Polyphenol Profile of Cistus × incanus L. and Its Relevance to Antioxidant Effect and α-Glucosidase Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reference Materials
2.2. General Equipment
2.3. Plant Material and Extracts
2.4. Preparative Separation of C. Incanus Flavonols
2.5. UHPLC-ESI-qTOF-MS and HPLC-DAD Experiments
2.6. Total Phenolic (TPC) and Flavonoid (TFC) Content
2.7. Antioxidant Activity Tests
2.7.1. DPPH and ABTS
2.7.2. FRAP
2.8. α-Glucosidase Inhibitory Assay
2.9. Kinetics of α-Glucosidase Inhibition
2.10. Statistical Analysis
3. Results and Discussion
3.1. C. Incanus Polyphenols
3.2. Quantification of Polyphenols, Flavonoids, Tannins, and Phenolic Acids in C. Incanus
3.2.1. Quantification of TPC and TFC
3.2.2. Quantification of Polyphenols, Flavonoids, Tannins, and Phenolic Acids by HPLC-DAD
3.3. In Vitro Antioxidant Potential of C. incanus
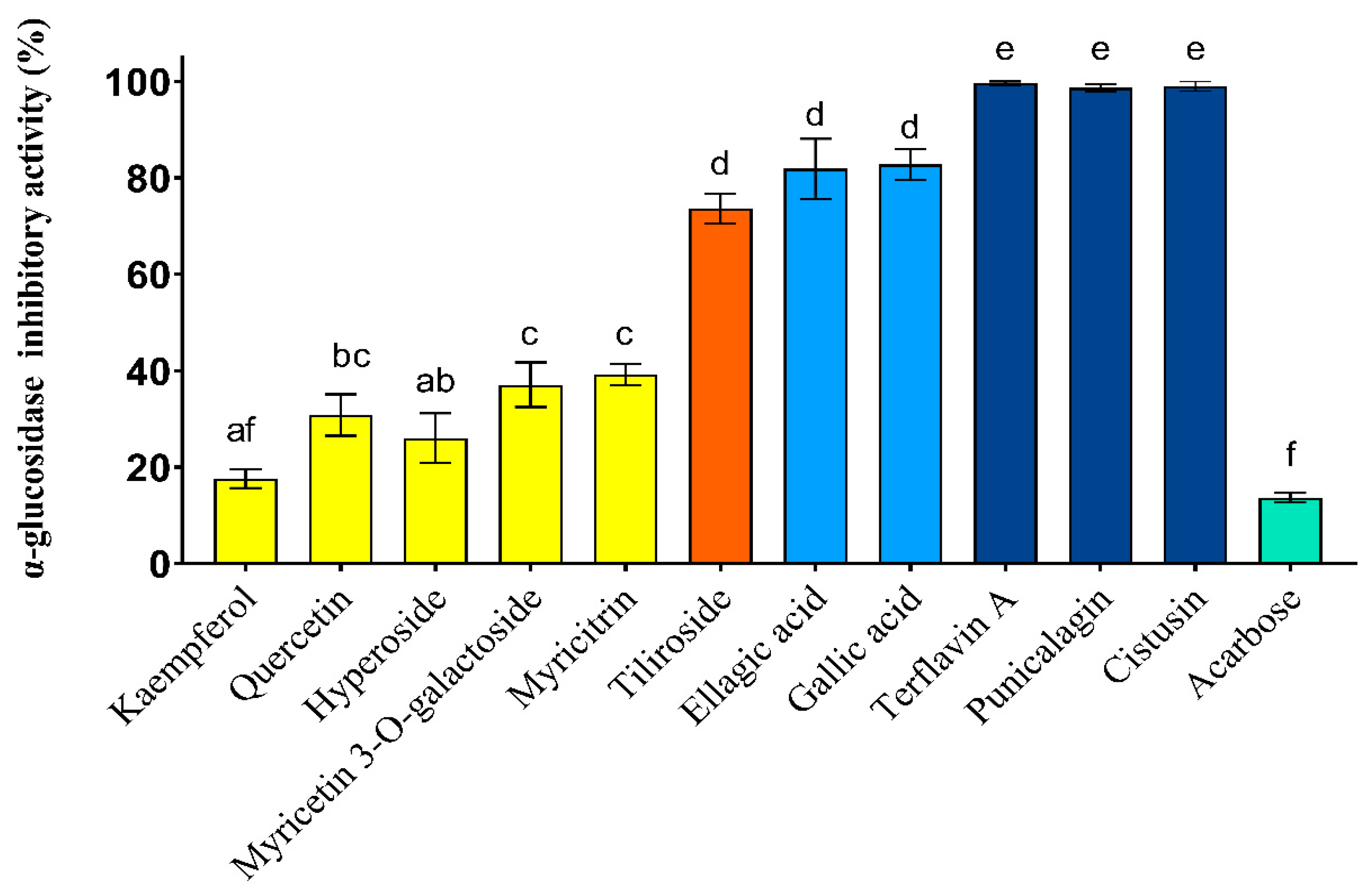
3.4. In Vitro α-Glucosidase Inhibitory Activity of C. incanus Extracts and Their Constituents
3.5. Mode of α-Glucosidase Inhibition by C. incanus Polyphenols and Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific opinion on the substantiation of health claims related to various food(S)/food constituent(s) and protection of cells from premature aging, antioxidant activity, antioxidant content and antioxidant properties, and protection of DNA, proteins and lipids from oxidative damage pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA J. 2010, 8, 1489. [Google Scholar] [CrossRef]
- Guzmán, B.; Vargas, P. Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences. Org. Divers. Evol. 2009, 9, 83–99. [Google Scholar] [CrossRef]
- Čarni, A.; Matevski, V.; Šilc, U. Morphological, chorological and ecological plasticity of Cistus incanus in the southern Balkans. Plant Biosyst. 2010, 144, 602–617. [Google Scholar] [CrossRef]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef]
- Tomou, E.-M.; Lytra, K.; Rallis, S.; Tzakos, A.G.; Skaltsa, H. An updated review of genus Cistus L. since 2014: Traditional uses, phytochemistry, and pharmacological properties. Phytochem. Rev. 2022, 21, 2049–2087. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus × incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Pennisi, G.; Attaguile, G.; Savoca, F.; Tita, B. Antiproliferative and cytotoxic activity of extracts from Cistus incanus L. and Cistus monspeliensis L. on human prostate cell lines. Nat. Prod. Res. 2011, 25, 188–202. [Google Scholar] [CrossRef]
- Bernacka, K.; Bednarska, K.; Starzec, A.; Mazurek, S.; Fecka, I. Antioxidant and antiglycation effects of Cistus × incanus water infusion, its phenolic components, and respective metabolites. Molecules 2022, 27, 2432. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Şüküroğlu, M.; Deliorman Orhan, D. In vivo and in vitro antidiabetic effect of Cistus laurifolius L. and detection of major phenolic compounds by UPLC-TOF-MS analysis. J. Ethnopharmacol. 2013, 146, 859–865. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef]
