Upcycling Quince Peel into Bioactive Ingredients and Fiber Concentrates through Multicomponent Extraction Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Experimental Design for Extraction Optimization
2.3. Extraction and Preparation of BEs and FCs
2.4. Determination of Experimental Responses
2.4.1. Extraction Yields
2.4.2. Phenolic Compounds
2.4.3. Organic Acids
2.4.4. Soluble Sugars
2.4.5. Dietary Fiber
2.4.6. Color Parameters
2.5. Extraction Process Modelling and Statistical Verification of the Models
2.6. Experimental Validation of the Models and Evaluation of Bioactive Properties of BEs Obtained under Optimized Conditions
2.6.1. Antioxidant Activity
2.6.2. Antimicrobial Activity
2.6.3. Evaluation of Statistical Differences between Extracts’ Bioactivity
3. Results & Discussion
3.1. Extraction Yields and Chemical Composition
3.1.1. Bioactive Extract (BE) Yields
3.1.2. Phenolic Compounds
3.1.3. Organic Acids
3.1.4. Soluble Sugars
3.1.5. Fiber Concentrates (FCs)
3.2. Models Fitting and Statistical Verification
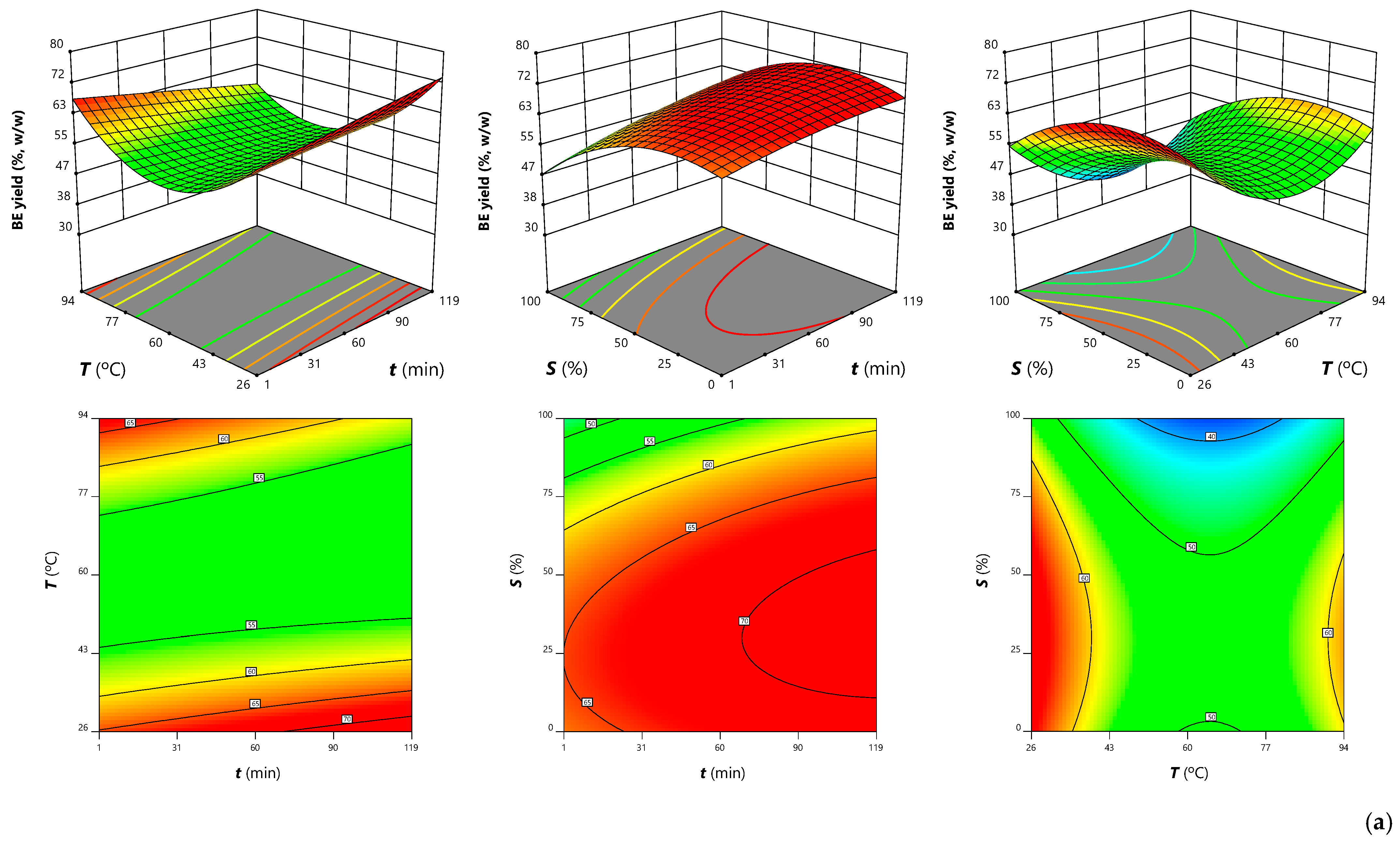
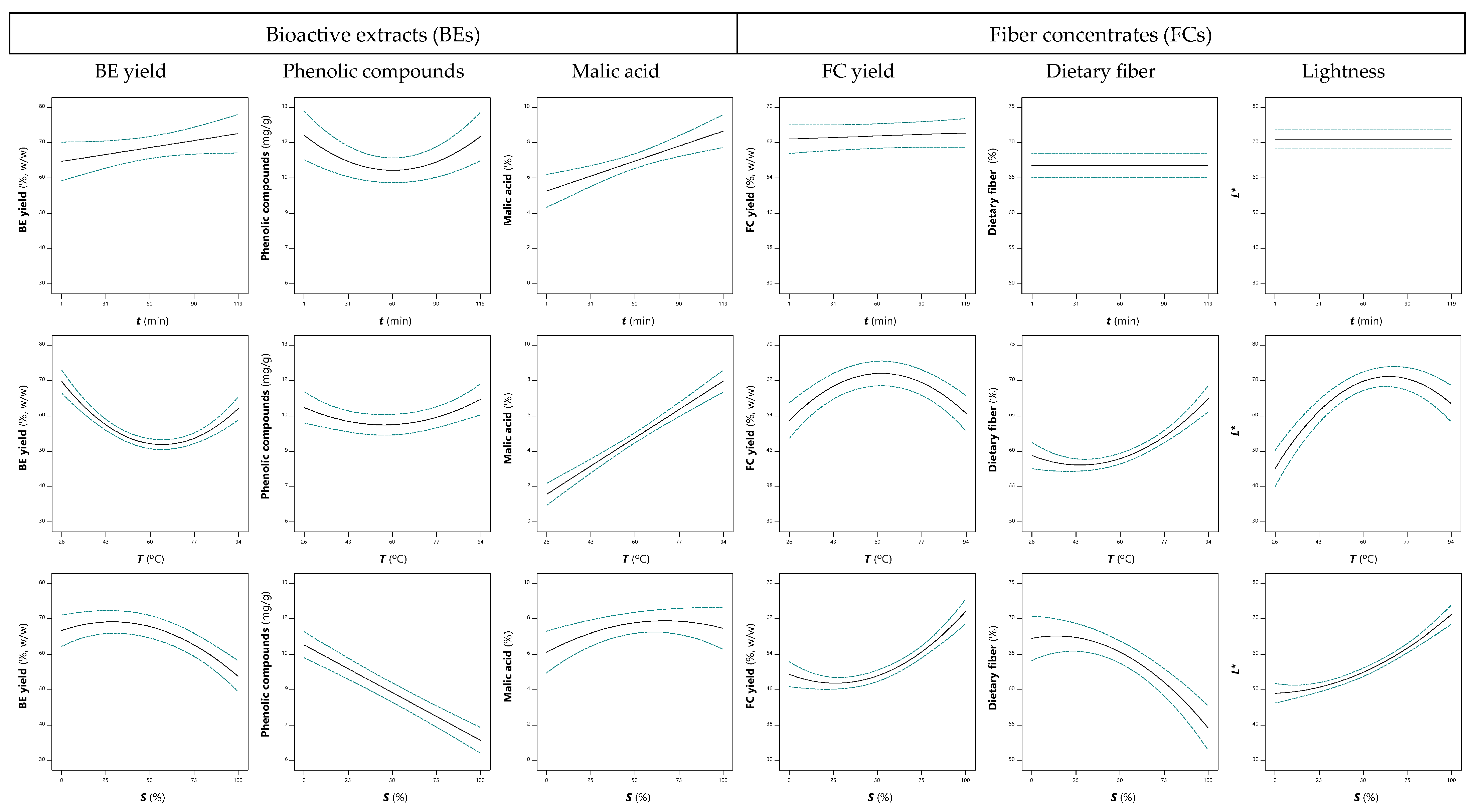
3.3. Effect of the Independent Variables and Optimal Extraction Conditions
3.3.1. Effects on Extraction Yield
3.3.2. Effects on Phenolic Compounds
3.3.3. Effects on Malic Acid and Other Organic Acids
3.3.4. Effects on Soluble Sugars
3.3.5. Effects on Fiber Concentrates (FCs)
3.4. Experimental Validation of the Predictive Models
3.5. Bioactive Properties of BEs Obtained under Optimized Conditions
3.5.1. Antioxidant Activity
3.5.2. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sustainability Pathways: Food Loss and Waste. Available online: https://www.fao.org/nr/sustainability/food-loss-and-waste/en/ (accessed on 21 July 2022).
- International Efforts on Wasted Food Recovery|US EPA. Available online: https://www.epa.gov/international-cooperation/international-efforts-wasted-food-recovery (accessed on 21 July 2022).
