Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. EV Isolation
2.4. Nanoparticle Tracking Analysis (NTA)
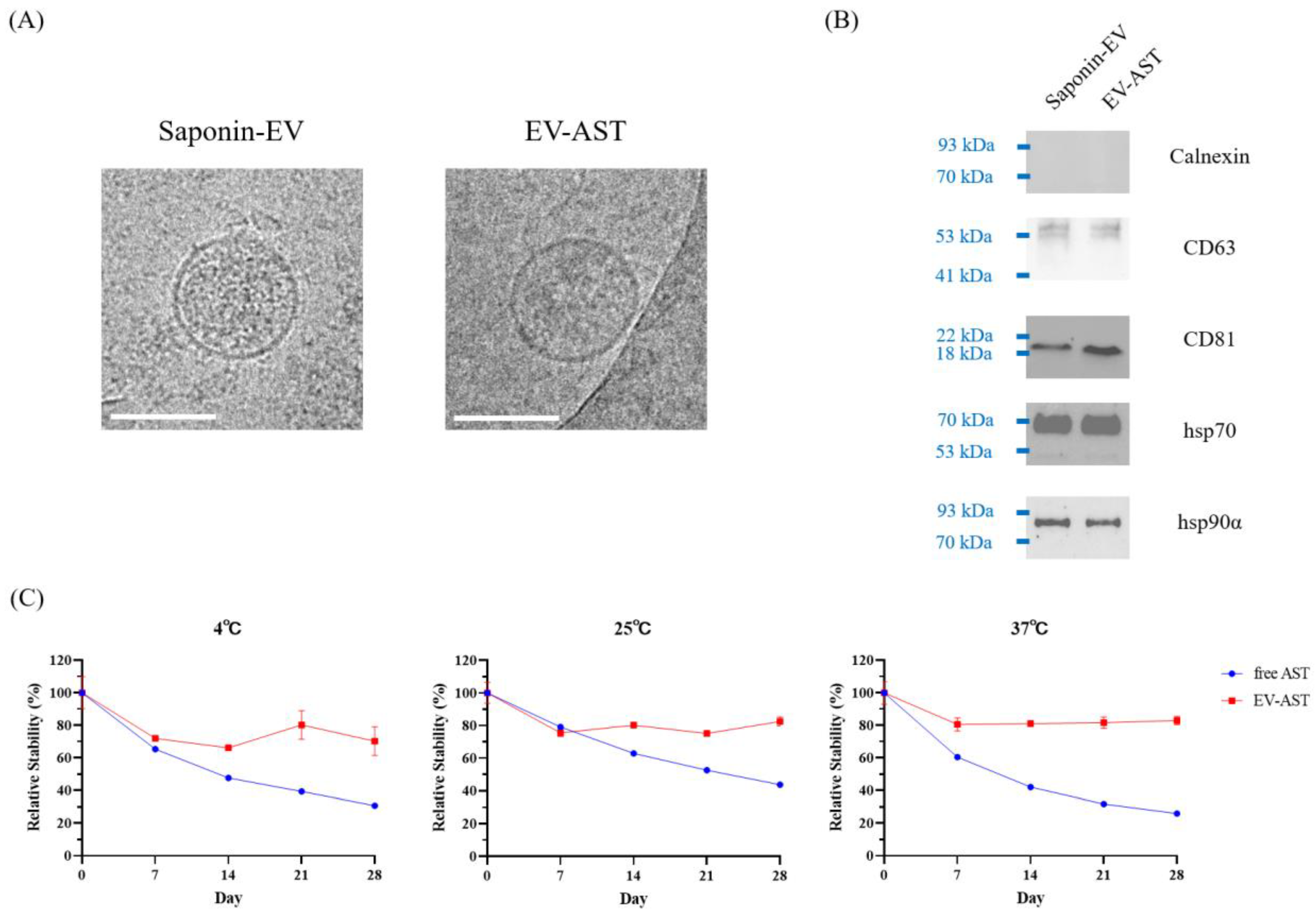
2.5. Western Blotting
2.6. Cryo-Transmission Electron Microscopy (Cryo-TEM)
2.7. Drug-Loading Methods
2.8. Determination of AST Content in EV-AST
2.9. Thermal Stability of EV-ASTs
2.10. ABTS Radical-Scavenging Assay
2.11. Cell-Viability Assay
2.12. Cellular Antioxidant Activity (CAA) Assay
2.13. Anti-Inflammatory Assay
2.14. mRNA Analysis
2.15. Statistical Analysis
3. Results and Discussion
3.1. Characterization of FBS-EVs
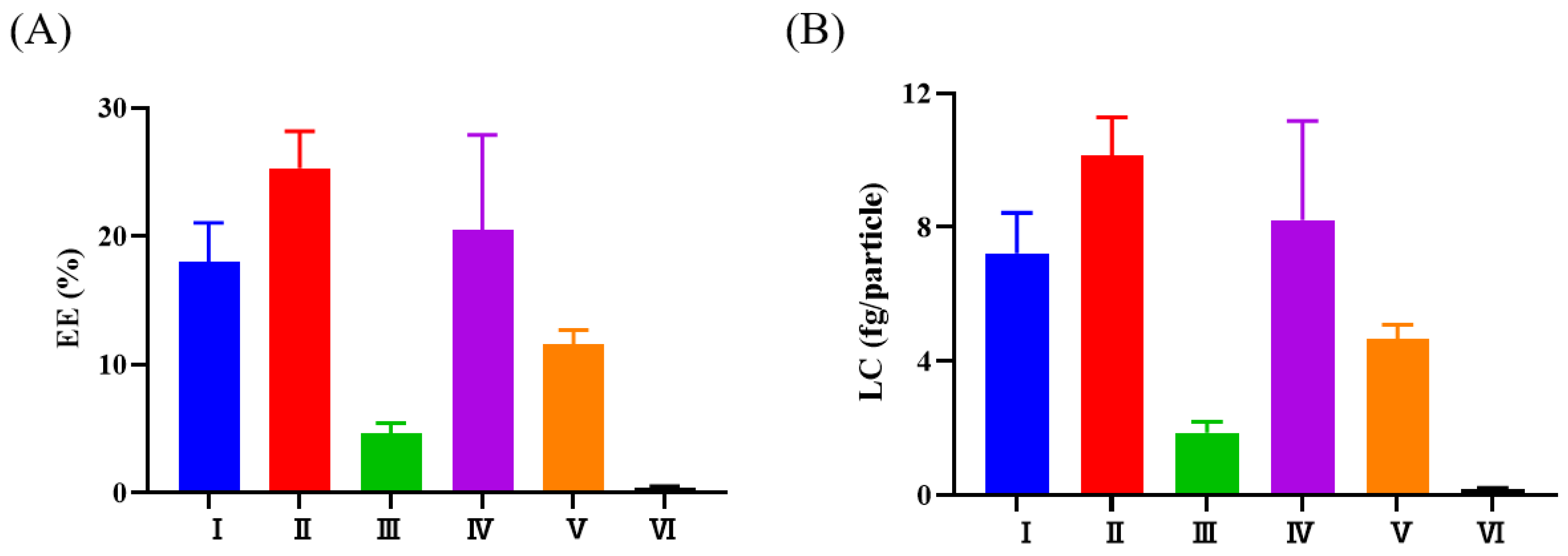
3.2. Selection of the Methods Used to Load AST into FBS-EVs
3.3. Characterization of EV-ASTs
3.4. In Vitro Antioxidant Activity of Free AST and EV-ASTs
3.5. Evaluation of Cytotoxicity in HaCaT and RAW 264.7 Cells
3.6. The CAA of Free AST and EV-ASTs
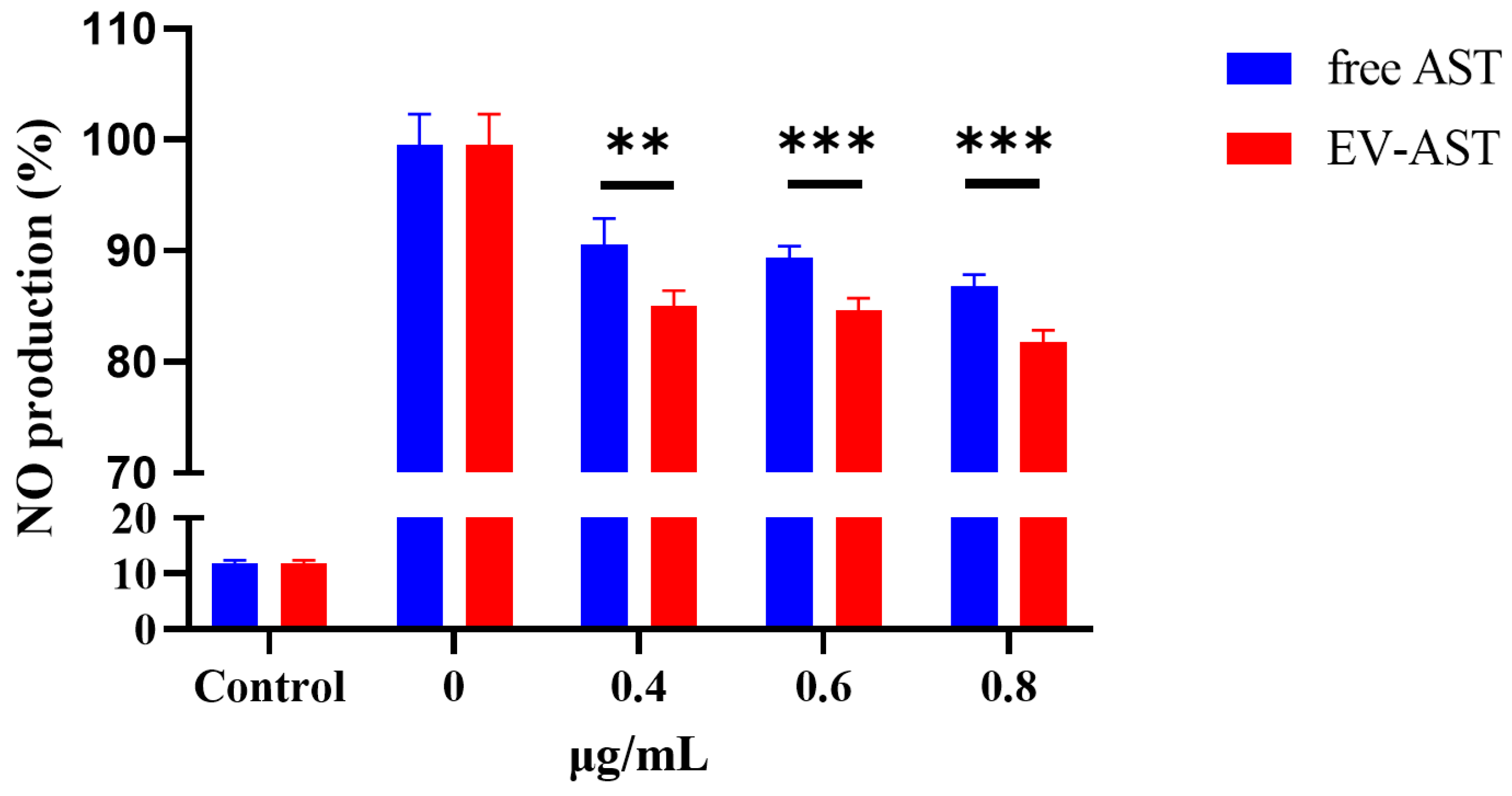
3.7. The Anti-Inflammatory Activity of Free AST and EV-AST
3.8. Assessment of Gene Expression Related to the Antioxidant and Anti-Inflammatory Activities of the EV-ASTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Yamashita, E. Let Astaxanthin Be Thy Medicine. PharmaNutrition 2015, 3, 115–122. [Google Scholar] [CrossRef]
- Yamashita, E. Astaxanthin as a Medical Food. Funct. Foods Health Dis. 2013, 3, 254. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Johnson, E.A.; An, G.-H. Astaxanthin from Microbial Sources. Crit. Rev. Biotechnol. 1991, 11, 297–326. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis Astaxanthin and Especially 9-Cis Astaxanthin Exhibits a Higher Antioxidant Activity In Vitro Compared to the All-Trans Isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- White, D.A.; Ørnsrud, R.; Davies, S.J. Determination of Carotenoid and Vitamin A Concentrations in Everted Salmonid Intestine Following Exposure to Solutions of Carotenoid in Vitro. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2003, 136, 683–692. [Google Scholar] [CrossRef]
- Wu, H.; Niu, H.; Shao, A.; Wu, C.; Dixon, B.; Zhang, J.; Yang, S.; Wang, Y. Astaxanthin as a Potential Neuroprotective Agent for Neurological Diseases. Mar. Drugs 2015, 13, 5750–5766. [Google Scholar] [CrossRef]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin Decreased Oxidative Stress and Inflammation and Enhanced Immune Response in Humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef]
