Omega-Class Glutathione Transferases Protect DNA from Oxidative Stress in Pathogenic Helminth Reproductive Cells
Abstract
1. Introduction
2. Results
2.1. CsGSTOs Catalyze Both Glutathionylation and Deglutathionylation
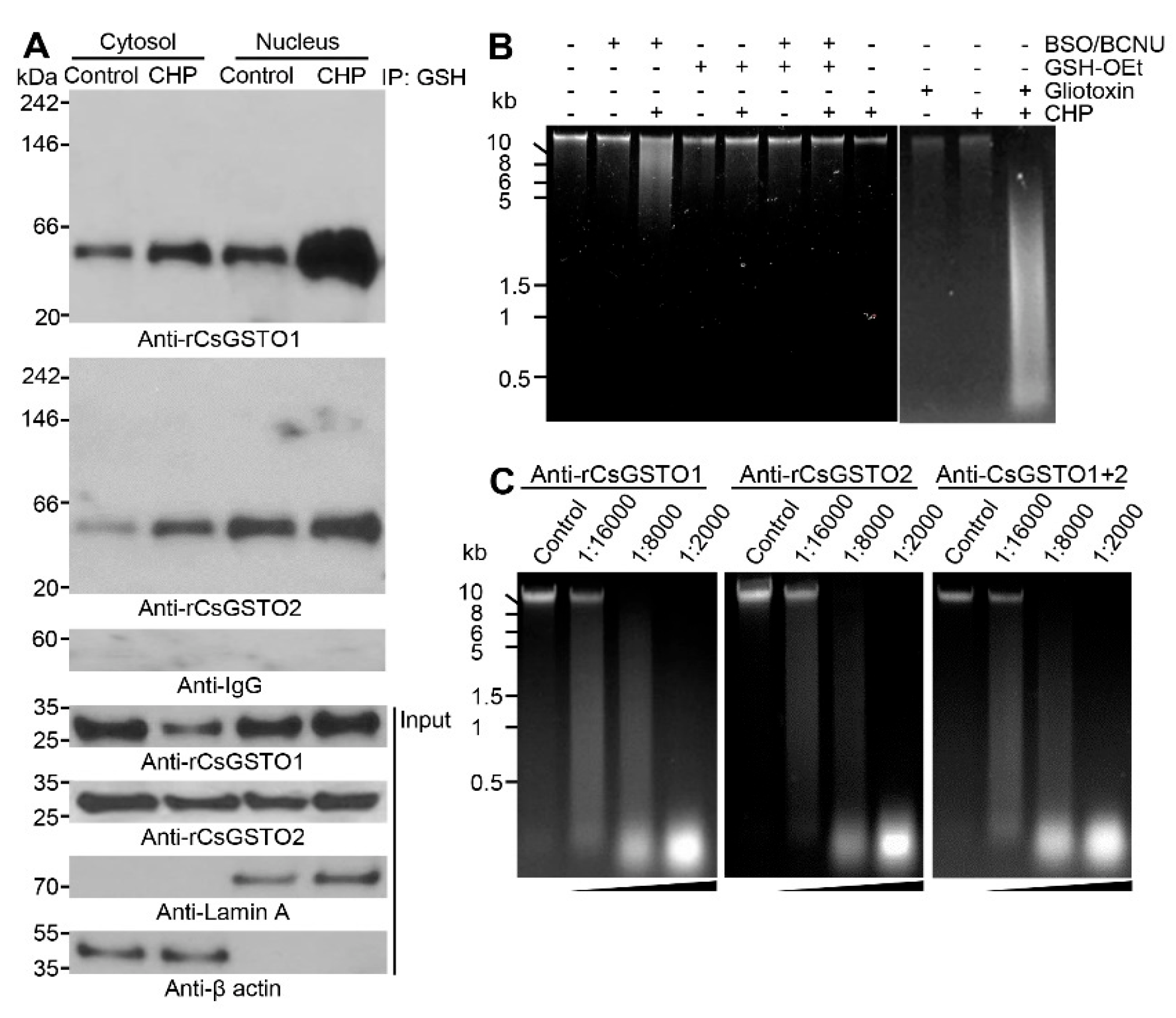
2.2. Cytosolic CsGSTOs Translocate to the Nucleus upon Oxidative Stimulation
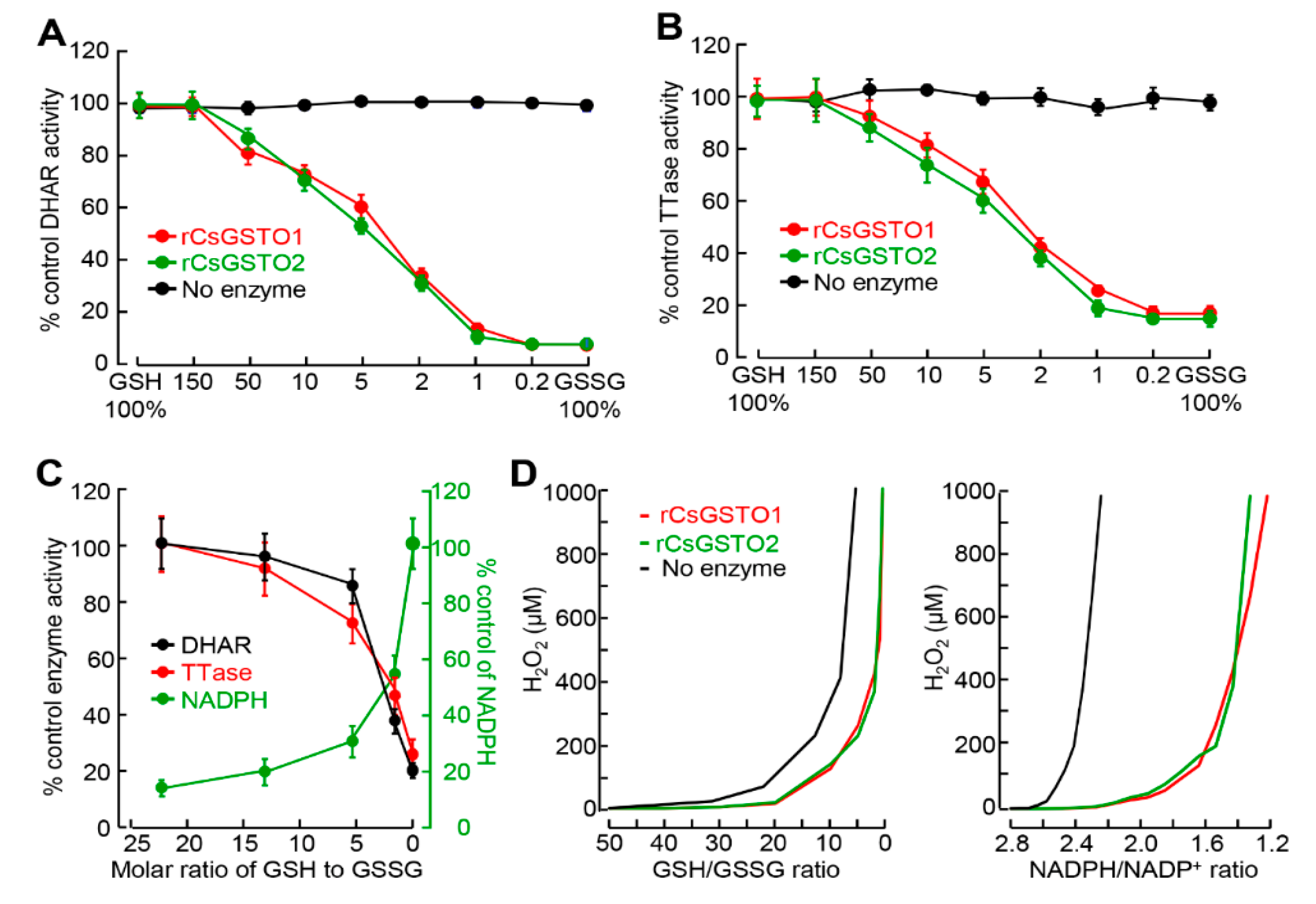
2.3. GSH and NADPH Redox Modify CsGSTO Function
2.4. The Nucleus Is Susceptible to Oxidative Stress and GSH Is Critical for Nuclear Transport of CsGSTOs
2.5. Gliotoxin Modulates CsGSTO Function
2.6. Nuclear Accumulation of Glutathionylated CsGSTOs Prevents Oxidative DNA Damage
3. Discussion
4. Materials and Methods
4.1. Parasite
4.2. Chemical Treatment and Sample Preparation
4.3. Recombinant Proteins and Specific Antibodies
4.4. Tryptophan Quenching Assay
4.5. GSTO Enzyme Assay
4.6. NADPH/NADP+ Ratio Measurement
4.7. Determination of GSH and GSSG Levels, and GSH/GSSG Ratio
4.8. PSSG Measurement
4.9. Quantitative Real-Time RT-PCR (qRT-PCR)
4.10. Western Blot
4.11. Immunoprecipitation
4.12. Amplex Ultra Red Assay
4.13. DNA Protection Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–58. [Google Scholar] [CrossRef]
- Laborde, E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ. 2010, 17, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.S.; Kim, J.G.; Kang, I.; Kong, Y. Omega-class glutathione transferases of carcinogenic liver fluke, Clonorchis sinensis, modulate apoptosis and differentiation of host cholangiocytes. Antioxidants 2021, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ketterer, B.; Tan, K.H.; Meyer, D.J.; Coles, B. Glutathione transferase: A possible role in the detoxification of DNA and lipid hydroperoxides. In Glutathione S-Transferase and Carcinogenesis, 1st ed.; Mantle, T.J., Pickett, D.B., Hayes, J.D., Eds.; Taylor and Francis: London, UK, 1987; pp. 149–163. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Kamada, K.; Goto, S.; Okunaga, T.; Ihara, Y.; Tsuji, K.; Kawai, Y.; Uchida, K.; Osawa, T.; Matsuo, T.; Nagata, I.; et al. Nuclear glutathione S-transferase pi prevents apoptosis by reducing the oxidative stress-induced formation of exocyclic DNA products. Free Radic. Biol. Med. 2004, 37, 1875–1884. [Google Scholar] [CrossRef]
