Food Wastes and Microalgae as Sources of Bioactive Compounds and Pigments in a Modern Biorefinery: A Review
Abstract
1. Introduction
2. Methodology
3. Innovative Extraction Methods
4. Bioactive Compounds
4.1. Bioactive Compounds from Food Wastes
4.1.1. Bioactive Compounds from Apples
4.1.2. Bioactive Compounds from Citrus
4.1.3. Bioactive Compounds from Cherries
4.1.4. Bioactive Compounds from Almond
4.1.5. Bioactive Compounds from Mango
4.2. Bioactive Compounds from Microalgae
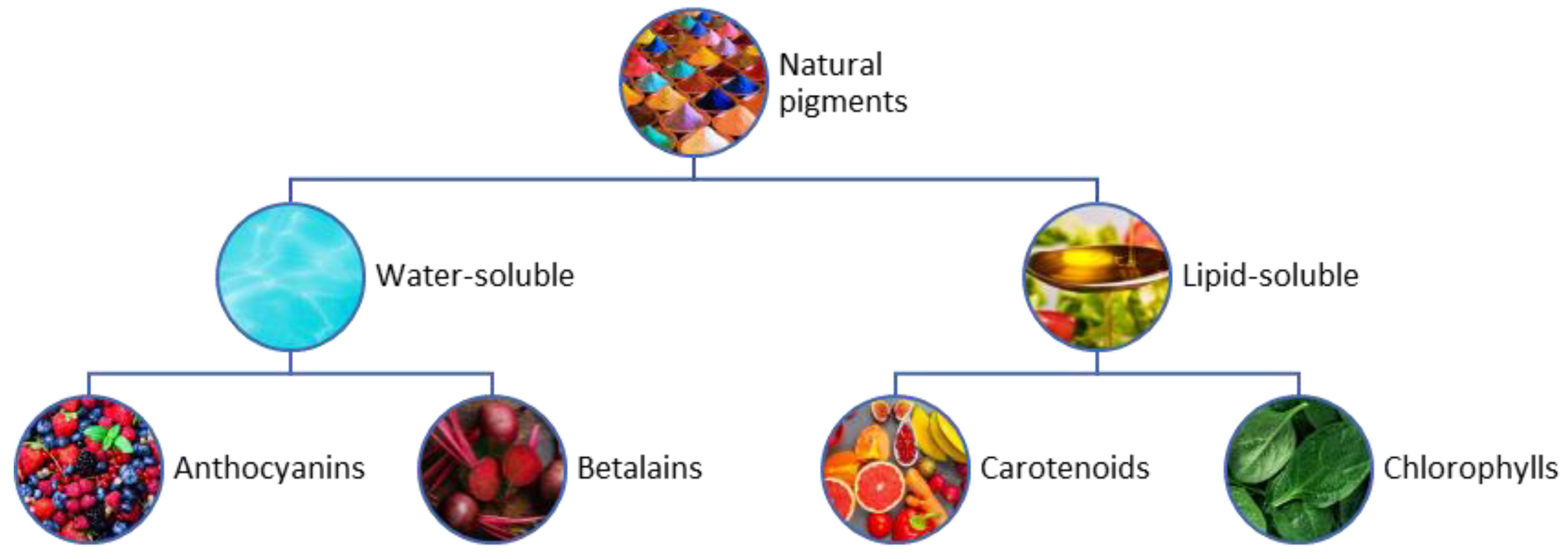
5. Bioactive Pigments
5.1. Bioactive Pigments from Food Wastes
5.2. Bioactive Pigments Derived from Microalgae
6. Market Analysis
6.1. Packaging Applications
6.2. Pharmaceutical Applications
6.3. Textile Applications
7. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
References
- Christopher, L.P. Integrated Forest Biorefineries: Current State and Development Potential; RSC Publishing: London, UK, 2012; Chapter 1; pp. 1–66. [Google Scholar] [CrossRef]
- United Nation. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2015. [Google Scholar]
- El-Rahman, M.A.; Darwish, S.; Megali, H.K.H.; El-Hakim, H.I.A. Characterization of β-carotene extracted from orange peels and its use as a natural colorant and antioxidant in ice cream. Egypt. J. Food Sci. 2019, 47, 173–185. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Varjani, S.; Kim, S.H.; Wong, J.W. Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour. Technol. 2021, 325, 124684. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2018, 233, 352–370. [Google Scholar] [CrossRef]
- Angiolillo, L.; Del Nobile, M.A.; Conte, A. The extraction of bioactive compounds from food residues using microwaves. Curr. Opin. Food Sci. 2015, 5, 93–98. [Google Scholar] [CrossRef]
- Oliveira, J. Determinantes que Influenciam a Decisão de Compra de Sumos de Fruta e Néctares: O Caso da Marca Compal. Master’s Thesis, Instituto Politécnico de Lisboa, Lisbon, Portugal, 2016. [Google Scholar]
- Pascoalino, L.A.; Reis, F.S.; Prieto, M.A.; Barreira, J.C.M.; Ferreira, I.C.F.R.; Barros, L. Valorization of bio-residues from the processing of main portuguese fruit crops: From discarded waste to health promoting compounds. Molecules 2021, 26, 2624. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-derived bioactive compounds with anti-lung cancer potential. Mar. Drugs 2020, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and future trends to produce carbohydrate enriched biomass, High-added value products and bioactive compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef]
- Rani, A.; Saini, K.C.; Bast, F.; Varjani, S.; Mehariya, S.; Bhatia, S.K.; Sharma, N.; Funk, C. A review on microbial products and their perspective application as antimicrobial agents. Biomolecules 2021, 11, 1860. [Google Scholar] [CrossRef]
- Villaró, S.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Acién-Fernández, G.; Lafarga, T. Microalgae Derived Astaxanthin: Research and Consumer Trends and Industrial Use as Food. Foods 2021, 10, 2303. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; LaRocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B.; Jambrak, A.R.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “green” and novel strategies for the extraction of bioactive added value compounds from citrus wastes—A review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Rezaei, K.; Haghighi, M.; Labbafi, M. Solvent and solvent to sample ratio as main parameters in the microwave-assisted extraction of polyphenolic compounds from apple pomace. Food Sci. Biotechnol. 2013, 22, 1–6. [Google Scholar] [CrossRef]
- Lu, W.; Alam, M.A.; Pan, Y.; Wu, J.; Wang, Z.; Yuan, Z. A new approach of microalgal biomass pretreatment using deep eutectic solvents for enhanced lipid recovery for biodiesel production. Bioresour. Technol. 2016, 218, 123–128. [Google Scholar] [CrossRef]
- Islam, S.U.; Shahid, M.; Mohammad, F. Perspectives for natural product based agents derived from industrial plants in textile applications—A review. J. Clean. Prod. 2013, 57, 2–18. [Google Scholar] [CrossRef]
