Abstract
Platycosides, saponins from balloon flower root (Platycodi radix), have diverse health benefits, such as antioxidant, anti-inflammatory, anti-tussive, anti-cancer, anti-obesity, anti-diabetes, and whitening activities. Deglycosylated platycosides, which show greater biological effects than glycosylated platycosides, are produced by the hydrolysis of glycoside moieties in glycosylated platycosides. In this review, platycosides are classified according to the chemical structures of the aglycone sapogenins and also divided into natural platycosides, including major, minor, and rare platycosides, depending on the content in Platycodi radix extract and biotransformed platycosides. The biological activities of platycosides are summarized and methods for deglycosylation of saponins, including physical, chemical, and biological methods, are introduced. The biotransformation of glycosylated platycosides into deglycosylated platycosides was described based on the hydrolytic pathways of glycosides, substrate specificity of glycosidases, and specific productivities of deglycosylated platycosides. Methods for producing diverse and/or new deglycosylated platycosides are also proposed.
1. Introduction
Platycodon grandiflorum (Jacq.) A.DC., a perennial herbaceous flowering plant species [1], belongs to the family Campanulaceae, is commonly known as the balloon flower, and is used as a traditional herbal medicine for the treatment of cough, phlegm, sore throat, lung abscess, chest pain, dysuria, and dysentery [2]. In Northeast Asia, P. grandiflorum root (Platycodi radix), an edible vegetable, is widely used as a food supplement to make side dishes (seasoned balloon flower root), desserts (balloon flower root sweet), teas (balloon flower root tea), flavored liquor, and traditional herbal medicines for the treatment of pulmonary diseases and respiratory disorders, including sore throat, bronchitis, tonsillitis, asthma, and tuberculosis [2,3,4,5].
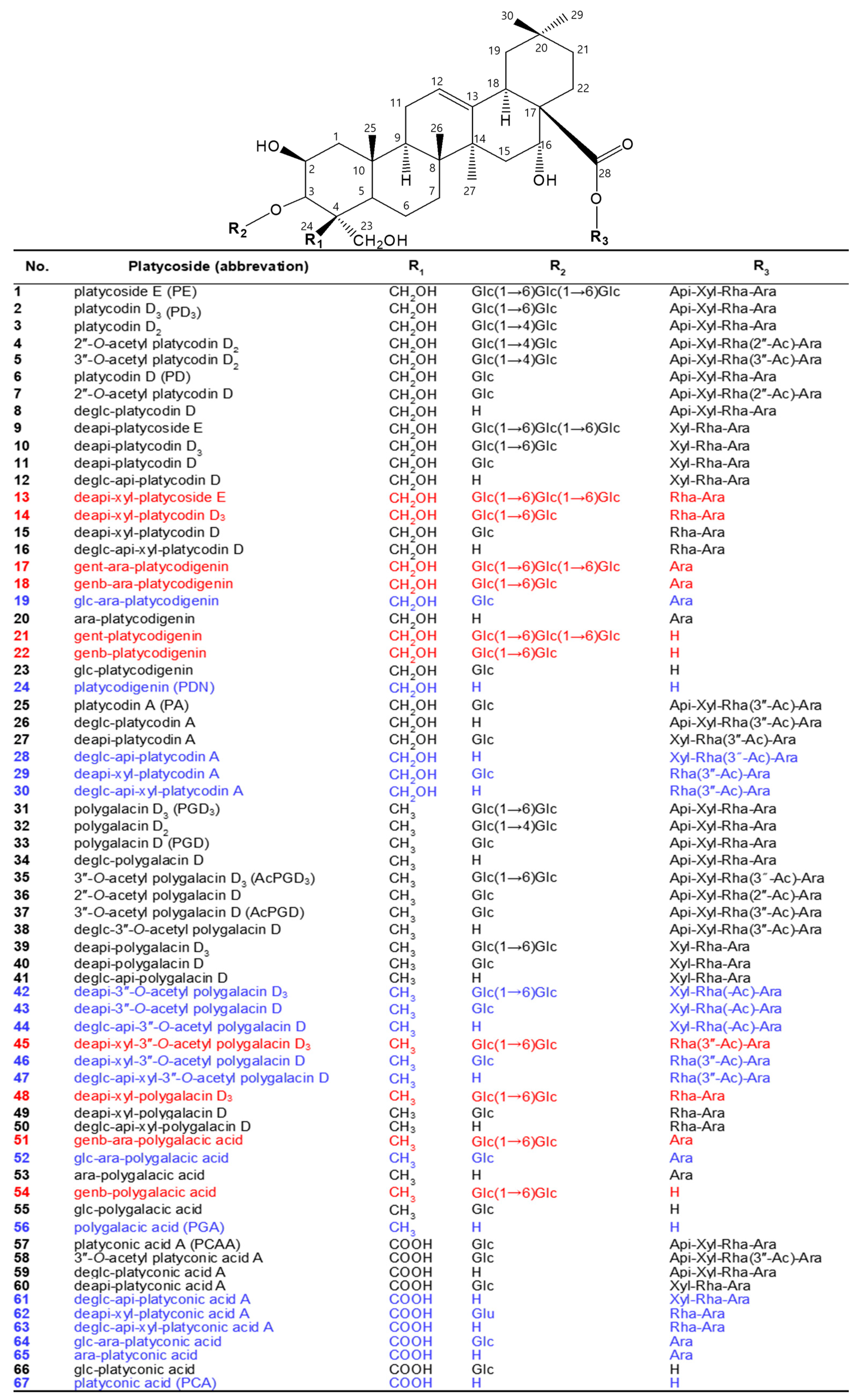
Platycosides, saponins of Platycodi radix, are the main components of Platycodi radix extract and the principal components responsible for these biological activities in P. grandiflorum [2,3]. They possess a wide range of biological properties, including not only antioxidant [6,7,8,9] but also anti-inflammatory [9,10,11,12,13], anti-tussive [14,15], anti-proliferative [16,17,18,19,20,21,22,23,24,25], anti-obesity [26,27,28,29,30,31,32], anti-diabetes [33,34,35], anti-allergic [36,37], immunomodulatory [38,39,40,41], cardiovascular protective [42,43,44], hepatoprotective [45,46], and whitening [9,47] effects. Platycosides are glycosylated compounds consisting of a non-glycoside component (aglycone) with a pentacyclic triterpene skeleton, and C-3 and C-28 glycoside groups (glycone) with 4~7 molecules, such as β-d-glucopyranose, β-d-apiofuranose, β-d-xylopyranose, α-l-rhamnopyranose, and α-l-arabinopyranose (Figure 1).
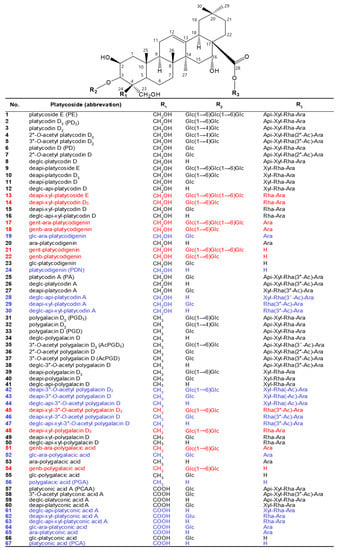
Figure 1.
Chemical structures of major and minor platycosides and their glycoside moiety-hydrolyzed platycosides. The functional groups at C-4 (R1) of PDN-type (platycosides 1−30), PGA-type (31−56), and PCA-type (57−67) platycosides are CH2OH, CH3, and COOH, respectively. Platycosides contain glycosides at C-3 and C-28. The glycosides at C-3 (R2) are H, Glc, Glc-Glc, and Glc-Glc-Glc, whereas those at C-28 (R3) are H, Ara, Rha-Ara or Rha(-Ac)-Ara, Xyl-Rha-Ara or Xyl-Rha(-Ac)-Ara, and Api-Xyl-Rha-Ara or Api-Xyl-Rha(-Ac)-Ara. Glc, β-d-glucopyranosyl-; Api, β-d-apiofuranosyl-; Xyl, β-d-xylopyranosyl-; Rha, α-l-rhamnopyranosyl-; Ara, α-l-arabinopyranosyl-; Ac, acetyl; genb, gentiobiosyl; gent, gentiotriosyl; deapi, deapiosylated; deglc, deglucosylated; deapi-xyl, deapiose-xylosylated; deglc-api, deglucose-apiosylated; and deglc-api-xyl, deglucose-apiose-xylosylated. Blue indicates unreported platycosides, which can be suggested by the reported glycosidases. Red indicates unreported platycosides, which cannot be suggested by the reported glycosidases.
The absorption of glycosylated saponins in the gastrointestinal tract is poor. In contrast, deglycosylated saponins are more readily absorbed in the human gastrointestinal tract into the bloodstream and function as active compounds because of their lower molecular weight and greater hydrophobicity [48,49]. The biological activities of deglycosylated saponins are superior to those of glycosylated saponins [4,48]. Therefore, many studies have focused on the hydrolysis of the glycoside moieties in glycosylated saponins using chemical, physical, and biological transformation techniques [48,50]. Platycosides from P. grandiflorum detected by high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR) contain more than 4 glycosides; thus, platycosides with less than 4 glycosides are few or absent in Platycodi radix [4,8,51,52,53,54,55,56,57], indicating that natural major platycosides are highly glycosylated saponins compared to natural ginseng saponins with 2~4 glycosides [48,49,58]. To acquire bioactive deglycosylated platycosides, hydrolysis of the glycoside moieties in natural glycosylated platycosides is required.
Reviews on platycosides have mainly focused on their pharmacological effects [2,3,53,59]. However, reviews specifically focusing on the biotransformation of platycosides have not been reported. The current review describes the transformation methods of platycosides by hydrolysis of their glycoside moieties for the production of bioactive deglycosylated platycosides. Furthermore, we propose the production of diverse and/or new deglycosylated platycosides using glycosidases.
2. Classification of Platycosides
Platycosides are structurally classified into platycodigenin (PDN)-, polygalacic acid (PGA)-, platyconic acid (PCA)-, methyl-PCA-, PCA lactone-, and other-type platycosides according to the chemical groups at C-4 of the aglycone sapogenins [2,3]. Platycosides are also divided into natural platycosides, including major, minor, and rare platycosides (depending on their content in Platycodi radix extract), and biotransformed platycosides. Platycosides determined as distinct peaks by HPLC-UV can be considered major platycosides, while platycosides detected by only HPLC-evaporative light scattering detector (ELSD) or LC-MS except for platycosides detected by HPLC-UV can be considered minor platycosides. In addition, residual platycosides as new unusual platycosides in nature can be defined as rare platycosides.
