Melatonin in Newborn Infants Undergoing Surgery: A Pilot Study on Its Effects on Postoperative Oxidative Stress
Abstract
:1. Introduction
Objective
2. Materials and Methods
2.1. Recruitment and Randomization
2.2. Interventions
2.3. Perioperative Management of Newborn Infants, Analgesia and Anesthesia
2.4. Melatonin and Oxidative Stress Biomarker Measurements
2.5. Statistics
3. Results
3.1. Melatonin Pre- and Postoperative Plasma Concentrations
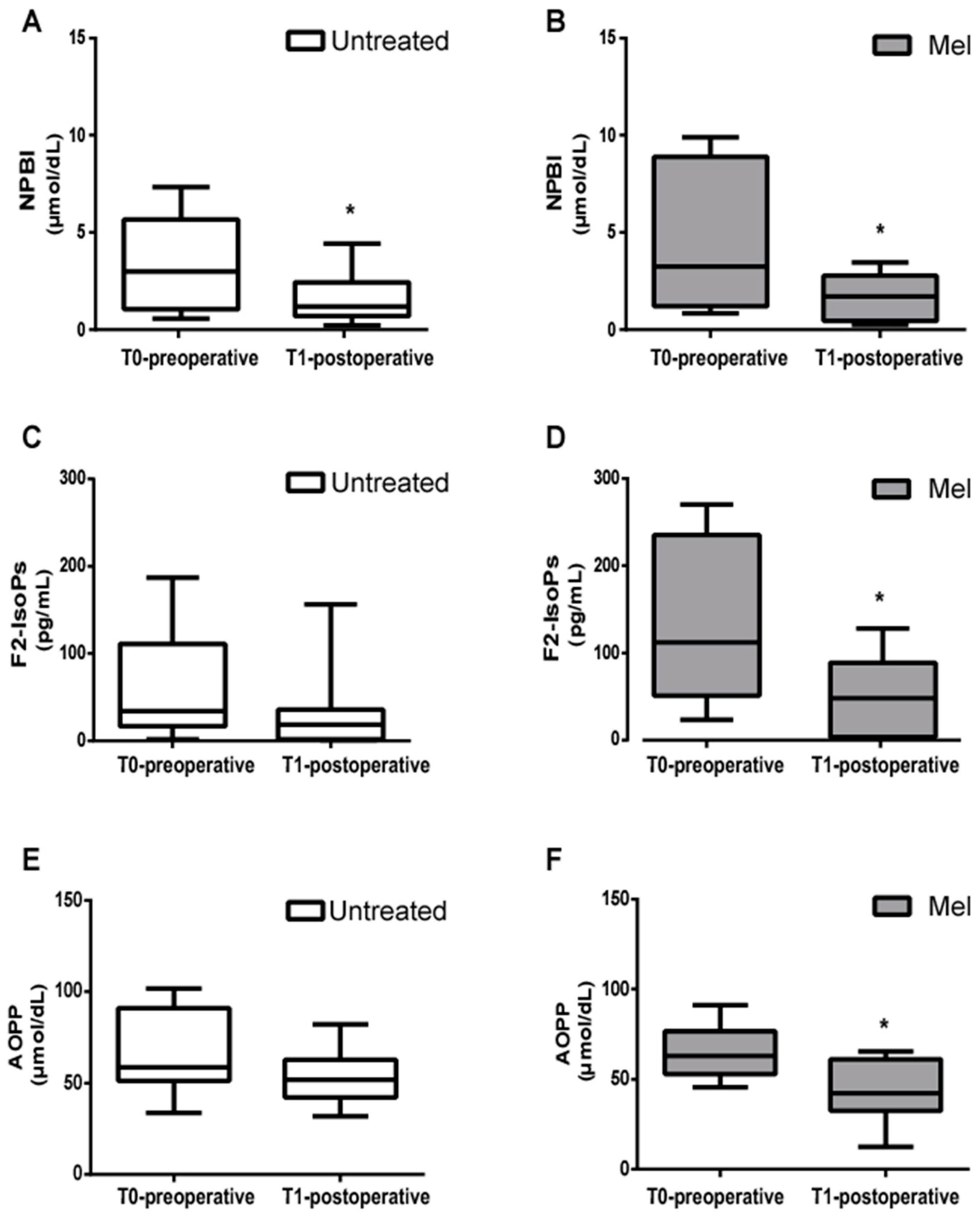
3.2. Pre- and Postoperative Blood Concentrations of Markers of Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kücükakin, B.; Gögenur, I.; Reiter, R.J.; Rosenberg, J. Oxidative stress in relation to surgery: Is there a role for the antioxidant melatonin? J. Surg. Res. 2009, 152, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Desborough, J.P. The stress response to trauma and surgery. Br. J. Anaesth. 2000, 85, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.G.; Hill, G.L. Metabolic response to severe injury. Br. J. Surg. 1998, 85, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Calvano, S.E.; Lowry, S.F. Inflammatory cytokines and cell response in surgery. Surgery 2000, 127, 117–126. [Google Scholar] [CrossRef]
- Gitto, E.; Romeo, C.; Reiter, R.J.; Impellizzeri, P.; Pesce, S.; Basile, M.; Antonuccio, P.; Trimarchi, G.; Gentile, C.; Barberi, I.; et al. Melatonin reduces oxidative stress in surgical neonates. J. Pediatr. Surg. 2004, 39, 184–189, Discussion 184–189. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Arrigo, T.; Barberi, I.; Reiter, R.J.; Gitto, E. Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid. Med. Cell. Longev. 2014, 2014, 358375. [Google Scholar] [CrossRef] [Green Version]
- Lembo, C.; Buonocore, G.; Perrone, S. Oxidative Stress in Preterm Newborns. Antioxidants 2021, 10, 1672. [Google Scholar] [CrossRef]
- Perrone, S.; Santacroce, A.; Longini, M.; Proietti, F.; Bazzini, F.; Buonocore, G. The Free Radical Diseases of Prematurity: From Cellular Mechanisms to Bedside. Oxid. Med. Cell. Longev. 2018, 2018, 7483062. [Google Scholar] [CrossRef] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Arrigo, T.; Cuppari, C.; Salpietro, C.; Gitto, E. Potential use of melatonin in procedural anxiety and pain in children undergoing blood withdrawal. J. Biol. Regul. Homeost. Agents 2015, 29, 509–514. [Google Scholar]
- Hardeland, R.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 313–316. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [Green Version]
- Fulia, F.; Gitto, E.; Cuzzocrea, S.; Reiter, R.J.; Dugo, L.; Gitto, P.; Barberi, S.; Cordaro, S.; Barberi, I. Increased levels of malondialdehyde and nitrite/nitrate in the blood of asphyxiated newborns: Reduction by melatonin. J. Pineal Res. 2001, 31, 343–349. [Google Scholar] [CrossRef]
