Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain
Abstract
1. Introduction
2. Vitamin C Turnover in Humans
3. Vitamin C and Neurotransmission
4. Vitamin C and Neuronal Calcium Signalling
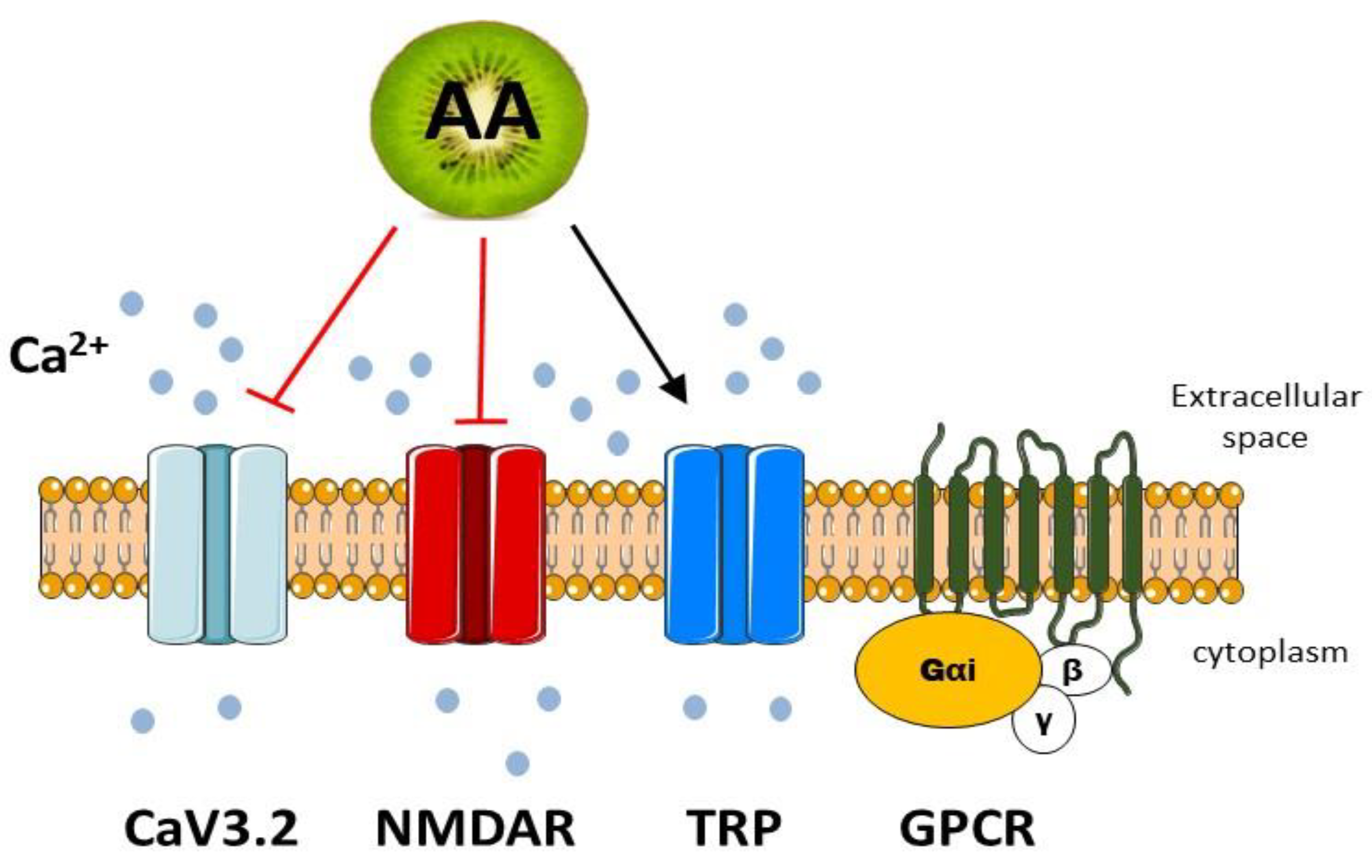
4.1. Vitamin C in the Regulation of Calcium Channels
4.1.1. Vitamin C in the Regulation of VGCC
4.1.2. Vitamin C in the Regulation of NMDARs
4.1.3. Vitamin C in the Regulation of TRP Channels
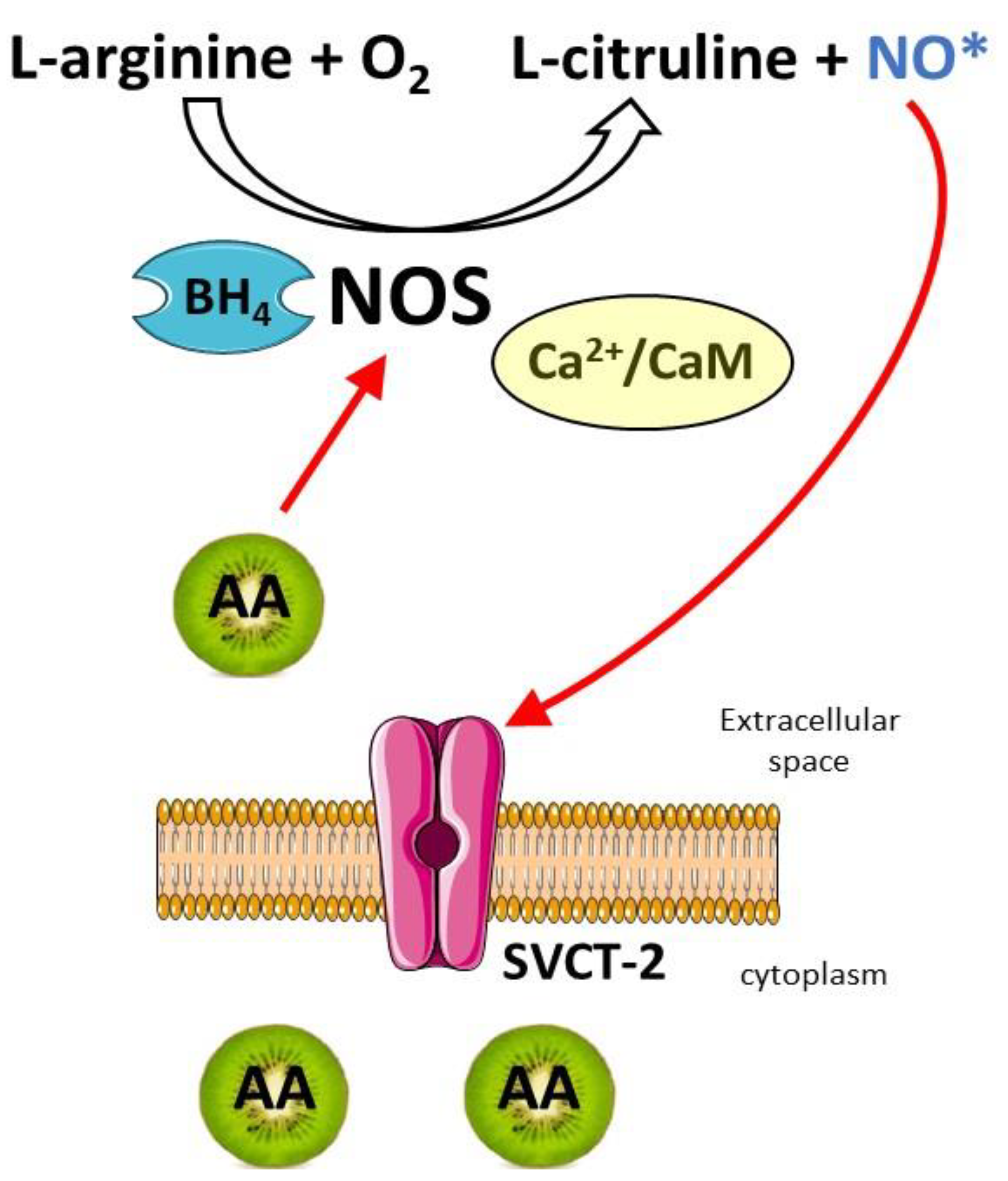
4.2. Vitamin C in the Regulation of NO
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piro, A.; Tagarelli, G.; Lagonia, P.; Tagarelli, A.; Quattrone, A. Casimir Funk: His discovery of the vitamins and their deficiency disorders. Ann. Nutr. Metab. 2010, 57, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000, 23, 209–216. [Google Scholar] [CrossRef] [PubMed]
- May, J.M. Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 2012, 56, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Pinto, A.; Acuña, A.I.; Beltrán, F.A.; Torres-Díaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxidative Med. Cell Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef] [PubMed]
- Mandl, J.; Szarka, A.; Bánhegyi, G. Vitamin C: Update on physiology and pharmacology. Br. J. Pharmacol. 2009, 157, 1097–1110. [Google Scholar] [CrossRef]
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health. Adv. Food Nutr. Res. 2018, 83, 281–310. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C-Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1510. [Google Scholar] [CrossRef]
- Ferreira, N.R.; Vitorino, C.; Fortuna, A. From antioxidant to neuromodulator: The role of ascorbate in the management of major depression disorder. Biochem. Pharmacol. 2022, 206, 115300. [Google Scholar] [CrossRef]
- Plevin, D.; Galletly, C. The neuropsychiatric effects of vitamin C deficiency: A systematic review. BMC Psychiatry 2020, 20, 315. [Google Scholar] [CrossRef]
- Berr, C. Oxidative stress and cognitive impairment in the elderly. J. Nutr. Health Aging 2002, 6, 261–266. [Google Scholar] [PubMed]
- Harrison, F.E. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J. Alzheimers Dis. 2012, 29, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, A.; Lykkesfeldt, J. Does vitamin C enhance nitric oxide bioavailability in a tetrahydrobiopterin-dependent manner? In vitro, in vivo and clinical studies. Nitric Oxide 2014, 36, 51–57. [Google Scholar] [CrossRef]
- Coker, S.J.; Smith-Díaz, C.C.; Dyson, R.M.; Vissers, M.C.M.; Berry, M.J. The Epigenetic Role of Vitamin C in Neurodevelopment. Int. J. Mol. Sci. 2022, 23, 1208. [Google Scholar] [CrossRef] [PubMed]
- Camarena, V.; Wang, G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, E.M.; Vivar, C.; Camandola, S. Physiology and pathology of calcium signaling in the brain. Front. Pharmacol. 2012, 3, 61. [Google Scholar] [CrossRef]
- Nanou, E.; Catterall, W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef]
- Hille, B.; Dickson, E.J.; Kruse, M.; Vivas, O.; Suh, B.C. Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 2015, 1851, 844–856. [Google Scholar] [CrossRef]