2.1. Chemical Structural Classification
Platycosides are pentacyclic triterpene sapogenins containing two glycosidic side chains at C-3 and C-28 (Figure 1). The aglycone sapogenins of platycosides contain methyl, carboxyl, hydroxymethyl, and other groups of C-4 (R1). In nature, one side chain is a moiety of 1~3 glucoside molecules (β-d-glucopyranosyl-β-d-glucopyranosyl-β-d-glucopyranosyl residues) linked to C-3 (R2) of the sapogenin by a glycosidic bond, whereas the other chain is an oligosaccharide moiety of arabinoside, rhamnoside or acetyl-rhamnoside, xyloside, and apioside sequentially (β-d-apiofuranosyl-β-d-xylopyranosyl-α-l-rhamnopyranosyl or acetyl-rhamnopyranosyl-α-l-arabinopyranosyl residues) bound to C-28 (R3) in the aglycone by an ester linkage [4]. Thus, platycosides have diverse chemical structures according to the groups of C-4 and the types and numbers of the glycosides attached to C-3 and C-28.
Depending on the chemical groups at C-4 of sapogenins, platycosides are divided into five types, including PDN- (R1 = CH2OH), PGA- (R1 = CH3), PCA- (R1 = COOH), methyl-PCA- (R1 = COOCH3), and PCA lactone- (R1 = -COO-, linked to C-2), and the other-type platycosides with different groups at C-2, C-14, C-16, C-17, and C-21 [2,3]. Although the contents of specific platycosides in Platycodi radix extracts depend on P. grandiflorum species, cultivation regions, and solvent extraction methods, the contents of PDN- (compounds 1–30), PGA- (compounds 31–56), PCA- (compounds 57–67), and other-type platycosides to total platycosides are 50−55%, 30−35%, 7−13%, and >1%, respectively [4,8,51,52,55]. PDN-, PGA-, and PCA-type platycosides are major and minor platycosides, whereas methyl- PCA-, PCA lactone-, and the other-type platycosides are rare platycosides.
2.2. Natural Platycosides
There are 11 major platycosides as determined by HPLC-UV, LC-MS, or NMR: platycoside E (PE), platycodin D3 (PD3), platycodin D (PD), deapiosylated PE (deapi-PE), deapi-PD3, deapi-PD, platycodin A (PA), polygalacin D3 (PGD3), polygalacin D (PGD), 3”-O-acetyl polygalacin D3 (AcPGD3), and platyconic acid A (PCAA) [54,60,61,62,63,64]. These major platycosides have been used as substrates for the production of deglycosylated platycosides by glycosidases. There are 11 minor platycosides as detected by only HPLC-ELSD or LC-MS: 2″-O-acetyl platycodin D2, 3″-O-acetyl platycodin D2, platycodin D2, deapi-platycodin D2, 2″-O-acetyl platycodin D, 3″-O-acetyl platycodin D, polygalacin D2, 2″-O-acetyl polygalacin D2, 2″-O-acetyl polygalacin D, 3″-O-acetyl polygalacin D (AcPGD), and 3″-O-acetyl platyconic acid A [55,57,65]. Based on the literature, 76 compounds of platycosides have been isolated from P. grandiflorum [3]. Thus, there are 54 reported compounds of rare platycosides.
2.3. Biotransformed Platycosides
Biotransformed platycosides are produced from natural major platycosides by the hydrolysis of the glycoside moieties using glycosidases, including recombinant enzymes, commercial enzymes, purified enzymes provided by reagent companies, crude enzymes, and cells containing enzymes with reactions such as partial deglucosylation of only outer and middle glucosides [51,62,66,67,68,69,70], deglucosylation [4,71], deglucose-apiose-xylosylation [72], deglucose-apiose-xylose-rhamnosylation [73], deapiosylation with partial deglucosylation [8], deapiose-xylosylation with partial deglucosylation [74,75,76], and deapiose-xylose-rhamnose-arabinosylation with partial deglucosylation [52] (Table 1). Nineteen biotransformed platycosides, including deglucosylated PD (deglc-PD), deglc-PA, deglc-AcPGD, deglc-PCAA, deapi-PGD, deapi-PA, deapi-PGD3, deapi-PGD, deapi-PCA, deapiose-xylosylated PD (deapi-xyl-PD), deapi-xyl-PGD, deglc-api-xyl-PD, deglc-api-PGD, deglc-api-xyl-PGD, glucosyl-PDN (glc-PDN), glc-PGA, glc-PCA, arabinosyl-PDN (ara-PDN), and ara-PGA, have been reported. In total, 18 biotransformed platycosides, including deapi-AcPGD3, deapi-AcPGD, deapi-xyl-PA, deapi-xyl-AcPGD, deapi-xyl-PCAA, deglc-api-PA, deglc-api-xyl-PA, deglc-api-AcPGD, deglc-api-xyl-AcPGD, deglc-api-PCAA, deglc-api-xyl-PCAA, glc-ara-PDN, glc-ara-PGA, glc-ara-PCA, ara-PCA, PDN, PGA, and PCA, were suggested by the reactions of reported enzymes with unused substrates, combination of reported enzymes, and new application of known enzymes to platycoside hydrolysis (blue color in Figure 1, Figure 2, Figure 3 and Figure 4). On the other hand, platycosides that could not be suggested by the reported glycosidases are marked with red color in Figure 1, Figure 2, Figure 3 and Figure 4. Currently, 37 platycosides, including biotransformed and suggested biotransformed platycosides, have been introduced.

Table 1.
Biosynthesis of biotransformed platycosides.

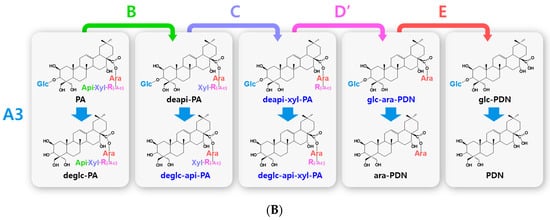
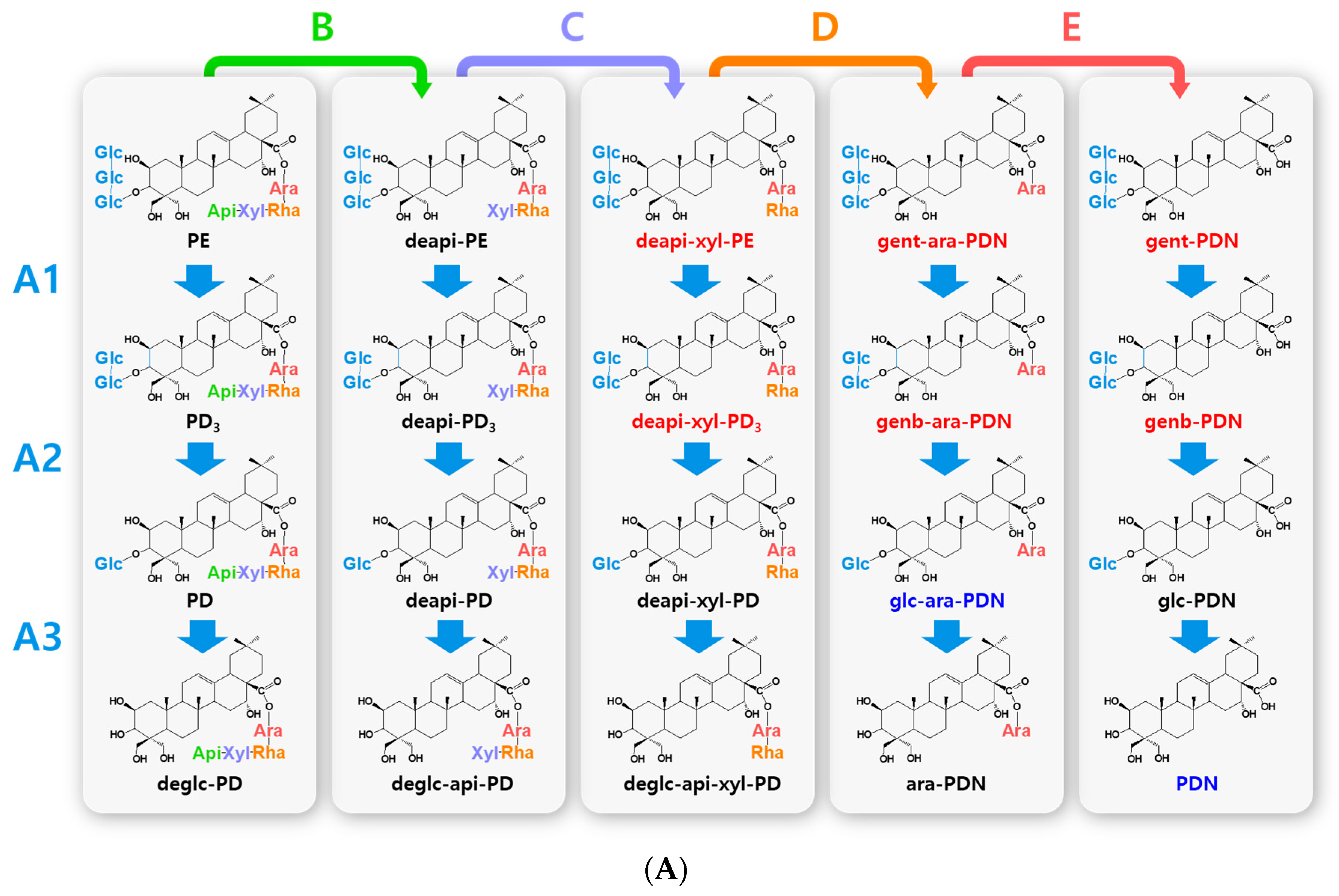
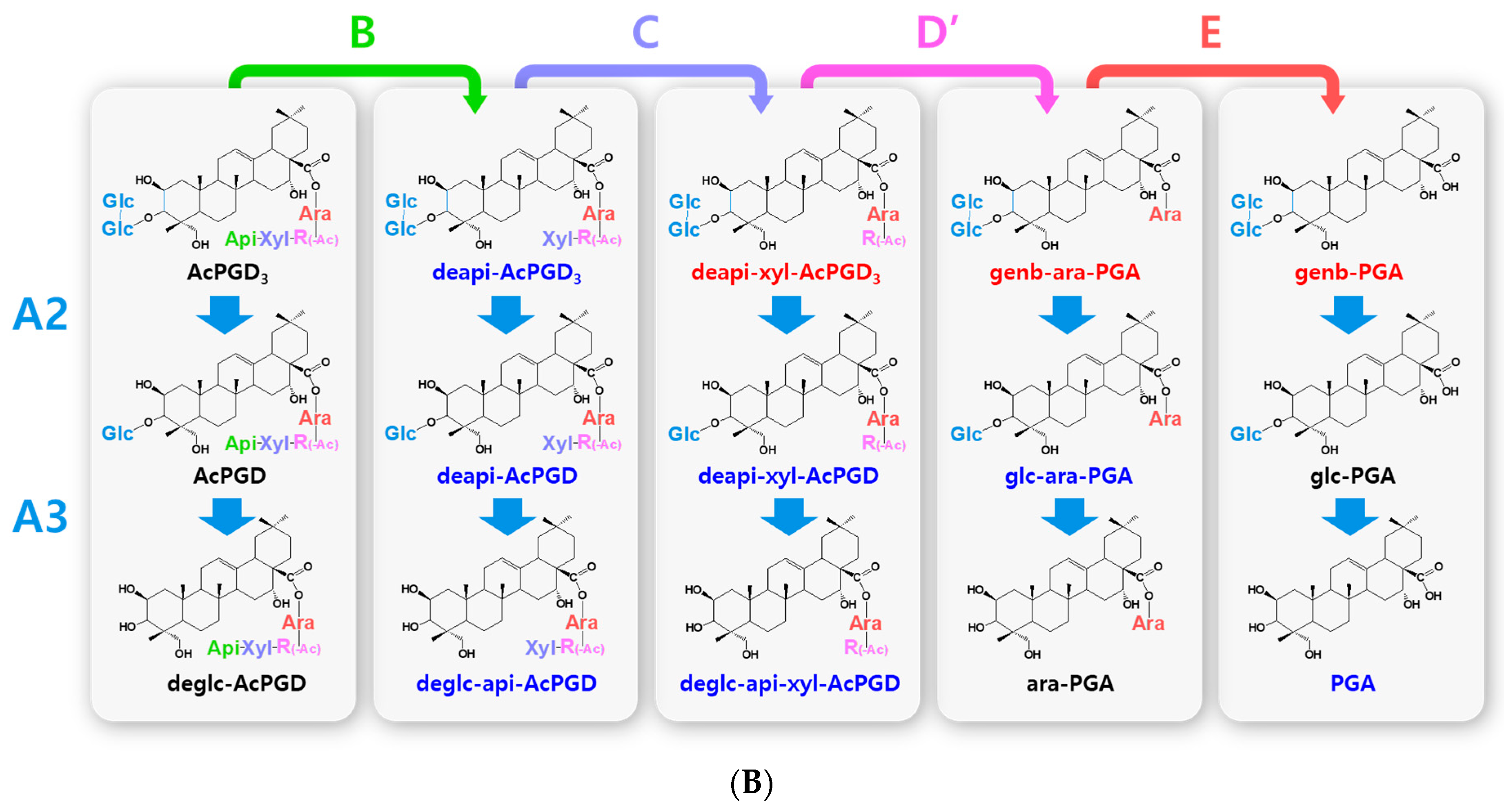
Figure 2.