- Valente, J.M.L.D. Subprodutos Alimentares: Novas Alternativas e Possíveis Aplicações Farmacêuticas. Ph.D. Thesis, Universidade Fernando Pessoa, Porto, Portugal, 2015. [Google Scholar]
- Yilmaz, E.; Soylak, M. Type of green solvents used in separation and preconcentration methods. In New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 5; pp. 207–266. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Which is the best food emerging solvent: IL, DES or NADES? Trends Food Sci. Technol. 2019, 90, 133–146. [Google Scholar] [CrossRef]
- De Morais, P.; Gonçalves, F.; Coutinho, J.A.P.; Ventura, S.P.M. Ecotoxicity of cholinium-based deep eutectic solvents. ACS Sustain. Chem. Eng. 2015, 3, 3398–3404. [Google Scholar] [CrossRef]
- Vanda, H.; Dai, Y.; Wilson, E.G.; Verpoorte, R.; Choi, Y.H. Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chim. 2018, 21, 628–638. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Dulyanska, Y.; Cruz-Lopes, L.P.; Esteves, B.; Ferreira, J.V.; Domingos, I.; Lima, M.J.; Correia, P.M.R.; Ferreira, M.; Fragata, A.; Barroca, M.J.; et al. Extraction of phenolic compounds from cherry seeds: A Preliminary Study. Agronomy 2022, 12, 1227. [Google Scholar] [CrossRef]
- Cristina-Gabriela, G.; Emilie, D.; Gabriel, L.; Claire, E. Bioactive Compounds Extraction from Pomace of Four Apple Varieties. 2012. Available online: https://www.researchgate.net/publication/288516820 (accessed on 30 September 2022).
- Carson, K.J.; Collins, J.L.; Penfield, M.P. Unrefined, Dried Apple Pomace as a Potential Food Ingredient. J. Food Sci. 1994, 59, 1213–1215. [Google Scholar] [CrossRef]
- Vilas-Boas, A.; Oliveira, A.; Ribeiro, T.; Ribeiro, S.; Nunes, C.; Gómez-García, R.; Nunes, J.; Pintado, M. Impact of extraction process in non-compliant ‘Bravo de Esmolfe’ apples towards the development of natural antioxidant extracts. Appl. Sci. 2021, 11, 5916. [Google Scholar] [CrossRef]
- García, Y.D.; Valles, B.S.; Lobo, A.P. Phenolic and antioxidant composition of by-products from the cider industry: Apple pomace. Food Chem. 2009, 117, 731–738. [Google Scholar] [CrossRef]
- Akšić, M.F.; Lazarević, K.; Šegan, S.; Natić, M.; Tosti, T.; Ćirić, I.; Meland, M. Assessing the fatty acid, carotenoid, and tocopherol compositions of seeds from apple cultivars (Malus domestica Borkh.) Grown in Norway. Foods 2021, 10, 1956. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.; Loos, H.M.; Bayha, S.; Carle, R.; Kammerer, D.R. Recovery and characterisation of coloured phenolic preparations from apple seeds. Food Chem. 2013, 136, 1277–1287. [Google Scholar] [CrossRef]
- Górnaś, P. Unique variability of tocopherol composition in various seed oils recovered from by-products of apple industry: Rapid and simple determination of all four homologues (α, β, γ and δ) by RP-HPLC/FLD. Food Chem. 2015, 172, 129–134. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food Ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef]
- Leontowicz, H.; Gorinstein, S.; Lojek, A.; Leontowicz, M.; Číž, M.; Soliva-Fortuny, R.; Park, Y.-S.; Jung, S.-T.; Trakhtenberg, S.; Martin-Belloso, O. Comparative content of some bioactive compounds in apples, peaches and pears and their influence on lipids and antioxidant capacity in rats. J. Nutr. Biochem. 2002, 13, 603–610. [Google Scholar] [CrossRef]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Citrus processing by-products: An overlooked repository of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2021, 63, 67–86. [Google Scholar] [CrossRef]
- Liang, X.; Ma, C.; Yan, X.; Liu, X.; Liu, F. Advances in research on bioactivity, metabolism, stability and delivery systems of lycopene. Trends Food Sci. Technol. 2019, 93, 185–196. [Google Scholar] [CrossRef]
- Triyono, B.; Prawisudha, P.; Mardiyati, M.; Pasek, A.D. Experimental Study on Utilization of Indonesian Non-Recycled Organic Waste as Renewable Solid Fuel Using Wet Torrefaction Process. Eng. J. 2018, 22, 81–92. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, H.; Sun, X.; Zhu, J.; Dai, H.; Zhang, Y. Nanocrystallization of anthocyanin extract from red-fleshed apple ‘QN-5’ Improved its antioxidant effect through enhanced stability and activity under stressful conditions. Molecules 2019, 24, 1421. [Google Scholar] [CrossRef]