- Gerber, U.; Gee, C.E.; Benquet, P. Metabotropic glutamate receptors: Intracellular signaling pathways. Curr. Opin. Pharmacol. 2007, 7, 56–61. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochim. Biophys. Acta 2009, 1793, 933–940. [Google Scholar] [CrossRef]
- Foskett, J.K.; White, C.; Cheung, K.H.; Mak, D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 2007, 87, 593–658. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA control of cell death and survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Patergnani, S.; Missiroli, S.; Morciano, G.; Rimessi, A.; Wieckowski, M.R.; Giorgi, C.; Pinton, P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium 2018, 69, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Calì, T.; Brini, M.; Carafoli, E. Regulation of Cell Calcium and Role of Plasma Membrane Calcium ATPases. Int. Rev. Cell Mol. Biol. 2017, 332, 259–296. [Google Scholar] [CrossRef]
- Tarasov, A.I.; Griffiths, E.J.; Rutter, G.A. Regulation of ATP production by mitochondrial Ca(2+). Cell Calcium 2012, 52, 28–35. [Google Scholar] [CrossRef]
- Brini, M.; Carafoli, E. Calcium pumps in health and disease. Physiol. Rev. 2009, 89, 1341–1378. [Google Scholar] [CrossRef]
- Strehler, E.E.; Thayer, S.A. Evidence for a role of plasma membrane calcium pumps in neurodegenerative disease: Recent developments. Neurosci. Lett. 2018, 663, 39–47. [Google Scholar] [CrossRef]
- Lytton, J. Na+/Ca2+ exchangers: Three mammalian gene families control Ca2+ transport. Biochem. J. 2007, 406, 365–382. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Trebak, M.; Perocchi, F.; Khananshvili, D.; Sekler, I. Crosslink between calcium and sodium signalling. Exp. Physiol. 2018, 103, 157–169. [Google Scholar] [CrossRef]
- Kelemen, K.; Szilágyi, T. New Approach for Untangling the Role of Uncommon Calcium-Binding Proteins in the Central Nervous System. Brain Sci. 2021, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Giorgi, C.; Pinton, P. Molecular mechanisms and consequences of mitochondrial permeability transition. Nat. Rev. Mol. Cell Biol. 2022, 23, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Fukuyama, R.; Minoshima, S.; Shimizu, N.; Yagi, K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J. Biol. Chem. 1994, 269, 13685–13688. [Google Scholar] [CrossRef] [PubMed]
- Linster, C.L.; Van Schaftingen, E. Vitamin C. Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef]
- Cahill, L.E.; El-Sohemy, A. Vitamin C transporter gene polymorphisms, dietary vitamin C and serum ascorbic acid. J. Nutr. Nutr. 2009, 2, 292–301. [Google Scholar] [CrossRef]
- Tsukaguchi, H.; Tokui, T.; Mackenzie, B.; Berger, U.V.; Chen, X.Z.; Wang, Y.; Brubaker, R.F.; Hediger, M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature 1999, 399, 70–75. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Naidu, K.A. Vitamin C in human health and disease is still a mystery? An overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef]
- Meredith, M.E.; May, J.M. Regulation of embryonic neurotransmitter and tyrosine hydroxylase protein levels by ascorbic acid. Brain Res. 2013, 1539, 7–14. [Google Scholar] [CrossRef]