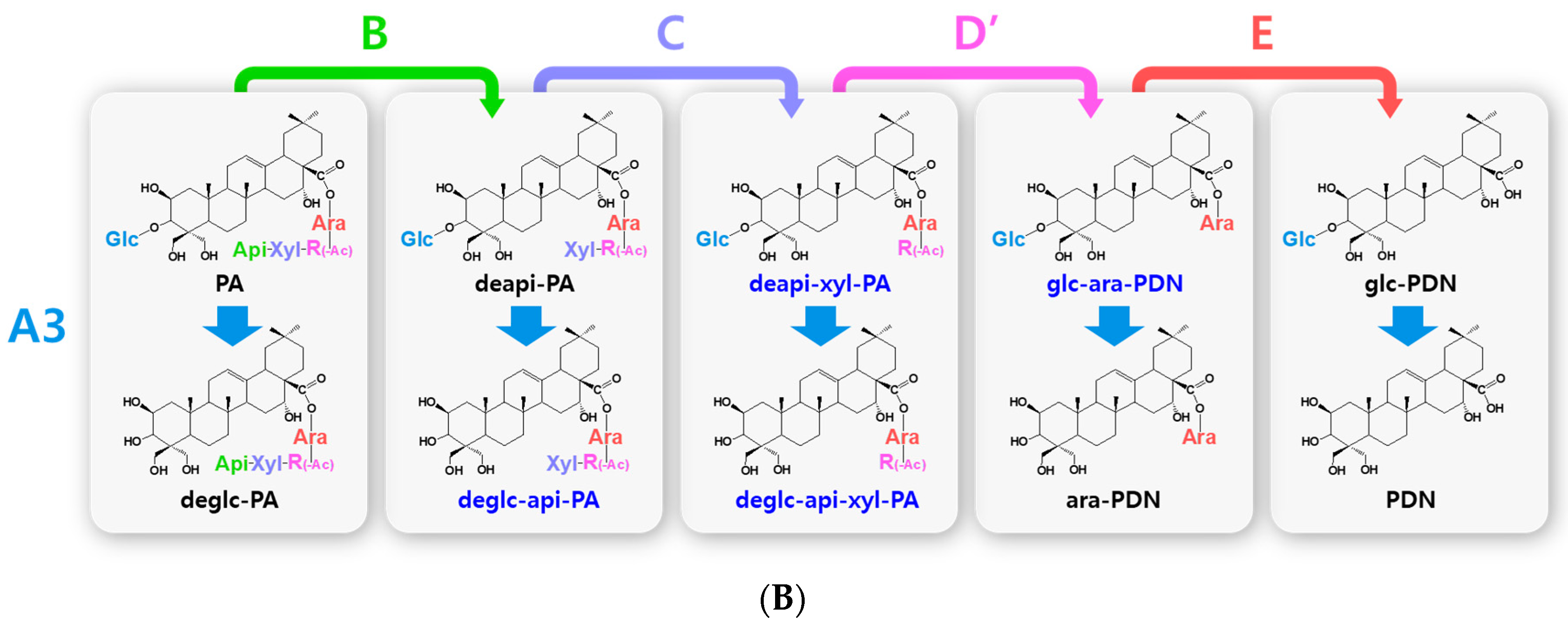
Hydrolytic pathways of the PDN-type platycosides by glycosidases. The hydrolytic pathways of the outer, middle, and inner glucoside residues linked to C-3 by β-d-glucosidases are assigned as A1, A2, and A3, respectively. The hydrolytic pathways of the four residues, including apioside, xyloside, rhamnoside or acetyl-rhamnoside, and arabinoside, linked to C-28 in PDN-type platycosides by β-d-apiosidase, β-d-xylosidase, α-l-rhamnosidase, and α-l-arabinosidase are assigned as B, C, D or D′, and E, respectively. (A) Hydrolytic pathways from PE to PDN. (B) Hydrolytic pathways from PA to PDN. R(-Ac), acetyl-α-l-rhamnopyranosyl-. Blue indicates unreported platycosides, which can be suggested by the reported glycosidases. Red indicates unreported platycosides, which cannot be suggested by the reported glycosidases.

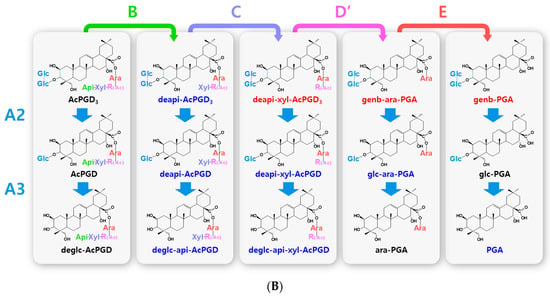
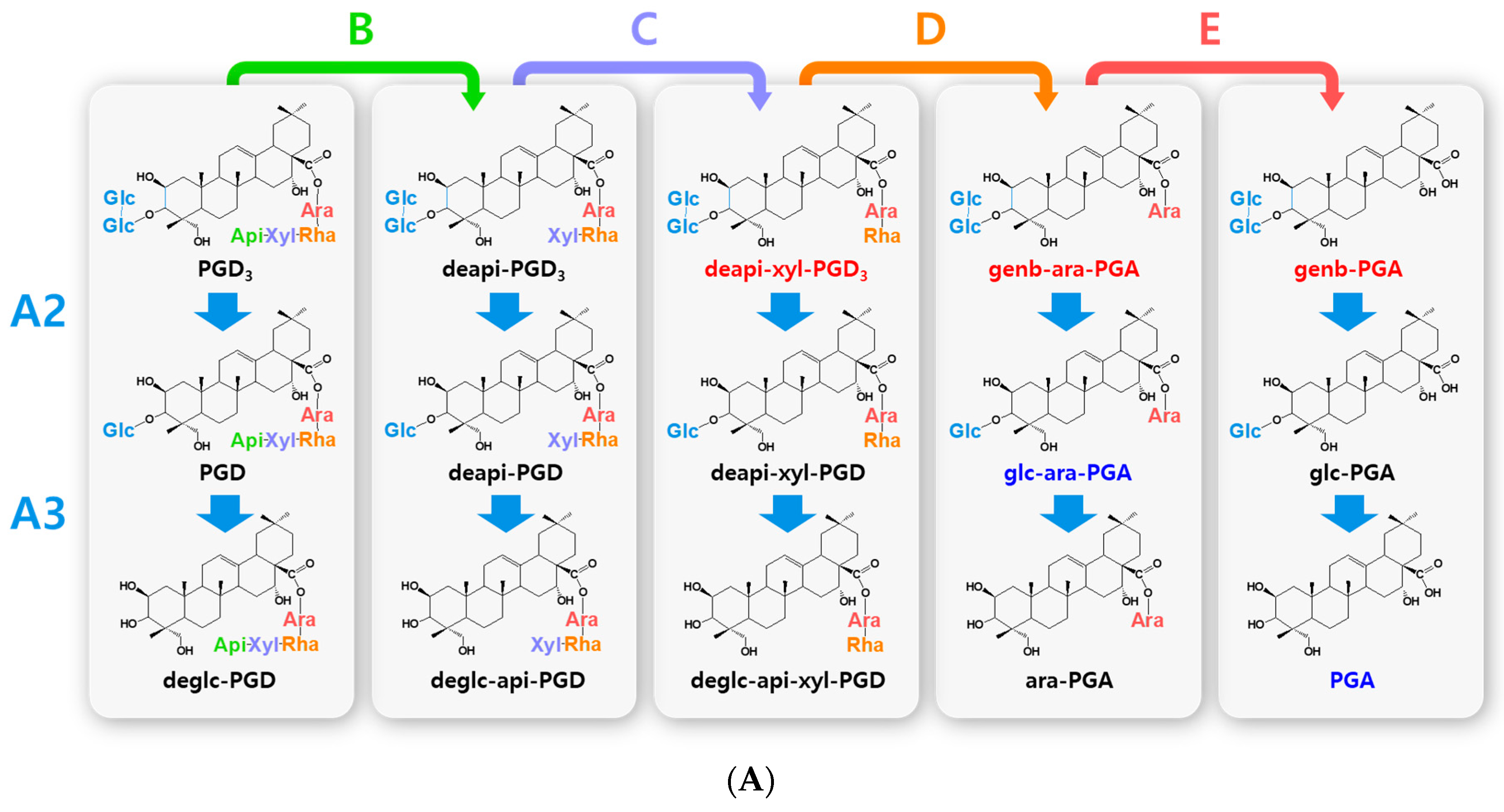
Figure 3.