- Górnaś, P.; Juhņeviča-Radenkova, K.; Radenkovs, V.; Mišina, I.; Pugajeva, I.; Soliven, A.; Segliņa, D. The impact of different baking conditions on the stability of the extractable polyphenols in muffins enriched by strawberry, sour cherry, raspberry or black currant pomace. LWT Food Sci. Technol. 2016, 65, 946–953. [Google Scholar] [CrossRef]
- Barreira, J.C.; Arraibi, A.A.; Ferreira, I.C. Bioactive and functional compounds in apple pomace from juice and cider manufacturing: Potential use in dermal formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lācis, G.; Siger, A.; Michalak, M.; Soliven, A.; Segliņa, D. Phenolic compounds in different fruit parts of crab apple: Dihydrochalcones as promising quality markers of industrial apple pomace by-products. Ind. Crop. Prod. 2015, 74, 607–612. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, L.-Z.; Zhao, L.; Shu, G.; Yuan, Z.-X.; Fu, H.-L.; Lv, C.; Lin, J.-C. Optimization of the ultrasound-assisted extraction of antioxidant phloridzin from Lithocarpus polystachyus Rehd. using response surface methodology. J. Sep. Sci. 2017, 40, 4329–4337. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Razzaghi, S.E.; Arabhosseini, A.; Turk, M.; Soubrat, T.; Cendres, A.; Kianmehr, M.H.; Perino, S.; Chemat, F. Operational efficiencies of six microwave based extraction methods for orange peel oil. J. Food Eng. 2018, 241, 26–32. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and Ultrasound-Assisted Extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Kimura, M.; Jorge, N. Phytochemicals and antioxidant activity of citrus seed oils. Food Sci. Technol. Res. 2012, 18, 399–404. [Google Scholar] [CrossRef]
- Russo, M.; Bonaccorsi, I.; Inferrera, V.; Dugo, P.; Mondello, L. Underestimated sources of flavonoids, limonoids and dietary fiber: Availability in orange’s by-products. J. Funct. Foods 2015, 12, 150–157. [Google Scholar] [CrossRef]
- Gonçalves, A.C.A. Sweet Cherries from Fundão as Health Promotors: Chemical Characterization and Biological Potential. Master Thesis, Universidade Da Beira Interior, Covilhã, Portugal, 2016. [Google Scholar]
- Wahib, W.; Maingonnat, J.F.; Fleury, U.; El-Maataoui, M.; Renard, C.M. Evolution of cherries texture in brine: Impact of harvest conditions during long-time storage. LWT Food Sci. Technol. 2017, 75, 243–250. [Google Scholar] [CrossRef]
- Farag, M.A.; Eldin, A.B.; Khalifa, I. Valorization and extraction optimization of Prunus seeds for food and functional food applications: A review with further perspectives. Food Chem. 2022, 388, 132955. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2021, 366, 130562. [Google Scholar] [CrossRef]
- Stryjecka, M.; Michalak, M.; Cymerman, J.; Kiełtyka-Dadasiewicz, A. Comparative Assessment of Phytochemical Compounds and Antioxidant Properties of Kernel Oil from Eight Sour Cherry (Prunus cerasus L.) Cultivars. Molecules 2022, 27, 696. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Raczyk, M.; Mišina, I.; Segliņa, D. Impact of Cultivar on Profile and Concentration of lipophilic bioactive compounds in kernel oils recovered from sweet cherry (Prunus avium L.) by-Products. Plant Foods Hum. Nutr. 2016, 71, 158–164. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Ćetković, G.; Čanadanović-Brunet, J.; Pajin, B.; Djilas, S.; Petrović, J.; Lončarević, I.; Stajčić, S.; Vulić, J. Sour cherry pomace extract encapsulated in whey and soy proteins: Incorporation in cookies. Food Chem. 2016, 207, 27–33. [Google Scholar] [CrossRef]
- Okur, I.; Baltacıoğlu, C.; Ağçam, E.; Baltacıoğlu, H.; Alpas, H. Evaluation of the effect of different extraction techniques on sour cherry pomace phenolic content and antioxidant activity and determination of phenolic compounds by FTIR and HPLC. Waste Biomass Valorization 2019, 10, 3545–3555. [Google Scholar] [CrossRef]
- Pasqualone, A.; Laddomada, B.; Spina, A.; Todaro, A.; Guzmàn, C.; Summo, C.; Mita, G.; Giannone, V. Almond by-products: Extraction and characterization of phenolic compounds and evaluation of their potential use in composite dough with wheat flour. LWT Food Sci. Technol. 2018, 89, 299–306. [Google Scholar] [CrossRef]