- May, J.M.; Qu, Z.C.; Nazarewicz, R.; Dikalov, S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res. Bull. 2013, 90, 35–42. [Google Scholar] [CrossRef]
- Rebec, G.V.; Pierce, R.C. A vitamin as neuromodulator: Ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog. Neurobiol. 1994, 43, 537–565. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, E.; Haga, Y.; Shinozuka, K.; Takeda, M. The effect of ascorbate on the acetylcholine release from guinea-pig ileal myenteric plexus. J. Pharm. Pharmacol. 1986, 38, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Yoshida, H. Ascorbic acid, an endogenous factor required for acetylcholine release from the synaptic vesicles. Jpn J. Pharmacol. 1980, 30, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Asher, A.; Pollard, H.; Zinder, O. Ascorbic acid and catecholamine secretion from cultured chromaffin cells. J. Biol. Chem. 1983, 258, 13111–13115. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, R.A. Ascorbic acid in the brain. Brain Res. Brain Res. Rev. 1993, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zeng, L.H.; Taniguchi, T.; Xie, Q.M. Activation of PKA and phosphorylation of sodium-dependent vitamin C transporter 2 by prostaglandin E2 promote osteoblast-like differentiation in MC3T3-E1 cells. Cell Death Differ. 2007, 14, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Johnson, D.; Ma, L.S.; Jarvis, S.M.; Wei-Jun, L. Regulation of the human vitamin C transporters expressed in COS-1 cells by protein kinase C [corrected]. Am. J. Physiol. Cell Physiol. 2002, 283, C1696–C1704. [Google Scholar] [CrossRef]
- Portugal, C.C.; da Encarnação, T.G.; Socodato, R.; Moreira, S.R.; Brudzewsky, D.; Ambrósio, A.F.; Paes-de-Carvalho, R. Nitric oxide modulates sodium vitamin C transporter 2 (SVCT-2) protein expression via protein kinase G (PKG) and nuclear factor-κB (NF-κB). J. Biol. Chem. 2012, 287, 3860–3872. [Google Scholar] [CrossRef]
- da Encarnação, T.G.; Portugal, C.C.; Nogueira, C.E.; Santiago, F.N.; Socodato, R.; Paes-de-Carvalho, R. Dopamine Promotes Ascorbate Release from Retinal Neurons: Role of D. Mol. Neurobiol. 2018, 55, 7858–7871. [Google Scholar] [CrossRef]
- Portugal, C.C.; da Encarnação, T.G.; Domith, I.; Dos Santos Rodrigues, A.; de Oliveira, N.A.; Socodato, R.; Paes-de-Carvalho, R. Dopamine-Induced Ascorbate Release From Retinal Neurons Involves Glutamate Release, Activation of AMPA/Kainate Receptors and Downstream Signaling Pathways. Front. Neurosci. 2019, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Portugal, C.C.; Miya, V.S.; Calaza, K.a.C.; Santos, R.A.; Paes-de-Carvalho, R. Glutamate receptors modulate sodium-dependent and calcium-independent vitamin C bidirectional transport in cultured avian retinal cells. J. Neurochem. 2009, 108, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Low, J.T.; Shukla, A.; Behrendorff, N.; Thorn, P. Exocytosis, dependent on Ca2+ release from Ca2+ stores, is regulated by Ca2+ microdomains. J. Cell Sci. 2010, 123, 3201–3208. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; Pozzan, T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef] [PubMed]