Hydrolytic pathways of the PGA-type platycosides by glycosidases. The hydrolytic pathways of the outer and inner glucoside residues linked to C-3 by β-d-glucosidases are assigned as A2 and A3, respectively. The hydrolytic pathways of the four residues, including apioside, xyloside, rhamnoside or acetyl-rhamnoside, and arabinoside, linked to C-28 in PDN-type platycosides by β-d-apiosidase, β-d-xylosidase, α-l-rhamnosidase, and α-l-arabinosidase are assigned as B, C, D or D′, and E, respectively. (A) Hydrolytic pathways from PGD3 to PGA. (B) Hydrolytic pathways from AcPGD3 to PGA. R(-Ac), acetyl-α-l-rhamnopyranosyl-. Blue indicates unreported platycosides, which can be suggested by the reported glycosidases. Red indicates unreported platycosides, which cannot be suggested by the reported glycosidases.
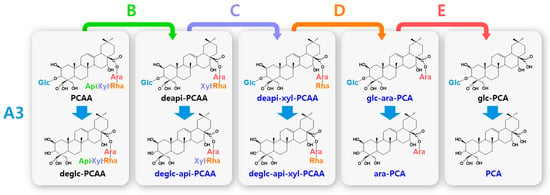
Figure 4.
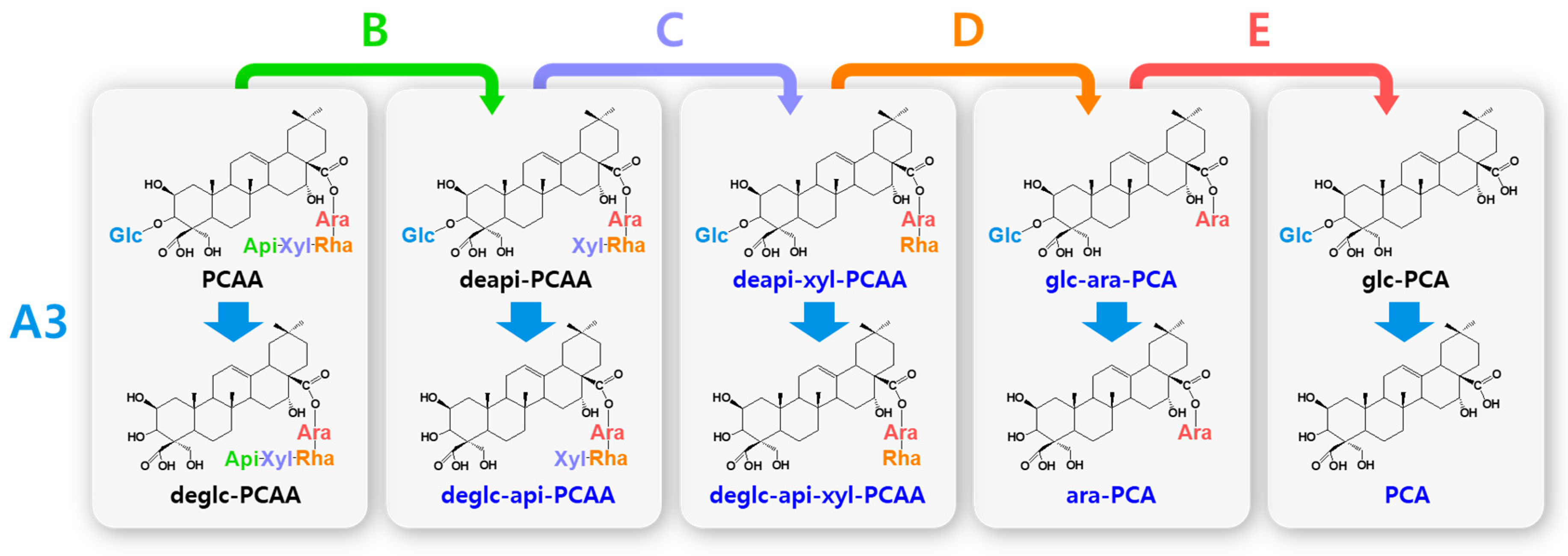
Hydrolytic pathways of the PCA-type platycosides by glycosidases. The hydrolytic pathways of the glucoside residue linked to C-3 by β-d-glucosidase are assigned as A3. The hydrolytic pathways of the four residues, including apioside, xyloside, rhamnoside, and arabinoside, linked to C-28 in PDN-type platycosides by β-d-apiosidase, β-d-xylosidase, α-l-rhamnosidase, and α-l-arabinosidase are assigned as B, C, D, and E, respectively. Blue indicates unreported platycosides, which can be suggested by the reported glycosidases. Red indicates unreported platycosides, which cannot be suggested by the reported glycosidases.
3. Biological Activities of Platycosides
3.1. Health Benefits
Platycosides have a wide range of pharmacological activities, including anti-inflammatory [9,10,11,12,13], apophlegmatic and anti-tussive [14,15], anti-cancer [16,17,18,19,20,21,22,23,24,25], anti-obesity [26,27,28,29,30,31,32], anti-diabetes [33,34,35], anti-allergic [36,37], immunomodulatory [38,39,40,41], cardiovascular protective [42,43,44], and hepatoprotective effects [45,46] and have been used as traditional herbal medicines in the pharmaceutical industry. Their antioxidant properties have also made them supplements in the food industry [6,7,8,9]. In addition, platycosides have been applied as ingredients in the cosmetic industry due to their anti-inflammatory, antioxidant, and whitening activities [9,47].
In vitro and in vivo results for the evaluation of biological activities of platycosides such as PD, PD3, PGD, deapi-PD, platycoside mixtures, and biotransformed platycosides are summarized in Table 2. The biological activities of the platycosides have been investigated using in vitro assays, in vitro cell lines, and in vivo animal models. For instance, the whitening effect is examined by tyrosinase inhibition using a tyrosinase inhibitor assay kit [9], the anticancer effect is studied by the inhibition of cancer cell proliferation using cancer cells [19,21,22], and the anti-inflammatory effect was evaluated by the reduction of pro-inflammatory cytokine levels using a lipopolysaccharide-induced mouse [13]. In vivo experiments were performed using natural platycosides, mainly PD and Platycodi radix extract as platycoside mixtures, whereas in vitro assay experiments were performed using biotransformed platycosides.

Table 2.
Biological activity of platycosides.
3.2. Biological Activities with Deglycosylation
In vitro anti-inflammatory activities of platycosides are evaluated using a lipoxygenase inhibitory assay [4,8,9]. The anti-inflammatory activities of platycodigenin-type platycosides followed the order glc-PDN (one glycoside) > deapi-PD (four glycosides) > deglc-PD (four glycosides) > PD (five glycosides) > PD3 (six glycosides) > PE (seven glycosides), indicating that the anti-inflammatory activity of platycodigenin-type platycosides increase as the number of glycosides decrease.
The antioxidant activities of platycosides have been estimated using a Trolox equivalent capacity assay or a total oxidant-scavenging capacity assay [6,8,9]. The antioxidant activities of platycodigenin-type platycosides using the Trolox equivalent capacity assay follow the order glc-PDN (one glycoside) > deapi-PD (four glycosides) > PD (five glycosides) > PD3 (six glycosides) > PE (seven glycosides). The antioxidant activity determined using the total oxidant scavenging capacity assay follows the order platycodigenin (no glycosides) > deapi-PE (six glycosides) > PD (five glycosides) > PE (seven glycosides). Thus, the antioxidant activities of platycodigenin-type platycosides are increased by decreasing the number of glycoside residues.
The tyrosinase inhibitor assay has been used to determine whitening activity [9]. The whitening activities as tyrosinase inhibitory activities of the platycodigenin-type platycosides follow the order glc-PDN (one glycosides) > PD (five glycosides) > PD3 (six glycosides) > PE (seven glycosides) [9]. The whitening activities of platycosides improve as the number of glycosides attached to the aglycone sapogenin decreases. The anti-inflammatory, antioxidant, and whitening activities of platycodigenin-type platycosides determined using assay kits increase as the number of glycosides attached to sapogenin decrease.
4. Deglycosylation Methods for Saponins
4.1. Physical Methods
Deglycosylated ginseng saponins have been prepared by steam heating, microwave heating, sulfur fumigation, and high hydrostatic pressure [48,50,71,75]. The deglycosylation of saponins using steam heating increases with increasing treatment temperature and time. However, the amount and production rate of deglycosylated saponins by steam heating are small and slow, respectively. Microwave heating is used as an efficient time-saving method to show a higher deglycosylation rate than steam heating [77]. Sulfur fumigation is a cheap method to decrease the drying time and generate new saponins but is environmentally harmful [78]. These physical methods cause structural changes in sapogenin structures by hydrolysis, dehydration, and decarboxylation, producing byproducts or new-type saponins [79,80] and they show low selectivity and have yet to be used in the deglycosylation of platycosides. However, the physical method using high hydrostatic pressure is used together with biological method such as enzymatic conversion to increase the productivity of deglycosylated saponins by increasing the activity and stability of glycosidases [71,75].
4.2. Chemical Methods
Under acidic and high-temperature conditions, glycosylated saponins such as natural ginsenosides are converted into deglycosylated saponins. Deglycosylation by acid hydrolysis is dependent on the pH, acid concentration, acid type, and treatment temperature and time. Deglycosylation increased with an increase in treatment temperature and time; however, the optimal pH is different for each saponin type [50]. Acids used in chemical transformation of saponins include acetic acid, formic acid, citric acid, lactic acid, tartaric acid, and hydrochloric acid. Acid hydrolysis causes epimerization, dehydration, and hydration reactions, resulting in the production of deglycosylated saponins and new saponins [81].
