DTPA-Bound Planar Catechin with Potent Antioxidant Activity Triggered by Fe3+ Coordination
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
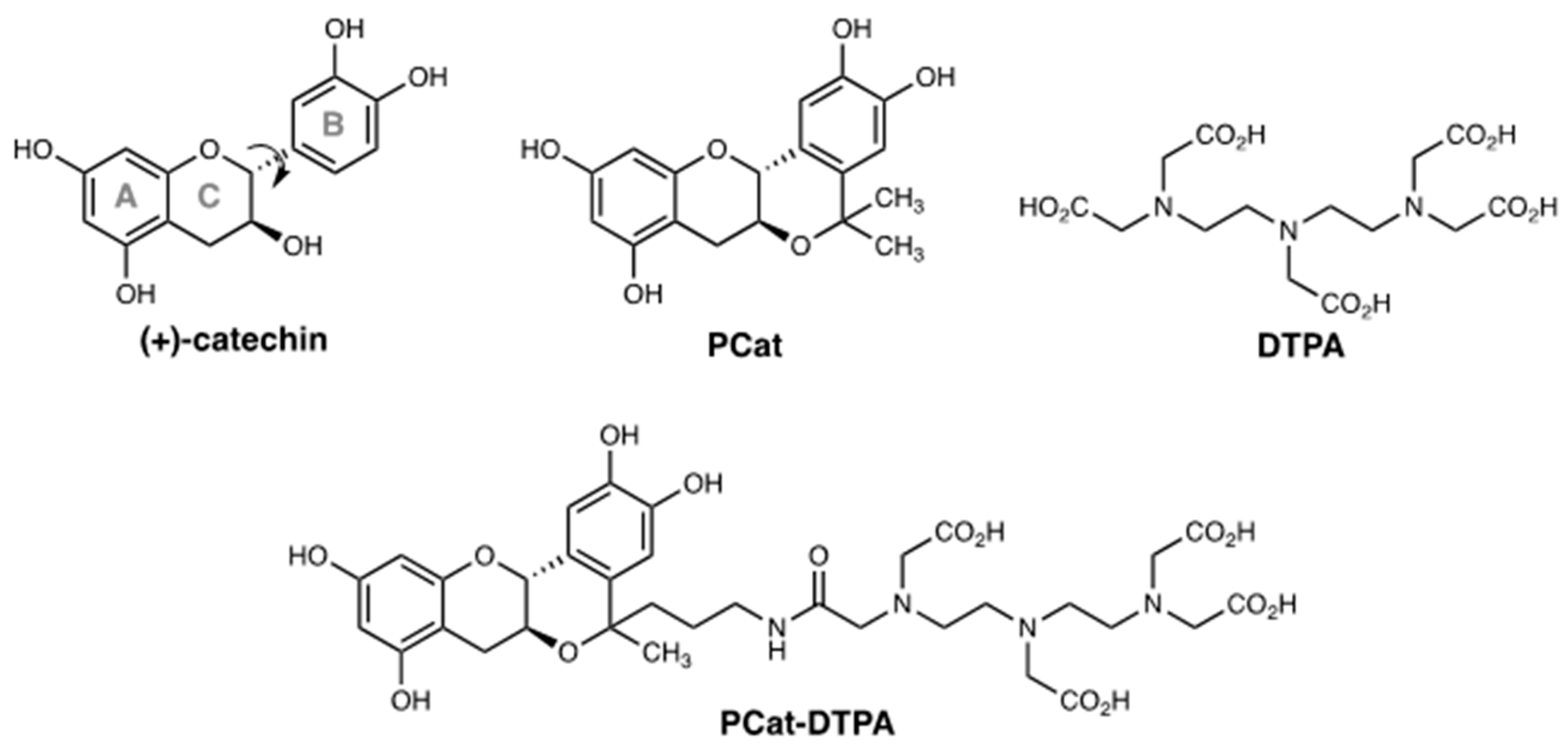
2.2. Synthesis of 2-PCat-DTPA Compounds
2.2.1. Ethyl 3-((6aS,12aR)-2,3,8,10-tetrahydroxy-5-methyl-5,6a,7,12a-tetrahydroisochromeno [4,3-b]chromen-5-yl)propanoate (1)
2.2.2. Ethyl 3-((6aS,12aR)-2,3,8,10-tetrakis(benzyloxy)-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propanoate (2)
2.2.3. 3-((6aS,12aR)-2,3,8,10-tetrakis(benzyloxy)-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propan-1-ol (3)