- Shirokova, O.M.; Pchelin, P.V.; Mukhina, I.V. MERCs. The Novel Assistant to Neurotransmission? Front. Neurosci. 2020, 14, 589319. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Missiroli, S.; Patergnani, S.; Duszynski, J.; Wieckowski, M.R.; Pinton, P. Mitochondria-associated membranes: Composition, molecular mechanisms, and physiopathological implications. Antioxid. Redox Signal. 2015, 22, 995–1019. [Google Scholar] [CrossRef]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in Health and Disease: Its Role in the Metabolism of Cells and Redox State in the Brain. Front. Physiol. 2015, 6, 397. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef]
- Dolphin, A.C. Voltage-gated calcium channels: Their discovery, function and importance as drug targets. Brain Neurosci. Adv. 2018, 2, 2398212818794805. [Google Scholar] [CrossRef]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient Receptor Potential (TRP) Channels. Subcell. Biochem. 2018, 87, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Perez-Reyes, E. Molecular characterization of T-type calcium channels. Cell Calcium 2006, 40, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Cheong, E.; Shin, H.S. T-type Ca2+ channels in normal and abnormal brain functions. Physiol. Rev. 2013, 93, 961–992. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, F.; Kawabata, A. T-type calcium channels: Functional regulation and implication in pain signaling. J. Pharmacol. Sci. 2013, 122, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The Physiology, Pathology, and Pharmacology of Voltage-Gated Calcium Channels and Their Future Therapeutic Potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef]
- Newman, M.; Connery, H.; Boyd, J. Opioids and Vitamin C: Known Interactions and Potential for Redox-Signaling Crosstalk. Antioxidants 2022, 11, 1267. [Google Scholar] [CrossRef]
- Nelson, M.T.; Joksovic, P.M.; Su, P.; Kang, H.W.; Van Deusen, A.; Baumgart, J.P.; David, L.S.; Snutch, T.P.; Barrett, P.Q.; Lee, J.H.; et al. Molecular mechanisms of subtype-specific inhibition of neuronal T-type calcium channels by ascorbate. J. Neurosci. 2007, 27, 12577–12583. [Google Scholar] [CrossRef]
- Nagasawa, K.; Tarui, T.; Yoshida, S.; Sekiguchi, F.; Matsunami, M.; Ohi, A.; Fukami, K.; Ichida, S.; Nishikawa, H.; Kawabata, A. Hydrogen sulfide evokes neurite outgrowth and expression of high-voltage-activated Ca2+ currents in NG108-15 cells: Involvement of T-type Ca2+ channels. J. Neurochem. 2009, 108, 676–684. [Google Scholar] [CrossRef]
- Tarui, T.; Fukami, K.; Nagasawa, K.; Yoshida, S.; Sekiguchi, F.; Kawabata, A. Involvement of Src kinase in T-type calcium channel-dependent neuronal differentiation of NG108-15 cells by hydrogen sulfide. J. Neurochem. 2010, 114, 512–519. [Google Scholar] [CrossRef]
- Okubo, K.; Nakanishi, H.; Matsunami, M.; Shibayama, H.; Kawabata, A. Topical application of disodium isostearyl 2-O-L-ascorbyl phosphate, an amphiphilic ascorbic acid derivative, reduces neuropathic hyperalgesia in rats. Br. J. Pharmacol. 2012, 166, 1058–1068. [Google Scholar] [CrossRef]