- Gitto, E.; Reiter, R.J.; Cordaro, S.P.; La Rosa, M.; Chiurazzi, P.; Trimarchi, G.; Gitto, P.; Calabrò, M.P.; Barberi, I. Oxidative and inflammatory parameters in respiratory distress syndrome of preterm newborns: Beneficial effects of melatonin. Am. J. Perinatol. 2004, 21, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Biran, V.; Decobert, F.; Bednarek, N.; Boizeau, P.; Benoist, J.-F.; Claustrat, B.; Barré, J.; Colella, M.; Frérot, A.; Garnotel, R.; et al. Melatonin Levels in Preterm and Term Infants and Their Mothers. Int. J. Mol. Sci. 2019, 20, 2077. [Google Scholar] [CrossRef] [Green Version]
- Cannavò, L.; Perrone, S.; Marseglia, L.; Viola, V.; Di Rosa, G.; Gitto, E. Potential benefits of melatonin to control pain in ventilated preterm newborns: An updated review. Pain Pract. 2022, 22, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Reiter, R.J.; Gitto, E. Potential Utility of Melatonin in Preeclampsia, Intrauterine Fetal Growth Retardation, and Perinatal Asphyxia. Reprod. Sci. 2016, 23, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Cornu-Labat, G.; Serra, M.; Smith, A.; McGregor, W.E.; Kasirajan, K.; Hirko, M.K.; Turner, J.J.; Rubin, J.R. Systemic consequences of oxidative stress fing aortic surgery correlate with the degree of antioxidant defenses. Ann. Vasc. Surg. 2000, 14, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Rulli, I.; Salvo, V.; Buonocore, G.; Reiter, R.J.; Gitto, E. Melatonin Secretion Is Increased in Children with Severe Traumatic Brain Injury. Int. J. Mol. Sci. 2017, 18, 1053. [Google Scholar] [CrossRef] [Green Version]
- Gögenur, I.; Ocak, U.; Altunpinar, O.; Middleton, B.; Skene, D.J.; Rosenberg, J. Disturbances in melatonin, cortisol and core body temperature rhythms after major surgery. World J. Surg. 2007, 31, 290–298. [Google Scholar] [CrossRef]
- Andersen, L.P.; Kücükakin, B.; Werner, M.U.; Rosenberg, J.; Gögenur, I. Absence of analgesic effect of intravenous melatonin administration during daytime after laparoscopic cholecystectomy: A randomized trial. J. Clin. Anesth. 2014, 26, 545–550. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Stamp, G.E.; Goble, F.C. Development of melatonin production in infants and the impact of prematurity. J. Clin. Endocrinol. Metab. 1992, 75, 367–369. [Google Scholar]