Deglycosylation via alkaline hydrolysis increases with increasing pH, pressure, temperature, and time. Alkaline hydrolysis requires strict treatment conditions because strong treatment conditions can cleave sapogenins [50]. Alkaline hydrolysis is an efficient method for the production of aglycone ginsenosides, such as protopanaxadiol and protopanaxatriol, because the production of sapogenins from natural ginseng saponins by alkaline hydrolysis has resulted in an 80% yield [82]. Therefore, alkaline hydrolysis can be applied to the production of aglycone platycosides such as PDN, PGA, and PCA. Alkaline hydrolysis also involves side reactions, such as epimerization, cyclization, and hydroxylation. The disadvantages of chemical methods are byproduct formation and the generation of environmental pollution.
4.3. Biological Methods
Physical and chemical methods for producing deglycosylated saponins have several limitations, such as low selectivity, generation of by-products, non-environment-friendly processes, and high energy consumption. To overcome these disadvantages, biological methods including enzyme conversion, cell conversion, and fermentation have been proposed [49]. The enzymatic conversion of saponins has been performed using recombinant enzymes [4,83], commercial enzymes [52,84], reagent enzymes [62,66,70], and native crude enzymes [8,85]. The conversion using recombinant enzymes showed the highest production of saponins compared to other enzymatic conversions. However, the application of recombinant enzymes in the food industry is limited. Enzymatic conversion exhibits high specificity, yield, and productivity for saponin deglycosylation. Moreover, this method is increasingly being recognized as a useful tool for structural modifications. However, it is less economical than fermentation because it requires enzyme purification and results in the loss of enzymes from cells during purification [86].
Cell conversion is the production of deglycosylated saponins by reactions using grown or washed microbial cells mixed with saponins, whereas fermentation involves cultivation of growing cells fed with saponins. Intestinal bacteria, yeast, fungi, and soil microorganisms have been used for the transformation of saponins [8,48,50,67,73]. Microbial transformation by intestinal bacteria requires expensive medium and exhibits low yield and poor productivity. Microorganisms isolated from soil-cultivated saponin-containing plants exhibit higher yields and productivity than intestinal bacteria [87,88,89]. However, soil microorganisms can be applied to foods only if they have been designated as generally regarded as safe (GRAS). GRAS microorganisms typically include human intestinal bacteria, lactic acid bacteria, and fungi. GRAS fungi are more suitable for the production than lactic acid bacteria because they are easier to grow in cheaper media and exhibit higher yields and productivities. Fermentation is more cost-effective than cell or enzymatic conversion because it does not require enzyme purification steps and can use both intracellular and extracellular enzymes. However, the productivity of deglycosylated saponins by fermentation is lower than that by cell conversion or enzymatic conversion. To increase the productivity and avoid the inhibition of saponins to cells during fermentation, a new strategy, such as continuous feeding of saponins in a fermenter, is required.
5. Biotransformation of Platycosides
5.1. Hydrolytic Pathways for Specific Glycosides
The hydrolytic pathways of glycosylated platycosides by β-d-glucosidase, β-d-apiosidase, β-d-xylosidase, α-l-rhamnosidase, and α-l-arabinosidase, which hydrolyze glucoside residues linked to C-3 and apioside, xyloside, rhamnoside, or acetyl-rhamnoside, and arabinoside residues linked to C-28 in the aglycone platycosides, respectively, are designated as A, B, C, D or D’, and E, respectively (Figure 2, Figure 3 and Figure 4). The hydrolytic pathways of glycosylated platycosides by β-d-glucosidases acting on the outer, middle, and inner glucoside residues at C-3 are designated as A1, A2, and A3, respectively.
Hydrolytic pathways of PDN-type platycosides by glycosidases have been proposed (Figure 2). In these pathways, PE and PA are converted into PDN by the reactions A1, A2, A3, B, C, D, and E, and A3, B, C, D’, and E, respectively, and 26 PDN-type platycosides are involved. The PGA-type platycoside, PGD3 or AcPGD3, is converted into PGA by the reactions of A2, A3, B, C, D or D’, and E, respectively, and 24 PGA-type platycosides were involved in the proposed hydrolytic pathways (Figure 3). Hydrolytic pathways of the PCA-type platycosides are proposed from PCCA to PCA via eight intermediate platycosides (Figure 4). In total, 60 platycosides are involved in the hydrolytic pathways from glycosylated platycosides to sapogenins.
The seven natural platycosides, platycodin D2 (compound 3), 2″-O-acetyl platycodin D2 (compound 4), 3″-O-acetyl platycodin D2 (compound 5), 2″-O-acetyl platycodin D (compound 7), polygalacin D2 (compound 32), 2″-O-acetyl polygalacin D (compound 36), and 3″-O-acetyl platyconic acid A (compound 58), are not involved in the hydrolytic pathways (Figure 1). The diverse specific hydrolytic pathways of glycosylated platycosides, including deapi-PE, PE, PD, PA, PGD3, AcPGD3, and PCAA, into deglycosylated platycosides by specific reagent, commercial, recombinant, and crude glycosidases, and cells containing glycosidase are summarized in Table 1.
5.2. Substrate Specificity of Glycosidases
The biosynthesis of deglycosylated platycosides by glycosidases with substrate specificity is shown in Table 1. β-Glucosidases with narrow substrate specificity for only the outer glucoside residue at C-3 by the reaction of A1 have not yet been reported. β-Glucosidases from Aspergillus usamii [68], Caldicellulosiruptor bescii [51], C. owensensis [69], and Cyberlindnera fabianii [67], cellulase from Trichoderma reesei [70], laminarinase from Trichoderma sp. [62], and snailase from snails [66] hydrolyze the outer and middle glucoside residues linked to C-3 of sapogenin by the reactions A1 and A2, respectively, but not the inner glucoside. β-Glucosidase from C. bescii converts PE, deapi-PE, PGD3, and AcPGD3 into PD, deapi-PD, PGD, and AcPGD, respectively. β-Glucosidase from Dictyoglomus turgidum and cellulase from A. niger as the commercial enzyme Pluszyme 2000P catalyze the hydrolysis of all three glucoside residues linked to C-3 of sapogenin by the reactions A1, A2, and A3 [4,71]. β-Glucosidase from D. turgidum converts PE, deapi-PE PGD3, AcPGD3 PA, and PCAA into deglc-PD, deglc-api-PE, deglu-PGD, deglu-AcPGD, deglu-PA, and deglu-PCAA, respectively. The β-glucosidases used in the biotransformation of platycosides hydrolyze the glucoside moiety at C-3 of sapogenin but not the glycoside moiety at C-28.
Crude enzyme from Rhizopus oryzae hydrolyzes not only the outer and middle glucoside residues at C-3 by the reactions A1 and A2 with β-glucosidase activity, respectively, but also the outer apioside at C-28 by the reaction of B with β-d-apiosidase activity [8]. The crude enzyme converts PE, PGD3, PA, and PCAA into deapi-PD, deapi-PGD, deapi-PA, and deapi-PCAA, respectively. The crude enzyme can be applied to the conversion of the unused substrates AcPGD3 and AcPGD into deapi-AcPGD3 and deapi-AcPGD, respectively. Pectinase from A. niger as the commercial enzyme Cytolase PCL5 hydrolyzes the outer and middle glucoside residues at C-3 by the reactions A1 and A2 with β-glucosidase activity, respectively, and the apioside and xyloside residues at C-28 by the reactions B and C, with β-d-apiosidase and β-d-xylosidase activities, respectively [74]. The commercial enzyme converts PE and PGD3 into deapi-xyl-PD and deapi-xyl-PGD, respectively. The enzyme can also be applied to the conversion of unused substrates AcPGD3, PA, and PCAA into deapi-xyl-AcPGD, deapi-xyl-PA, and deapi-xyl-PCAA, respectively. α-l-Rhamnosidases, which hydrolyze rhamnoside in platycosides by the reaction of D, have not been used in the biotransformation of platycosides to date. α-l-Rhamnosidases hydrolyze rhamnosides, but not glucosides [35], suggesting that these enzymes can be applied to the production of new platycosides glc-ara-PDN, glc-ara-PGA, glc-ara-PCA, and ara-PCA from deapi-xyl-PD, deapi-xyl-PGD, deapi-xyl-PCAA, and deglc-api-xyl-PCAA, respectively. Pectinase from A. aculeatus as the commercial enzyme Pectinex Ultra SP-L with broad substrate specificity hydrolyzes the outer and middle glucoside residues at C-3 by the reactions A1 and A2, respectively, and the oligosaccharide moiety (apiosyl-xylosyl-rhamnosyl or acetyl-rhamnosyl-arabinosyl residues) at C-28 by the reactions B and E with β-d-apiosidase and α-l-arabinosidase activities, respectively [52]. The enzyme converts PE, PGD3, AcPGD3, PA, and PCAA into glc-PDN, glc-PGA, glc-PGA, glc-PDN, and glc-PCA, respectively.
Crude enzyme from A. tubingensis has β-glucosidase, β-d-apiosidase, and β-d-xylosidase activities [72]. The multi-hydrolytic activities, which may be because the crude enzyme contains several glycosidases [85]. The crude enzyme hydrolyzes all three glucoside residues at C-3 by the reactions A1, A2, and A3 with β-glucosidase activity and the apioside and xyloside residues at C-28 by the reactions B and C with β-d-apiosidase and β-d-xylosidase activities, respectively. The crude enzyme converts PE and PGD3 into deglc-api-xyl-PD and deglc-api-xyl-PGD, respectively. The crude enzyme can also be used to convert the unused substrates PA, AcPGD3, and PCAA into deglc-api-xyl-PA, deglc-api-xyl-AcPGD, and deglc-api-xyl-PCAA, respectively. The use of a combination of enzymes or cells with multiple enzymes is a good tool to produce new highly deglycosylated platycosides. If the combination of A. aculeatus pectinase and D. turgidum β-glucosidase are applied to the hydrolysis of the glycosylated platycosides PE, PA, PGD3, AcPGD3, and PCAA, all residues linked to C-3 and C-28 will be hydrolyzed into PDN, PDN, PGA, PGA, and PCA, respectively, by the reactions A1, A2, A3, B, C, D or D’, and E. The human intestinal bacterium Bacteroides converts PD into ara-PDN by the reactions A1, A2, A3, B, C, and D [73]. The cells also convert deglc-api-PD into deglc-api-PGD by dihydroxylation, which is converted into api-PGD.