- Takahashi, T.; Aoki, Y.; Okubo, K.; Maeda, Y.; Sekiguchi, F.; Mitani, K.; Nishikawa, H.; Kawabata, A. Upregulation of Ca(v)3.2 T-type calcium channels targeted by endogenous hydrogen sulfide contributes to maintenance of neuropathic pain. Pain 2010, 150, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, S.M.; Jevtovic-Todorovic, V. T-type voltage-gated calcium channels as targets for the development of novel pain therapies. Br. J. Pharmacol. 2011, 163, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, S.M.; Jevtovic-Todorovic, V. Redox regulation of neuronal voltage-gated calcium channels. Antioxid. Redox Signal. 2014, 21, 880–891. [Google Scholar] [CrossRef]
- Glasgow, N.G.; Siegler Retchless, B.; Johnson, J.W. Molecular bases of NMDA receptor subtype-dependent properties. J. Physiol. 2015, 593, 83–95. [Google Scholar] [CrossRef]
- Paoletti, P. Molecular basis of NMDA receptor functional diversity. Eur. J. Neurosci. 2011, 33, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Toutenhoofd, S.L.; Strehler, E.E. The calmodulin multigene family as a unique case of genetic redundancy: Multiple levels of regulation to provide spatial and temporal control of calmodulin pools? Cell Calcium 2000, 28, 83–96. [Google Scholar] [CrossRef]
- Spratt, D.E.; Newman, E.; Mosher, J.; Ghosh, D.K.; Salerno, J.C.; Guillemette, J.G. Binding and activation of nitric oxide synthase isozymes by calmodulin EF hand pairs. FEBS J. 2006, 273, 1759–1771. [Google Scholar] [CrossRef]
- Shen, X.; Valencia, C.A.; Szostak, J.W.; Dong, B.; Liu, R. Scanning the human proteome for calmodulin-binding proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 5969–5974. [Google Scholar] [CrossRef]
- Colomer, J.; Means, A.R. Physiological roles of the Ca2+/CaM-dependent protein kinase cascade in health and disease. Subcell Biochem. 2007, 45, 169–214. [Google Scholar] [CrossRef]
- Means, A.R. The Year in Basic Science: Calmodulin kinase cascades. Mol. Endocrinol. 2008, 22, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Boczek, T.; Radzik, T.; Ferenc, B.; Zylinska, L. The Puzzling Role of Neuron-Specific PMCA Isoforms in the Aging Process. Int. J. Mol. Sci. 2019, 20, 6338. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, K.P.; Ikura, M. Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 2002, 108, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Vetter, S.W.; Leclerc, E. Novel aspects of calmodulin target recognition and activation. Eur. J. Biochem. 2003, 270, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Travica, N.; Ried, K.; Sali, A.; Scholey, A.; Hudson, I.; Pipingas, A. Vitamin C Status and Cognitive Function: A Systematic Review. Nutrients 2017, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Kornhuber, J.; Mack-Burkhardt, F.; Riederer, P. Regional distribution of [3H]MK-801 binding sites in the human brain. Brain Res. 1989, 489, 397–399. [Google Scholar] [CrossRef]