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174. [Google Scholar] [CrossRef]
- Espinosa, E.; Rincón, E.; Morcillo-Martín, R.; Rabasco-Vílchez, L.; Rodríguez, A. Orange Peel Waste Biorefinery in Multi-Component Cascade Approach: Polyphenolic Compounds and Nanocellulose for Food Packaging. Ind. Crops Prod. 2022, 187, 115413. [Google Scholar] [CrossRef]
- Ebrahimian, F.; Denayer, J.F.M.; Karimi, K. Potato Peel Waste Biorefinery for the Sustainable Production of Biofuels, Bioplastics, and Biosorbents. Bioresour. Technol. 2022, 360, 127609. [Google Scholar] [CrossRef]
- Arun, K.B.; Madhavan, A.; Anoopkumar, A.N.; Surendhar, A.; Liz Kuriakose, L.; Tiwari, A.; Sirohi, R.; Kuddus, M.; Rebello, S.; Kumar Awasthi, M.; et al. Integrated Biorefinery Development for Pomegranate Peel: Prospects for the Production of Fuel, Chemicals and Bioactive Molecules. Bioresour. Technol. 2022, 362, 127833. [Google Scholar] [CrossRef]
- Torres-Sciancalepore, R.; Fernandez, A.; Asensio, D.; Riveros, M.; Fabani, M.P.; Fouga, G.; Rodriguez, R.; Mazza, G. Kinetic and Thermodynamic Comparative Study of Quince Bio-Waste Slow Pyrolysis before and after Sustainable Recovery of Pectin Compounds. Energy Convers. Manag. 2022, 252, 115076. [Google Scholar] [CrossRef]
- Al-Zughbi, I.; Krayem, M. Quince Fruit Cydonia Oblonga Mill Nutritional Composition, Antioxidative Properties, Health Benefits and Consumers Preferences towards Some Industrial Quince Products: A Review. Food Chem. 2022, 393, 133362. [Google Scholar] [CrossRef]
- Othman, S.; Añibarro-Ortega, M.; Dias, M.I.; Ćirić, A.; Mandim, F.; Soković, M.; Ferreira, I.C.F.R.; Pinela, J.; Barros, L. Valorization of Quince Peel into Functional Food Ingredients: A Path towards “Zero Waste” and Sustainable Food Systems. Heliyon 2022, 8, e11042. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic Composition, Antioxidant Activity, and Polyphenol Oxidase (PPO) Activity of Quince (Cydonia oblonga Miller) Varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.I.G.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Antimicrobial Activity of Tunisian Quince (Cydonia oblonga Miller) Pulp and Peel Polyphenols Extracts. J. Agric. Food Chem. 2007, 55, 963–969. [Google Scholar] [CrossRef]
- Stojanović, B.T.; Mitić, S.S.; Stojanović, G.S.; Mitić, M.N.; Kostić, D.A.; Paunović, D.; Arsić, B.B.; Pavlović, A.N. Phenolic Profiles and Metal Ions Analyses of Pulp and Peel of Fruits and Seeds of Quince (Cydonia oblonga Mill.). Food Chem. 2017, 232, 466–475. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of Phenolics and Dietary Fibre Extraction from Date Seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Akram, M.; Al-Zuhair, S.; Elnajjar, E.; Munir, M.T. Subcritical Water Extraction of Phenolics, Antioxidants and Dietary Fibres from Waste Date Pits. J. Environ. Chem. Eng. 2020, 8, 104490. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Fernández-Ochoa, Á.; Cádiz-Gurrea, M.L.; Lozano-Sánchez, J.; Oliver-Simancas, R.; Alañón, M.E.; Castangia, I.; Segura-Carretero, A.; Arráez-Román, D. Application of Response Surface Methodologies to Optimize High-Added Value Products Developments: Cosmetic Formulations as an Example. Antioxidants 2022, 11, 1552. [Google Scholar] [CrossRef]
- Silva, A.R.; Pinela, J.; García, P.A.; Ferreira, I.C.F.R.; Barros, L. Cytinus hypocistis (L.) L.: Optimised Heat/Ultrasound-Assisted Extraction of Tannins by Response Surface Methodology. Sep. Purif. Technol. 2021, 276, 119358. [Google Scholar] [CrossRef]
- Rocha, R.; Pinela, J.; Abreu, R.M.V.; Añibarro-Ortega, M.; Pires, T.C.S.P.; Saldanha, A.L.; Alves, M.J.; Nogueira, A.; Ferreira, I.C.F.R.; Barros, L. Extraction of Anthocyanins from Red Raspberry for Natural Food Colorants Development: Processes Optimization and In Vitro Bioactivity. Processes 2020, 8, 1447. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic Profile and Antioxidant Activity of Coleostephus myconis (L.) Rchb.f.: An Underexploited and Highly Disseminated Species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Use of UFLC-PDA for the Analysis of Organic Acids in Thirty-Five Species of Food and Medicinal Plants. Food Anal. Methods 2013, 6, 1337–1344. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and Bioactive Characterization of the Aromatic Plant: Levisticum Officinale W.D.J. Koch: A Comprehensive Study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 20th ed.; Latimer, G.W., Ed.; AOAC International: Gaithersburg, MD, USA, 2016; ISBN 0935584870. [Google Scholar]