- Funke, I.; Melzig, M.F. Effect of different phenolic compounds on α-amylase activity: Screening by microplate-reader based kinetic assay. Pharmazie 2005, 60, 796–797. [Google Scholar] [PubMed]
- Gori, A.; Nascimento, L.B.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and diurnal variation in leaf phenolics of three medicinal mediterranean wild species: What is the best harvesting moment to obtain the richest and the most antioxidant extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef] [PubMed]
- Dimcheva, V.; Kaloyanov, N.; Karsheva, M. The polyphenol composition of Cistus incanus L., Trachystemon orientalis L. and Melissa officinalis L. infusions by HPLC-DAD method. Open J. Anal. Bioanal. Chem. 2019, 3, 31–38. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Ekiert, H. In vitro shoot cultures of pink rock-rose (Cistus × incanus L.) as a potential source of phenolic compounds. Acta Soc. Bot. Pol. 2017, 86. [Google Scholar] [CrossRef]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, C. The polyphenolic composition of Cistus incanus herbal tea and its antibacterial and anti-adherent activity against Streptococcus mutans. Planta Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef]
- Gori, A.; Ferrini, F.; Marzano, M.; Tattini, M.; Centritto, M.; Baratto, M.; Pogni, R.; Brunetti, C. Characterisation and antioxidant activity of crude extract and polyphenolic rich fractions from C. incanus leaves. Int. J. Mol. Sci. 2016, 17, 1344. [Google Scholar] [CrossRef] [PubMed]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Frankowski, R. Cistus incanus a promising herbal tea rich in bioactive compounds: LC–MS/MS determination of catechins, flavonols, phenolic acids and alkaloids—A comparison with Camellia sinensis, Rooibos and Hoan Ngoc herbal tea. J. Food Compos. Anal. 2018, 74, 71–81. [Google Scholar] [CrossRef]
- Fecka, I.; Włodarczyk, M.; Starzec, A. Isolation and structure elucidation of cistusin: A new ellagitannin from Cistus × incanus L. leaves. Ind. Crops Prod. 2020, 158, 112971. [Google Scholar] [CrossRef]
- Starzec, A.; Włodarczyk, M.; Urbanowicz, I.; Fecka, I. Charakterystyka, potencjał leczniczy i prozdrowotny Cistus × incanus L. [Characteristics, therapeutic and health-promoting potential of Cistus × incanus L.]. Farm. Pol. 2021, 76, 647–664. [Google Scholar] [CrossRef]
- Viapiana, A.; Konopacka, A.; Waleron, K.; Wesolowski, M. Cistus incanus L. commercial products as a good source of polyphenols in human diet. Ind. Crops Prod. 2017, 107, 297–304. [Google Scholar] [CrossRef]
