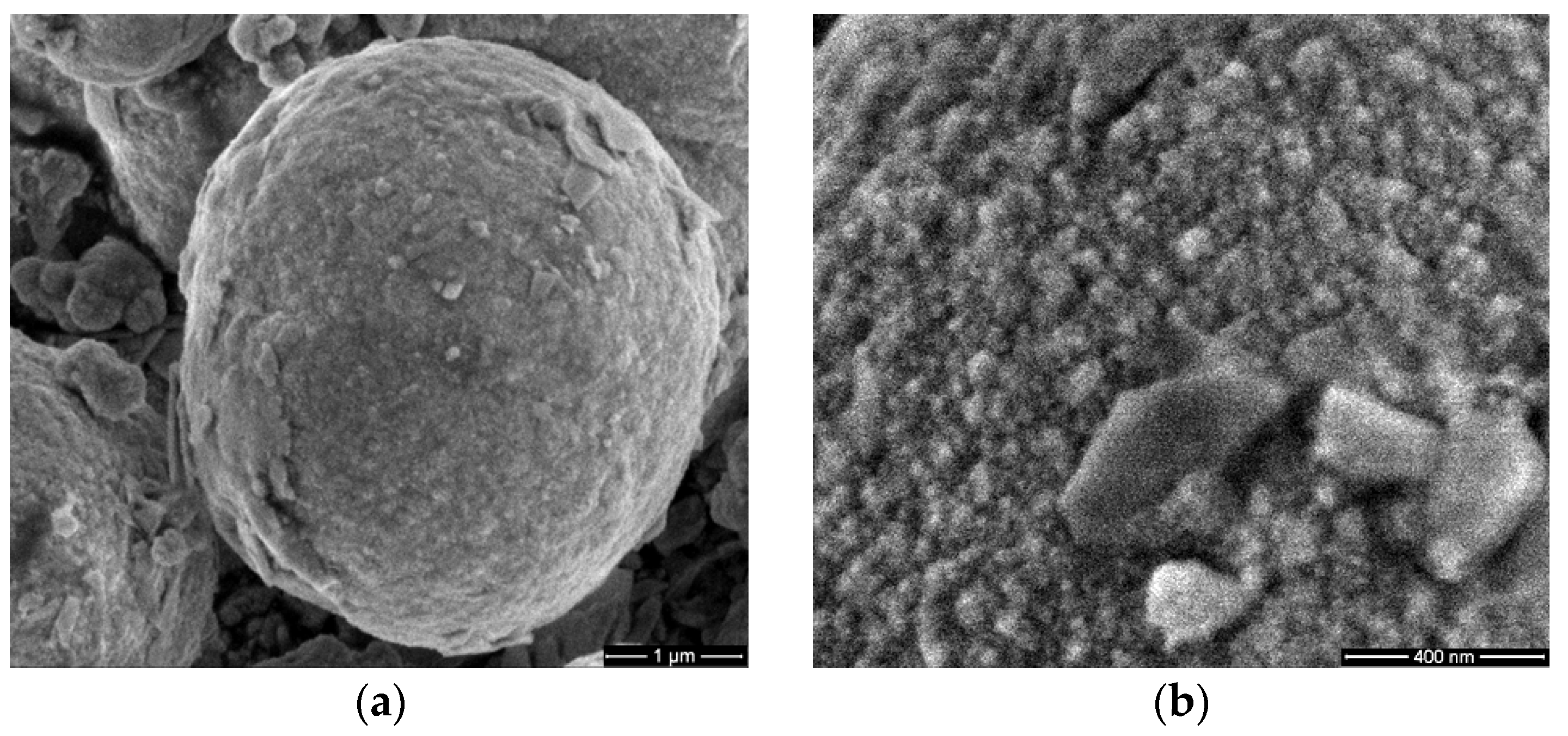
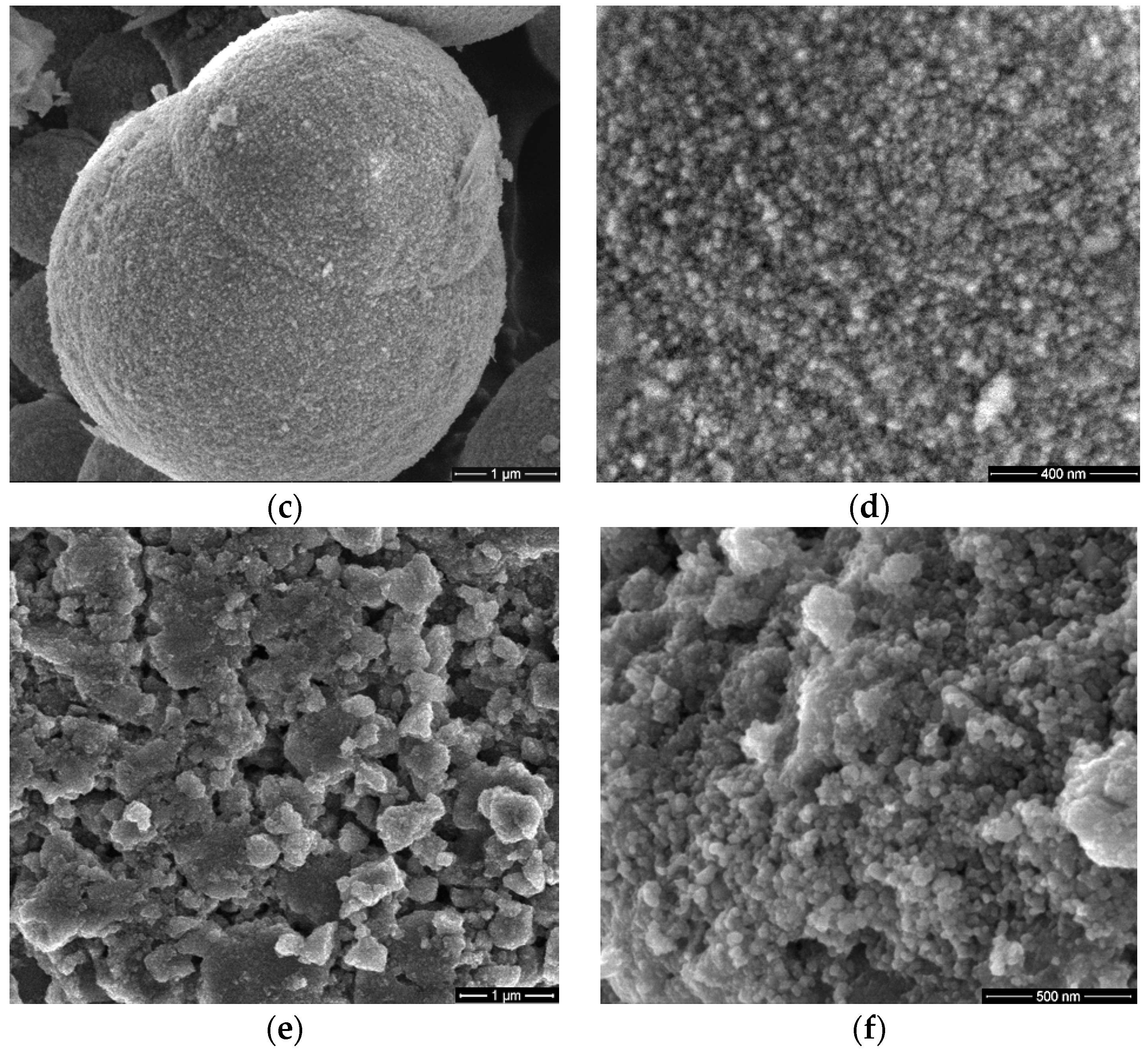
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. Dark field microscopy
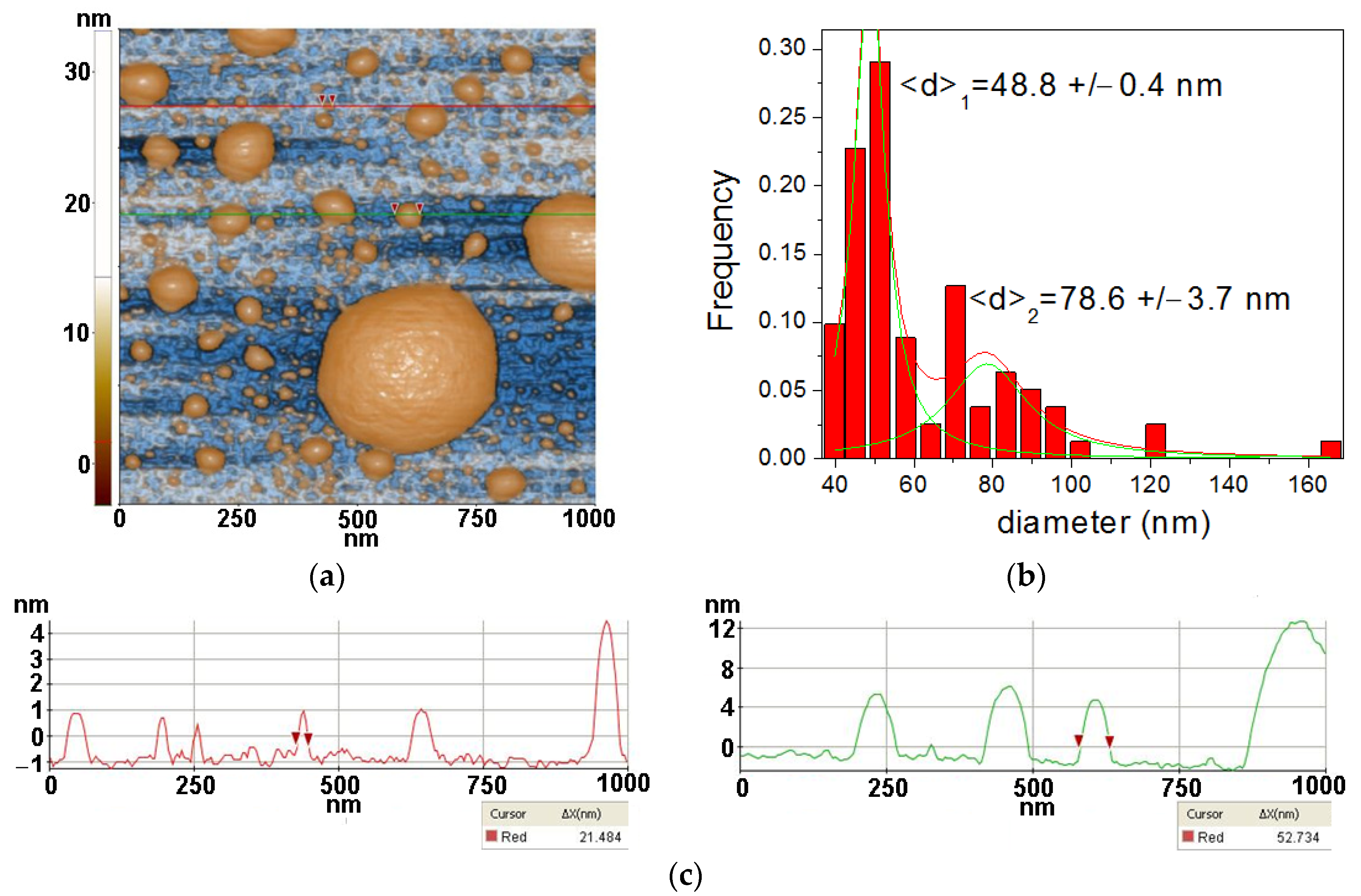
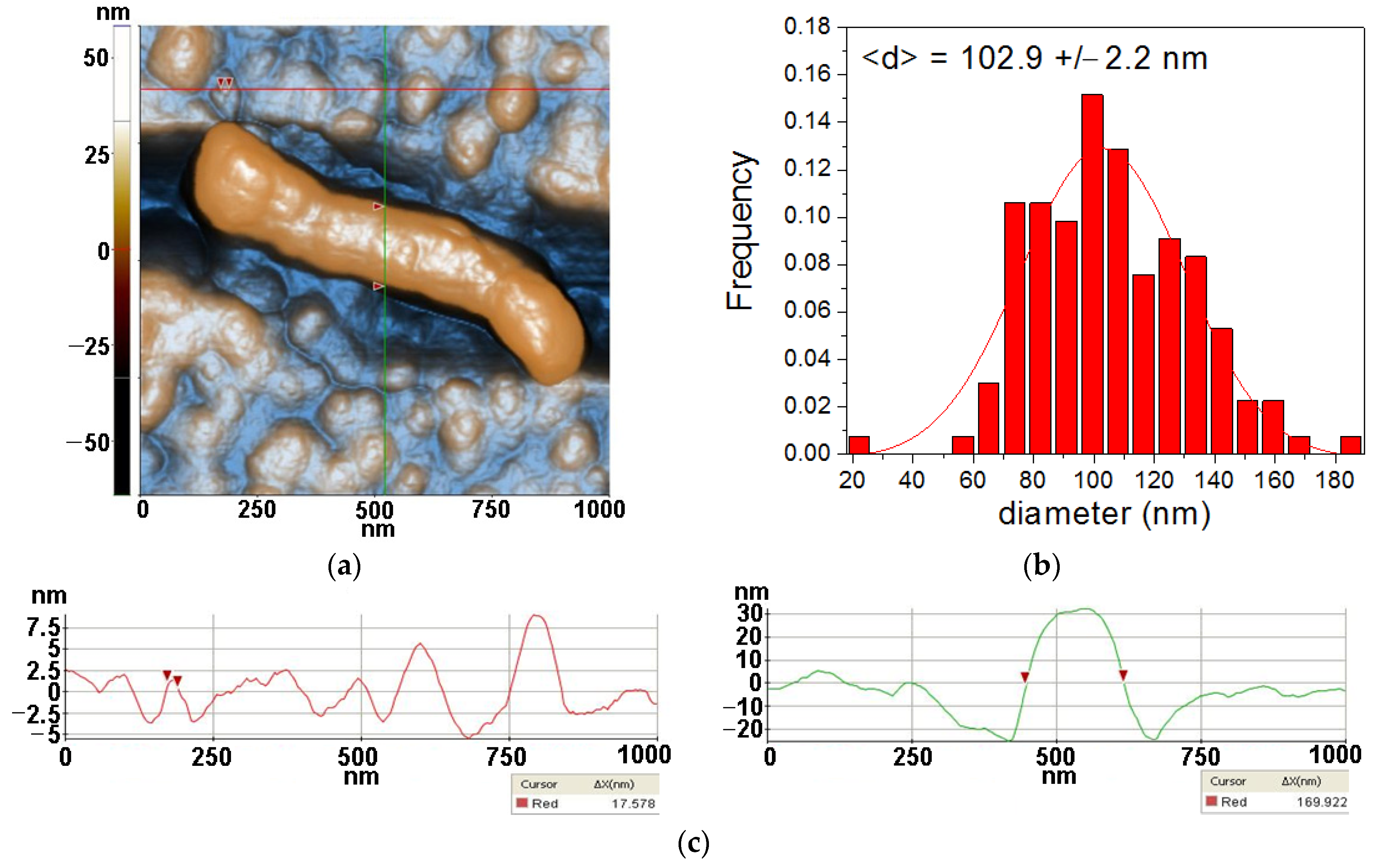
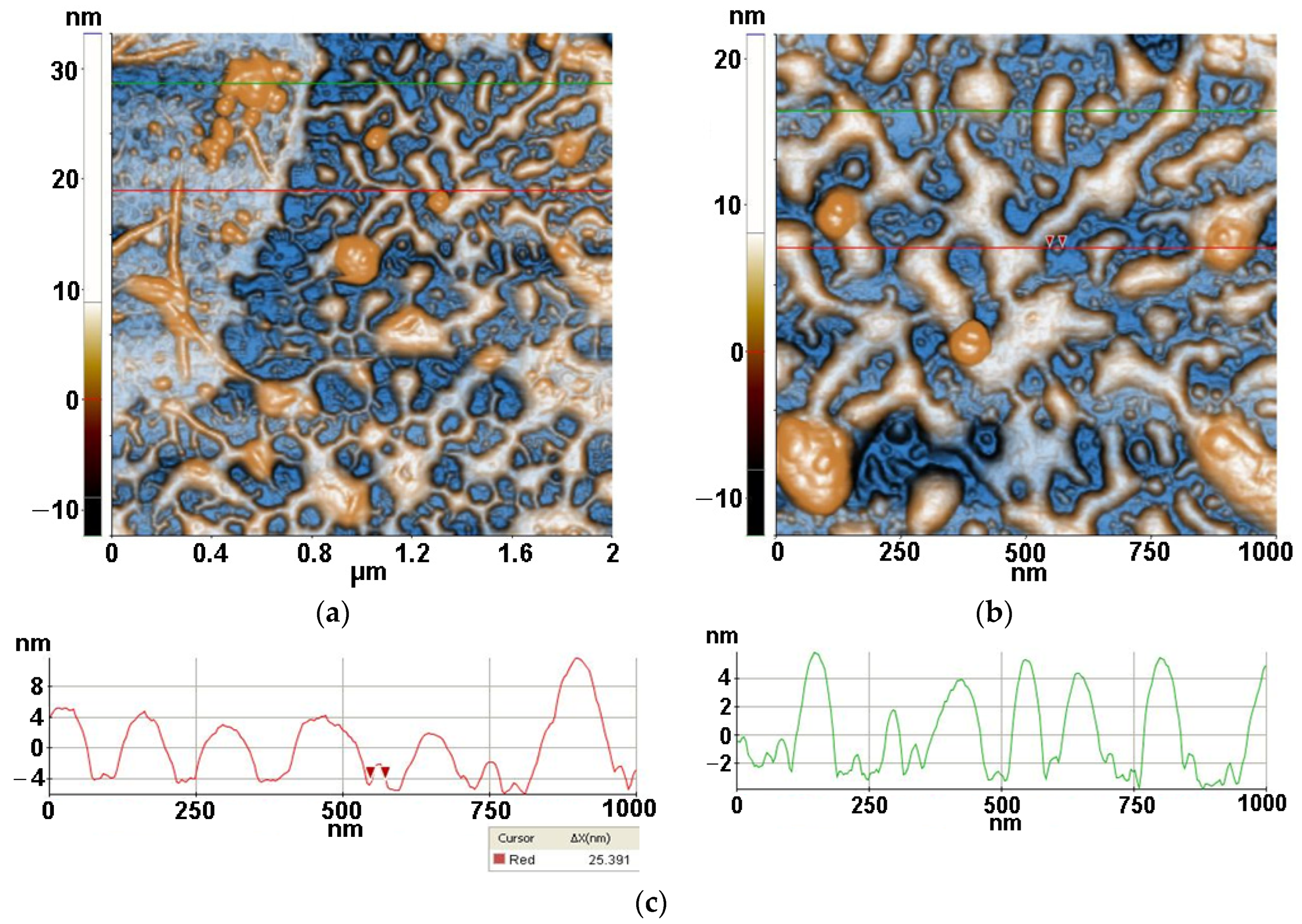
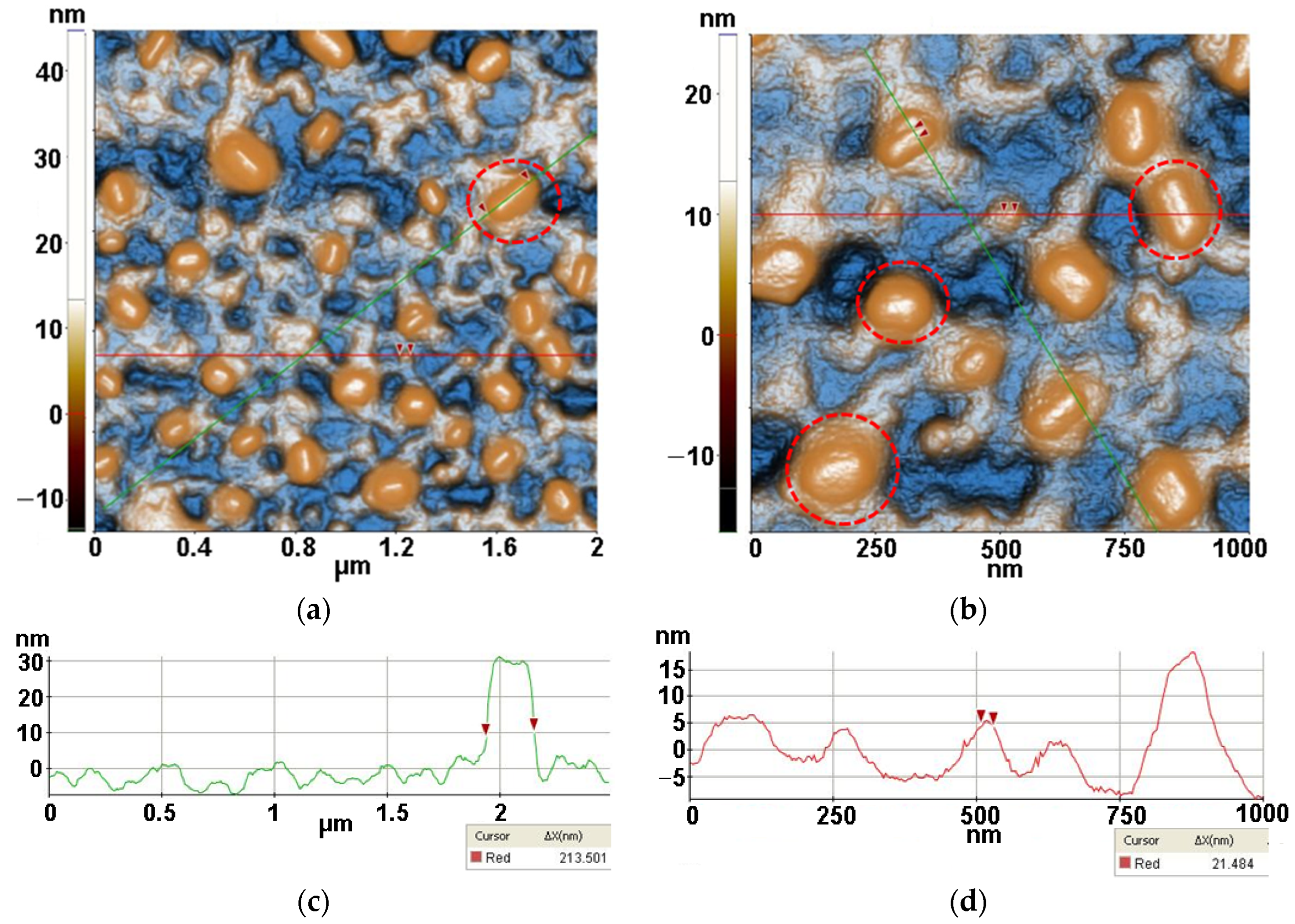
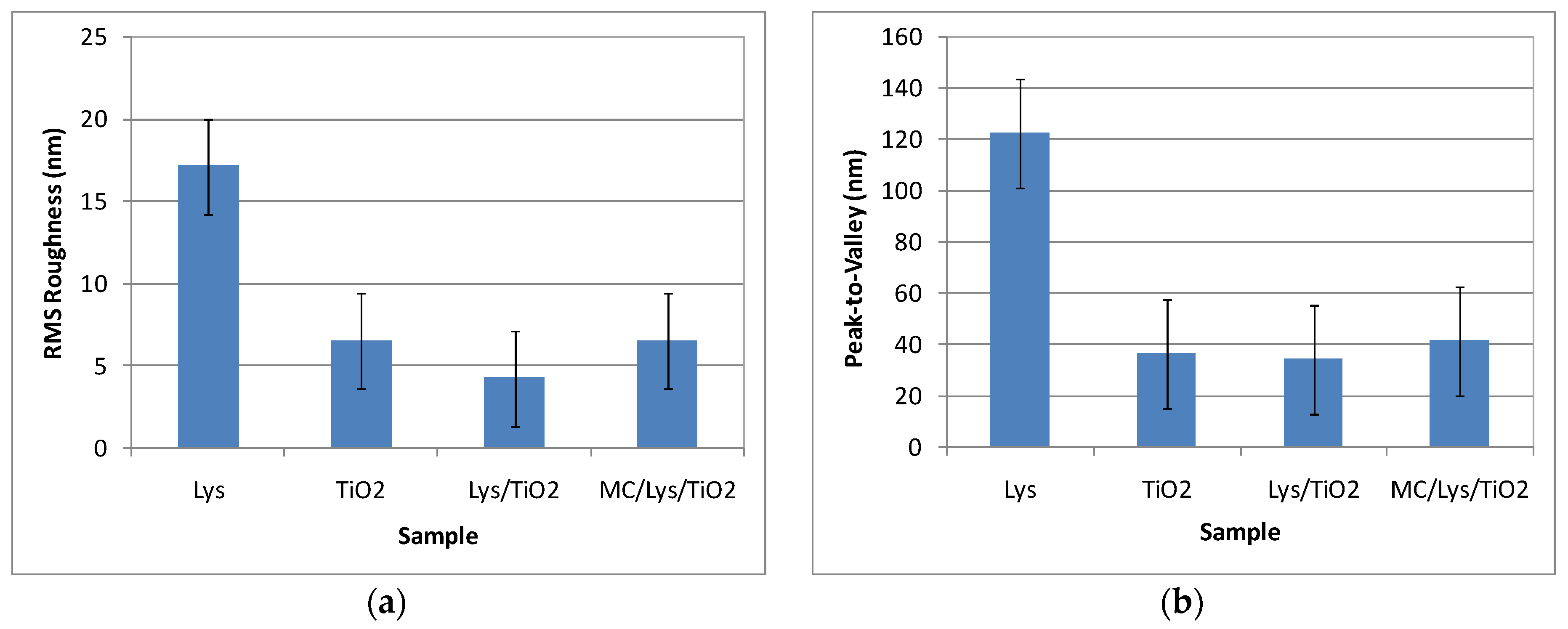
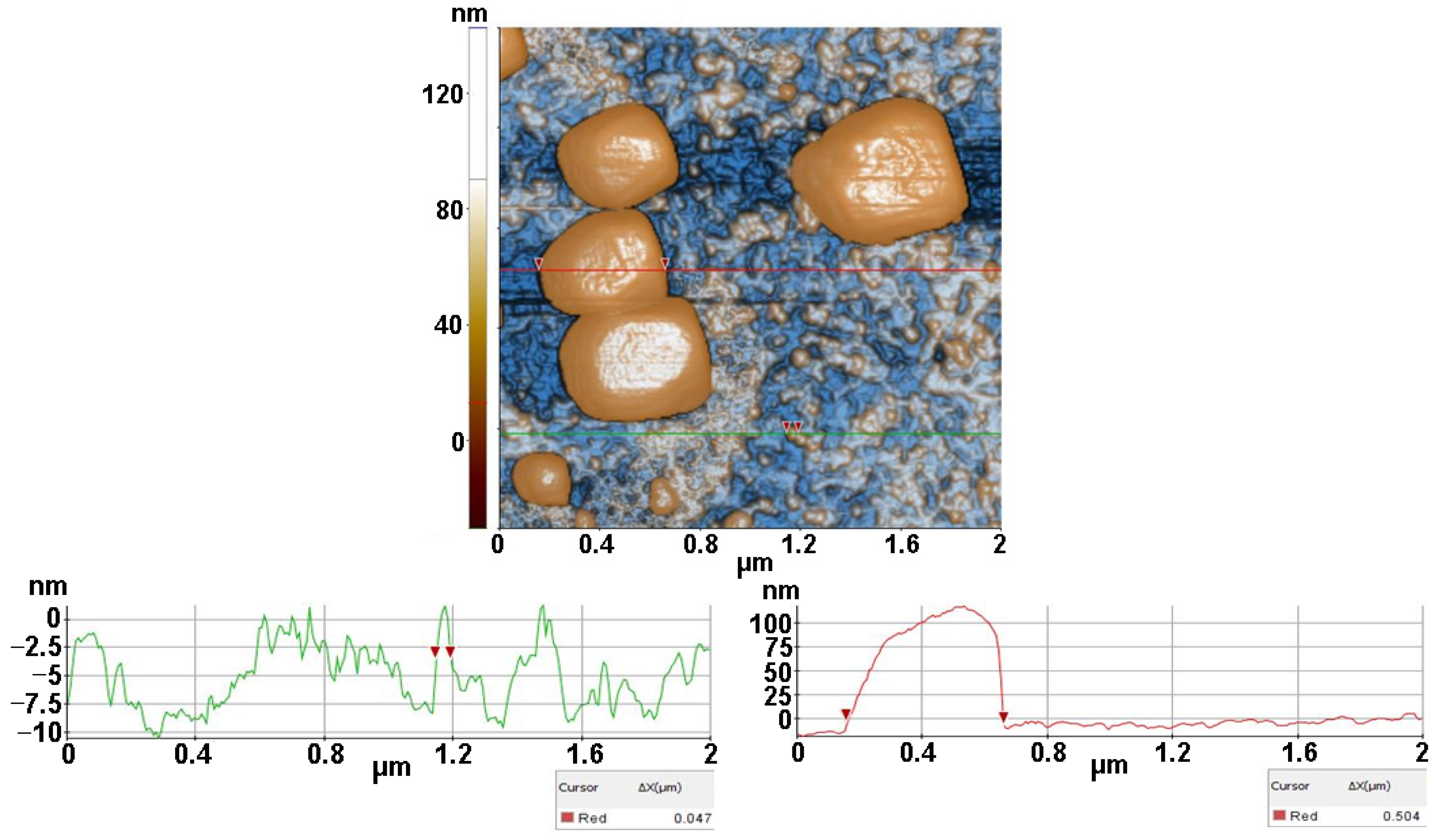
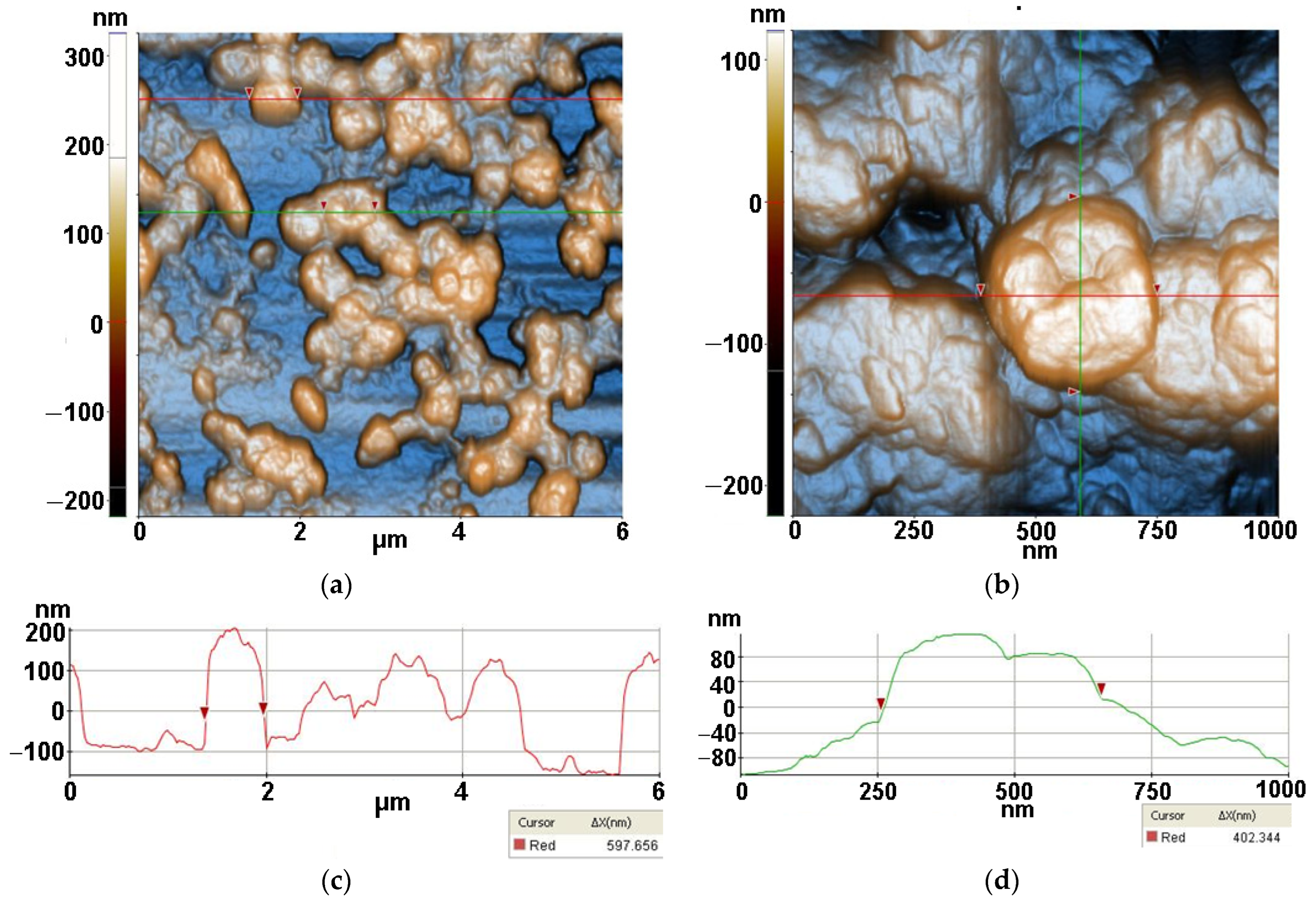
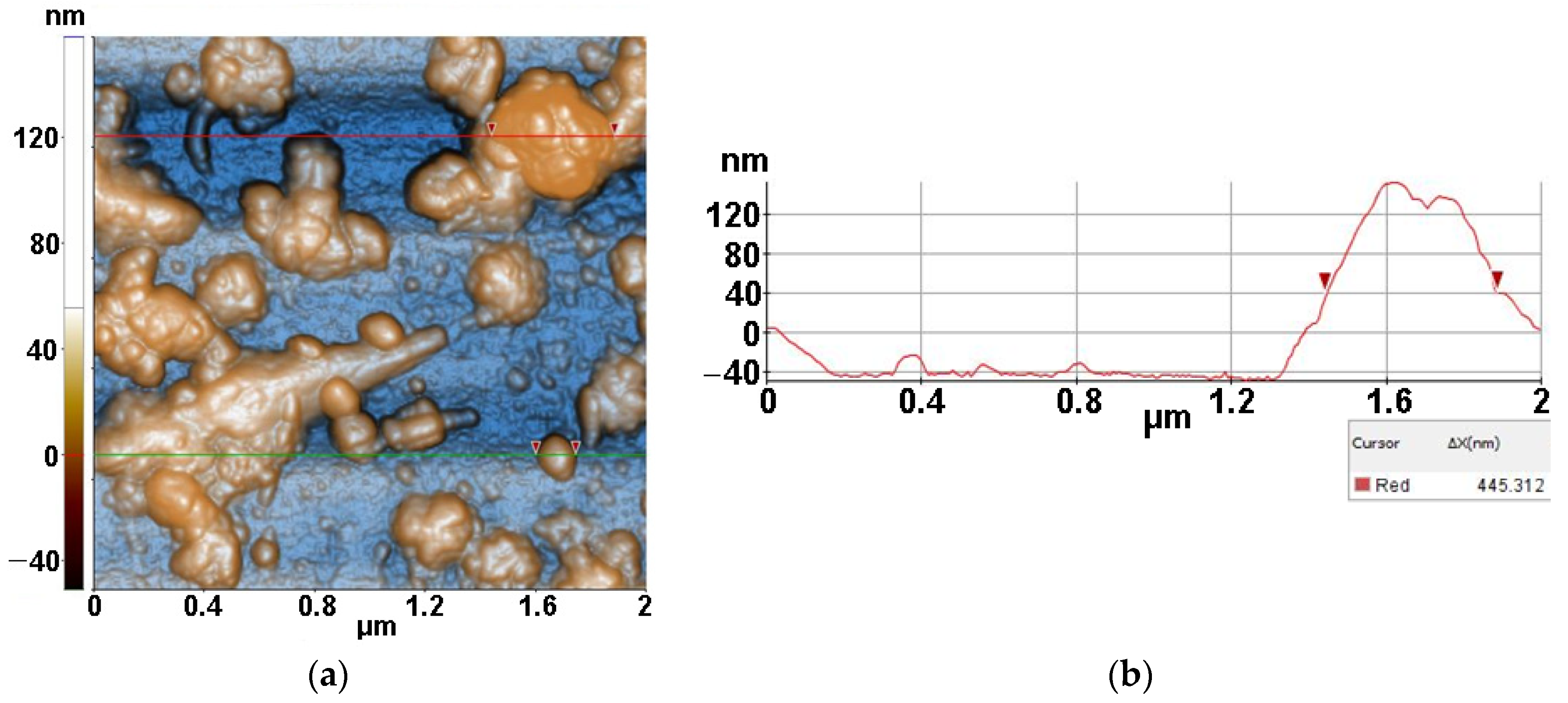
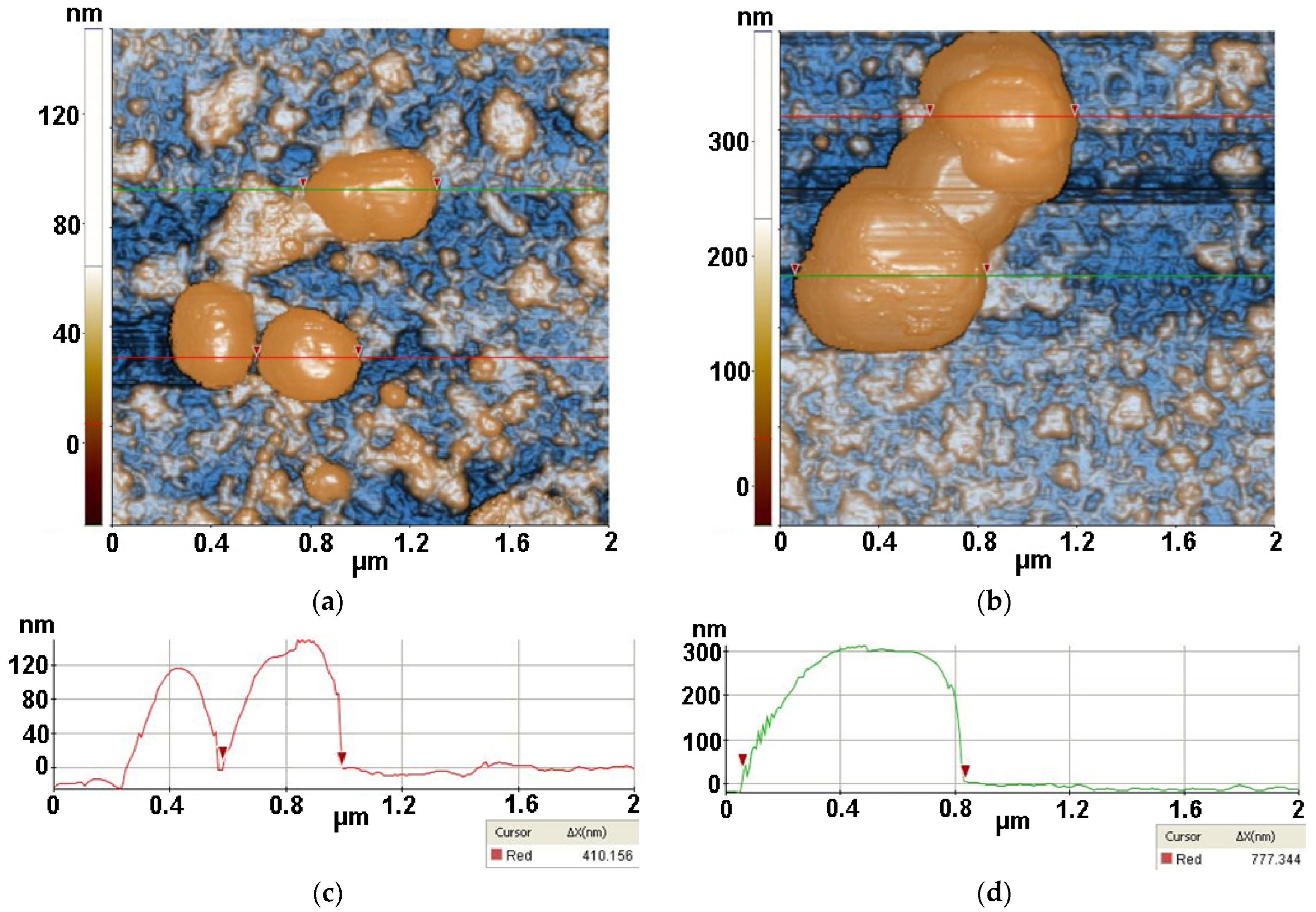
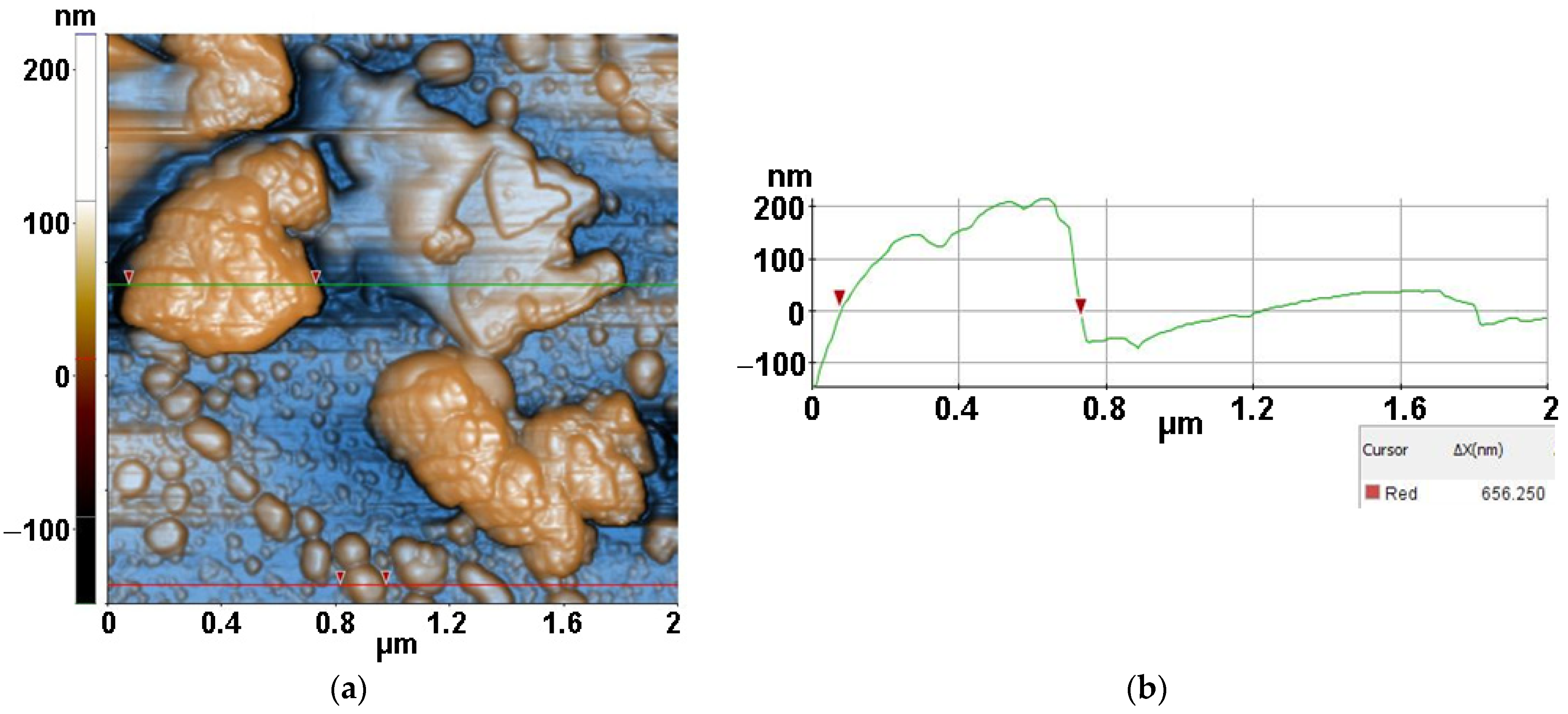
2.3.3. Atomic force microscopy (AFM)
2.3.4. Diffuse Reflectance UV-Vis
2.3.5. Reactive Oxygen Species (ROS) Generation under Visible Light Irradiation
2.3.6. X-ray Fluorescence (XRF) Characterization for Ion Releasing Measurements
2.4. Antimicrobial Activity Assay
2.4.1. Antimicrobial Activity Assay for the Inorganic Samples (ZnSe, ZnSe-TiO2 and TiO2) and Their Hybrid Complex with Lysozyme (Lys/ZnSe, Lys/ZnSe-TiO2 and Lys/TiO2)
2.4.2. Antimicrobial Activity Assay for the Samples of Interest Assisted by Visible Light Irradiation (λ > 420 nm)
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM)
3.2. Dark Field Microscopy (Cyto Viva)
3.3. Atomic force microscopy
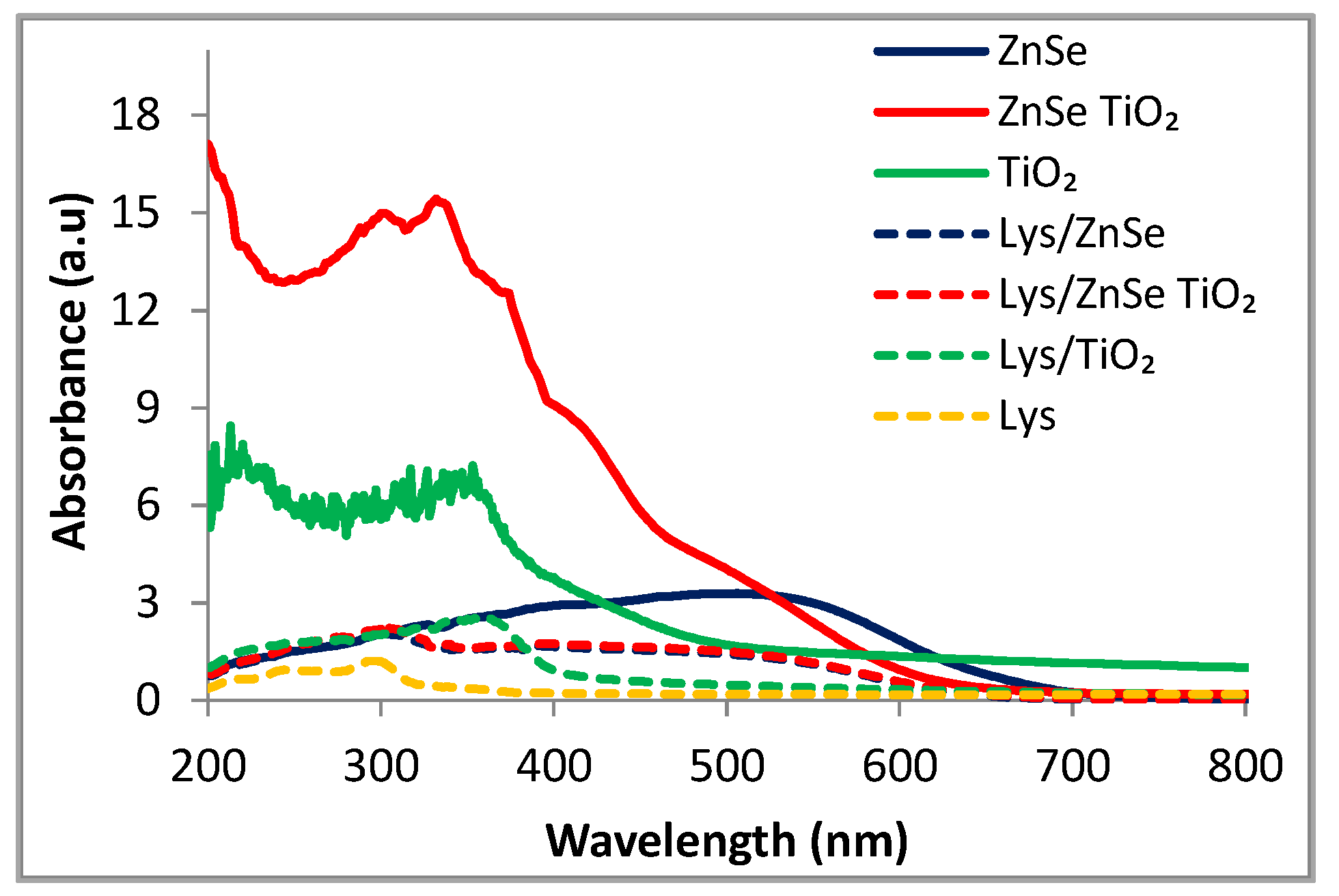
3.4. UV-Vis Spectroscopy