2.2.4. tert-Butyl (tert-butoxycarbonyl)(3-((6aS,12aR)-2,3,8,10-tetrakis(benzyloxy)-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propyl)carbamate (4)
2.2.5. 3-((6aS,12aR)-2,3,8,10-tetrakis(benzyloxy)-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromen-5-yl)propan-1-amine (5)
2.2.6. (6aS,12aR)-5-(5-oxo-7,10,13,13-tetrakis(carboxymethyl)-4,7,10,13-tetra-azatridecane)-5-methyl-5,6a,7,12a-tetrahydroisochromeno[4,3-b]chromene-2,3,8,10-tetraol (PCat-DTPA)
2.3. 2,2-Diphenyl-1-picrylhydrazyl (DPPH•)-Scavenging Assay
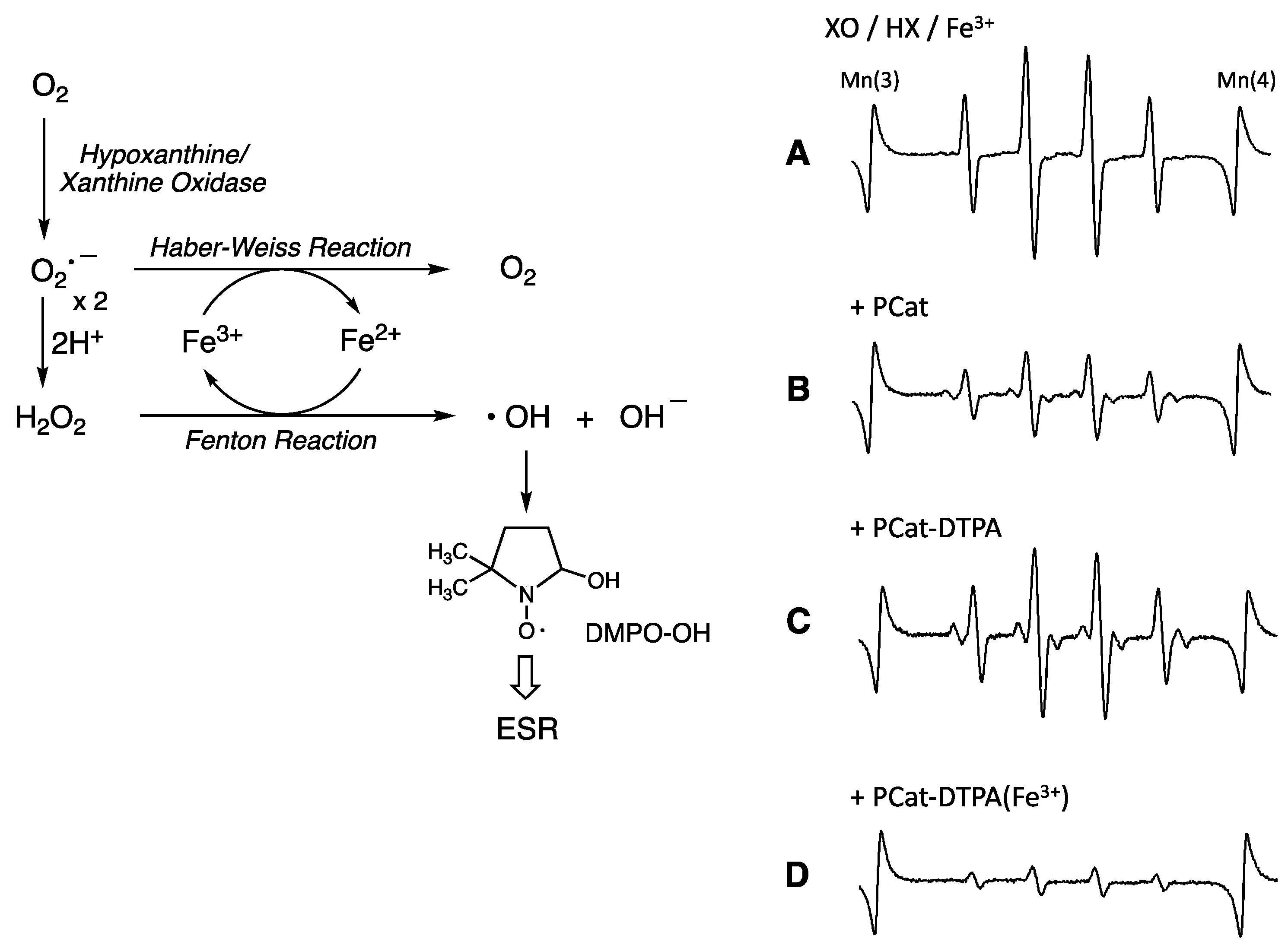
2.4. Hydroxyl Radical Scavenging Assay