- Kuchta, A.; Konopacka, A.; Waleron, K.; Viapiana, A.; Wesołowski, M.; Dąbkowski, K.; Ćwiklińska, A.; Mickiewicz, A.; Śledzińska, A.; Wieczorek, E.; et al. The effect of Cistus incanus herbal tea supplementation on oxidative stress markers and lipid profile in healthy adults. Cardiol. J. 2021, 28, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I. Qualitative and quantitative determination of hydrolysable tannins and other polyphenols in herbal products from meadowsweet and dog rose. Phytochem. Anal. 2009, 20, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Sahebkar, A.; Azizi, N.; Emami, S.A. Everlasting flowers: Phytochemistry and pharmacology of the genus Helichrysum. Ind. Crops Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Lavault, M.; Richomme, P. Constituents of Helichrysum stoechas variety olonnense. Chem. Nat. Compd. 2004, 40, 118–121. [Google Scholar] [CrossRef]
- Krebs, K.G.; Matern, J. Über die Inhaltsstoffe von Menyanthes trifoliata L. 3. Mitt.: Zur Identität von Trifoliosid und Trifolin. Arch. Pharm. 1958, 291, 163–165. [Google Scholar] [CrossRef]
- Hilbert, G.; Temsamani, H.; Bordenave, L.; Pedrot, E.; Chaher, N.; Cluzet, S.; Delaunay, J.-C.; Ollat, N.; Delrot, S.; Mérillon, J.-M.; et al. Flavonol profiles in berries of wild Vitis accessions using liquid chromatography coupled to mass spectrometry and nuclear magnetic resonance spectrometry. Food Chem. 2015, 169, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yanagida, A.; Komeya, S.; Kawana, M.; Honma, D.; Tagashira, M.; Kanda, T.; Shibusawa, Y. Comprehensive separation and structural analyses of polyphenols and related compounds from bracts of hops (Humulus lupulus L.). J. Agric. Food Chem. 2014, 62, 2198–2206. [Google Scholar] [CrossRef]
- Kim, H.J.; Woo, E.-R.; Park, H. A novel lignan and flavonoids from Polygonum aviculare. J. Nat. Prod. 1994, 57, 581–586. [Google Scholar] [CrossRef]
- Dare, A.P.; Tomes, S.; McGhie, T.K.; Klink, J.W.; Sandanayaka, M.; Hallett, I.C.; Atkinson, R.G. Overexpression of chalcone isomerase in apple reduces phloridzin accumulation and increases susceptibility to herbivory by two-spotted mites. Plant J. 2020, 103, 293–307. [Google Scholar] [CrossRef]
- Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Tannins and related compounds. XL. Revision of the structures of punicalin and punicalagin, and isolation and characterization of 2-O-galloylpunicalin from the bark of Punica granatum L. Chem. Pharm. Bull. 1986, 34, 650–655. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of polyphenolic composition of a herbal medicinal product − peppermint tincture. Molecules 2020, 25, 69. [Google Scholar] [CrossRef]