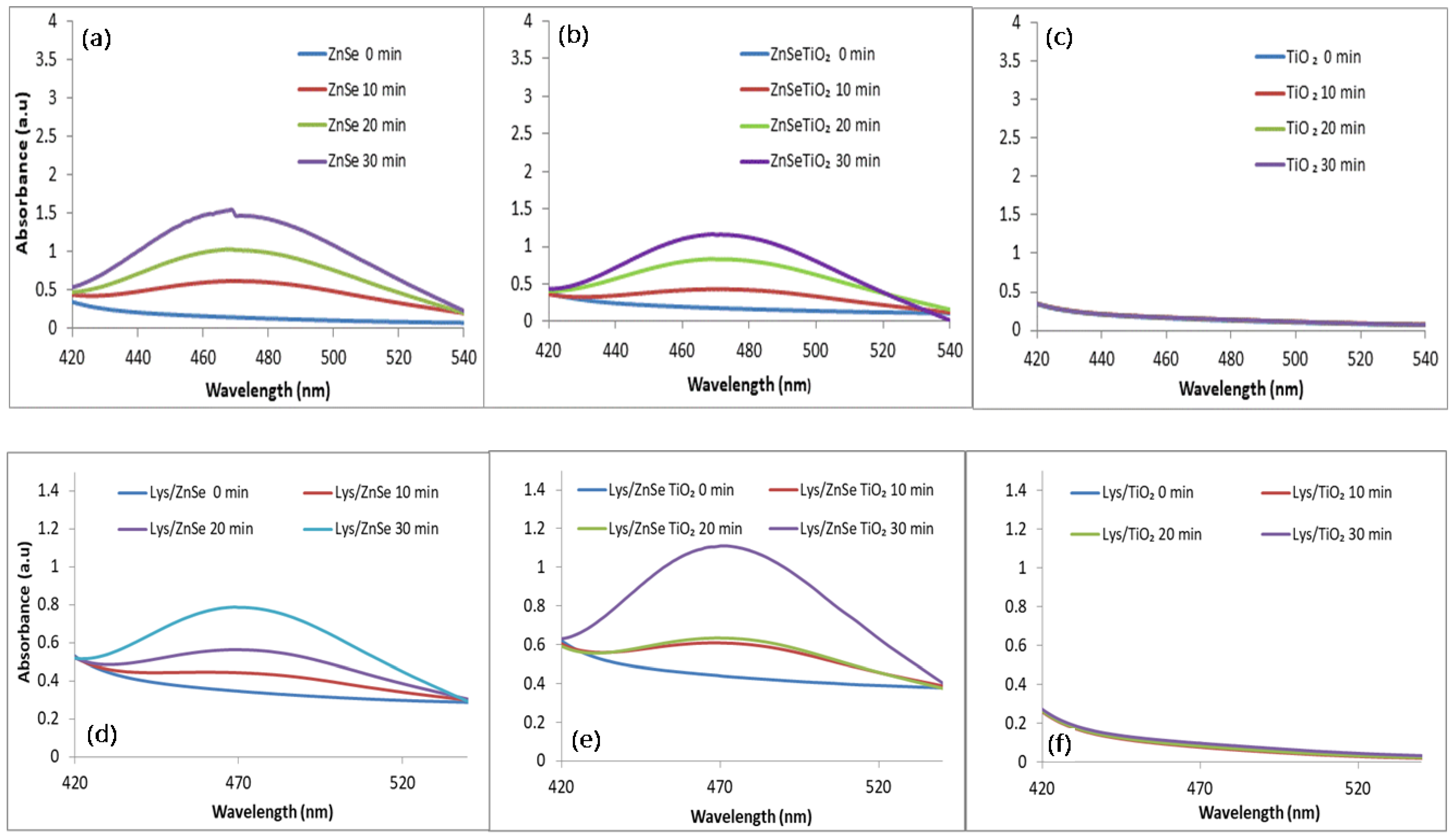
3.5. ROS Generation under Visible Light Irradiation (λ > 420 nm)
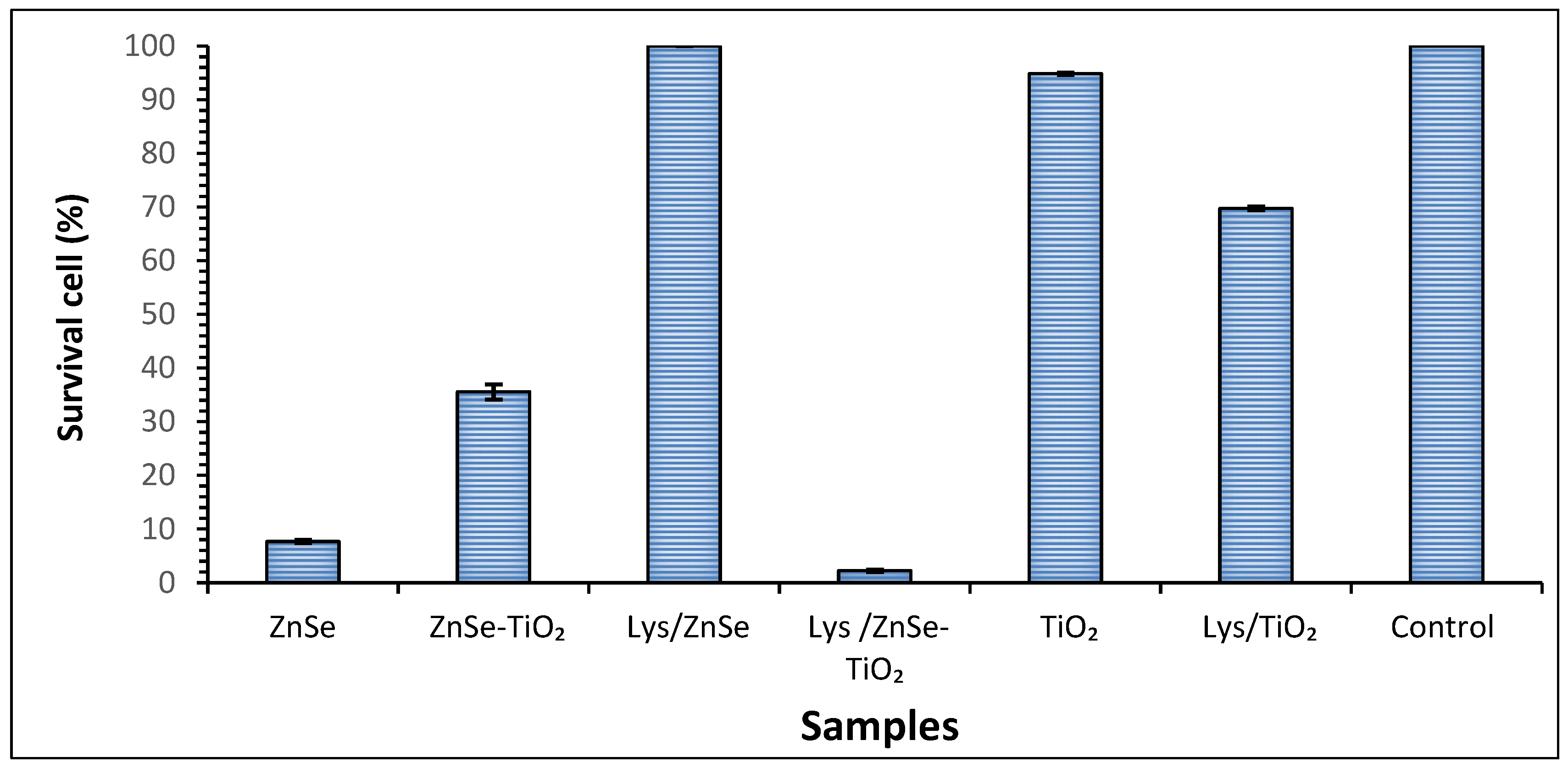
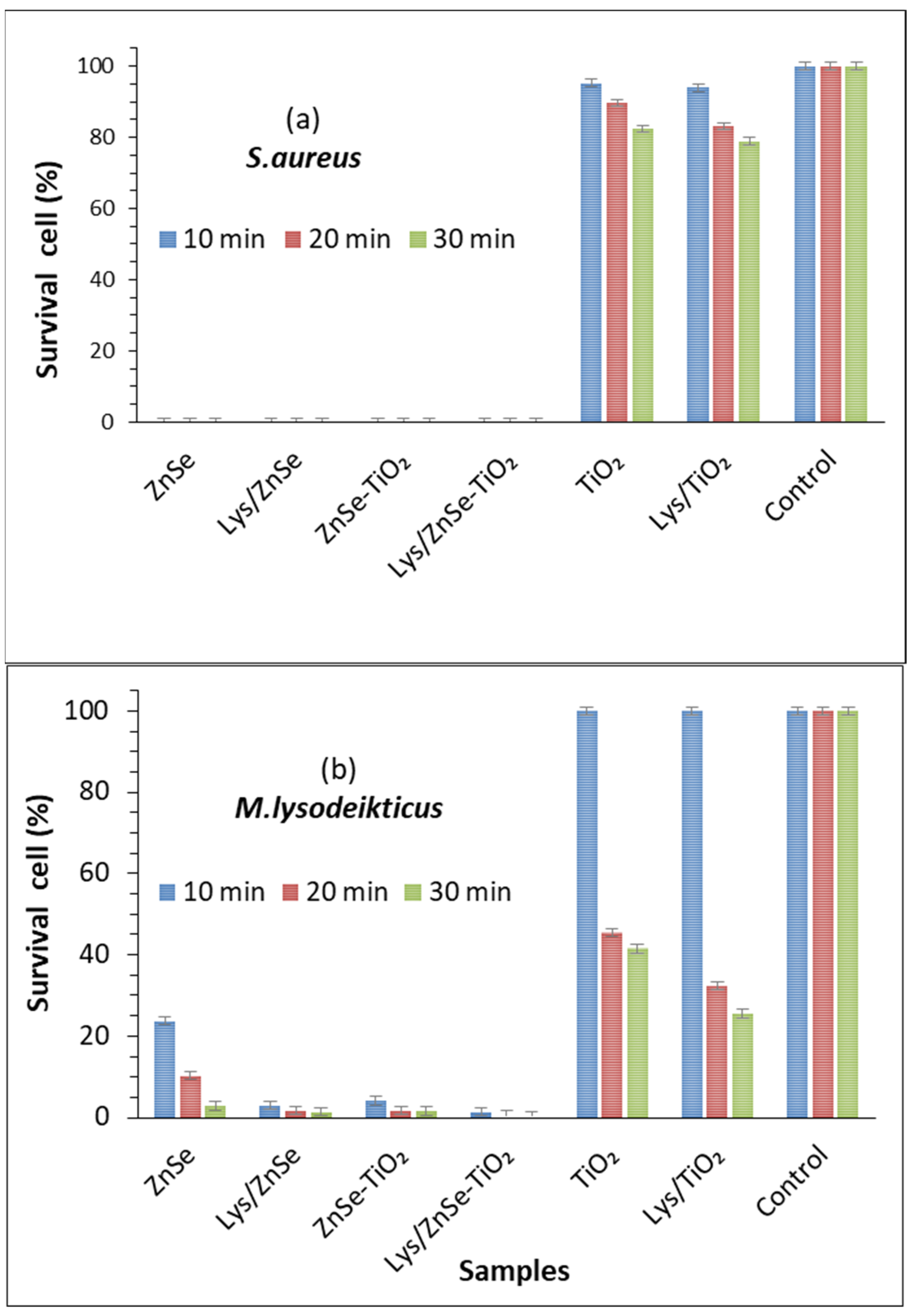
3.6. Antimicrobial Activity Assay for the Inorganic Samples (ZnSe, ZnSe-TiO2, TiO2) and Their Hybrid Complex with Lysozyme (Lys/ZnSe, Lys/ZnSe-TiO2 and Lys/TiO2) against S. aureus
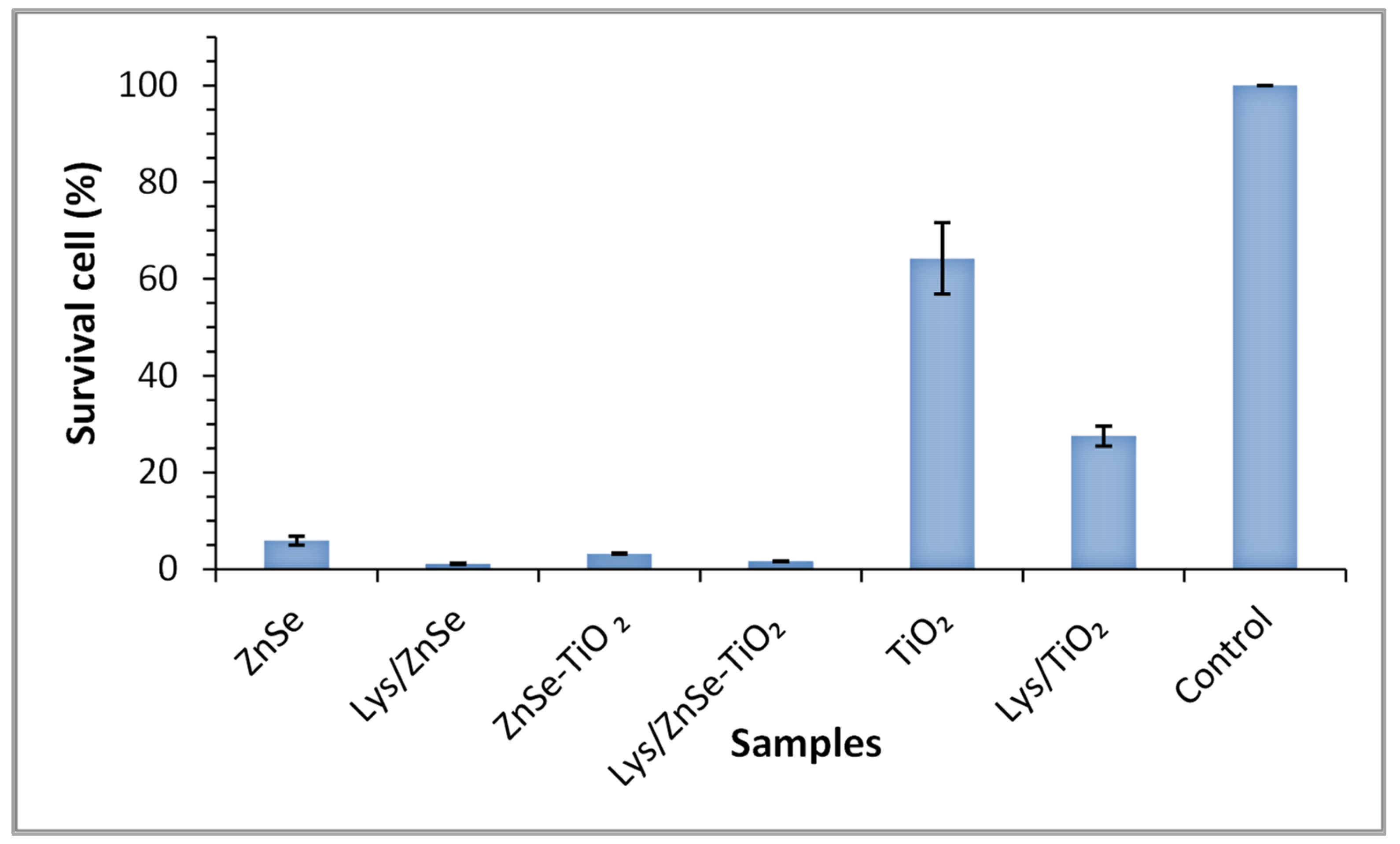
3.7. Antimicrobial Activity Assay for the Inorganic Samples (ZnSe, ZnSe-TiO2 and TiO2) and Their Hybrid Complex with Lysozyme (Lys/ZnSe, Lys/ZnSe-TiO2 and Lys/TiO2) against M. lysodeikticus
3.8. Antibacterial Activity of the Inorganic and Hybrid Materials (ZnSe, ZnSe-TiO2, TiO2 Lys/ZnSe, Lys/ZnSe-TiO2 and Lys/TiO2) Exposed to Visible Light Irradiation against S. aureus and M. lysodeikticus
- (i)
- h⁺ + -OH (surface hydroxyl) → •OH
- (ii)
- O2 + e− → (O2•−)ads (on semiconductor surface); (O2•−)ads + 2H⁺+e− → H2O2 → 2•OH
- (iii)
- H2O + hυ → •OH
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mei, L.; Zhu, S.; Liu, Y.; Yin, W.; Gu, Z.; Zhao, Y. An overview of the use of nanozymes in antibacterial applications. Chem. Eng. J. 2021, 418, 129431. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Cao, L. Immobilised enzymes: Science or art? Curr. Opin. Chem. Biol. 2005, 9, 217–226. [Google Scholar] [CrossRef]