- Goričar, K.; Kovač, V.; Jazbec, J.; Zakotnik, B.; Lamovec, J.; Dolžan, V. Genetic variability of DNA repair mechanisms and glutathione-S-transferase genes influences treatment outcome in osteosarcoma. Cancer Epidemiol. 2015, 10, 182–188. [Google Scholar] [CrossRef]
- Board, P.G.; Coggan, M.; Chelvanayagam, G.; Easteal, S.; Jermiin, L.S.; Schulte, G.K.; Danley, D.E.; Hoth, L.R.; Griffor, M.C.; Kamath, A.V.; et al. Identification, characterization, and crystal structure of the omega class glutathione transferase. J. Biol. Chem. 2000, 275, 24798–24806. [Google Scholar] [CrossRef]
- Board, P.G. The omega-class glutathione transferases: Structure, function, and genetics. Drug Metab. Rev. 2011, 43, 226–235. [Google Scholar] [CrossRef]
- Menon, D.; Board, P.G. A role for glutathione transferase Omega 1 (GSTO1-1) in the glutathionylation cycle. J. Biol. Chem. 2013, 288, 25769–25779. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, G.; Giustarini, D.; Milzani, A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem. Sci. 2009, 34, 85–96. [Google Scholar] [CrossRef]
- Whitbread, A.K.; Masoumi, M.; Tetlow, N.; Schmuck, E.; Coggan, M.; Board, P.G. Characterization of the omega class of glutathione transferases. Methods Enzymol. 2005, 401, 78–99. [Google Scholar] [PubMed]
- Torgerson, P.R.; Devleesschauwer, B.; Praet, N.; Speybroeck, N.; Willingham, A.L.; Kasuga, F.; Rokni, M.B.; Zhou, X.N.; Fèvre, E.M.; Sripa, B.; et al. World health organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001920. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.B.; Utzinger, J.; Keiser, J.; Zhou, X.N. Clonorchiasis. Lancet 2016, 387, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factor. Liver Int. 2019, 39, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. WHO International Agency for Research on Cancer Monograph Working Group, A review of human carcinogens-Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Lancet Oncol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.S.; Hong, S.M.; Lee, K.T.; Lee, J.G.; Choi, C.H.; Heo, J.S.; Choi, D.W.; Choi, D.; Lim, J.H. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008, 543, 589–598. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Adams, A.M. Foodborne trematodes. In Foodborne Parasites, 1st ed.; Ortega, Y.R., Ed.; Springer: Berlin, Germany, 2006; pp. 168–173. [Google Scholar]