- Garcia-Perez, P.; Xiao, J.; Munekata, P.E.S.; Lorenzo, J.M.; Barba, F.J.; Rajoka, M.S.R.; Barros, L.; Mascoloti Sprea, R.; Amaral, J.S.; Prieto, M.A.; et al. Revalorization of almond by-products for the design of novel functional foods: An updated review. Foods 2021, 10, 1823. [Google Scholar] [CrossRef]
- Gallardo-Rivera, C.T.; Lu, A.; Treviño-Garza, M.Z.; García-Márquez, E.; Amaya-Guerra, C.; Aguilera, C.; Báez-González, J.G. Valorization of almond (prunus serotina) by obtaining bioactive compounds. Front. Nutr. 2021, 8, 663953. [Google Scholar] [CrossRef]
- Delgado, C.O.; Fleuri, L.F. Orange and mango by-products: Agro-industrial waste as source of bioactive compounds and botanical versus commercial description—A review. Food Rev. Int. 2015, 32, 1–14. [Google Scholar] [CrossRef]
- Siddiq, M.; Brecht, J.K.; Sidhu, J.S. Handbook of Mango Fruit: Production, Postharvest Science, Processing Technology and Nutrition; Wiley-Blackwell: New York, NY, USA, 2017; pp. 1–320. [Google Scholar]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.; Casas, L.; Mantell, C.; de la Ossa, E.M. Impregnation of mango leaf extract into a polyester textile using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of bioactive compounds from mango (Mangifera indica L. var. Carabao) seed kernel with ethanol–water binary solvent systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Ajila, C.; Naidu, K.; Bhat, S.; Rao, U.P. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Coelho, E.M.; de Souza, M.E.A.O.; Corrêa, L.C.; Viana, A.C.; de Azevêdo, L.C.; Lima, M.D.S. Bioactive compounds and antioxidant activity of mango peel liqueurs (Mangifera indica L.) produced by different methods of maceration. Antioxidants 2019, 8, 102. [Google Scholar] [CrossRef]
- Manhongo, T.; Chimphango, A.; Thornley, P.; Röder, M. An economic viability and environmental impact assessment of mango processing waste-based biorefineries for co-producing bioenergy and bioactive compounds. Renew. Sustain. Energy Rev. 2021, 148, 111216. [Google Scholar] [CrossRef]
- Ventura, S.; Nobre, B.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.; Palavra, A. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 461–483. [Google Scholar] [CrossRef]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.A.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V.; et al. Scenedesmus obliquus microalga-based biorefinery—From brewery effluent to bioactive compounds, biofuels and biofertilizers—Aiming at a circular bioeconomy. Biofuels Bioprod. Biorefining 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Fu, W.; Nelson, D.; Yi, Z.; Xu, M.; Khraiwesh, B.; Jijakli, K.; Chaiboonchoe, A.; Alzahmi, A.; Al-Khairy, D.; Brynjolfsson, S.; et al. Bioactive Compounds from Microalgae: Current Development and Prospects. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 54, pp. 199–225. [Google Scholar]
- Kholany, M.; Coutinho, J.A.P.; Ventura, S.P.M. Carotenoid production from microalgae: The portuguese Scenario. Molecules 2022, 27, 2540. [Google Scholar] [CrossRef]
- Prabandono, K.; Amin, S. Biofuel Production from Microalgae. In Handbook of Marine Microalgae: Biotechnology Advances; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 145–158. [Google Scholar] [CrossRef]
- Santoro, I.; Nardi, M.; Benincasa, C.; Costanzo, P.; Giordano, G.; Procopio, A.; Sindona, G. Sustainable and selective extraction of lipids and bioactive compounds from microalgae. Molecules 2019, 24, 4347. [Google Scholar] [CrossRef]
- Liu, X.; Yu, D.; Luo, H.; Li, C. Green solvents for lipid extraction from microalgae to produce biodiesel. Front. Chem. 2022, 10, 1–5. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; López, V.H.; Rodríguez-Rodríguez, J.; Alemán-Nava, G.S.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.; Parra-Saldívar, R. supercritical carbon dioxide and microwave-assisted extraction of functional lipophilic compounds from arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef]