- Anastasescu, C.; Anastasescu, M.; Balint, I.; Zaharescu, M. SiO2 based materials for immobilization of enzymes. In Nanomaterials—Toxicity, Human Health and Environment; InTechOpen: London, UK, 2019; ISBN 978-1-78984-617-1. [Google Scholar] [CrossRef]
- Jolles, P. Lysozymes: A chapter of molecular biology. Angew. Chem. Int. Ed. Engl. 1969, 8, 227–294. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, J.; Huang, M.; Xu, Q.; Ma, M. The changes of secondary structures and properties of lysozyme along with the egg storage. Int. J. Biol. Macromol. 2016, 92, 600–606. [Google Scholar] [CrossRef]
- Calderon, C.; Abuin, E.; Lissi, E.; Montecinos, R. Effect of human serum albumin on the kinetics of 4-methylumbelliferyl-β-D-N-N′-N″ triacetylchitotrioside hydrolysis catalyzed by hen egg white lysozyme. Protein J. 2011, 30, 367–373. [Google Scholar] [CrossRef]
- Chifor, E.; Bordeianu, I.; Anastasescu, C.; Calderon-Moreno, J.M.; Bratan, V.; Eftemie, D.-I.; Anastasescu, M.; Preda, S.; Plavan, G.; Pelinescu, D.; et al. Bioactive coatings based on nanostructured TiO2 modified with noble metal nanoparticles and lysozyme for Ti dental implants. Nanomaterials 2022, 12, 3186. [Google Scholar] [CrossRef]