- Majewska, M.D.; Bell, J.A.; London, E.D. Regulation of the NMDA receptor by redox phenomena: Inhibitory role of ascorbate. Brain Res. 1990, 537, 328–332. [Google Scholar] [CrossRef]
- Majewska, M.D.; Bell, J.A. Ascorbic acid protects neurons from injury induced by glutamate and NMDA. Neuroreport 1990, 1, 194–196. [Google Scholar] [CrossRef]
- Domith, I.; Socodato, R.; Portugal, C.C.; Munis, A.F.; Duarte-Silva, A.T.; Paes-de-Carvalho, R. Vitamin C modulates glutamate transport and NMDA receptor function in the retina. J. Neurochem. 2018, 144, 408–420. [Google Scholar] [CrossRef]
- Godoy, A.; Ormazabal, V.; Moraga-Cid, G.; Zúñiga, F.A.; Sotomayor, P.; Barra, V.; Vasquez, O.; Montecinos, V.; Mardones, L.; Guzmán, C.; et al. Mechanistic insights and functional determinants of the transport cycle of the ascorbic acid transporter SVCT2. Activation by sodium and absolute dependence on bivalent cations. J. Biol. Chem. 2007, 282, 615–624. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef] [PubMed]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef]
- Morgado-Bernal, I. Learning and memory consolidation: Linking molecular and behavioral data. Neuroscience 2011, 176, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fraga, D.B.; Costa, A.P.; Olescowicz, G.; Camargo, A.; Pazini, F.L.; E Freitas, A.; Moretti, M.; S Brocardo, P.; S Rodrigues, A.L. Ascorbic acid presents rapid behavioral and hippocampal synaptic plasticity effects. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 96, 109757. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; Rodrigues, A.L.S. Functional role of ascorbic acid in the central nervous system: A focus on neurogenic and synaptogenic processes. Nutr. Neurosci. 2022, 25, 2431–2441. [Google Scholar] [CrossRef]
- Tveden-Nyborg, P.; Johansen, L.K.; Raida, Z.; Villumsen, C.K.; Larsen, J.O.; Lykkesfeldt, J. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am. J. Clin. Nutr. 2009, 90, 540–546. [Google Scholar] [CrossRef]
- Karamian, R.; Komaki, A.; Salehi, I.; Tahmasebi, L.; Komaki, H.; Shahidi, S.; Sarihi, A. Vitamin C reverses lead-induced deficits in hippocampal synaptic plasticity in rats. Brain Res. Bull. 2015, 116, 7–15. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef]
- Ambudkar, I.S.; Bandyopadhyay, B.C.; Liu, X.; Lockwich, T.P.; Paria, B.; Ong, H.L. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium 2006, 40, 495–504. [Google Scholar] [CrossRef]
- Bollimuntha, S.; Pani, B.; Singh, B.B. Neurological and Motor Disorders: Neuronal Store-Operated Ca. Adv. Exp. Med. Biol. 2017, 993, 535–556. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.M.; Das, A.B. Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Herst, P.M.; Broadley, K.W.; Harper, J.L.; McConnell, M.J. Pharmacological concentrations of ascorbate radiosensitize glioblastoma multiforme primary cells by increasing oxidative DNA damage and inhibiting G2/M arrest. Free Radic. Biol. Med. 2012, 52, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Uetaki, M.; Tabata, S.; Nakasuka, F.; Soga, T.; Tomita, M. Metabolomic alterations in human cancer cells by vitamin C-induced oxidative stress. Sci. Rep. 2015, 5, 13896. [Google Scholar] [CrossRef]