- Lechner, S.; Müller-Ladner, U.; Schlottmann, K.; Jung, B.; McClelland, M.; Rüschoff, J.; Welsh, J.; Schölmerich, J.; Kullmann, F. Bile acids mimic oxidative stress induced upregulation of thioredoxin reductase in colon cancer cell lines. Carcinogenesis 2002, 23, 1281–1288. [Google Scholar] [CrossRef]
- Bae, Y.A.; Kim, J.G.; Kong, Y. Phylogenetic characterization of Clonorchis sinensis proteins homologous to the sigma-class glutathione transferase and their differential expression profiles. Mol. Biochem. Parasitol. 2016, 206, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.A.; Ahn, D.W.; Lee, E.G.; Kim, S.H.; Cai, G.B.; Kang, I.; Sohn, W.M.; Kong, Y. Differential activation of diverse glutathione transferases of Clonorchis sinensis in response to the host bile and oxidative stressors. PLoS Negl. Trop. Dis. 2013, 7, e2211. [Google Scholar] [CrossRef]
- Kim, J.G.; Ahn, C.S.; Kim, S.H.; Bae, Y.A.; Kwon, N.Y.; Kang, I.; Yang, H.J.; Sohn, W.M.; Kong, Y. Clonorchis sinensis omega-class glutathione transferases play major roles in the protection of the reproductive system during maturation and the response to oxidative stress. Parasit. Vectors 2016, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Colombo, R.; Petralia, S.; Giampaoletti, S.; Milzani, A.; Rossi, R. Protein glutathionylation in erythrocytes. Clin. Chem. 2003, 49, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Anathy, V.; Lahue, K.G.; Chapman, D.G.; Chia, S.B.; Casey, D.T.; Aboushousha, R.; van der Velden, J.L.J.; Elko, E.; Hoffman, S.M.; McMillan, D.H.; et al. Reducing protein oxidation reverses lung fibrosis. Nat. Med. 2018, 24, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.D.; Bridge, W.J. The glutathione system and the related thiol network in Caenorhabditis elegans. Redox. Biol. 2019, 24, 101171. [Google Scholar] [CrossRef] [PubMed]
- Townsend, D.M.; Manevich, Y.; He, L.; Hutchens, S.; Pazoles, C.J.; Tew, K.D. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009, 284, 436–445. [Google Scholar] [CrossRef]