- Pinela, J.; Barreira, J.C.M.; Barros, L.; Cabo Verde, S.; Antonio, A.L.; Carvalho, A.M.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Suitability of Gamma Irradiation for Preserving Fresh-Cut Watercress Quality during Cold Storage. Food Chem. 2016, 206, 50–58. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical Features and Bioactivities of Cornflower (Centaurea cyanus L.) Capitula: The Blue Flowers and the Unexplored Non-Edible Part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Soković, M.; van Griensven, L.J.L.D. Antimicrobial Activity of Essential Oils and Their Components against the Three Major Pathogens of the Cultivated Button Mushroom, Agaricus bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Marin, P.D.; Brkić, D.; Griensven, L.J.L.D. van Antibacterial Effects of the Essential Oils of Commonly Consumed Medicinal Herbs Using an In Vitro Model. Molecules 2010, 15, 7532–7546. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Santos-Buelga, C. Polyphenols and Human Beings: From Epidemiology to Molecular Targets. Molecules 2021, 26, 4218. [Google Scholar] [CrossRef]
- Karar, M.G.E.; Pletzer, D.; Jaiswal, R.; Weingart, H.; Kuhnert, N. Identification, Characterization, Isolation and Activity against Escherichia Coli of Quince (Cydonia oblonga) Fruit Polyphenols. Food Res. Int. 2014, 65, 121–129. [Google Scholar] [CrossRef]
- Szychowski, P.J.; Lech, K.; Sendra-Nadal, E.; Hernández, F.; Figiel, A.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Kinetics, Biocompounds, Antioxidant Activity, and Sensory Attributes of Quinces as Affected by Drying Method. Food Chem. 2018, 255, 157–164. [Google Scholar] [CrossRef]
- Wojdyło, A.; Teleszko, M.; Oszmiański, J. Antioxidant Property and Storage Stability of Quince Juice Phenolic Compounds. Food Chem. 2014, 152, 261–270. [Google Scholar] [CrossRef]
- Zapata, P.J.; Martínez-Esplá, A.; Gironés-Vilaplana, A.; Santos-Lax, D.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A. Phenolic, Volatile, and Sensory Profiles of Beer Enriched by Macerating Quince Fruits. LWT 2019, 103, 139–146. [Google Scholar] [CrossRef]
- Dai, Z.; Zhou, H.; Zhang, S.; Gu, H.; Yang, Q.; Zhang, W.; Dong, W.; Ma, J.; Fang, Y.; Jiang, M.; et al. Current Advance in Biological Production of Malic Acid Using Wild Type and Metabolic Engineered Strains. Bioresour. Technol. 2018, 258, 345–353. [Google Scholar] [CrossRef]
- Rodríguez-Guisado, I.; Hernández, F.; Melgarejo, P.; Legua, P.; Martínez, R.; Martínez, J.J. Chemical, Morphological and Organoleptical Characterisation of Five Spanish Quince Tree Clones (Cydonia oblonga Miller). Sci. Hortic. 2009, 122, 491–496. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Mendes, G.C.; Seabra, R.M.; Ferreira, M.A. Study of the Organic Acids Composition of Quince (Cydonia oblonga Miller) Fruit and Jam. J. Agric. Food Chem. 2002, 50, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, P.J.; Munera-Picazo, S.; Szumny, A.; Carbonell-Barrachina, Á.A.; Hernández, F. Quality Parameters, Bio-Compounds, Antioxidant Activity and Sensory Attributes of Spanish Quinces (Cydonia oblonga Miller). Sci. Hortic. 2014, 165, 163–170. [Google Scholar] [CrossRef]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) Scientific Opinion on the Re-Evaluation of Beetroot Red (E162) as a Food Additive. EFSA J. 2015, 13, 4318. [CrossRef]
- Galanakis, C.M. Food Waste Recovery: Prospects and Opportunities. In Sustainable Food Systems from Agriculture to Industry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 401–419. ISBN 9780128119358. [Google Scholar]
- EFSA Scientific Opinion on Dietary Reference Values for Folate. EFSA J. 2014, 12, 3893.