- Dragoumi, M.; Dragoumis, D.; Karatzoglou, S.; Spiridakis, I.; Chitoglou-Makedou, A.; Giakoumettis, G.; Alexidis, P.; Tremmas, I.; Papageorgiou, I.; Drevelegas, K.; et al. The Fluctuations of Melatonin and Copeptin Levels in Blood Serum During Surgical Stress Regarding the Pediatric Population. Curr. Pediatr. Rev. 2021, 17, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Yoshitaka, S.; Egi, M.; Morimatsu, H.; Kanazawa, T.; Toda, Y.; Morita, K. Perioperative plasma melatonin concentration in postoperative critically ill patients: Its association with delirium. J. Crit. Care 2013, 28, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Reber, A.; Huber, P.R.; Ummenhofer, W.; Gürtler, C.M.; Zurschmiede, C.; Drewe, J.; Schneider, M. General anaesthesia for surgery can influence circulating melatonin during daylight hours. Acta Anaesthesiol. Scand. 1998, 42, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Barzegari, M.; Aghapour, B.; Adeli, S.; Khademi, F.; Musazadeh, V.; Jamilian, P.; Jamilian, P.; Fakhr, L.; Chehregosha, F.; et al. Melatonin effectiveness in amelioration of oxidative stress and strengthening of antioxidant defense system: Findings from a systematic review and dose-response meta-analysis of controlled clinical trials. Clin. Nutr. ESPEN 2022, 48, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Gitto, E.; Marseglia, L.; D’Angelo, G.; Manti, S.; Crisafi, C.; Montalto, A.S.; Impellizzeri, P.; Reiter, R.J.; Romeo, C. Melatonin versus midazolam premedication in children undergoing surgery: A pilot study. J. Paediatr. Child Health 2016, 52, 291–295. [Google Scholar] [CrossRef]
- Marseglia, L.; Gitto, E.; Laschi, E.; Giordano, M.; Romeo, C.; Cannavò, L.; Toni, A.L.; Buonocore, G.; Perrone, S. Antioxidant Effect of Melatonin in Preterm Newborns. Oxid. Med. Cell. Longev. 2021, 2021, 6308255. [Google Scholar] [CrossRef]
- Balduini, W.; Weiss, M.D.; Carloni, S.; Rocchi, M.; Sura, L.; Rossignol, C.; Longini, M.; Bazzini, F.; Perrone, S.; Ott, D.; et al. Melatonin pharmaco- kinetics and dose extrapolation after enteral infusion in neonates subjected to hypothermia. J. Pineal Res. 2019, 66, e12565. [Google Scholar] [CrossRef]
- Perrone, S.; Longini, M.; Marzocchi, B.; Picardi, A.; Bellieni, C.V.; Proietti, F.; Rodriguez, A.; Turrisi, G.; Buonocore, G. Effects of lutein on oxidative stress in the term newborn: A pilot study. Neonatology 2010, 97, 36–40. [Google Scholar] [CrossRef]
- Perrone, S.; Tei, M.; Longini, M.; Santacroce, A.; Turrisi, G.; Proietti, F.; Felici, C.; Picardi, A.; Bazzini, F.; Vasarri, P.; et al. Lipid and protein oxidation in newborn infants after lutein administration. Oxid. Med. Cell. Longev. 2014, 2014, 781454. [Google Scholar] [CrossRef] [Green Version]
- Carloni, S.; Proietti, F.; Rocchi, M.; Longini, M.; Marseglia, L.; D’Angelo, G.; Balduini, W.; Gitto, E.; Buonocore, G. Melatonin Pharmacokinetics Following Oral Administration in Preterm Neonates. Molecules 2017, 22, 2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manti, S.; Licari, A. How to obtain informed consent for research. Breathe 2018, 14, 145–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.P.; Gögenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef]