- Mendiola, J.A.; García-Martínez, D.; Rupérez, F.J.; Martín-Álvarez, P.J.; Reglero, G.; Cifuentes, A.; Barbas, C.; Ibañez, E.; Señoráns, F.J. Enrichment of vitamin E from spirulina platensis microalga by SFE. J. Supercrit. Fluids 2008, 43, 484–489. [Google Scholar] [CrossRef]
- Rao, M.P.N.; Xiao, M.; Li, W.-J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Britton, G. The Biochemistry of Natural Pigments; Cambridge University Press: Cambrige, UK, 1983. [Google Scholar]
- Sánchez-Muñoz, S.; Mariano-Silva, G.; Leite, M.O.; Mura, F.B.; Verma, M.L.; da Silva, S.S.; Chandel, A.K. Production of fungal and bacterial pigments and their applications. In Biotechnological Production of Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 327–361. [Google Scholar] [CrossRef]
- Choo, W.S.; Dufossé, L.; Morales-Oyervides, L. Sustainable Production of Bioactive Pigments. Frontiers Media SA: Lausanne, Switzerland, 2021. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of food industry waste for high-value products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef]
- Wani, F.A.; Rashid, R.; Jabeen, A.; Brochier, B.; Yadav, S.; Aijaz, T.; Makroo, H.A.; Dar, B.N. Valorisation of food wastes to produce natural pigments using non-thermal novel extraction methods: A review. Int. J. Food Sci. Technol. 2021, 56, 4823–4833. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, J.; Xie, S.-Y.; Zhang, T.-Y.; Soladoye, O.P.; Aluko, R.E. Red Beetroot Betalains: Perspectives on extraction, processing, and potential health benefits. J. Agric. Food Chem. 2020, 68, 11595–11611. [Google Scholar] [CrossRef]
- Sampaio, S.L.; Lonchamp, J.; Dias, M.I.; Liddle, C.; Petropoulos, S.A.; Glamočlija, J.; Alexopoulos, A.; Santos-Buelga, C.; Ferreira, I.C.; Barros, L. Anthocyanin-rich extracts from purple and red potatoes as natural colourants: Bioactive properties, application in a soft drink formulation and sensory analysis. Food Chem. 2020, 342, 128526. [Google Scholar] [CrossRef]
- Murador, D.C.; Braga, A.R.C.; Martins, P.; Mercadante, A.Z.; de Rosso, V.V. Ionic liquid associated with ultrasonic-assisted extraction: A new approach to obtain carotenoids from orange peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef]
- Kodal, S.P.; Aksu, Z. Phenolic pigment extraction from orange peels: Kinetic modeling. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August–2 September 2017. [Google Scholar]
- Milea, A.; Vasile, A.M.; Cîrciumaru, A.; Dumitrașcu, L.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Valorizations of sweet cherries skins phytochemicals by extraction, microencapsulation and development of value-added food products. Foods 2019, 8, 188. [Google Scholar] [CrossRef]
- Chedea, V.S.; Kefalas, P.; Socaciu, C. patterns of carotenoid pigments extracted from two orange peel wastes (valencia and navel var.). J. Food Biochem. 2010, 34, 101–110. [Google Scholar] [CrossRef]
- Murador, D.C.; Salafia, F.; Zoccali, M.; Martins, P.L.G.; Ferreira, A.G.; Dugo, P.; Mondello, L.; de Rosso, V.V.; Giuffrida, D. Green Extraction Approaches for Carotenoids and Esters: Characterization of Native Composition from Orange Peel. Antioxidants 2019, 8, 613. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosière, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural deep eutectic solvent formulations for spirulina: Preparation, intensification, and skin impact. Algal Res. 2021, 56, 102317. [Google Scholar] [CrossRef]
- Schumann, R.; Häubner, N.; Klausch, S.; Karsten, U. Chlorophyll extraction methods for the quantification of green microalgae colonizing building facades. Int. Biodeterior. Biodegrad. 2005, 55, 213–222. [Google Scholar] [CrossRef]
- Fan, C.; Liu, Y.; Shan, Y.; Cao, X. A priori design of new natural deep eutectic solvent for lutein recovery from microalgae. Food Chem. 2021, 376, 131930. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food waste biorefinery: Sustainable strategy for circular bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Global Market Insights (GMI). Global Carotenoids Market. 2022. Available online: https://www.gminsights.com/industry-analysis/beta-carotene-market (accessed on 17 January 2023).