- Larsericsdotter, H.; Oscarsson, S.; Buijs, J. Thermodynamic analysis of proteins adsorbed on silica particles: Electrostatic effects. J. Colloid Interface Sci. 2001, 237, 98–103. [Google Scholar] [CrossRef]
- Shareghi, B.; Farhadian, S.; Zamani, N.; Salavati-Niasari, M.; Moshtaghi, H.; Gholamrezaei, S. Investigation the activity and stability of lysozyme on presence of magnetic nanoparticles. J. Ind. Eng. Chem. 2015, 21, 862–867. [Google Scholar] [CrossRef]
- Jeon, H.-J.; Yi, S.-C.; Oh, S.-G. Preparation and antibacterial effects of Ag–SiO2 thin films. Biomaterials 2003, 24, 4921–4928. [Google Scholar] [CrossRef]
- Guo, C.; Guo, X.; Chu, W.; Jiang, N.; Li, H. FTIR-ATR study for adsorption of trypsin in aqueous environment on bare and TiO2 coated ZnSe surfaces. Chin. Chem. Lett. 2020, 31, 150–154. [Google Scholar] [CrossRef]
- Mir, I.A.; Rawat, K.; Solanki, P.R.; Bohidar, H.B. ZnSe core and ZnSe@ZnS core-shell quantum dots as platform for folic acid sensing. J. Nanopart. Res. 2017, 19, 260. [Google Scholar] [CrossRef]
- Sun, C.; Gu, Y.; Wen, W.; Zhao, L. ZnSe based semiconductor core-shell structures: From preparation to application. Opt. Mater. 2018, 81, 12–22. [Google Scholar] [CrossRef]