- Zhang, X.-S.; Zhang, X.; Wu, Q.; Li, W.; Zhang, Q.-R.; Wang, C.-X.; Zhou, X.-M.; Li, H.; Shi, J.-X.; Zhou, M.-L. Astaxanthin Alleviates Early Brain Injury Following Subarachnoid Hemorrhage in Rats: Possible Involvement of Akt/Bad Signaling. Mar. Drugs 2014, 12, 4291–4310. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic Benefits of Astaxanthin on Humans Subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Miyachi, M.; Matsuno, T.; Asano, K.; Mataga, I. Anti-Inflammatory Effects of Astaxanthin in the Human Gingival Keratinocyte Line NDUSD-1. J. Clin. Biochem. Nutr. 2015, 56, 171–178. [Google Scholar] [CrossRef]
- Park, S.-A.; Ahn, J.-B.; Choi, S.-H.; Lee, J.-S.; Lee, H.G. The Effects of Particle Size on the Physicochemical Properties of Optimized Astaxanthin-Rich Xanthophyllomyces Dendrorhous-Loaded Microparticles. LWT-Food Sci. Technol. 2014, 55, 638–644. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, S.; McClements, D.J.; Wang, D.; Xu, Y. Design of Astaxanthin-Loaded Core–Shell Nanoparticles Consisting of Chitosan Oligosaccharides and Poly(Lactic-Co-Glycolic Acid): Enhancement of Water Solubility, Stability, and Bioavailability. J. Agric. Food Chem. 2019, 67, 5113–5121. [Google Scholar] [CrossRef]
- Galarza, J.I.; Arredondo Vega, B.O.; Villón, J.; Henríquez, V. Deesterification of Astaxanthin and Intermediate Esters from Haematococcus Pluvialis Subjected to Stress. Biotechnol. Rep. 2019, 23, e00351. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, J.; Zhong, B.; Huang, J.; Jiang, L.; Jiang, Y.; Yuan, J.; Sun, J.; Dai, L.; Yang, C.; et al. Exosomes as Potential Sources of Biomarkers in Colorectal Cancer. Cancer Lett. 2020, 476, 13–22. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.; Park, J.S.; Kim, J.H.; Lee, E.S.; Cha, B.S.; Park, K.S. DNA Barcode-Based Detection of Exosomal MicroRNAs Using Nucleic Acid Lateral Flow Assays for the Diagnosis of Colorectal Cancer. Talanta 2022, 242, 123306. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Kwon, W.Y.; Park, K.S. Exosomes for Non-Invasive Cancer Monitoring. Biotechnol. J. 2019, 14, 1800430. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Kwon, W.Y.; Park, K.S. A Simple Approach for Rapid and Cost-Effective Quantification of Extracellular Vesicles Using a Fluorescence Polarization Technique. J. Biol. Eng. 2019, 13, 31. [Google Scholar] [CrossRef]