- Ezekoye, V.; Adinde, R.; Ezekoye, D.; Ofomatah, A. Syntheses and characterization of biodiesel from citrus sinensis seed oil. Sci. Afr. 2019, 6, e00217. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. Application of natural extracts as active ingredient in biopolymer based packaging systems. J. Food Sci. Technol. 2022, 1–15. [Google Scholar] [CrossRef]
- Kuntzler, S.G.; de Almeida, A.C.A.; Costa, J.A.V.; de Morais, M.G. Polyhydroxybutyrate and phenolic compounds microalgae electrospun nanofibers: A novel nanomaterial with antibacterial activity. Int. J. Biol. Macromol. 2018, 113, 1008–1014. [Google Scholar] [CrossRef]
- Karaduman, F.R.; Çulha, S.T.; Horzum, N. Algal nanofibers: Current status and recent developments. Mater. Today Commun. 2022, 33, 104248. [Google Scholar] [CrossRef]
- Moreira, J.B.; Terra, A.L.M.; Costa, J.A.V.; de Morais, M.G. Development of pH indicator from PLA/PEO ultrafine fibers containing pigment of microalgae origin. Int. J. Biol. Macromol. 2018, 118, 1855–1862. [Google Scholar] [CrossRef]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial textile: Recent developments and functional perspective. Polym. Bull. 2021, 79, 5747–5771. [Google Scholar] [CrossRef] [PubMed]
- Provin, A.P.; de Aguiar Dutra, A.R. Circular economy for fashion industry: Use of waste from the food industry for the production of biotextiles. Technol. Forecast. Soc. Chang. 2021, 169, 120858. [Google Scholar] [CrossRef]
- Singhee, D. Review on Natural Dyes for Textiles from Wastes. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Verma, M.; Gahlot, N.; Singh, S.S.J.; Rose, N.M. UV protection and antibacterial treatment of cellulosic fibre (cotton) using chitosan and onion skin dye. Carbohydr. Polym. 2021, 257, 117612. [Google Scholar] [CrossRef]
- Moldovan, S.; Ferrandiz, M.; Franco, E.; Mira, E.; Capablanca, L.; Bonet, M. Printing of cotton with eco-friendly, red algal pigment from Gracilaria sp. IOP Conf. Series Mater. Sci. Eng. 2017, 254, 192011. [Google Scholar] [CrossRef]
- Algae Planet. Vollebak Launches Black Algae Dyed T-Shirt Commercially. Available online: https://algaeplanet.com/vollebak-launches-black-algae-dyed-t-shirt-commercially/ (accessed on 16 December 2022).
- Balakrishnan, N.K.; Koenig, K.; Seide, G. The effect of dye and pigment concentrations on the diameter of melt-electrospun polylactic acid fibers. Polymers 2020, 12, 2321. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, H.J.; Jang, J.Y.; Shin, H.S. Development of coaxial alginate-PCL nanofibrous dressing for controlled release of Spirulina extract. J. Biomater. Sci. Polym. Ed. 2018, 29, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.-M.; Min, S.K.; Lee, H.C.; Kwon, Y.S.; Jung, M.H.; Shin, H.S. Spirulina-PCL nanofiber wound dressing to improve cutaneous wound healing by enhancing antioxidative mechanism. J. Nanomater. 2016, 2016, 6135727. [Google Scholar] [CrossRef]
| Search Terms | Science Direct | Web of Science |
|---|---|---|
| “green extraction” AND “microalgae” AND “bioactive compounds” | 245 | 118 |
| “green extraction” AND “food wastes” AND “bioactive compounds” | 240 | 442 |
| “green extraction” AND “microalgae” AND “bioactive pigments” | 7 | 21 |
| “green extraction” AND “food wastes” AND “bioactive pigments” | 7 | 342 |
| “NADES“ AND “microalgae” AND “bioactive compounds” | 39 | 0 |
| “NADES“ AND “food wastes” AND “bioactive compounds” | 49 | 0 |
| Total | 587 | 923 |
| Method | Advantages | Disadvantages | |
|---|---|---|---|
| Conventional | Maceration | Simple method Can used for bulk and small extractions. | Often presents low extraction yield Time consuming Large quantities of solvent |
| Soxhlet | Simple method No filtration of extract required | Large volumes of solvent Long extraction times | |
| Non-conventional | UAE | Quicker extraction times Higher efficiency Low temperatures Lower energy costs Higher extraction yield | Energy loss may occur due to wastage of ultrasonic energy though ultrasonic bath |
| MAE | Simple method Low volumes of solvent required Low extraction times needed | May require filtration after extraction High instrument cost | |
| SC | High extraction yield Environmentally friendly process No product degradation Faster process | Use of co-solvents Extraction depends on flow rate High cost of equipment High pressure | |