- Hofmann, A.; Pettenkofer, C. Surface orientation dependent band alignment for CuInSe2-ZnSe-ZnO. Appl. Phys. Lett. 2011, 98, 113503. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Yang, X.; Qu, Y.; Li, Q. Nanostructured ZnSe anchored on grapheme nanosheets with superior electrochemical properties for lithium ion batteries. Electrochim. Acta 2015, 168, 285–291. [Google Scholar] [CrossRef]
- Anastasescu, C.; Gifu, I.C.; Negrila, C.; Socoteanu, R.; Atkinson, I.; Calderon-Moreno, J.M.; Munteanu, C.; Plavan, G.; Strungaru, S.A.; Cheatham, B.; et al. Morpho-structural properties of ZnSe, TiO2-ZnSe materials and enzymatic activity of their bioinorganic hybrids with lysozyme. Mat. Sci. Eng. B 2021, 272, 115350. [Google Scholar] [CrossRef]
- Ibrahim, H.R.; Matsuzaki, T.; Aoki, T. Genetic evidence that antibacterial activity of lysozyme is independent ofits catalytic function. FEBS Lett. 2001, 506, 27–32. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the mechanism of antibacterial activity of ZnO: Surface, defects mediated reactive oxygen species even in the dark, release of metallic ions and electrostatic interaction of investigated material with cell membrane. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO nanomaterials: Current advancements in antibacterial mechanisms and applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Kumaravel, V.; Nair, K.M.; Mathew, S.; Bartlett, J.; Kennedy, J.E.; Manning, H.G.; Whelan, B.J.; Leyland, N.S.; Pillai, S.C. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem. Eng. J. 2021, 416, 129071. [Google Scholar] [CrossRef]