- Scharf, D.H.; Brakhage, A.A.; Mukherjee, P.K. Gliotoxin-bane or boon? Environ. Microbiol. 2016, 18, 1096–1109. [Google Scholar] [CrossRef]
- Couto, N.; Malys, N.; Gaskell, S.J.; Barber, J. Partition and turnover of glutathione reductase from Saccharomyces cerevisiae: A proteomic approach. J. Proteome Res. 2013, 12, 2885–2894. [Google Scholar] [CrossRef]
- Paardekooper, L.M.; van Vroonhoven, E.; Beest, M.T.; van den Bogaart, G. Radical stress is more cytotoxic in the nucleus than in other organelles. Int. J. Mol. Sci. 2019, 20, 4147. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Structure, function and disease relevance of Omega-class glutathione transferases. Arch. Toxicol. 2016, 90, 1049–1067. [Google Scholar] [CrossRef]
- Xiao, Z.; La Fontaine, S.; Bush, A.I.; Wedd, A.G. Molecular mechanisms of glutaredoxin enzymes: Versatile hubs for thiol-disulfide exchange between protein thiols and glutathione. J. Mol. Biol. 2019, 431, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.; Pal, R.; Qu, Y.; Liu, X.; Yu, H.; Shiao, S.L.; Wang, X.; Smith, E.O.; Cui, X.; Rodney, G.G.; et al. Nuclear glutaredoxin 3 is critical for protection against oxidative stress-induced cell death. Free Radic. Biol. Med. 2015, 85, 197–206. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Zhao, S.; Xu, W.; Li, Y.; Zhao, P.; Wang, D.; Cheng, H.; Ke, Y.; Zhang, X. Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat. Commun. 2012, 12, 7094. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Jakhar, R.; Bhardwaj, M.; Kang, C.S. Glutathione-S-transferase omega 1 (GSTO1-1) acts as mediator of signaling pathways involved in aflatoxin B1-induced apoptosis-autophagy crosstalk in macrophages. Free Radic. Biol. Med. 2015, 89, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Belcastro, E.; Gaucher, C.; Corti, A.; Leroy, P.; Lartaud, I.; Pompella, A. Regulation of protein function by S-nitrosation and S-glutathionylation: Processes and targets in cardiovascular pathophysiology. J. Biol. Chem. 2017, 398, 1267–1293. [Google Scholar] [CrossRef]