- Brownlee, I.A.; Chater, P.I.; Pearson, J.P.; Wilcox, M.D. Dietary Fibre and Weight Loss: Where Are We Now? Food Hydrocoll. 2017, 68, 186–191. [Google Scholar] [CrossRef]
- Cho, S.S.; Qi, L.; Fahey, G.C., Jr.; Klurfield, D.M. Consumption of Cereal Fiber, Mixtures of Whole Grains and Bran, and Whole Grains and Risk Reduction in Type 2 Diabetes, Obesity, and Cardiovascular Disease. Am. J. Clin. Nutr. 2013, 98, 594–619. [Google Scholar] [CrossRef]
- Słowiński, M.; Miazek, J.; Dasiewicz, K.; Chmiel, M. The Effect of the Addition of Fiber Preparations on the Color of Medium-Grounded Pasteurized and Sterilized Model Canned Meat Products. Molecules 2021, 26, 2247. [Google Scholar] [CrossRef]
- Livingston, G.E. Malic Acid-Fructose Reaction. J. Am. Chem. Soc. 1953, 75, 1342–1344. [Google Scholar] [CrossRef]
- Afifi, H.S.; Hashim, I.B.; Altubji, S.I. Optimizing Extraction Conditions of Crude Fiber, Phenolic Compounds, Flavonoids and Antioxidant Activity of Date Seed Powder. J. Food Sci. Technol. 2017, 54, 4149–4161. [Google Scholar] [CrossRef]
- Aydar, A.Y. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials. In Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes; Silva, V., Ed.; InTech: Charlotte, NC, USA, 2018; pp. 157–169. [Google Scholar] [CrossRef]
- Forni, E.; Penci, M.; Polesello, A. A Preliminary Characterization of Some Pectins from Quince Fruit (Cydonia oblonga Mill.) and Prickly Pear (Opuntia ficus-indica) Peel. Carbohydr. Polym. 1994, 23, 231–234. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on the Re-Evaluation of Ascorbic Acid (E 300), Sodium Ascorbate (E 301) and Calcium Ascorbate (E 302) as Food Additives. EFSA J. 2015, 13, 4087. [CrossRef]
- EFSA Scientific Opinion on the Re-evaluation of Sulfur Dioxide (E 220), Sodium Sulfite (E 221), Sodium Bisulfite (E 222), Sodium Metabisulfite (E 223), Potassium Metabisulfite (E 224), Calcium Sulfite (E 226), Calcium Bisulfite (E 227) and Potassium Bisulfite (E 228) as Food Additives. EFSA J. 2018, 14, 4438. [CrossRef]
- Atabo, S.; Umar, I.A.; James, D.B.; Mamman, A.I. Sickled Erythrocytes Reversal and Membrane Stabilizing Compounds in Telfairia Occidentalis. Scientifica 2016, 2016, 1568061. [Google Scholar] [CrossRef] [PubMed]
- Pauline, N.; Cabral, B.N.P.; Anatole, P.C.; Jocelyne, A.M.V.; Bruno, M.; Jeanne, N.Y. The In Vitro Antisickling and Antioxidant Effects of Aqueous Extracts Zanthoxyllum Heitzii on Sickle Cell Disorder. BMC Complement. Altern. Med. 2013, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M. Protective Effect of Quince (Cydonia oblonga Miller) Fruit against Oxidative Hemolysis of Human Erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef]
- Alesiani, D.; Canini, A.; D’Abrosca, B.; DellaGreca, M.; Fiorentino, A.; Mastellone, C.; Monaco, P.; Pacifico, S. Antioxidant and Antiproliferative Activities of Phytochemicals from Quince (Cydonia vulgaris) Peels. Food Chem. 2010, 118, 199–207. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Martins, R.C.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) Fruit Characterization Using Principal Component Analysis. J. Agric. Food Chem. 2005, 53, 111–122. [Google Scholar] [CrossRef]