- Rossi, J.A.; Singleton, V.L. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Anonym. Birch leaf (Betulae folium) monograph. In European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2020; pp. 1345–1347. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Chen, L.; Kang, Y.-H. Antioxidant activities of Agrimonia pilosa Ledeb: In vitro comparative activities of its different fractions. Korean J. Plant Resour. 2014, 27, 642–649. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rouzbehan, S.; Moein, S.; Homaei, A.; Moein, M.R. Kinetics of α-glucosidase inhibition by different fractions of three species of Labiatae extracts: A new diabetes treatment model. Pharm. Biol. 2017, 55, 1483–1488. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Wu, S.-H.; Hsieh, J.-F. Isolation and characterization of α-glucosidase inhibitory constituents from Rhodiola crenulata. Food Res. Int. 2014, 57, 8–14. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, K.W.; Wu, D.-T.; Liu, H.-Y.; Li, H.-B.; Zhang, J.-R.; Gan, R.-Y. Pomegranate peel-derived punicalagin: Ultrasonic-assisted extraction, purification, and its α-glucosidase inhibitory mechanism. Food Chem. 2022, 374, 131635. [Google Scholar] [CrossRef]
- Gürbüz, P.; Demirezer, L.Ö.; Güvenalp, Z.; Kuruüzüm-Uz, A.; Kazaz, C. Isolation and structure elucidation of uncommon secondary metabolites from Cistus salviifolius L. Rec. Nat. Prod. 2015, 9, 175–183. [Google Scholar]
- Riehle, P.; Rusche, N.; Saake, B.; Rohn, S. Influence of the leaf content and herbal particle size on the presence and extractability of quantitated phenolic compounds in Cistus incanus herbal teas. J. Agric. Food Chem. 2014, 62, 10978–10988. [Google Scholar] [CrossRef]
- Petereit, F.; Kolodziej, H.; Nahrstedt, A. Flavan-3-ols and proanthocyanidins from Cistus incanus. Phytochemistry 1991, 30, 981–985. [Google Scholar] [CrossRef]
- Dimcheva, V.; Karsheva, M. Antioxidant activity and polyphenolic content of the Bulgarian wild herb Cistus incanus L. stored under different conditions. J. Chem. Technol. Metall. 2017, 52, 781–790. [Google Scholar]
- Dimcheva, V.; Karsheva, M. Cistus incanus from Strandja Mountain as a source of bioactive antioxidants. Plants 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Waed, A.; Ghalia, S.; Adawia, K. Evaluation of radical scavenging activity, Total phenolics and total flavonoids contents of Cistus species in Syria. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1071–1077. [Google Scholar]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Vlachos, D.; Malisova, S.; Lindberg, F.A.; Karaniki, G. Glycemic Index (GI) or Glycemic Load (GL) and dietary interventions for optimizing postprandial hyperglycemia in patients with T2 diabetes: A review. Nutrients 2020, 12, 1561. [Google Scholar] [CrossRef] [PubMed]
- Kaku, K. Efficacy of voglibose in type 2 diabetes. Expert Opin. Pharmacother. 2014, 15, 1181–1190. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-diabetic activities of phenolic compounds in muscadine against alpha-glucosidase and pancreatic lipase. LWT—Food Sci. Technol. 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Benalla, W.; Bellahcen, S.; Bnouham, M. Antidiabetic medicinal plants as a source of alpha glucosidase inhibitors. Curr. Diabetes Rev. 2010, 6, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, I.; Benariba, N.; Adjdir, S.; Fekhikher, Z.; Daoud, I.; Terki, M.; Benramdane, H.; Ghalem, S. In silico evaluation of phenolic compounds as inhibitors of A-amylase and A-glucosidase. J. Biomol. Struct. Dyn. 2021, 39, 816–822. [Google Scholar] [CrossRef]