- Safaeipour, M.; Jauregui, J.; Castillo, S.; Bekarian, M.; Esparza, D.; Sanchez, M.; Stemp, E.D.A. Glutathione directly intercepts DNA radicals to inhibit oxidative DNA-protein cross-linking induced by the one-electron oxidation of guanine. Biochemistry 2019, 58, 4621–4631. [Google Scholar] [CrossRef]
- Burmeister, C.; Lüersen, K.; Heinick, A.; Hussein, A.; Domagalski, M.; Walter, R.D.; Liebau, E. Oxidative stress in Caenorhabditis elegans: Protective effects of the Omega class glutathione transferase (GSTO-1). FASEB J. 2008, 22, 343–354. [Google Scholar] [CrossRef]
- Adam, G.C.; Sorensen, E.J.; Cravatt, B.F. Proteomic profiling of mechanistically distinct enzyme classes using a common chemotype. Nat. Biotechnol. 2002, 20, 805–809. [Google Scholar] [CrossRef]
- Djukic, T.; Simic, T.; Pljesa-Ercegovac, M.; Matic, M.; Suvakov, S.; Coric, V.; Dragicevic, D.; Savic-Radojevic, A. Upregulated glutathione transferase omega-1 correlates with progression of urinary bladder carcinoma. Redox. Rep. 2017, 22, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Manupati, K.; Debnath, S.; Goswami, K.; Bhoj, P.S.; Chanda, H.S.; Bahekar, S.P.; Das, A. Glutathione S-transferase omega 1 inhibition activates JNK-mediated apoptotic response in breast cancer stem cells. FEBS J. 2019, 286, 2167–2192. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, Y.; Sato, Y.; Takanashi, K.; Hayashi, T.; Kubo-Birukawa, N.; Shimizu, F.; Fujitani, N.; Shimoyama, R.; Kukitsu, T.; Kurata, W.; et al. A CRAF/glutathione-S-transferase P1 complex sustains autocrine growth of cancers with KRAS and BRAF mutations. Proc. Natl. Acad. Sci. USA 2020, 117, 19435–19445. [Google Scholar] [CrossRef] [PubMed]
- Young, N.D.; Nagarajan, N.; Lin, S.J.; Korhonen, P.K.; Jex, A.R.; Hall, R.S.; Safavi-Hemami, H.; Kaewkong, W.; Bertrand, D.; Gao, S.; et al. The Opisthorchis viverrini genome provides insights into life in the bile duct. Nat. Commun. 2014, 5, 4378. [Google Scholar] [CrossRef]
- Kim, J.G.; Ahn, C.S.; Sripa, B.; Eom, K.S.; Kang, I.; Sohn, W.M.; Nawa, Y.; Kong, Y. Clonorchis sinensis omega-class glutathione transferases are reliable biomarkers for serodiagnosis of clonorchiasis and opisthorchiasis. Clin. Microbiol. Infect. 2019, 25, 109.e1–109.e6. [Google Scholar] [CrossRef]