5.3. Quantitative Biotransformation
A summary of the quantitative biotransformation of glycosylated platycosides, such as PE, PA, PGD3, and PCAA, into deglycosylated platycosides (such as PD, deglc-PD, deapi-PD, deapi-PA, deapi-PGD, deapi-PCAA, deapi-xyl-PD, deapi-xyl-PGD, deglc-api-xyl-PD, deglc-api-xyl-PGD, glc-PDN, glc-PGA, and glc-PGA) by glycosidases, including reagent, recombinant, commercial, and crude glycosidases, is shown in Table 3. Quantitative biotransformation is essential for industrial production of deglycosylated platycosides. In the production of PD from PE, snailase shows the highest concentration and volumetric productivity among the PD-producing β-glucosidases [66]. However, the specific productivity of PD by recombinant β-glucosidase from C. bescii is significantly higher than that of PD by snailase [51]. Recombinant β-glucosidase from D. turgidum shows the highest specific productivity for the production of deglycosylated platycosides among the deglycosylated platycoside-producing enzymes [4]. These results indicate that the recombinant glycosidases are the most efficient biocatalysts for platycoside hydrolysis compared to reagent, commercial, and crude glycosidases. However, recombinant glycosidases have limited food applications.

Table 3.
Quantitative biotransformation of glycosylated platycosides into deglycosylated platycosides by glycosidases.
Commercial enzymes such as Pluszyme 2000P [71], Cytolase PCL5 [74], and Pectinex Ultra SP-L [52] exhibit higher specific productivity and lower cost than reagents such as cellulase [70], laminarinase [62], and snailase [66], indicating that commercial enzymes are more feasible for the industrial production of deglycosylated platycosides. The specific productivities of the commercial enzymes followed the order Pluszyme 2000P (deglucosylation) > Cytolase PCL5 (deapiose-xylosylation) > Pectinex Ultra SP-L (deapiose-xylose-rhamnosylation). These enzymes convert PE (seven glycosides) to deglc-PD (four glycosides), deapi-xyl-PD (three glycosides), and glc-PDN (one glycoside). Therefore, the specific productivity decreases with increasing degree of deglycosylation.
6. Conclusions and Future Perspectives
In this review, we describe the biotransformation of platycosides based on their hydrolytic pathways, glycosidases, and specific productivity of deglycosylated platycosides. However, the number of reports on the transformation of balloon flower root saponins are significantly fewer than those of ginseng saponins. Therefore, transformation methods for glycosylated ginsenosides can be applied for the transformation of glycosylated platycosides to produce diverse deglycosylated platycosides. Physical methods, including steam heating, microwave heating, and sulfur fumigation [48,50], and chemical methods, including acid and alkaline hydrolysis, which have been used in the hydrolysis of glycosylated ginsenosides, can be applied to the hydrolysis of glycosylated platycosides [50,81].
Currently, the pool of glycosidases that hydrolyze glycosylated platycosides is too small. Cloning of glycosidase genes and discovery of natural glycosidases are needed to expand the pool to produce diverse platycosides. To produce new platycosides by specific hydrolysis of the glycoside moieties in platycosides, different types of glycosidases with narrow substrate specificities are required. For instance, the discovery of β-glucosidases that hydrolyze only the outer glucoside residue at C-3, and β-d-apiosidase, β-d-xylosidase, α-l-rhamnosidase, and α-l-arabinosidase, which hydrolyze apioside, xyloside, rhamnoside, and arabinoside, respectively, but no glucosides are needed. To obtain highly deglycosylated platycosides, such as glc-ara-platycodigenin and ara-platycodigenin, glycosidases with broad substrate specificity that hydrolyze platycosides through different pathways are also required.
Microorganisms involved in saponin transformation include soil fungi and bacteria, intestinal bacteria, and GRAS fungi [48,50]. The use of GRAS microorganisms and enzymes originating from GRAS microorganisms for saponin transformation is an appropriate method for food applications. Although glycosidases from GRAS fungi such as A. aculeatus [52], A. niger [74,75,76], A. tubingensis [72], A. usamii [68], R. oryzae [8], and T. reesei [70] are used in the biotransformation of platycosides, only one human intestinal bacterium, Bacteroides [73] is applied in biotransformation. Thus, more diverse GRAS human intestinal bacteria, such as lactic acid bacteria, should be applied in the biotransformation of platycosides because they can metabolize orally ingested saponins and can be used safely in a variety of foods [48,90].
We propose methods for producing diverse and/or new deglycosylated platycosides that may contribute to the development of biotransformation processes to produce bioactive deglycosylated platycosides. The biological effects of platycosides are mainly attributed to natural platycosides. However, the effects of biotransformed platycosides using cell lines and animal models have not yet been investigated. Recently, biotransformed platycosides have increasingly been reported. Thus, biotransformed platycosides will allow for the initiation of pharmacological studies at the cell and animal levels, and platycosides with improved bioactivity are expected to be developed through pharmacological studies.
Author Contributions
Conceptualization, K.-C.S. and D.-K.O.; data curation, K.-C.S. and D.-K.O.; funding acquisition, K.-C.S. and D.-K.O.; project administration, D.-K.O.; writing—original draft, K.-C.S. and D.-K.O.; writing—reviewing and editing, K.-C.S. and D.-K.O. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, and Forestry [821022-3] of the Ministry of Agriculture, Food, and Rural Affairs and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2020R1I1A1A01063145), Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This paper was supported by the KU Research Professor Program of Konkuk University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yan, Y.; Xue, J.; Wu, J.; Yoo, D.; Lee, S.; Kim, Y.; Uddin, M.; Park, S. Variation of triterpenoid saponin content in Platycodon grandiflorum (Jacq.) ADC. Asian J. Chem. 2012, 24, 1268–1270. [Google Scholar]
- Zhang, L.; Wang, Y.; Yang, D.; Zhang, C.; Zhang, N.; Li, M.; Liu, Y. Platycodon grandifloras—An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015, 164, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Huang, M.Y.; Yang, Y.; Huang, M.Q.; Shi, J.J.; Zou, L.; Lu, J.J. Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. 2020, 327, 127029. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, T.H.; Shin, K.C.; Ko, Y.J.; Oh, D.K. Biotransformation of food-derived saponins, platycosides, into deglucosylated saponins including deglucosylated platycodin D and their anti-inflammatory activities. J. Agric. Food Chem. 2019, 67, 1470–1477. [Google Scholar] [CrossRef]
- Ma, C.H.; Gao, Z.J.; Zhang, J.J.; Zhang, W.; Shao, J.H.; Hai, M.R.; Chen, J.W.; Yang, S.C.; Zhang, G.H. Candidate genes involved in the biosynthesis of triterpenoid saponins in Platycodon grandiflorum identified by transcriptome analysis. Front. Plant Sci. 2016, 7, 673. [Google Scholar] [CrossRef]
- Ryu, C.S.; Kim, C.H.; Lee, S.Y.; Lee, K.S.; Choung, K.J.; Song, G.Y.; Kim, B.H.; Ryu, S.Y.; Lee, H.S.; Kim, S.K. Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem. 2012, 132, 333–337. [Google Scholar] [CrossRef]
- Luo, H.; Lin, S.; Ren, F.; Wu, L.; Chen, L.; Sun, Y. Antioxidant and antimicrobial capacity of Chinese medicinal herb extracts in raw sheep meat. J. Food Prot. 2007, 70, 1440–1445. [Google Scholar] [CrossRef]
- Shin, K.C.; Kil, T.G.; Lee, T.E.; Oh, D.K. Production of bioactive deapiosylated platycosides from glycosylated platycosides in balloon flower root using the crude enzyme from the food-available fungus Rhizopus oryzae. J. Agric. Food Chem. 2021, 69, 4766–4777. [Google Scholar] [CrossRef]
- Ju, J.H.; Lee, T.E.; Lee, J.; Kim, T.H.; Shin, K.C.; Oh, D.K. Improved bioactivity of 3-O-β-D-glucopyranosyl platycosides in biotransformed platycodon grandiflorum root extract by pectinase from Aspergillus aculeatus. J. Microbiol. Biotechnol. 2021, 31, 847–854. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, Y.P.; Kim, D.H.; HAN, E.H.; Chung, Y.C.; Roh, S.H.; Jeong, H.G. Inhibitory effect of the saponins derived from roots of Platycodon grandiflorum on carrageenan-induced inflammation. Biosci. Biotechnol. Biochem. 2006, 70, 858–864. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, I.G.; Kim, S.B.; Choi, A.J. Chemical characterization of balloon flower (Platycodon grandiflorum) sprout extracts and their regulation of inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 murine macrophage cells. Food Sci. Nutr. 2020, 8, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Guo, Y.; Yang, H. Platycodin D protects against cigarette smoke-induced lung inflammation in mice. Int. Immunopharmacol. 2017, 47, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Fu, Y.; Lu, X.; Zhang, Z.; Zhang, W.; Cao, Y.; Zhang, N. Protective effects of platycodin D on lipopolysaccharide-induced acute lung injury by activating LXRα-ABCA1 signaling pathway. Front. Immunol. 2016, 7, 644. [Google Scholar] [CrossRef]
- Ryu, J.; Lee, H.J.; Park, S.H.; Kim, J.; Lee, D.; Lee, S.K.; Kim, Y.S.; Hong, J.H.; Seok, J.H.; Lee, C.J. Effects of the root of Platycodon grandiflorum on airway mucin hypersecretion in vivo and platycodin D3 and deapi-platycodin on production and secretion of airway mucin in vitro. Phytomedicine 2014, 21, 529–533. [Google Scholar] [CrossRef]