- Ali Asgar, M. Anti-diabetic potential of phenolic compounds: A review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Khwaja, N.U.D.; Arunagirinathan, G. Efficacy and cardiovascular safety of alpha glucosidase inhibitors. Curr. Drug Saf. 2021, 16, 122–128. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Chaturvedi, N.; Singh, A.; Mishra, A. Characterization, inhibitory activity and mechanism of polyphenols from faba bean (gallic-acid and catechin) on α-glucosidase: Insights from molecular docking and simulation study. Prep. Biochem. Biotechnol. 2020, 50, 123–132. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Bellesia, A.; Verzelloni, E.; Tagliazucchi, D. Pomegranate ellagitannins inhibit α-glucosidase activity in vitro and reduce starch digestibility under simulated gastro-intestinal conditions. Int. J. Food Sci. Nutr. 2015, 66, 85–92. [Google Scholar] [CrossRef]
- Lee, D.Y.; Kim, H.W.; Yang, H.; Sung, S.H. Hydrolyzable tannins from the fruits of Terminalia chebula Retz and their α-glucosidase inhibitory activities. Phytochemistry 2017, 137, 109–116. [Google Scholar] [CrossRef]
- Yuca, H.; Özbek, H.; Demirezer, L.Ö.; Kasil, H.G.; Güvenalp, Z. trans-Tiliroside: A potent α-glucosidase inhibitor from the leaves of Elaeagnus angustifolia L. Phytochemistry 2021, 188, 112795. [Google Scholar] [CrossRef]
- Indrianingsih, A.W.; Tachibana, S.; Dewi, R.T.; Itoh, K. Antioxidant and α-glucosidase inhibitor activities of natural compounds isolated from Quercus gilva Blume leaves. Asian Pac. J. Trop. Biomed. 2015, 5, 748–755. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The impact of ellagitannins and their metabolites through gut microbiome on the gut health and brain wellness within the gut-brain axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef] [PubMed]
| Origin | HPLC-DAD Method | Spectrophotometric Method | |||||
|---|---|---|---|---|---|---|---|
| SPP Sum of Polyphenols | SF Sum of Flavonoids | SET Sum of Ellagitannins | SPA Sum of Phenolic Acids | TPC | TFC | ||
| [mg/g d.w.] | [mg/g d.w.] | [mg/g d.w.] | [mg/g d.w.] | [mg GAE/g d.w.] | [mg ME/g d.w.] | ||
| All (n = 52) | Mean | 101.65 | 17.68 | 73.24 | 10.73 | 349.59 | 39.71 |
| SD | 41.07 | 2.28 | 39.06 | 2.83 | 137.26 | 12.05 | |
| Median | 90.43 | 18.12 | 65.43 | 10.44 | 372.95 | 36.79 | |
| Max | 228.75 | 22.89 | 189.36 | 20.92 | 623.40 | 72.12 | |
| Min | 54.35 | 12.13 | 26.48 | 4.68 | 13.62 | 21.45 | |
| Turkey (n = 23) | Mean | 123.71 | 18.42 | 94.10 | 11.18 | 354.80 | 44.68 |