- Gouveia, M.J.; Pakharukova, M.Y.; Laha, T.; Sripa, B.; Maksimova, G.A.; Rinaldi, G.; Brindley, P.J.; Mordvinov, V.A.; Amaro, T.; Santos, L.L.; et al. Infection with Opisthorchis felineus induces intraepithelial neoplasia of the biliary tract in a rodent model. Carcinogenesis 2017, 38, 929–937. [Google Scholar] [CrossRef]
- Sripa, B.; Tangkawattana, S.; Brindley, P.J. Update on pathogenesis of opisthorchiasis and cholangiocarcinoma. Adv. Parasitol. 2018, 102, 97–113. [Google Scholar]
- Pakharukova, M.Y.; Zaparina, O.G.; Kapushchak, Y.K.; Baginskaya, N.V.; Mordvinov, V.A. Opisthorchis felineus infection provokes time-dependent accumulation of oxidative hepatobiliary lesions in the injured hamster liver. PLoS ONE 2019, 14, e0216757. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Prim. 2021, 7, 65. [Google Scholar] [CrossRef]
- Petoniemi, M.; Karala, A.R.; Jurvansuu, J.K.; Kinnula, V.L.; Ruddock, L.W. Insights into deglutathionylation reactions. Different intermediates in the glutaredoxin and protein disulfide isomerase catalyzed reactions are defined by the gamma-linkage present in glutathione. J. Biol. Chem. 2006, 281, 33107–33114. [Google Scholar]
- Livak, K.L.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-G.; Kang, I.; Ahn, C.-S.; Sohn, W.-M.; Kong, Y. Omega-Class Glutathione Transferases Protect DNA from Oxidative Stress in Pathogenic Helminth Reproductive Cells. Antioxidants 2023, 12, 560. https://doi.org/10.3390/antiox12030560
Kim J-G, Kang I, Ahn C-S, Sohn W-M, Kong Y. Omega-Class Glutathione Transferases Protect DNA from Oxidative Stress in Pathogenic Helminth Reproductive Cells. Antioxidants. 2023; 12(3):560. https://doi.org/10.3390/antiox12030560
Chicago/Turabian StyleKim, Jeong-Geun, Insug Kang, Chun-Seob Ahn, Woon-Mok Sohn, and Yoon Kong. 2023. "Omega-Class Glutathione Transferases Protect DNA from Oxidative Stress in Pathogenic Helminth Reproductive Cells" Antioxidants 12, no. 3: 560. https://doi.org/10.3390/antiox12030560
APA StyleKim, J.-G., Kang, I., Ahn, C.-S., Sohn, W.-M., & Kong, Y. (2023). Omega-Class Glutathione Transferases Protect DNA from Oxidative Stress in Pathogenic Helminth Reproductive Cells. Antioxidants, 12(3), 560. https://doi.org/10.3390/antiox12030560