- Lee, E.G.; Kim, K.H.; Hur, J.; Kang, J.Y.; Lee, H.Y.; Lee, S.Y. Platycodin D attenuates airway inflammation via suppression Th2 transcription factor in a murine model of acute asthma. J. Asthma 2022, 59, 1279–1289. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, G.Y.; Li, W.; Choi, B.T.; Kim, N.D.; Kang, H.S.; Choi, Y.H. Implication of intracellular ROS formation, caspase-3 activation and Egr-1 induction in platycodon D-induced apoptosis of U937 human leukemia cells. Biomed. Pharmacother. 2009, 63, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Kim, J.Y.; Kim, Y.S.; Chung, Y.C.; Hahm, K.S.; Jeong, H.G. Augmentation of macrophage functions by an aqueous extract isolated from Platycodon grandiflorum. Cancer Lett. 2001, 166, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Yim, N.H.; Hwang, Y.w.; Liang, C.; Ma, J.Y. A platycoside-rich fraction from the root of Platycodon grandiflorum enhances cell death in A549 human lung carcinoma cells via mainly AMPK/mTOR/AKT signal-mediated autophagy induction. J. Ethnopharmacol. 2016, 194, 1060–1068. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Choi, S.U.; Kim, J.S.; Lee, H.S.; Roh, S.H.; Jeong, Y.C.; Kim, Y.K.; Ryu, S.Y. Isolation of a new saponin and cytotoxic effect of saponins from the root of Platycodon grandiflorum on human tumor cell lines. Planta Med. 2005, 71, 566–568. [Google Scholar] [CrossRef]
- Khan, M.; Maryam, A.; Zhang, H.; Mehmood, T.; Ma, T. Killing cancer with platycodin D through multiple mechanisms. J. Cell. Mol. Med. 2016, 20, 389–402. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yoo, D.S.; Cha, M.R.; Choi, C.W.; Kim, Y.S.; Choi, S.U.; Lee, K.R.; Ryu, S.Y. Antiproliferative effects of saponins from the roots of Platycodon grandiflorum on cultured human tumor cells. J. Nat. Prod. 2010, 73, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Kim, A.K. Platycodin D induces apoptosis in MCF-7 human breast cancer cells. J. Med. Food 2010, 13, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, M.; Zheng, W.; Liu, Y. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, suppresses the growth and invasion of human oral squamous cell carcinoma cells via the NF-kappaB pathway. J. Biochem. Mol. Toxicol. 2017, 31, e21934. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Lu, Z.L.; Wang, J.J.; Zhou, R.; Guo, J.; Liu, J.; Sun, H.L.; Wang, H.; Song, W.; Yang, J.; et al. Platycodin D, a metabolite of Platycodin grandiflorum, inhibits highly metastatic MDA-MB-231 breast cancer growth in vitro and in vivo by targeting the MDM2 oncogene. Oncol. Rep. 2016, 36, 1447–1456. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Chen, X.; Ma, D.L.; Leung, C.H.; Lu, J.J. Platycodin D potentiates proliferation inhibition and apoptosis induction upon AKT inhibition via feedback blockade in non-small cell lung cancer cells. Sci. Rep. 2016, 6, 37997. [Google Scholar] [CrossRef]
- Zhao, H.; Harding, S.; Marinangeli, C.; Kim, Y.; Jones, P. Hypocholesterolemic and anti-obesity effects of saponins from Platycodon grandiflorum in hamsters fed atherogenic diets. J. Food Sci. 2008, 73, H195–H200. [Google Scholar] [CrossRef]
- Han, L.K.; Zheng, Y.N.; Xu, B.J.; Okuda, H.; Kimura, Y. Saponins from Platycodi radix ameliorate high fat diet–induced obesity in mice. J. Nutr. 2002, 132, 2241–2245. [Google Scholar] [CrossRef]
- Hwang, K.A.; Hwang, Y.J.; Im, P.R.; Hwang, H.J.; Song, J.; Kim, Y.J. Platycodon grandiflorum extract reduces high-fat diet-induced obesity through regulation of adipogenesis and lipogenesis pathways in mice. J. Med. Food 2019, 22, 993–999. [Google Scholar] [CrossRef]
- Zhao, H.L.; Kim, Y.S. Determination of the kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1,2-diglyceride-based colorimetric assay. Arch. Pharm. Res. 2004, 27, 968–972. [Google Scholar] [CrossRef]
- Lee, E.J.; Kang, M.; Kim, Y.S. Platycodin D inhibits lipogenesis through AMPKα-PPARγ2 in 3T3-L1 cells and modulates fat accumulation in obese mice. Planta Med. 2012, 78, 1536–1542. [Google Scholar] [CrossRef]
- Kim, H.L.; Park, J.; Jung, Y.; Ahn, K.S.; Um, J.Y. Platycodin D, a novel activator of AMP-activated protein kinase, attenuates obesity in db/db mice via regulation of adipogenesis and thermogenesis. Phytomedicine 2019, 52, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Kim, J.W.; Jung, G.W.; Park, D.C.; Moon, S.B.; Cho, H.R.; Ku, S.K.; Choi, J.S. Synergic antiobesity effects of bitter melon water extract and platycodin-D in genetically obese mice. J. Environ. Biol. 2018, 39, 603–611. [Google Scholar] [CrossRef]
- Ahn, Y.M.; Kim, S.K.; Kang, J.S.; Lee, B.C. Platycodon grandiflorum modifies adipokines and the glucose uptake in high-fat diet in mice and L6 muscle cells. J. Pharm. Pharmacol. 2012, 64, 697–704. [Google Scholar] [CrossRef]
- Zheng, J.; He, J.; Ji, B.; Li, Y.; Zhang, X. Antihyperglycemic effects of Platycodon grandiflorum (Jacq.) A. DC. extract on streptozotocin-induced diabetic mice. Plant Foods Hum. Nutr. 2007, 62, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.J.; Liu, H.B.; Wang, P.; Guan, H.S. A study on the antioxidant activity and tissues selective inhibition of lipid peroxidation by saponins from the roots of Platycodon grandiflorum. Am. J. Chin. Med. 2009, 37, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.C.; Kang, O.H.; Choi, J.G.; Lee, Y.S.; Brice, O.O.; Jung, H.J.; Hong, S.H.; Lee, Y.M.; Shin, D.W.; Kim, Y.S. Anti-allergic activity of a platycodon root ethanol extract. Inter. J. Mol. Sci. 2010, 11, 2746–2758. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, Y.H.; Kang, H.S.; Choi, B.T. An aqueous extract of Platycodi radix inhibits LPS-induced NF-κB nuclear translocation in human cultured airway epithelial cells. Inter. J. Mol. Med. 2004, 13, 843–847. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, Y.P.; Sun, H.X.; Li, D. Contribution of the glycidic moieties to the haemolytic and adjuvant activity of platycodigenin-type saponins from the root of Platycodon grandiflorum. Vaccine 2008, 26, 3452–3460. [Google Scholar] [CrossRef]
- Noh, E.M.; Kim, J.M.; Lee, H.Y.; Song, H.K.; Joung, S.O.; Yang, H.J.; Kim, M.J.; Kim, K.S.; Lee, Y.R. Immuno-enhancement effects of Platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model. BMC Complement. Altern. Med. 2019, 19, 322. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Yan, P.; Cheng, G.; Wang, C.; Geng, N.; Wang, X.; Liu, J. Effects of polysaccharides from Platycodon grandiflorum on immunity-enhancing activity in vitro. Molecules 2017, 22, 1918. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lin, Y.C.; Kuo, W.W.; Shen, C.Y.; Cheng, Y.C.; Lin, Y.M.; Chang, R.L.; Padma, V.V.; Huang, C.Y.; Huang, C.Y. Platycodin D reverses pathological cardiac hypertrophy and fibrosis in spontaneously hypertensive rats. Am. J. Chin. Med. 2018, 46, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, G.; Zhu, W.; Wen, W.; Zhang, F.; Yuan, J.; An, L. Anti-atherosclerotic activity of platycodin D derived from roots of Platycodon grandiflorum in human endothelial cells. Biol. Pharm. Bull. 2012, 35, 1216–1221. [Google Scholar] [CrossRef]
- Luo, Q.; Wei, G.; Wu, X.; Tang, K.; Xu, M.; Wu, Y.; Liu, Y.; Li, X.; Sun, Z.; Ju, W.; et al. Platycodin D inhibits platelet function and thrombus formation through inducing internalization of platelet glycoprotein receptors. J. Transl. Med. 2018, 16, 311. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.L.; Liu, Y.; Leng, J.; Zhang, J.; He, Y.F.; Chen, C.; Wang, Z.; Li, W. Platycodin D protects acetaminophen-induced hepatotoxicity by inhibiting hepatocyte MAPK pathway and apoptosis in C57BL/6J mice. Biomed. Pharmacother. 2018, 107, 867–877. [Google Scholar] [CrossRef]
- Liu, Y.M.; Cong, S.; Cheng, Z.; Hu, Y.X.; Lei, Y.; Zhu, L.L.; Zhao, X.K.; Mu, M.; Zhang, B.F.; Fan, L.D.; et al. Platycodin D alleviates liver fibrosis and activation of hepatic stellate cells by regulating JNK/c-JUN signal pathway. Eur. J. Pharmacol. 2020, 876, 172946. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.T.; Shao, S.; Xiao, F.Q.; Zhang, H.Y.; Zhang, R.R.; Wang, M.; Li, G.Z.; Yan, M.M. Platycodon grandiflorum extract: Chemical composition and whitening, antioxidant, and anti-inflammatory effects. RSC Adv. 2021, 11, 10814–10826. [Google Scholar] [CrossRef]
- Park, C.S.; Yoo, M.H.; Noh, K.H.; Oh, D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl. Microbiol. Biot. 2010, 87, 9–19. [Google Scholar] [CrossRef]
- Shin, K.C.; Oh, D.K. Classification of glycosidases that hydrolyze the specific positions and types of sugar moieties in ginsenosides. Crit. Rev. Biotechnol. 2016, 36, 1036–1049. [Google Scholar] [CrossRef]
- Zheng, M.M.; Xu, F.X.; Li, Y.J.; Xi, X.Z.; Cui, X.W.; Han, C.C.; Zhang, X.L. Study on transformation of ginsenosides in different methods. Biomed. Res. Int. 2017, 2017, 8601027. [Google Scholar] [CrossRef]