- Cha, B.S.; Park, K.S.; Park, J.S. Signature MRNA Markers in Extracellular Vesicles for the Accurate Diagnosis of Colorectal Cancer. J. Biol. Eng. 2020, 14, 4. [Google Scholar] [CrossRef]
- Bagheri Hashkavayi, A.; Cha, B.S.; Lee, E.S.; Kim, S.; Park, K.S. Advances in Exosome Analysis Methods with an Emphasis on Electrochemistry. Anal. Chem. 2020, 92, 12733–12740. [Google Scholar] [CrossRef]
- Lee, E.S.; Cha, B.S.; Kim, S.; Park, K.S. Synthesis of Exosome-Based Fluorescent Gold Nanoclusters for Cellular Imaging Applications. Int. J. Mol. Sci. 2021, 22, 4433. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Cha, B.S.; Lee, E.S.; Park, K.S. Dual Rolling Circle Amplification-Enabled Ultrasensitive Multiplex Detection of Exosome Biomarkers Using Electrochemical Aptasensors. Anal. Chim. Acta 2022, 1205, 339762. [Google Scholar] [CrossRef]
- Fujita, D.; Arai, T.; Komori, H.; Shirasaki, Y.; Wakayama, T.; Nakanishi, T.; Tamai, I. Apple-Derived Nanoparticles Modulate Expression of Organic-Anion-Transporting Polypeptide (OATP) 2B1 in Caco-2 Cells. Mol. Pharm. 2018, 15, 5772–5780. [Google Scholar] [CrossRef]
- Patel, M.M.; Patel, B.M. Crossing the Blood–Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017, 31, 109–133. [Google Scholar] [CrossRef]
- Hood, J.L.; Wickline, S.A. A Systematic Approach to Exosome-Based Translational Nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 458–467. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv. Mater. 2019, 31, 1802896. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome Engineering: Current Progress in Cargo Loading and Targeted Delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Ho, E.A. Challenges in the Development and Establishment of Exosome-Based Drug Delivery Systems. J. Control. Release 2021, 329, 894–906. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.v.; et al. Exosomes as Drug Delivery Vehicles for Parkinson’s Disease Therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Eitan, E.; Zhang, S.; Witwer, K.W.; Mattson, M.P. Extracellular Vesicle–Depleted Fetal Bovine and Human Sera Have Reduced Capacity to Support Cell Growth. J. Extracell. Vesicles 2015, 4, 26373. [Google Scholar] [CrossRef]