| Waste | Extraction Method | Bioactive Compounds Detected | Ref. |
|---|---|---|---|
| Pomace | Maceration, 60 min, Water | Polyphenols: gallic acid, catechin, chlorogenic acid, and rutin | [26] |
| UAE, 30 min, Water | |||
| MAE, 1.5 min, Water | |||
| PLE, 5 min | |||
| Conventional extraction with temperature, 20 min, 80 °C, Ethanol/Water (50:50) | Polyphenols: hydroxycinnamic acids, flavanols, and chalcone (phoretin and phloridzin) | [28] | |
| UAE, 5 min, 20 °C, Acetone/Water (70:30) | Polyphenols: flavanols, dihydrochalcones (phloridzin and phloretin-20-xyloglucoside), flavonols and cinnamic acids (chlorogenic and caffeic acids) | [29] | |
| Seed | UAE, 15 min, 40 Hz, Hexane/Ethanol/Acetone | Tocopherols (Vitamin E) | [30] |
| Maceration, 2 h, 25 °C, Acetone/Water (60:40) | Polyphenols: phloridzin, quercetin, and epicatechin | [31] | |
| UAE, 5 min, 35 °C, n-hexane | Tocopherols (Vitamin E) | [32] | |
| Peel | Maceration, Acetone, 5 min | Phenolic compounds: gallic acid, catechin | [33] |
| Turbo-extraction, 30 min, 40 °C, Ethanol | Phenolic compounds: phenolic acids | [34] |
| Waste | Extraction Method | Bioactive Compounds Detected | Ref. |
|---|---|---|---|
| Peel | UAE and PEF, Ethanol, 15–180 min, 20–80 °C | Hesperidin, polyphenols and vitamin C | [46] |
| UAE, 400 W, 30 min, 50% Ethanol | Vitamin C and phenolics (mainly hesperidin) | [47] | |
| Seed | Soxhlet, 40–60 °C, 6 h, Petroleum ether | Tocopherols | [48] |
| Maceration, Methanol | Limonoids and phenolic compounds: flavanones, phenolic acids | [49] |
| Waste | Extraction Method | Bioactive Compounds Detected | Ref. |
|---|---|---|---|
| Seeds | Maceration, Ethanol/Water, 80 °C | Phenolic compounds, flavonoids and flavonols | [25] |
| Soxhlet, 6 h, Diethyl ether | Tocochromanols and polyphenols | [54] | |
| UAE, 5 min, 35 °C, n-hexane | Tocochromanols (tocopherol and sitosterol) | [55] | |
| Pomace | Maceration, 2 h, Ethanol/Water (80:20) | Phenolic compounds | [50] |
| Maceration, 2 h, Ethanol/Water (50/50) | Polyphenols | [56] | |
| MAE, 90 s, 900 W, 60 °C | Phenolic compounds | [57] | |
| Maceration, 30 min, 50 °C, Methanol/water (80/20) | |||
| UAE, 5, 10, 15 min, 24 kHz, 400 W, Room temperature, Methanol/Water (80/20) |
| Waste | Extraction Method | Bioactive Compounds Detected |
|---|---|---|
| Hull | Maceration, Ethanol/Acetone, 24 h | Phenolic and flavonoid compounds |
| UAE, 51.2% Ethanol, 40 kHz, 300 W, 13 min | Phenolic acids and catechin | |
| Shell | Soxhlet | Phenolic compounds |
| SFE, Petroleum ether, 40–60 °C, 90 min, 11 kPa | Lignin | |
| Kernel | MAE, NaOH, 2450 MHz, 800 W, 23–67 °C, 3 min | Phenolic compounds (Lignans) |
| SFE, butane, −0.09 MPa | Phenolic, phylosterol, tocopherol and tocotrienol compounds | |
| Skin | UAE, Water, 20 kHz, 100 W, 20 min | Phenolic compounds, lipids, and proteins. |
| MAE, 70% Ethanol, 2450 MHz, 100 W, 60 s | Flavonol rutinosides |
| Waste | Extraction Method | Bioactive Compounds Detected | Ref. |
|---|---|---|---|
| Leaves | Enhanced solvent extraction using a mixture of CO2/Methanol (50%) at 120 bar and 100 °C | Polyphenols | [63] |
| Kernel | Maceration, 1 h, Room temperature, Ethanol/Water | Polyphenols (gallic acid, caffeic acid, rutin and penta-O-galloyl-b-D-glucose) | [64] |
| Peel | 80% Acetone | Polyphenols | [65] |
| Alcoholic maceration and maceration with pectinase | Flavanols | [66] |
| Microalgae. | Extraction Method | Bioactive Compounds Detected | Ref. |
|---|---|---|---|
| Chlorella sp. | Maceration, DES | Lipids | [16] |
| UAE, IL | Lipids | [74] | |
| Spirulina platensis | SFE, CO2 and Ethanol/Water, 50 min | Tocopherols and fatty acids | [75] |
| MAE, Methanol/Ethyl acetate/Light petroleum, 400 W, 1 bar, 15 min | Tocopherols and fatty acids | ||
| SFE, CO2 and Ethanol, 75 min | Tocopherols | [76] |
| Waste | Extraction Method | Reported Yield | Ref. |
|---|---|---|---|
| Apple peel | Solvent extraction, 80% acetone | Cyanidin: 169.7 g/100 g of dry peel | [33] |
| Apple seeds | UAE, Hexane/Acetone, 15 min, 40 Hz | β-carotene: 1.370–25.800 μg/g of dry weight Lycopene: 0.080–5.370 µg/g of dry weight | [30] |
| Orange peel | UAE, Ionic liquid extraction: [BMIM][Cl] | Carotenoid: 32.08 ± 2.05 μg/g of dry peel | [87] |
| Orange peel | UAE, Acetone | Carotenoid: 7.88 ± 0.59 μg/g of dry peel | [87] |