- Quek, J.-A.; Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. Visible light responsive flower-like ZnO in photocatalytic antibacterial mechanism towards Enterococcus faecalis and Micrococcus luteus. J. Photochem. Photobiol. B Biol. 2018, 187, 66–75. [Google Scholar] [CrossRef]
- Neagu, S.; Anastasescu, C.; Balint, I.; Zaharescu, M.; Ardelean, I.; Enache, M. The response of Escherichia Coli cells to the action of ZnSe based materials. Rom. J. Mater. 2019, 49, 322–330. [Google Scholar]
- Soheyli, E.; Sahraei, R.; Nabiyouni, G.; Hatamnia, A.A.; Rostamzad, A.; Soheyli, S. Aqueous-based synthesis of Cd-free and highly emissive Fe-doped ZnSe(S)/ZnSe(S) core/shell quantum dots with antibacterial activity. J. Colloid Interface Sci. 2018, 529, 520–530. [Google Scholar] [CrossRef]
- Anastasescu, C.; Spataru, N.; Culita, D.; Atkinson, I.; Spataru, T.; Bratan, V.; Munteanu, C.; Anastasescu, M.; Negrila, C.; Balint, I. Chemically assembled light harvesting CuOx-TiO2 p–n heterostructures. Chem. Eng. J. 2015, 281, 303–311. [Google Scholar] [CrossRef]