- Jessberger, N.; Dietrich, R.; Granum, P.E.; Märtlbauer, E. The Bacillus cereus Food Infection as Multifactorial Process. Toxins 2020, 12, 701. [Google Scholar] [CrossRef]
- Hodge, R.P.; Harris, C.M.; Harris, T.M. Verrucofortine, a Major Metabolite of Penicillium Verrucosum Var. Cyclopium, the Fungus That Produces the Mycotoxin Verrucosidin. J. Nat. Prod. 1988, 51, 66–73. [Google Scholar] [CrossRef]
- Stojkovic, D.; Jevremovic, K.; Smiljkovic, M.; Živkovic, J.; Sokovic, M. Inhibition of Microbial Biofilm Formation by Cydonia oblonga Mill. Fruit Peel and Leaf Ethanolic Extracts. Lek. Sirovine 2018, 38, 58–61. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial Activity of Malic Acid against Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O157:H7 in Apple, Pear and Melon Juices. Food Control 2009, 20, 105–112. [Google Scholar] [CrossRef]




| Independent Variables | Symbols | Units | Levels 1 | ||||
|---|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | +1 | +1.68 | |||
| X1: Time | t | min | 1 | 25 | 60 | 95 | 119 |
| X2: Temperature | T | °C | 26 | 40 | 60 | 80 | 94 |
| X3: EtOH percentage | S | %, v/v | 0 | 20 | 50 | 80 | 100 |
| Run | Experimental Domain | Bioactive Extract (BE) Dependent Variables | Fiber Concentrate (FC) Dependent Variables | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t (min) | T (°C) | S (%) | BE yield (%) | PC (mg/g) | PA (mg/g) | F3O (mg/g) | FL (mg/g) | MA (g/100 g) | FC yield (%) | DF (g/100 g) | L* | a* | b* | RGB Color | |
| 1 | 25 | 40 | 20 | 58.55 | 9.77 | 3.26 | 4.36 | 2.15 | 6.43 | 43.66 | 57.84 | 59.66 | 7.48 | 21.22 | |
| 2 | 95 | 40 | 20 | 59.12 | 9.99 | 3.72 | 4.17 | 2.10 | 4.59 | 43.08 | 56.78 | 56.75 | 7.50 | 21.14 | |
| 3 | 25 | 80 | 20 | 57.89 | 9.48 | 3.39 | 4.08 | 2.02 | 6.43 | 42.11 | 64.44 | 37.24 | 6.85 | 13.97 | |
| 4 | 95 | 80 | 20 | 53.24 | 10.13 | 3.74 | 4.45 | 1.94 | 6.63 | 46.76 | 62.65 | 35.62 | 5.44 | 11.59 | |
| 5 | 25 | 40 | 80 | 50.31 | 6.86 | 2.26 | 2.68 | 1.92 | 0.59 | 51.90 | 57.27 | 55.88 | 7.20 | 20.91 | |
| 6 | 95 | 40 | 80 | 54.12 | 7.95 | 2.76 | 3.38 | 1.81 | 0.79 | 51.59 | 58.66 | 56.02 | 7.62 | 21.57 | |
| 7 | 25 | 80 | 80 | 50.59 | 7.10 | 2.20 | 2.88 | 2.02 | 4.32 | 49.41 | 57.73 | 59.51 | 7.92 | 21.57 | |
| 8 | 95 | 80 | 80 | 47.30 | 8.89 | 3.08 | 3.85 | 1.96 | 6.18 | 52.70 | 57.53 | 58.60 | 8.14 | 19.93 | |
| 9 | 1 | 60 | 50 | 48.36 | 8.93 | 2.82 | 3.86 | 2.25 | 3.95 | 51.64 | 57.21 | 58.35 | 6.52 | 21.61 | |
| 10 | 119 | 60 | 50 | 48.62 | 10.04 | 3.71 | 4.23 | 2.10 | 5.07 | 50.25 | 58.64 | 56.80 | 6.31 | 20.41 | |
| 11 | 60 | 26 | 50 | 67.09 | 8.87 | 3.26 | 3.76 | 1.85 | 2.90 | 39.82 | 59.83 | 51.76 | 6.81 | 22.31 | |
| 12 | 60 | 94 | 50 | 59.19 | 9.04 | 3.39 | 3.83 | 1.82 | 6.97 | 40.81 | 65.55 | 34.09 | 8.51 | 20.18 | |
| 13 | 60 | 60 | 0 | 49.69 | 9.78 | 3.56 | 4.13 | 2.09 | 6.06 | 49.18 | 55.33 | 55.90 | 7.43 | 21.07 | |
| 14 | 60 | 60 | 100 | 34.47 | 6.50 | 2.00 | 2.62 | 1.88 | 1.63 | 65.53 | 52.46 | 70.62 | 6.63 | 22.25 | |
| 15 | 60 | 60 | 50 | 49.69 | 8.43 | 2.90 | 3.50 | 2.03 | 4.60 | 50.31 | 59.95 | 54.21 | 7.49 | 21.30 | |
| 16 | 60 | 60 | 50 | 51.82 | 7.67 | 2.61 | 3.08 | 1.98 | 4.72 | 48.18 | 58.11 | 56.62 | 7.71 | 21.39 | |
| 17 | 60 | 60 | 50 | 53.32 | 8.48 | 3.03 | 3.40 | 2.05 | 5.37 | 46.68 | 59.35 | 56.42 | 6.91 | 20.79 | |