| SD | 47.53 | 1.96 | 45.40 | 3.20 | 156.50 | 13.48 | |
| Median | 105.47 | 18.62 | 76.22 | 11.15 | 374.13 | 44.67 | |
| Max | 228.75 | 22.89 | 189.36 | 20.92 | 609.58 | 72.12 | |
| Min | 55.35 | 14.54 | 27.79 | 4.68 | 13.62 | 23.20 | |
| Albania (n = 10) | Mean | 80.18 | 17.33 | 51.82 | 11.03 | 327.70 | 33.47 |
| SD | 21.27 | 2.52 | 18.86 | 2.18 | 115.45 | 6.13 | |
| Median | 78.60 | 17.82 | 50.07 | 10.54 | 358.91 | 34.06 | |
| Max | 124.68 | 21.13 | 92.78 | 15.82 | 472.57 | 42.12 | |
| Min | 54.76 | 12.64 | 29.17 | 8.67 | 94.36 | 22.75 | |
| Greece (n = 3) | Mean | 92.27 | 17.85 | 64.14 | 10.27 | 403.43 | 40.38 |
| SD | 28.00 | 0.70 | 27.28 | 1.38 | 50.76 | 7.34 | |
| Median | 83.23 | 17.79 | 55.53 | 9.90 | 374.92 | 40.56 | |
| Max | 123.67 | 18.58 | 94.69 | 11.80 | 462.03 | 47.63 | |
| Min | 69.90 | 17.19 | 42.21 | 9.11 | 373.34 | 32.95 | |
| Origin | HHDP-Glc 1,2 | Punicalin 1,2 | Gallic Acid | Punicalagin 1 | Terflavin A 1 | Cistusin 1 | Catechin | Epicatechin | Myricetin-3-O- galactoside | Myricetin-3-O- glucoside 3 | Ellagic Acid | Myricetin-3-O- arabinoside 3 | Myricitrin | Hyperoside | Isoquercitrin | Quercetin-3-O- arabinoside 4 | Quercitrin | Kaempferol-3-O- glucoside | Helichrysoside 1,5 | Tiliroside 1 | Coumaroyl- tiliroside 1,5 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg/g d.w] | ||||||||||||||||||||||
| All (n = 52) | Mean | 4.62 | 0.41 | 7.29 | 29.41 | 14.01 | 24.79 | 1.14 | 1.11 | 2.66 | 0.21 | 3.44 | 0.63 | 3.80 | 3.22 | 0.30 | 0.27 | 0.11 | 0.09 | 0.40 | 2.42 | 1.32 |
| SD | 2.36 | 0.33 | 2.12 | 18.14 | 4.98 | 15.70 | 0.48 | 0.43 | 0.95 | 0.33 | 1.00 | 0.32 | 1.83 | 0.64 | 0.27 | 0.30 | 0.20 | 0.13 | 0.08 | 0.76 | 0.56 | |
| Median | 4.30 | 0.39 | 7.10 | 25.77 | 13.28 | 20.67 | 1.15 | 1.09 | 2.74 | 0.04 | 3.36 | 0.60 | 3.53 | 3.04 | 0.22 | 0.22 | LOQ | 0.02 | 0.39 | 2.24 | 1.16 | |
| Max | 13.05 | 1.06 | 16.11 | 82.86 | 33.08 | 74.93 | 2.27 | 2.44 | 4.54 | 1.26 | 5.59 | 1.32 | 8.59 | 5.07 | 0.98 | 1.10 | 0.97 | 0.46 | 0.65 | 4.38 | 2.72 | |
| Min | 0.68 | LOQ | 3.53 | 7.77 | 6.51 | 5.55 | 0.42 | 0.42 | LOQ | LOQ | 1.15 | LOQ | 0.39 | 2.46 | LOQ | LOQ | LOQ | LOQ | 0.21 | 1.25 | 0.55 | |
| Turkey (n = 23) | Mean | 6.05 | 0.58 | 7.45 | 37.96 | 16.25 | 33.27 | 1.13 | 1.30 | 2.84 | 0.28 | 3.73 | 0.65 | 3.48 | 3.39 | 0.33 | 0.32 | 0.12 | 0.12 | 0.38 | 2.58 | 1.49 |
| SD | 2.75 | 0.30 | 2.50 | 21.72 | 5.98 | 17.71 | 0.56 | 0.43 | 0.91 | 0.34 | 1.12 | 0.32 | 1.91 | 0.70 | 0.31 | 0.32 | 0.25 | 0.16 | 0.08 | 0.85 | 0.55 | |
| Median | 6.22 | 0.49 | 7.17 | 29.00 | 15.45 | 26.00 | 1.17 | 1.35 | 2.91 | 0.13 | 3.54 | 0.61 | 3.38 | 3.22 | 0.25 | 0.28 | LOQ | 0.03 | 0.38 | 2.54 | 1.52 | |
| Max | 13.05 | 1.06 | 16.11 | 82.86 | 33.08 | 74.93 | 2.27 | 2.44 | 4.16 | 1.26 | 5.52 | 1.28 | 8.59 | 5.07 | 0.98 | 1.10 | 0.97 | 0.46 | 0.61 | 4.38 | 2.48 | |