- Whitehouse, D.J. Surface Characterization and Roughness Measurements in Engineering. In Photomechanics; Rastogi, P.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 77, pp. 413–461. [Google Scholar]
- Balint, I.; Miyazaki, A.; Aika, K.-I. Alumina dissolution during impregnation with PdCl42- in acid pH range. Chem. Mater. 2001, 13, 932–938. [Google Scholar] [CrossRef]
- Moriyama, T.; Morikawa, A. Sample preparation for X-ray fluorescence analysis. VII. Liquid sample. Rigaku J.—Tech. Artic. 2017, 33, 24–29. [Google Scholar]
- Anastasescu, C.; Negrila, C.; Angelescu, D.G.; Atkinson, I.; Anastasescu, M.; Spataru, N.; Zaharescu, M.; Balint, I. Distinct and interrelated facets bound to photocatalysis and ROS generation on insulators and semiconductors: Cases of SiO2, TiO2 and their composite SiO2-TiO2. Catal. Sci. Technol. 2018, 8, 5657–5668. [Google Scholar] [CrossRef]
- Preda, S.; Anastasescu, C.; Balint, I.; Umek, P.; Sluban, M.; Negrila, C.; Angelescu, D.G.; Bratan, V.; Rusu, A.; Zaharescu, M. Charge separation and ROS generation on tubular sodium titanates exposed to simulated solar light. Appl. Surf. Sci. 2019, 470, 1053–1063. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anastasescu, C.; Neagu, S.; Preda, S.; Culita, D.; Stancu, M.; Banciu, C.; Munteanu, C.; Bratan, V.; Calderon-Moreno, J.M.; State, R.; et al. Antibacterial Activity of ZnSe, ZnSe-TiO2 and TiO2 Particles Tailored by Lysozyme Loading and Visible Light Irradiation. Antioxidants 2023, 12, 691. https://doi.org/10.3390/antiox12030691