- Lehrich, B.; Liang, Y.; Khosravi, P.; Federoff, H.; Fiandaca, M. Fetal Bovine Serum-Derived Extracellular Vesicles Persist within Vesicle-Depleted Culture Media. Int. J. Mol. Sci. 2018, 19, 3538. [Google Scholar] [CrossRef]
- Ochieng, J.; Pratap, S.; Khatua, A.K.; Sakwe, A.M. Anchorage-Independent Growth of Breast Carcinoma Cells Is Mediated by Serum Exosomes. Exp. Cell Res. 2009, 315, 1875–1888. [Google Scholar] [CrossRef]
- Dong, M.; Wu, S.; Xu, H.; Yu, X.; Wang, L.; Bai, H.; Niu, W. FBS-Derived Exosomes as a Natural Nano-Scale Carrier for Icariin Promote Osteoblast Proliferation. Front. Bioeng. Biotechnol. 2021, 9, 615920. [Google Scholar] [CrossRef]
- Cha, B.S.; Lee, E.S.; Kim, S.; Kim, J.M.; Hwang, S.H.; Oh, S.S.; Park, K.S. Simple Colorimetric Detection of Organophosphorus Pesticides Using Naturally Occurring Extracellular Vesicles. Microchem. J. 2020, 158, 105130. [Google Scholar] [CrossRef]
- Emelyanov, A.; Shtam, T.; Kamyshinsky, R.; Garaeva, L.; Verlov, N.; Miliukhina, I.; Kudrevatykh, A.; Gavrilov, G.; Zabrodskaya, Y.; Pchelina, S.; et al. Cryo-Electron Microscopy of Extracellular Vesicles from Cerebrospinal Fluid. PLoS ONE 2020, 15, e0227949. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.-W.; Chan, L.-C.; Wei, Y.; Hsu, J.-M.; Xia, W.; Cha, J.-H.; Hou, J.; Hsu, J.L.; Sun, L.; et al. Exosomal PD-L1 Harbors Active Defense Function to Suppress T Cell Killing of Breast Cancer Cells and Promote Tumor Growth. Cell Res. 2018, 28, 862–864. [Google Scholar] [CrossRef]
- Ban, J.-J.; Lee, M.; Im, W.; Kim, M. Low PH Increases the Yield of Exosome Isolation. Biochem. Biophys. Res. Commun. 2015, 461, 76–79. [Google Scholar] [CrossRef]
- Tang, X.; Chang, C.; Guo, J.; Lincoln, V.; Liang, C.; Chen, M.; Woodley, D.T.; Li, W. Tumour-Secreted Hsp90α on External Surface of Exosomes Mediates Tumour-Stromal Cell Communication via Autocrine and Paracrine Mechanisms. Sci. Rep. 2019, 9, 15108. [Google Scholar] [CrossRef]