| Min | 1.97 | LOQ | 3.53 | 8.46 | 7.13 | 8.93 | 0.42 | 0.42 | 0.49 | LOQ | 1.15 | 0.02 | 0.39 | 2.47 | LOQ | LOQ | LOQ | LOQ | 0.21 | 1.25 | 0.55 | |
| Albania (n = 10) | Mean | 3.14 | 0.17 | 7.76 | 19.95 | 13.08 | 15.49 | 1.30 | 0.74 | 2.83 | 0.17 | 3.26 | 0.71 | 4.34 | 3.04 | 0.20 | 0.23 | 0.13 | 0.02 | 0.38 | 2.21 | 1.02 |
| SD | 1.20 | 0.20 | 1.50 | 8.04 | 3.55 | 7.64 | 0.38 | 0.21 | 0.68 | 0.37 | 0.75 | 0.37 | 1.28 | 0.41 | 0.16 | 0.25 | 0.13 | 0.04 | 0.05 | 0.69 | 0.55 | |
| Median | 3.32 | 0.08 | 7.54 | 19.56 | 12.78 | 15.87 | 1.27 | 0.71 | 2.81 | LOQ | 2.93 | 0.71 | 4.85 | 3.13 | 0.24 | 0.17 | 0.08 | LOQ | 0.38 | 2.11 | 0.88 | |
| Max | 4.52 | 0.59 | 10.86 | 33.05 | 20.39 | 34.24 | 1.81 | 1.08 | 3.92 | 1.03 | 4.96 | 1.28 | 5.80 | 3.82 | 0.45 | 0.69 | 0.34 | 0.14 | 0.45 | 3.99 | 2.54 | |
| Min | 0.68 | LOQ | 5.89 | 8.87 | 8.09 | 6.64 | 0.75 | 0.47 | 1.66 | LOQ | 2.53 | LOQ | 1.63 | 2.47 | LOQ | LOQ | LOQ | LOQ | 0.31 | 1.36 | 0.58 | |
| Greece (n = 3) | Mean | 4.39 | 0.21 | 7.25 | 26.46 | 13.34 | 19.74 | 1.18 | 1.19 | 2.61 | 0.39 | 3.02 | 0.64 | 3.82 | 3.15 | 0.38 | 0.36 | 0.03 | LOQ | 0.33 | 2.55 | 1.23 |
| SD | 0.17 | 0.20 | 0.77 | 17.16 | 4.39 | 9.84 | 0.44 | 0.35 | 0.62 | 0.67 | 0.72 | 0.21 | 1.04 | 0.62 | 0.26 | 0.36 | 0.04 | LOQ | 0.07 | 0.82 | 0.59 | |
| Median | 4.45 | 0.14 | 7.37 | 18.79 | 12.84 | 14.20 | 1.25 | 1.01 | 2.67 | LOQ | 2.69 | 0.70 | 4.30 | 3.01 | 0.26 | 0.35 | LOQ | LOQ | 0.36 | 2.13 | 0.97 | |
| Max | 4.53 | 0.43 | 7.95 | 46.11 | 17.96 | 31.10 | 1.58 | 1.60 | 3.19 | 1.16 | 3.85 | 0.82 | 4.54 | 3.83 | 0.67 | 0.73 | 0.08 | LOQ | 0.38 | 3.49 | 1.91 | |
| Min | 4.20 | 0.05 | 6.42 | 14.47 | 9.23 | 13.92 | 0.71 | 0.97 | 1.96 | LOQ | 2.53 | 0.41 | 2.62 | 2.61 | 0.21 | LOQ | LOQ | LOQ | 0.25 | 2.02 | 0.81 | |
| Origin | DPPH | ABTS | FRAP | ||||
|---|---|---|---|---|---|---|---|
| Inhibition | GAE | Inhibition | GAE | Fe(II) | GAE | ||
| [%] | [mM/g d.w.] | [%] | [mM/g d.w.] | [mM/g d.w.] | [mM/g d.w.] | ||
| All (n = 52) | Mean | 78.55 ± 5.22 | 151.30 ± 10.06 | 29.34 ± 16.70 | 3.17 ± 1.80 | 204.84 ± 51.65 | 45.41 ± 11.45 |
| Median | 79.38 | 152.90 | 25.63 | 2.77 | 205.15 | 45.47 | |
| Min | 68.95 | 132.82 | 6.21 | 0.67 | 109.67 | 24.31 | |
| Max | 89.08 | 171.59 | 68.70 | 7.41 | 336.75 | 74.64 | |
| Turkey (n = 23) | Mean | 79.73 ± 4.69 | 153.57 ± 9.59 | 38.22 ± 18.50 | 4.12 ± 2.00 | 230.63 ± 49.30 | 51.12 ± 10.93 |
| Median | 80.96 | 155.95 | 36.67 | 3.96 | 225.46 | 49.98 | |
| Min | 69.34 | 133.56 | 10.28 | 1.11 | 133.27 | 29.54 | |
| Max | 85.93 | 165.52 | 68.70 | 7.41 | 336.75 | 74.64 | |
| Albania (n = 10) | Mean | 78.95 ± 4.95 | 152.08 ± 9.54 | 19.55 ± 10.37 | 2.11 ± 1.17 | 175.22 ± 41.29 | 38.84 ± 9.15 |
| Median | 80.54 | 155.13 | 18.00 | 1.94 | 168.47 | 37.34 | |
| Min | 69.80 | 134.45 | 6.21 | 0.67 | 109.67 | 24.31 | |
| Max | 86.70 | 167.00 | 40.81 | 4.40 | 234.12 | 51.89 | |