Anastasescu C, Neagu S, Preda S, Culita D, Stancu M, Banciu C, Munteanu C, Bratan V, Calderon-Moreno JM, State R, et al. Antibacterial Activity of ZnSe, ZnSe-TiO2 and TiO2 Particles Tailored by Lysozyme Loading and Visible Light Irradiation. Antioxidants. 2023; 12(3):691. https://doi.org/10.3390/antiox12030691
Chicago/Turabian StyleAnastasescu, Crina, Simona Neagu, Silviu Preda, Daniela Culita, Mihaela Stancu, Cristian Banciu, Cornel Munteanu, Veronica Bratan, Jose Maria Calderon-Moreno, Razvan State, and et al. 2023. "Antibacterial Activity of ZnSe, ZnSe-TiO2 and TiO2 Particles Tailored by Lysozyme Loading and Visible Light Irradiation" Antioxidants 12, no. 3: 691. https://doi.org/10.3390/antiox12030691
APA StyleAnastasescu, C., Neagu, S., Preda, S., Culita, D., Stancu, M., Banciu, C., Munteanu, C., Bratan, V., Calderon-Moreno, J. M., State, R., Anastasescu, M., Enache, M., Balint, I., & Zaharescu, M. (2023). Antibacterial Activity of ZnSe, ZnSe-TiO2 and TiO2 Particles Tailored by Lysozyme Loading and Visible Light Irradiation. Antioxidants, 12(3), 691. https://doi.org/10.3390/antiox12030691