- Wang, A.Q.; Wei, B.P.; Zhang, Y.; Wang, Y.J.; Xu, L.; Lan, K. An ultra-high sensitive bioanalytical method for plasma melatonin by liquid chromatography-tandem mass spectrometry using water as calibration matrix. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2259–2264. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casetta, B.; Longini, M.; Proietti, F.; Perrone, S.; Buonocore, G. Development of a fast and simple LC-MS/ MS method for measuring the F2-isoprostanes in newborns. J. Matern. Fetal Neonatal Med. 2012, 25, 114–118. [Google Scholar] [CrossRef]
- Paffetti, P.; Perrone, S.; Longini, M.; Ferrari, A.; Tanganelli, D.; Marzocchi, B.; Buonocore, G. Non-protein-bound iron detection in small samples of biological fluids and tissues. Biol. Trace Elem. Res. 2006, 112, 221–232. [Google Scholar] [CrossRef]
- Marseglia, L.; D’Angelo, G.; Manti, S.; Aversa, S.; Reiter, R.J.; Antonuccio, P.; Centorrino, A.; Romeo, C.; Impellizzeri, P.; Gitto, E. Oxidative Stress-Mediated Damage in Newborns with Necrotizing Enterocolitis: A Possible Role of Melatonin. Am. J. Perinatol. 2015, 32, 905–909. [Google Scholar]
- Gögenur, I. Postoperative circadian disturbances. Dan. Med. Bull. 2010, 57, B4205. [Google Scholar]
- Yin, Y.Q.; Luo, A.L.; Guo, X.Y.; Li, L.H.; Ren, H.Z.; Ye, T.H.; Huang, Y.G. Perioperative melatonin circadian secretion in patients undergoing coronary artery bypass grafting surgery. Zhonghua Yi Xue Za Zhi 2004, 84, 456–459. [Google Scholar]
- Cronin, A.J.; Keifer, J.C.; Davies, M.F.; King, T.S.; Bixler, E.O. Melatonin secretion after surgery. Lancet 2000, 356, 1244–1245. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Luo, A.L.; Ren, H.Z.; Yie, T.H.; Huang, Y.G. Perioperative melatonin secretion rhyme in patients undergoing coronary artery bypass grafting surgery. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2003, 25, 594–598. [Google Scholar]
- Kennaway, D.J.; Hugel, H.M. Melatonin binding sites: Are they receptors? Mol. Cell Endocrinol. 1992, 88, C1–C9. [Google Scholar] [CrossRef]
- Sadeh, A. Sleep and melatonin in infants: A preliminary study. Sleep 1997, 20, 185–191. [Google Scholar] [PubMed]
- Perrone, S.; Giordano, M.; De Bernardo, G.; Lugani, P.; Sarnacchiaro, P.; Stazzoni, G.; Buonocore, G.; Esposito, S.; Tataranno, M.L.; National Study Group of Neonatal Clinical Biochemistry of the Italian Society of Neonatology. Management of oxygen saturation monitoring in preterm newborns in the NICU: The Italian picture. Ital. J. Pediatr. 2021, 47, 104. [Google Scholar] [CrossRef]