| Greece (n = 3) | Mean | 78.56 ± 5.93 | 151.32 ± 11.42 | 26.01 ± 10.37 | 2.81 ± 1.12 | 223.50 ± 39.89 | 49.54 ± 8.84 |
| Median | 81.85 | 157.65 | 29.28 | 3.16 | 205.29 | 45.50 | |
| Min | 71.71 | 138.13 | 14.40 | 1.55 | 195.97 | 43.44 | |
| Max | 82.11 | 158.16 | 34.35 | 3.71 | 269.24 | 59.68 | |
| Method | Group of Compounds | Linear Regression | Spearman’s Rank Order Correlation |
|---|---|---|---|
| R | R | ||
| ABTS | Sum of polyphenols (SPP) | 0.41 | 0.47 |
| Sum of flavonoids (SF) | 0.47 | 0.52 | |
| Sum of ellagitannins (SET) | 0.37 | 0.43 | |
| Sum of phenolic acids (SPA) | 0.34 | 0.41 | |
| Total phenolic content (TPC) | 0.37 | 0.38 | |
| Total flavonoid content (TFC) | 0.47 | 0.57 | |
| DPPH | Sum of polifenolic (SPP) | 0.37 | 0.34 |
| Sum of flavonoids (SF) | 0.31 | 0.28 | |
| Sum of ellagitannins (SET) | 0.33 | 0.31 | |
| Sum of phenolic acids (SPA) | 0.47 | 0.39 | |
| Total phenolic content (TPC) | 0.33 | 0.35 | |
| Total flavonoid content (TFC) | 0.62 | 0.57 | |
| FRAP | Sum of polyphenols (SPP) | 0.68 | 0.69 |
| Sum of flavonoids (SF) | 0.46 | 0.52 | |
| Sum of ellagitannins (SET) | 0.66 | 0.65 | |
| Sum of phenolic acids (SPA) | 0.45 | 0.46 | |
| Total phenolic content (TPC) | 0.51 | 0.55 | |
| Total flavonoid content (TFC) | 0.78 | 0.77 |
| Compound | Molar Mass [g/mol] | Inhibition Type | IC50 [µg/mL] | IC50 [µM] |
|---|---|---|---|---|
| Gallic acid | 170.1 | mixed | 116.70 | 685.99 |
| Ellagic acid | 302.2 | mixed | 3.27 | 10.81 |
| Cistusin | 1252.8 | mixed | 0.83 | 0.66 |
| Punicalagin | 1084.7 | competitive | 1.23 | 1.14 |
| Terflavin A | 1086.7 | mixed | 1.19 | 1.10 |
| Tiliroside | 594.5 | mixed | 168.50 | 283.47 |
| Acarbose | 645.6 | competitive | 2.16 1 | 3.34 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starzec, A.; Włodarczyk, M.; Kunachowicz, D.; Dryś, A.; Kepinska, M.; Fecka, I. Polyphenol Profile of Cistus × incanus L. and Its Relevance to Antioxidant Effect and α-Glucosidase Inhibition. Antioxidants 2023, 12, 553. https://doi.org/10.3390/antiox12030553
Starzec A, Włodarczyk M, Kunachowicz D, Dryś A, Kepinska M, Fecka I. Polyphenol Profile of Cistus × incanus L. and Its Relevance to Antioxidant Effect and α-Glucosidase Inhibition. Antioxidants. 2023; 12(3):553. https://doi.org/10.3390/antiox12030553
Chicago/Turabian StyleStarzec, Aneta, Maciej Włodarczyk, Dominika Kunachowicz, Andrzej Dryś, Marta Kepinska, and Izabela Fecka. 2023. "Polyphenol Profile of Cistus × incanus L. and Its Relevance to Antioxidant Effect and α-Glucosidase Inhibition" Antioxidants 12, no. 3: 553. https://doi.org/10.3390/antiox12030553
APA StyleStarzec, A., Włodarczyk, M., Kunachowicz, D., Dryś, A., Kepinska, M., & Fecka, I. (2023). Polyphenol Profile of Cistus × incanus L. and Its Relevance to Antioxidant Effect and α-Glucosidase Inhibition. Antioxidants, 12(3), 553. https://doi.org/10.3390/antiox12030553