- Asea, A.; Jean-Pierre, C.; Kaur, P.; Rao, P.; Linhares, I.M.; Skupski, D.; Witkin, S.S. Heat Shock Protein-Containing Exosomes in Mid-Trimester Amniotic Fluids. J. Reprod. Immunol. 2008, 79, 12–17. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, D.; Ma, X.; Wang, J.; Hou, W.; Zhang, W. Exosomes as Drug Carriers for Cancer Therapy and Challenges Regarding Exosome Uptake. Biomed. Pharmacother. 2020, 128, 110237. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted Doxorubicin-Loaded Mesenchymal Stem Cells-Derived Exosomes as a Versatile Platform for Fighting against Colorectal Cancer. Life Sci. 2020, 261, 118369. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Wang, T.; Yin, W.; Tran, T.T.D.; Barua, H.T.; Zhang, Y.; Midge, S.B.; Nguyen, T.N.G.; Lee, B.-J.; Duan, W. Development of a Nanoamorphous Exosomal Delivery System as an Effective Biological Platform for Improved Encapsulation of Hydrophobic Drugs. Int. J. Pharm. 2019, 566, 697–707. [Google Scholar] [CrossRef]
- Hood, J.L. Post Isolation Modification of Exosomes for Nanomedicine Applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef]
- Shany, S.; Bernheimer, A.W.; Grushoff, P.S.; Kim, K.-S. Evidence for Membrane Cholesterol as the Common Binding Site for Cereolysin, Streptolysin O and Saponin. Mol. Cell Biochem. 1974, 3, 179–186. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Tu, C.; Chen, X.; Yang, B.; Huo, Y.; Li, Y.; Chen, A.-Z.; Lan, P.; Zhang, Y.S.; et al. High-Throughput Single-Cell Analysis of Exosome Mediated Dual Drug Delivery, In Vivo Fate and Synergistic Tumor Therapy. Nanoscale 2020, 12, 13742–13756. [Google Scholar] [CrossRef]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. MiRNA in Plasma Exosome Is Stable under Different Storage Conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Walker, R.B.; Everette, J.D. Comparative Reaction Rates of Various Antioxidants with ABTS Radical Cation. J. Agric. Food Chem. 2009, 57, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Furger, C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants 2021, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The Exosome Journey: From Biogenesis to Uptake and Intracellular Signalling. Cell Commun. Signal. 2021, 19, 47. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering Exosomes as Refined Biological Nanoplatforms for Drug Delivery. Acta Pharm. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef]