- Bellieni, C.V.; Tei, M.; Cornacchione, S.; Di Lucia, S.; Nardi, V.; Verrotti, A.; Buonocore, G. Pain perception in NICU: A pilot questionnaire. J. Matern. Fetal Neonatal Med. 2018, 31, 1921–1923. [Google Scholar] [CrossRef] [PubMed]
- Slater, L.; Asmerom, Y.; Boskovic, D.S.; Bahjri, K.; Plank, M.S.; Angeles, K.R.; Phillips, R.; Deming, D.; Ashwal, S.; Hougland, K.; et al. Procedural pain and oxidative stress in premature neonates. J. Pain 2012, 13, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Tortora, D.; Severino, M.; Di Biase, C.; Malova, M.; Parodi, A.; Minghetti, D.; Traggiai, C.; Uccella, S.; Boeri, L.; Morana, G.; et al. Early pain exposure influences functional brain connectivity in very preterm neonates. Front. Neurosci. 2019, 13, 899. [Google Scholar] [CrossRef]
- Chau, C.M.Y.; Ranger, M.; Bichin, M.; Park, M.T.; Amaral, R.S.C.; Chakravarty, M.; Poskitt, K.; Synnes, A.R.; Miller, S.; Grunau, R.E. Hippocampus, amygdala, and thalamus volumes in very preterm children at 8 years: Neonatal pain and genetic variation. Front. Behav. Neurosci. 2019, 13, 51. [Google Scholar] [CrossRef]
- Gitto, E.; Aversa, S.; Salpietro, C.D.; Barberi, I.; Arrigo, T.; Trimarchi, G.; Reiter, R.J.; Pellegrino, S. Pain in neonatal intensive care: Role of melatonin as an analgesic antioxidant. J. Pineal Res. 2012, 52, 291–295. [Google Scholar] [CrossRef]
- Marseglia, L.; Aversa, S.; Barberi, I.; Salpietro, C.D.; Cusumano, E.; Speciale, A.; Saija, A.; Romeo, C.; Trimarchi, G.; Reiter, R.J.; et al. High endogenous melatonin levels in critically ill children: A pilot study. J. Pediatr. 2013, 162, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Kücükakin, B.; Wilhelmsen, M.; Lykkesfeldt, J.; Reiter, R.J.; Rosenberg, J.; Gögenur, I. No effect of melatonin to modify surgical-stress response after major vascular surgery: A randomised placebo-controlled trial. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 461–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longini, M.; Belvisi, E.; Proietti, F.; Bazzini, F.; Buonocore, G.; Perrone, S. Oxidative Stress Biomarkers: Establishment of Reference Values for Isoprostanes, AOPP, and NPBI in Cord Blood. Mediat. Inflamm. 2017, 2017, 1758432. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Laschi, E.; Buonocore, G. Oxidative stress biomarkers in the perinatal period: Diagnostic and prognostic value. Semin. Fetal Neonatal Med. 2020, 25, 101087. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Negro, S.; Laschi, E.; Calderisi, M.; Giordano, M.; De Bernardo, G.; Parigi, G.; Toni, A.L.; Esposito, S.; Buonocore, G. Metabolomic Profile of Young Adults Born Preterm. Metabolites 2021, 11, 697. [Google Scholar] [CrossRef]