| Orange peel | Soxhlet, Ethanol, 79 °C, 40:1 liquid/solid ratio | Phenolic pigment: 57 g/g of dry peel | [88] |
| Orange peel | UAE | β-carotene: 0.63 mg/100 g of dry peel | [47] |
| Orange peel | Solvent extraction, Ethanol: hexane (4:3) | β-carotene: 4.99 mg/100 g of dry peel | [83] |
| Orange peel | UAE and PEF, Ethanol, 20–80 °C, 15–180 min | Carotenoids: 52.98 μg/g of dry peel | [46] |
| Orange seeds | Soxhlet, petroleum ether, 40–60 °C, 6 h | Carotenoids: 0.32 mg/kg of dry mass | [48] |
| Mango peel | Solvent extraction, 80% acetone | Anthocyanins:203 ± 5.03; 326 ± 3.05 μg/g of dry peel Carotenoids: 73.5 ± 0.53; 81.0 ± 0.42 μg/g of dry peel | [65] |
| Sweet Cherry Skin | UAE, 70% Ethanol, 40 kHz, 100 W, 40 °C | Carotenoids: 12.2 mg/g of dry skin | [89] |
| Cherrie seed | Soxhlet, 6 h, diethyl ether | Carotenoids: 0.56–1.61 mg/100 g of dry mass | [54] |
| Cherrie pomace | Stirring, Ethanol/Water (80:20), 2 h | Anthocyanins: 1076.97–2183.55 µg/g of dry weight | [50] |
| Red beetroot | Maceration, methanol/water (80/20) | Betalains: 0.47 mg betanin/100 g and 0.26 mg betanin/100 g | [85] |
| Red beetroot | UAE, 37 and 52 °C, 165 W, 25 kHz, 90 min | Betalains: 4.20 and 2.80 mg/g of betacyanins and betaxanthins | [85] |
| Microalgae | Extraction Method | Reported Yield | Ref. |
|---|---|---|---|
| Chlorella sp. | Maceration, Acetone | Chlorophyll-a: 6 mg/L | [94] |
| Stichococcus sp. | Maceration, Acetone | Chlorophyll-a: 7 mg/L | [94] |
| Spirulina sp. | UAE, NADES: Glycerol/glucose/water | Chlorophylls: 0.50 mg/g Carotenoids: 0.22 mg/g Phycocyanin: 3.96 mg/g | [93] |
| MAE, Methanol/Ethyl acetate/Light petroleum, 400 W, 1 bar, 15 min | Carotenoids: 629 ± 0.13 µg/g | [75] | |
| SFE, CO2 and Ethanol/Water, 50 min | Carotenoids: 283 ± 0.10 µg/g | ||
| Scenedesmus sp. | UAE, 70 min, 60 °C, 40 KHz, 300 W, NADES: Fen-Thy. | Carotenoid (Lutein): 4.4 mg/g | [95] |
| Waste | Application | Ref. |
|---|---|---|
| Apple peel | Anthocyanins impregnation for pH-sensitive packaging to evaluate food freshness and quality | [84] |
| Microalgae | Phenolic compounds extracted from Spirulina sp. were incorporated in the production of nanofibers with antibacterial activity | [101] |
| Microalgae | Phycocyanin derived from Spirulina sp. as bioindicator in food packaging due to changing pH | [103] |
| Waste | Application | Ref. |
|---|---|---|
| Apple pomace | Di-hydrochalcones used in treatment of type-2 diabetes, obesity, or hyperglycaemia attenuation | [40] |
| Apple pomace | Phenolic compounds in regulation of sebum production | [40] |
| Apple seeds | Tocopherols (Vitamin E) prevention of heart disease and prostate cancer | [30] |
| Mango leaves | Mangiferin in prevention of chronic diseases including cancer and neurodegenerative and cardiovascular diseases | [65] |
| Almond | α-eleostearic acid which is effective in the suppression of the growth of cancer cells | [60] |
| Cherry seeds | Phenolic compounds in various pharmaceutical applications | [25] |
| Cherry pomace | Inhibition of α-glucosidase, treatment of inflammatory diseases (as diabetes, gout, and arthritis), hemolytic anemia, cancer, neurological and cardiovascular pathologies | [50] |
| Microalgae | Carotenoids in photoprotection of the skin against UV light, prevention of liver fibrosis and cancer (colorectal) | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.; Sales, H.; Pontes, R.; Nunes, J.; Gouveia, I. Food Wastes and Microalgae as Sources of Bioactive Compounds and Pigments in a Modern Biorefinery: A Review. Antioxidants 2023, 12, 328. https://doi.org/10.3390/antiox12020328
Martins R, Sales H, Pontes R, Nunes J, Gouveia I. Food Wastes and Microalgae as Sources of Bioactive Compounds and Pigments in a Modern Biorefinery: A Review. Antioxidants. 2023; 12(2):328. https://doi.org/10.3390/antiox12020328
Chicago/Turabian StyleMartins, Rodrigo, Hélia Sales, Rita Pontes, João Nunes, and Isabel Gouveia. 2023. "Food Wastes and Microalgae as Sources of Bioactive Compounds and Pigments in a Modern Biorefinery: A Review" Antioxidants 12, no. 2: 328. https://doi.org/10.3390/antiox12020328
APA StyleMartins, R., Sales, H., Pontes, R., Nunes, J., & Gouveia, I. (2023). Food Wastes and Microalgae as Sources of Bioactive Compounds and Pigments in a Modern Biorefinery: A Review. Antioxidants, 12(2), 328. https://doi.org/10.3390/antiox12020328