- Oronowicz, J.; Reinhard, J.; Reinach, P.S.; Ludwiczak, S.; Luo, H.; Omar Ba Salem, M.H.; Kraemer, M.M.; Biebermann, H.; Kakkassery, V.; Mergler, S. Ascorbate-induced oxidative stress mediates TRP channel activation and cytotoxicity in human etoposide-sensitive and -resistant retinoblastoma cells. Lab. Investig. 2021, 101, 70–88. [Google Scholar] [CrossRef]
- Naylor, J.; Al-Shawaf, E.; McKeown, L.; Manna, P.T.; Porter, K.E.; O’Regan, D.; Muraki, K.; Beech, D.J. TRPC5 channel sensitivities to antioxidants and hydroxylated stilbenes. J. Biol. Chem. 2011, 286, 5078–5086. [Google Scholar] [CrossRef]
- Cui, M.; Gosu, V.; Basith, S.; Hong, S.; Choi, S. Polymodal Transient Receptor Potential Vanilloid Type 1 Nocisensor: Structure, Modulators, and Therapeutic Applications. Adv. Protein Chem. Struct. Biol. 2016, 104, 81–125. [Google Scholar] [CrossRef]
- Kim, K.S.; Yoo, H.Y.; Park, K.S.; Kim, J.K.; Zhang, Y.H.; Kim, S.J. Differential effects of acute hypoxia on the activation of TRPV1 by capsaicin and acidic pH. J. Physiol. Sci. 2012, 62, 93–103. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, D.Y. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide 2009, 20, 223–230. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, N.; Kourosh-Arami, M.; Nadjafi, S.; Ashtari, B. Structure, Distribution, Regulation, and Function of Splice Variant Isoforms of Nitric Oxide Synthase Family in the Nervous System. Curr. Protein Pept. Sci. 2022, 23, 510–534. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Sah, A.N.; Bawari, S.; Nabavi, S.F.; Dehpour, A.R.; Shirooie, S.; Braidy, N.; Fiebich, B.L.; Vacca, R.A.; Nabavi, S.M. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr. Neuropharmacol. 2021, 19, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991, 14, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Knott, A.B.; Bossy-Wetzel, E. Nitric oxide in health and disease of the nervous system. Antioxid. Redox Signal. 2009, 11, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Clifford, D.B.; Zorumski, C.F. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science 1992, 257, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Zorumski, C.F. Nitric oxide and long-term synaptic depression in the rat hippocampus. Neuroreport 1993, 4, 1131–1134. [Google Scholar]
- Lourenço, C.F.; Ledo, A.; Barbosa, R.M.; Laranjinha, J. Neurovascular-neuroenergetic coupling axis in the brain: Master regulation by nitric oxide and consequences in aging and neurodegeneration. Free Radic. Biol. Med. 2017, 108, 668–682. [Google Scholar] [CrossRef]
- Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000, 347 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Rodríguez-Crespo, I.; Moënne-Loccoz, P.; Loehr, T.M.; Ortiz de Montellano, P.R. Endothelial nitric oxide synthase: Modulations of the distal heme site produced by progressive N-terminal deletions. Biochemistry 1997, 36, 8530–8538. [Google Scholar] [CrossRef]