| MEL * Group (n = 10) | Untreated Group (n = 13) | p-Value | |
|---|---|---|---|
| Gestational Age (wks) * | 38.26 ± 3.40 | 37.13 ± 2.64 | NS * |
| Birth weight (g) * | 2920.10 ± 635.23 | 2865.35 ± 498.34 | NS |
| Gender (F;M) * | 6 (F); 4 (M) | 8 (F); 5 (M) | NS |
| Age at surgery (days of life) | 10 ± 7 | 7 ± 5 | NS |
| Average no. of minutes before surgery (time of sampling) | 240 ± 85 | 255 ± 45 | NS |
| Average time at intervention (melatonin or placebo, hours) | 8 ± 1.5 | 8 ± 2 | NS |
| Enterostomy | 4 | 6 | -- |
| Omphalocele | 1 | 0 | -- |
| Meconium ileus | 2 | 3 | -- |
| Severe hydronephrosis | 1 | 2 | -- |
| Sacrococcygeal teratoma | 0 | 1 | -- |
| Intestinal duplication | 1 | 1 | -- |
| Biomarker | Untreated | Mel | p-Value |
|---|---|---|---|
| NPBI T0 | 3.24 (2.45); 2.99 (1.05–5.66) | 4.69 (3.85); 3.24 (1.20–8.89) | ns |
| NPBI T1 | 1.70 (1.32); 1.19 (0.69–2.43) § | 1.65 (1.18); 1.71 (0.43–2.77) * | ns |
| F2–IsoPs T0 | 57.35 (59.92); 33.90 (16.60–110.80) | 128.40 (92.30); 112.00 (51.00–235.22) | 0.035 |
| F2–IsoPs T1 | 29.47 (43.17); 18.30 (1.91–35.55) | 50.25 (47.47); 47.75 (3.51–88.70) ** | ns |
| AOPP T0 | 67.83 (23.18); 58.70 (51.28–90.78) | 65.18 (15.50); 62.89 (52.89–76.50) | ns |
| AOPP T1 | 52.84 (14.13); 51.82 (42.17–62.83) | 43.98 (17.92); 42.33 (32.52–61.01) *** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, S.; Romeo, C.; Marseglia, L.; Manti, S.; Rizzo, C.; Carloni, S.; Albertini, M.C.; Balduini, W.; Buonocore, G.; Weiss, M.D.; et al. Melatonin in Newborn Infants Undergoing Surgery: A Pilot Study on Its Effects on Postoperative Oxidative Stress. Antioxidants 2023, 12, 563. https://doi.org/10.3390/antiox12030563
Perrone S, Romeo C, Marseglia L, Manti S, Rizzo C, Carloni S, Albertini MC, Balduini W, Buonocore G, Weiss MD, et al. Melatonin in Newborn Infants Undergoing Surgery: A Pilot Study on Its Effects on Postoperative Oxidative Stress. Antioxidants. 2023; 12(3):563. https://doi.org/10.3390/antiox12030563
Chicago/Turabian StylePerrone, Serafina, Carmelo Romeo, Lucia Marseglia, Sara Manti, Cristina Rizzo, Silvia Carloni, Maria Cristina Albertini, Walter Balduini, Giuseppe Buonocore, Michael D. Weiss, and et al. 2023. "Melatonin in Newborn Infants Undergoing Surgery: A Pilot Study on Its Effects on Postoperative Oxidative Stress" Antioxidants 12, no. 3: 563. https://doi.org/10.3390/antiox12030563
APA StylePerrone, S., Romeo, C., Marseglia, L., Manti, S., Rizzo, C., Carloni, S., Albertini, M. C., Balduini, W., Buonocore, G., Weiss, M. D., & Gitto, E. (2023). Melatonin in Newborn Infants Undergoing Surgery: A Pilot Study on Its Effects on Postoperative Oxidative Stress. Antioxidants, 12(3), 563. https://doi.org/10.3390/antiox12030563