| 18 | 60 | 60 | 50 | 51.73 | 7.71 | 2.64 | 3.07 | 2.00 | 4.57 | 48.27 | 57.86 | 57.30 | 7.22 | 21.29 | |
| 19 | 60 | 60 | 50 | 49.46 | 7.80 | 2.64 | 3.07 | 2.09 | 4.75 | 50.54 | 57.94 | 58.92 | 7.05 | 21.40 | |
| 20 | 60 | 60 | 50 | 52.03 | 8.17 | 2.88 | 3.34 | 1.94 | 5.41 | 47.97 | 60.48 | 58.33 | 7.20 | 21.54 | |
| Coefficients | BE Yield | PC | PA | F3O | FL | MA | FC Yield | DF | L* | a* | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | b0 | 50.89 ± 0.65 | 8.05 ± 0.11 | 2.77 ± 0.05 | 3.28 ± 0.07 | 2.01 ± 0.01 | 4.83 ± 0.10 | 49.05 ± 0.59 | 58.79 ± 0.36 | 56.86 ± 0.57 | 7.23 ± 0.14 |
| Linear terms | b1 (t) | −0.23 ± 0.51 * | 0.41 ± 0.08 | 0.27 ± 0.04 | 0.18 ± 0.05 | −0.04 ± 0.01 | 0.17 ± 0.09 * | 0.35 ± 0.46 * | ns | ns | −0.08 ± 0.11 * |
| b2 (T) | −1.93 ± 0.51 | 0.10 ± 0.08 * | 0.05 ± 0.04 * | 0.06 ± 0.05 * | −0.01 ± 0.01 * | 1.32 ± 0.09 | 0.18 ± 0.46 * | 1.57 ± 0.28 | −4.91 ± 0.44 | 0.25 ± 0.11 | |
| b3 (S) | 3.81 ± 0.51 | −1.03 ± 0.08 | −0.47 ± 0.04 | −0.50 ± 0.05 | −0.06 ± 0.01 | −1.44 ± 0.09 | 4.21 ± 0.46 | −1.12 ± 0.28 | 4.80 ± 0.44 | 0.02 ± 0.11 * | |
| Quadratic terms | b11 (tt) | ns | 0.48 ± 0.08 | 0.15 ± 0.04 | 0.27 ± 0.05 | 0.06 ± 0.01 | ns | ns | ns | ns | −0.24 ± 0.11 |
| b22 (TT) | ns | 0.29 ± 0.08 | 0.17 ± 0.04 | 0.18 ± 0.05 | −0.06 ± 0.01 | ns | −3.40 ± 0.44 | 1.55 ± 0.27 | −5.37 ± 0.43 | ns | |
| b33 (SS) | −2.67 ± 0.49 | ns | ns | ns | ns | −0.35 ± 0.09 | 3.63 ± 0.44 | −1.56 ± 0.27 | 1.82 ± 0.43 | ns | |
| Interaction terms | b12 (tT) | −1.54 ± 0.67 | ns | ns | ns | ns | 0.46 ± 0.13 | 1.10 ± 0.59 | ns | ns | ns |
| b13 (tS) | ns | 0.25 ± 0.11 | ns | 0.19 ± 0.07 | 0.07 ± 0.01 | 0.46 ± 0.13 | ns | ns | ns | ns | |
| b23 (TS) | ns | ns | ns | ns | ns | 0.89 ± 0.13 | ns | −1.64 ± 0.37 | 6.22 ± 0.58 | 0.49 ± 0.14 | |
| Modeling statistics | |||||||||||
| Model F-value | 34.18 | 38.18 | 49.38 | 26.43 | 27.81 | 71.54 | 31.97 | 28.10 | 108.00 | 4.63 | |
| Model p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0099 | |
| Lack-of-Fit | 0.2237 | 0.7923 | 0.8772 | 0.5805 | 0.9774 | 0.6356 | 0.3454 | 0.6731 | 0.5502 | 0.1723 | |
| R2 | 0.9404 | 0.9463 | 0.9463 | 0.9242 | 0.9277 | 0.9766 | 0.9365 | 0.9094 | 0.9749 | 0.6913 | |
| Adj R2 | 0.9129 | 0.9215 | 0.9272 | 0.8893 | 0.8944 | 0.9629 | 0.9072 | 0.8770 | 0.9657 | 0.5342 | |
| Ad. Precision | 27.29 | 20.83 | 25.04 | 17.17 | 19.14 | 28.23 | 24.41 | 23.56 | 40.83 | 7.77 | |
| C.V. (%) | 3.64 | 3.59 | 4.65 | 5.24 | 1.84 | 7.77 | 3.48 | 1.76 | 3.01 | 5.56 | |
| Phenolic Extraction | Malic Acid Extraction | Phenolic/Malic Acid Extraction | |
|---|---|---|---|
| BE yield (%, w/w) | 48 ± 3 a | 49 ± 3 a | 50 ± 2 a |
| Phenolic compounds (mg/g BE) | 9.3 ± 0.6 a | 8.1± 0.4 b | 8.1 ± 0.6 b |
| Phenolic acids (mg/g BE) | 2.8 ± 0.3 b | 3.5 ± 0.2 a | 3.1 ± 0.3 a,b |
| Flavan-3-ols (mg/g BE) | 4.2 ± 0.4 a | 3.0 ± 0.2 b | 3.7 ± 0.3 b |
| Flavonols (mg/g BE) | 2.3 ± 0.3 a | 1.62 ± 0.01 b | 1.30 ± 0.03 c |
| Malic acid (g/100 g BE) | 4.5 ± 0.2 c | 7.6 ± 0.4 a | 6.0 ± 0.1 b |
| FC yield (%, w/w) | 51 ± 1 a | 50 ± 8 a | 47 ± 1 a |
| L* value | 54.9 ± 0.7 a | 35.8 ± 0.2 c | 41.4 ± 1.1 b |
| a* value | 7.0 ± 0.2 a | 7.5 ± 0.9 a | 8.2 ± 0.2 a |
| b* value | 23.3 ± 0.5 a | 17.1 ± 0.3 c | 19.4 ± 0.5 b |
| RGB color |