- Ghosh, D.K.; Wu, C.; Pitters, E.; Moloney, M.; Werner, E.R.; Mayer, B.; Stuehr, D.J. Characterization of the inducible nitric oxide synthase oxygenase domain identifies a 49 amino acid segment required for subunit dimerization and tetrahydrobiopterin interaction. Biochemistry 1997, 36, 10609–10619. [Google Scholar] [CrossRef] [PubMed]
- Steinert, J.R.; Chernova, T.; Forsythe, I.D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 2010, 16, 435–452. [Google Scholar] [CrossRef] [PubMed]
- Porasuphatana, S.; Tsai, P.; Rosen, G.M. The generation of free radicals by nitric oxide synthase. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2003, 134, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Nitric oxide and neuronal death. Nitric Oxide 2010, 23, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.A.; Jasiulionis, M.G.; Melo, F.H.M. The Role of the BH4 Cofactor in Nitric Oxide Synthase Activity and Cancer Progression: Two Sides of the Same Coin. Int. J. Mol. Sci. 2021, 22, 9546. [Google Scholar] [CrossRef]
- Ferreira, N.R.; Lourenço, C.F.; Barbosa, R.M.; Laranjinha, J. Coupling of ascorbate and nitric oxide dynamics in vivo in the rat hippocampus upon glutamatergic neuronal stimulation: A novel functional interplay. Brain Res. Bull. 2015, 114, 13–19. [Google Scholar] [CrossRef]
- Ward, M.S.; Lamb, J.; May, J.M.; Harrison, F.E. Behavioral and monoamine changes following severe vitamin C deficiency. J. Neurochem. 2013, 124, 363–375. [Google Scholar] [CrossRef]
- Riveros, M.E.; Ávila, A.; Schruers, K.; Ezquer, F. Antioxidant Biomolecules and Their Potential for the Treatment of Difficult-to-Treat Depression and Conventional Treatment-Resistant Depression. Antioxidants 2022, 11, 540. [Google Scholar] [CrossRef]
- Maratha, S.; Sharma, V.; Walia, V. Antidepressant Like Effect of Ascorbic Acid in Mice: Possible Involvement of NO-sGC-cGMP Signaling. Neurochem. Res. 2022, 47, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Mohd Idris, R.A.; Ahmed, N.; Ahmad, S.; Murtadha, A.H.; Tengku Din, T.A.D.A.; Yean, C.Y.; Wan Abdul Rahman, W.F.; Mat Lazim, N.; Uskoković, V.; et al. High-Dose Vitamin C for Cancer Therapy. Pharmaceuticals 2022, 15, 711. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Huang, Y.; Cao, D.; Qiu, S.; Chen, B.; Li, J.; Bao, Y.; Wei, Q.; Han, P.; Liu, L. Vitamin C Intake and Cancers: An Umbrella Review. Front. Nutr. 2021, 8, 812394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zylinska, L.; Lisek, M.; Guo, F.; Boczek, T. Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain. Antioxidants 2023, 12, 231. https://doi.org/10.3390/antiox12020231
Zylinska L, Lisek M, Guo F, Boczek T. Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain. Antioxidants. 2023; 12(2):231. https://doi.org/10.3390/antiox12020231
Chicago/Turabian StyleZylinska, Ludmila, Malwina Lisek, Feng Guo, and Tomasz Boczek. 2023. "Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain" Antioxidants 12, no. 2: 231. https://doi.org/10.3390/antiox12020231
APA StyleZylinska, L., Lisek, M., Guo, F., & Boczek, T. (2023). Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain. Antioxidants, 12(2), 231. https://doi.org/10.3390/antiox12020231