- Diniyah, N.; Alam, M.B.; Choi, H.J.; Lee, S.H. Lablab Purpureus Protects Hacat Cells from Oxidative Stress-Induced Cell Death through Nrf2-Mediated Heme Oxygenase-1 Expression via the Activation of P38 and Erk1/2. Int. J. Mol. Sci. 2020, 21, 8583. [Google Scholar] [CrossRef]
- Han, M.; Lee, D.; Lee, S.H.; Kim, T.H. Oxidative Stress and Antioxidant Pathway in Allergic Rhinitis. Antioxidants 2021, 10, 1266. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, J.; Jin, C.; Wu, S.; Lu, X.; Hu, X.; Sun, Y.; Cai, Y. The Effect of Nuclear Factor Erythroid 2-Related Factor/Antioxidant Response Element Signalling Pathway in the Lanthanum Chloride-Induced Impairment of Learning and Memory in Rats. J. Neurochem. 2017, 140, 463–475. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, J.M.; Kim, S.; Yoon, M.J.; Park, K.S. Chemical Transformation of Astaxanthin from Haematococcus Pluvialis Improves Its Antioxidative and Anti-Inflammatory Activities. ACS Omega 2020, 5, 19120–19130. [Google Scholar] [CrossRef]
- Dong, J.; Li, J.; Cui, L.; Wang, Y.; Lin, J.; Qu, Y.; Wang, H. Cortisol Modulates Inflammatory Responses in LPS-Stimulated RAW264.7 Cells via the NF-ΚB and MAPK Pathways. BMC Vet. Res. 2018, 14, 30. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, Y.J.; Cha, B.S.; Kim, D.; Lee, E.S.; Kim, S.; Han, J.; Shin, J.; Kim, S.; Park, K.S. Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin. Antioxidants 2023, 12, 473. https://doi.org/10.3390/antiox12020473
Jang YJ, Cha BS, Kim D, Lee ES, Kim S, Han J, Shin J, Kim S, Park KS. Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin. Antioxidants. 2023; 12(2):473. https://doi.org/10.3390/antiox12020473
Chicago/Turabian StyleJang, Young Jun, Byung Seok Cha, Doyeon Kim, Eun Sung Lee, Seokjoon Kim, Jinjoo Han, Jiye Shin, Seokhwan Kim, and Ki Soo Park. 2023. "Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin" Antioxidants 12, no. 2: 473. https://doi.org/10.3390/antiox12020473
APA StyleJang, Y. J., Cha, B. S., Kim, D., Lee, E. S., Kim, S., Han, J., Shin, J., Kim, S., & Park, K. S. (2023). Extracellular Vesicles, as Drug-Delivery Vehicles, Improve the Biological Activities of Astaxanthin. Antioxidants, 12(2), 473. https://doi.org/10.3390/antiox12020473