| Bioassays | Bioactive Extracts (BEs) | Positive Controls | |||||
|---|---|---|---|---|---|---|---|
| Phenolic BE | Malic Acid BE | Phenolic/Malic Acid BE | E302 | E223 | Trolox | ||
| TBARS (EC50, µg/mL) | 83 ± 1 d | 26 ± 1 b | 45 ± 2 c | 284 ± 9 f | 229 ± 9 e | 5.4 ± 0.3 a | |
| OxHLIA | Δt 60 min | 187 ± 9 d | 105 ± 3 c | 223 ± 7 e | 41 ± 1 b | na | 21.7 ± 0.4 a |
| (IC50 µg/mL) | Δt 120 min | 471 ± 12 d | 234 ± 5 c | 458 ± 9 d | 84 ± 2 b | na | 43 ± 1 a |
| Microorganisms | Bioactive Extracts (BEs) | Positive Controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic BE | Malic Acid BE | Phenolic/Malic Acid BE | E211 | E224 | Streptomycin | Ampicillin | ||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Gram-positive bacteria | ||||||||||||||
| Staphylococcus aureus | 4 | 8 | 1 | 2 | 4 | 8 | 4 | 4 | 1 | 1 | 0.04 | 0.1 | 0.25 | 0.45 |
| Bacillus cereus | 1 | 2 | 1 | 2 | 1 | 2 | 0.5 | 0.5 | 2 | 4 | 0.1 | 0.2 | 0.25 | 0.4 |
| Listeria monocytogenes | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 0.5 | 1 | 0.2 | 0.3 | 0.4 | 0.5 |
| Gram-negative bacteria | ||||||||||||||
| Escherichia coli | 1 | 2 | 1 | 2 | 4 | 8 | 1 | 2 | 0.5 | 1 | 0.2 | 0.3 | 0.4 | 0.5 |
| Salmonella Typhimurium | 2 | 4 | 1 | 2 | 4 | 8 | 1 | 2 | 1 | 1 | 0.2 | 0.3 | 0.75 | 1.2 |
| Enterobacter cloacae | 1 | 2 | 0.5 | 1 | 4 | 8 | 2 | 4 | 0.5 | 0.5 | 0.2 | 0.3 | 0.25 | 0.5 |
| Ketoconazole | Bifonazole | |||||||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| Aspergillus fumigatus | 1 | 2 | 1 | 2 | 2 | 4 | 1 | 2 | 1 | 1 | 0.25 | 0.5 | 0.15 | 0.2 |
| Aspergillus niger | 2 | 4 | 1 | 2 | 2 | 4 | 1 | 2 | 1 | 1 | 0.2 | 0.5 | 0.15 | 0.2 |
| Aspergillus versicolor | >8 | >8 | 1 | 2 | 4 | 8 | 2 | 2 | 1 | 1 | 0.2 | 0.5 | 0.1 | 0.2 |
| Penicillium funiculosum | 2 | 4 | 2 | 4 | 2 | 4 | 1 | 2 | 0.5 | 0.5 | 0.2 | 0.5 | 0.2 | 0.25 |
| Penicillium verrucosum var. cyclopium | >8 | >8 | >8 | >8 | >8 | >8 | 2 | 4 | 1 | 1 | 0.2 | 0.3 | 0.1 | 0.2 |
| Trichoderma viride | 2 | 4 | 2 | 4 | 2 | 4 | 1 | 2 | 0.5 | 0.5 | 2.5 | 3.5 | 0.2 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.; Añibarro-Ortega, M.; Kostić, M.; Nogueira, A.; Soković, M.; Pinela, J.; Barros, L. Upcycling Quince Peel into Bioactive Ingredients and Fiber Concentrates through Multicomponent Extraction Processes. Antioxidants 2023, 12, 260. https://doi.org/10.3390/antiox12020260
Pereira A, Añibarro-Ortega M, Kostić M, Nogueira A, Soković M, Pinela J, Barros L. Upcycling Quince Peel into Bioactive Ingredients and Fiber Concentrates through Multicomponent Extraction Processes. Antioxidants. 2023; 12(2):260. https://doi.org/10.3390/antiox12020260
Chicago/Turabian StylePereira, Alexis, Mikel Añibarro-Ortega, Marina Kostić, António Nogueira, Marina Soković, José Pinela, and Lillian Barros. 2023. "Upcycling Quince Peel into Bioactive Ingredients and Fiber Concentrates through Multicomponent Extraction Processes" Antioxidants 12, no. 2: 260. https://doi.org/10.3390/antiox12020260
APA StylePereira, A., Añibarro-Ortega, M., Kostić, M., Nogueira, A., Soković, M., Pinela, J., & Barros, L. (2023). Upcycling Quince Peel into Bioactive Ingredients and Fiber Concentrates through Multicomponent Extraction Processes. Antioxidants, 12(2), 260. https://doi.org/10.3390/antiox12020260