- Kil, T.G.; Kang, S.H.; Kim, T.H.; Shin, K.C.; Oh, D.K. Enzymatic biotransformation of balloon flower root saponins into bioactive platycodin D by deglucosylation with Caldicellulosiruptor bescii β-glucosidase. Int. J. Mol. Sci. 2019, 20, 3854. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.H.; Kang, S.H.; Kim, T.H.; Shin, K.C.; Oh, D.K. Biotransformation of glycosylated saponins in balloon flower root extract into 3-O-β-ᴅ-glucopyranosyl platycosides by deglycosylation of pectinase from Aspergillus aculeatus. J. Microbiol. Biotechnol. 2020, 30, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Nyakudya, E.; Jeong, J.H.; Lee, N.K.; Jeong, Y.S. Platycosides from the roots of Platycodon grandiflorum and their health benefits. Prev. Nutr. Food Sci. 2014, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.W.; Na, Y.C.; Seo, J.J.; Kim, S.N.; Linhardt, R.J.; Kim, Y.S. Qualitative and quantitative determination of ten major saponins in Platycodi Radix by high performance liquid chromatography with evaporative light scattering detection and mass spectrometry. J. Chromatogr. A 2006, 1135, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.S.; Choi, Y.H.; Cha, M.R.; Lee, B.H.; Kim, S.J.; Yon, G.H.; Hong, K.S.; Jang, Y.S.; Lee, H.S.; Kim, Y.S.; et al. HPLC-ELSD analysis of 18 platycosides from balloon flower roots (Platycodi Radix) sourced from various regions in Korea and geographical clustering of the cultivation areas. Food Chem. 2011, 129, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ji, S.H.; Kim, G.S.; Song, K.S.; Um, Y.; Kim, O.T.; Lee, Y.; Hong, C.P.; Shin, D.H.; Kim, C.K.; et al. Global profiling of various metabolites in Platycodon grandiflorum by UPLC-QTOF/MS. Int. J. Mol. Sci. 2015, 16, 26786–26796. [Google Scholar] [CrossRef]
- Yu, J.; Chang, X.; Peng, H.; Wang, X.; Wang, J.; Peng, D.; Gui, S. A strategy based on isocratic and linear-gradient high-speed counter-current chromatography for the comprehensive separation of platycosides from Platycodi radix. Anal. Methods 2021, 13, 477–483. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, K.C.; Lee, G.W.; Oh, D.K. Production of aglycone protopanaxatriol from ginseng root extract using Dictyoglomus turgidum β-glycosidase that specifically hydrolyzes the xylose at the C-6 position and the glucose in protopanaxatriol-type ginsenosides. Appl. Microbiol. Biotechnol. 2014, 98, 3659–3667. [Google Scholar] [CrossRef]
- Li, Q.; Yang, T.; Zhao, S.; Zheng, Q.; Li, Y.; Zhang, Z.; Sun, X.; Liu, Y.; Zhang, Y.; Xie, J. Distribution, biotransformation, pharmacological effects, metabolic mechanism and safety evaluation of Platycodin DA comprehensive review. Curr. Drug Metab. 2022, 23, 21–29. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.S.; Wang, Z.; Zheng, Y.N. Isolation and purification of saponins from Platycodon grandiflorum by semi-preparative high performance liquid chromatography and LC/ESI-MS. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 547–557. [Google Scholar] [CrossRef]
- Wang, L.; Fu, H.; Li, J.; Chen, L.; Yang, J.; Zhong, L.; Xiao, X.; Feng, Y.; Luo, Y. Ultra-high-performance liquid chromatography quadrupole time-of-flight tandem mass spectrometry coupled with three-step data post-processing techniques for comprehensive profiling of the multiple components in Fufang Xianzhuli Ye. Phytochem. Anal. 2023, 34, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.K.; Ha, I.J.; Kim, Y.S.; Na, Y.C. Glycosylated platycosides: I dentification by enzymatic hydrolysis and structural determination by LC–MS/MS. J. Sep. Sci. 2014, 37, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Kaneiwa, Y.; Shoji, J.; Shibata, S. Studies on the saponins of the root of Platycodon grandiflorum A. De Candolle. I. Isolation and the structure of platycodin-D. Chem. Pharm. Bull. 1975, 23, 2965–2972. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Tori, K.; Tozyo, T.; Yoshimura, Y. Structures of polygalacin-D and -D2, platycodin-D and -D2, and their monoacetates, saponins isolated from Platycodon grandiflorum A. DC., determined by carbon-13 nuclear magnetic resonance spectroscopy. Chem. Pharm. Bull. 1978, 26, 674–677. [Google Scholar] [CrossRef]
- Ha, I.J.; Kang, M.; Na, Y.C.; Park, Y.; Kim, Y.S. Preparative separation of minor saponins from platycodi radix by high-speed counter-current chromatography. J. Sep. Sci. 2011, 34, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, L.C.; Wang, Z.; Zheng, Y.N.; Liang, J.; Wang, H. Response surface methodology to optimize enzymatic preparation of deapio-platycodin D and platycodin D from radix Platycodi. Inter. J. Mol. Sci. 2012, 13, 4089–4100. [Google Scholar] [CrossRef]
- Lee, N.K.; Nyakudya, E.; Jeong, Y.S. Bioconversion of Platycodon grandiflorum saponins by the platycodin D-converting microorganism, yeast Cyberlindnera fabianii. J. Food Biochem. 2016, 40, 358–365. [Google Scholar] [CrossRef]
- Ahn, H.J.; You, H.J.; Park, M.S.; Johnston, T.V.; Ku, S.; Ji, G.E. Biocatalysis of platycoside E and platycodin D3 using fungal extracellular β-glucosidase responsible for rapid platycodin D production. Inter. J. Mol. Sci. 2018, 19, 2671. [Google Scholar] [CrossRef]
- Shin, K.C.; Seo, M.J.; Kim, D.W.; Yeom, S.J.; Kim, Y.S. Characterization of β-glycosidase from Caldicellulosiruptor owensensis and its application in the production of platycodin D from balloon flower leaf. Catalysts 2019, 9, 1025. [Google Scholar] [CrossRef]
- Ha, I.J.; Ha, Y.W.; Kang, M.; Lee, J.; Park, D.; Kim, Y.S. Enzymatic transformation of platycosides and one-step separation of platycodin D by high-speed countercurrent chromatography. J. Sep. Sci. 2010, 33, 1916–1922. [Google Scholar] [CrossRef]
- Shin, K.C.; Kim, D.W.; Oh, Y.J.; Seo, M.J.; Na, C.S.; Kim, Y.S. Improved production of deglucosylated platycodin D from saponins from balloon flower leaf by a food-grade enzyme using high hydrostatic pressure. Heliyon 2021, 7, e08104. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Kil, T.G.; Kang, S.H.; Oh, D.K. Production of deglucose-apiose-xylosylated platycosides from glycosylated platycosides by crude enzyme from Aspergillus tubingensis. J. Microbiol. Biotechnol. 2022, 32, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.W.; Na, Y.C.; Ha, I.J.; Kim, D.H.; Kim, Y.S. Liquid chromatography/mass spectrometry-based structural analysis of new platycoside metabolites transformed by human intestinal bacteria. J. Pharm. Biomed. 2010, 51, 202–209. [Google Scholar] [CrossRef]
- Shin, K.C.; Kim, D.W.; Woo, H.S.; Oh, D.K.; Kim, Y.S. Conversion of glycosylated platycoside E to deapiose-xylosylated platycodin D by Cytolase PCL5. Inter. J. Mol. Sci. 2020, 21, 1207. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Seo, M.J.; Oh, Y.J.; Kim, D.W.; Na, C.S.; Kim, Y.S. Improved biotransformation of platycoside e into deapiose-xylosylated platycodin D by Cytolase PCL5 under high hydrostatic pressure. Appl. Sci. 2021, 11, 10623. [Google Scholar] [CrossRef]
- Wie, H.J.; Zhao, H.L.; Chang, J.H.; Kim, Y.S.; Hwang, I.K.; Ji, G.E. Enzymatic modification of saponins from Platycodon grandiflorum with Aspergillus niger. J. Agric. Food Chem. 2007, 55, 8908–8913. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, L.; Qu, C.; Meng, X.; Zhang, H. Microwave degradation of floatation-enriched ginsenoside extract from Panax quinquefolium L. leaf. J. Agric. Food Chem. 2009, 57, 10252–10260. [Google Scholar] [CrossRef]
- Kan, W.L.; Ma, B.; Lin, G. Sulfur fumigation processing of traditional chinese medicinal herbs: Beneficial or detrimental? Front. Pharmacol. 2011, 2, 84. [Google Scholar] [CrossRef]
- Lee, S.M.; Shon, H.J.; Choi, C.S.; Hung, T.M.; Min, B.S.; Bae, K. Ginsenosides from heat processed ginseng. Chem. Pharm. Bull. 2009, 57, 92–94. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, L.Y.; Shen, H.; Xu, J.; Li, S.L.; Jia, X.B.; Cai, H.; Cai, B.C.; Yan, R. Influence of sulphur-fumigation on the quality of white ginseng: A quantitative evaluation of major ginsenosides by high performance liquid chromatography. Food Chem. 2012, 135, 1141–1147. [Google Scholar] [CrossRef]
- Wu, W.; Qin, Q.; Guo, Y.; Sun, J.; Liu, S. Studies on the chemical transformation of 20S-protopanaxatriol (PPT)-type ginsenosides Re, Rg2, and Rf using rapid resolution liquid chromatography coupled with quadruple-time-of-flight mass spectrometry (RRLC-Q-TOF-MS). J. Agric. Food Chem. 2012, 60, 10007–10014. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.F.; Garle, M.; Lund, E.; Bjorkhem, I.; Eneroth, P. Analysis of ginsenosides by chromatography and mass spectrometry: Release of 20 S-protopanaxadiol and 20 S-protopanaxatriol for quantitation. Anal. Biochem. 1993, 210, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.C.; Kim, T.H.; Choi, J.H.; Oh, D.K. Complete biotransformation of protopanaxadiol-type ginsenosides to 20-O-β-glucopyranosyl-20(S)-protopanaxadiol using a novel and thermostable β-glucosidase. J. Agric. Food Chem. 2018, 66, 2822–2829. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Lim, S.; Kim, S.O.; Ahn, S.H.; Choi, Y.J. Optimization of enzymatic treatment for compound K production from white ginseng extract by response surface methodology. Biosci. Biotechnol. Biochem. 2013, 77, 1138–1140. [Google Scholar] [CrossRef]
- Kim, S.A.; Jeong, E.B.; Oh, D.K. Complete bioconversion of protopanaxadiol-type ginsenosides to compound K by extracellular enzymes from the isolated strain Aspergillus tubingensis. J. Agric. Food Chem. 2021, 69, 315–324. [Google Scholar] [CrossRef]
- Jeong, E.B.; Kim, S.A.; Shin, K.C.; Oh, D.K. Biotransformation of protopanaxadiol-type ginsenosides in Korean ginseng extract into food-available compound K by an extracellular enzyme from Aspergillus niger. J. Microbiol. Biotechnol. 2020, 30, 1560–1567. [Google Scholar] [CrossRef]
- Han, Y.; Sun, B.; Hu, X.; Zhang, H.; Jiang, B.; Spranger, M.I.; Zhao, Y. Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil-cultivated ginseng. J. Agric. Food Chem. 2007, 55, 9373–9379. [Google Scholar] [CrossRef]
- Chi, H.; Ji, G.E. Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol. Lett. 2005, 27, 765–771. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, Q.; Li, J.Y.; Zhang, X.C.; Zhou, P. Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp. 229. J. Appl. Microbiol. 2008, 104, 699–706. [Google Scholar] [CrossRef]
- Ku, S. Finding and producing probiotic glycosylases for the biocatalysis of ginsenosides: A mini review. Molecules 2016, 21, 645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).