2.5. Inhibition Assay of DNA Strand Breaks
2.6. Calculation Methods
3. Results and Discussion
3.1. Chemistry
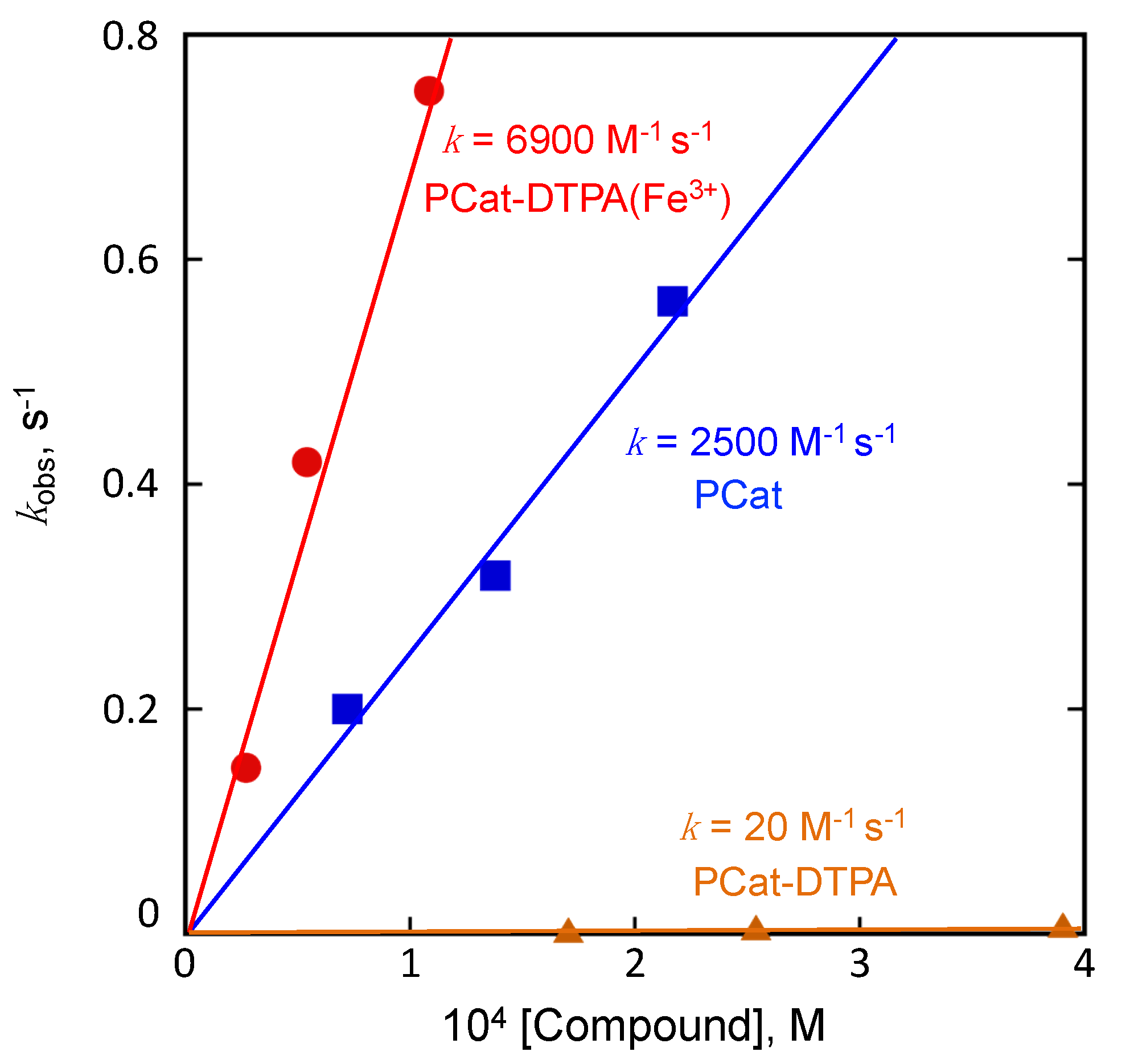
3.2. Radical Scavenging Activity of PCat-DTPA toward DPPH•
3.3. Antioxidant Activity of PCat-DTPA toward Free Radicals Generated from the Fe-XO/HX System
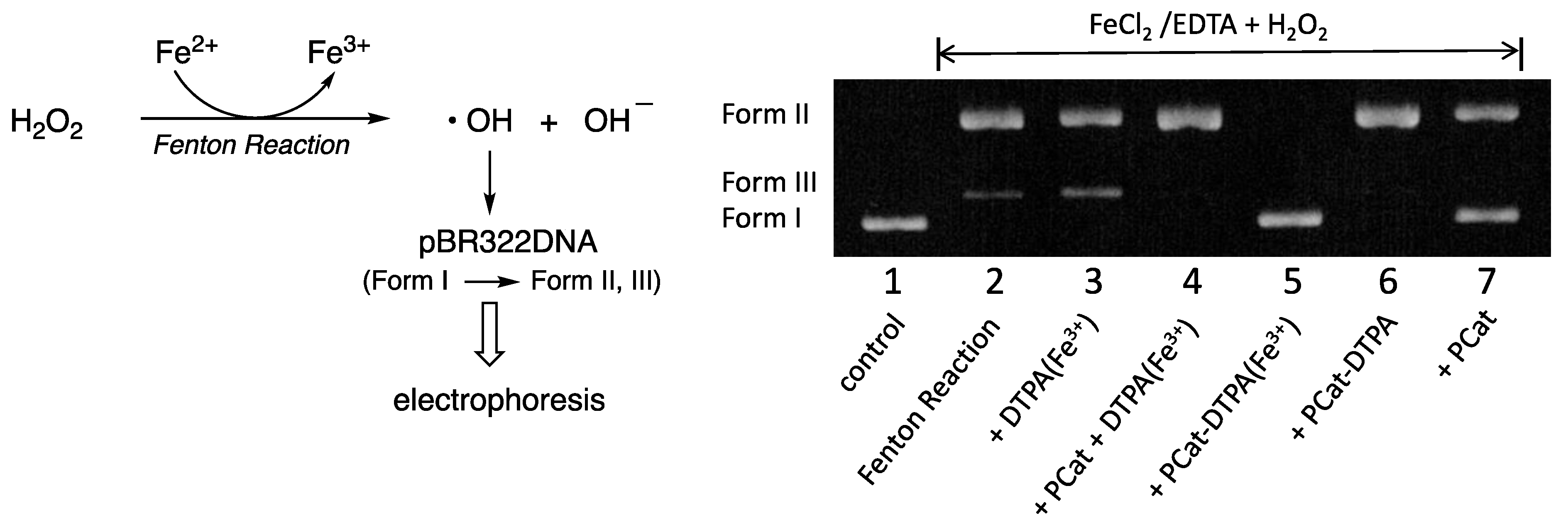
3.4. Effect of PCat-DTPA on Oxidative Damage of pBR322DNA by Fenton Reaction
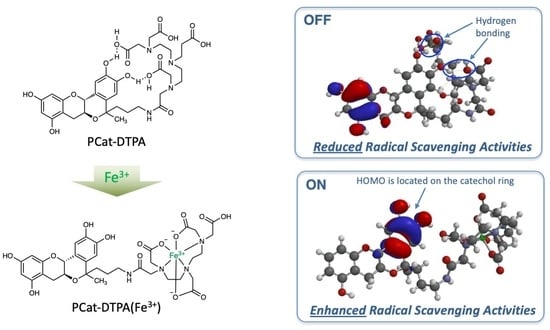
3.5. DFT Calculations for PCat-DTPA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Leyane, T.S.; Jere, S.W.; Houreld, N.N. Oxidative Stress in Ageing and Chronic Degenerative Pathologies: Molecular Mechanisms Involved in Counteracting Oxidative Stress and Chronic Inflammation. Int. J. Mol. Sci. 2022, 23, 7273. [Google Scholar] [CrossRef] [PubMed]
- Umare, M.D.; Wankhede, N.L.; Bajaj, K.K.; Trivedi, R.V.; Taksande, B.G.; Umekar, M.J.; Mahore, J.G.; Kale, M.B. Interweaving of reactive oxygen species and major neurological and psychiatric disorders. Ann. Pharm. Françaises 2022, 80, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Shohag, S.; Akhter, S.; Islam, S.; Sarker, T.; Sifat, M.K.; Rahman, M.M.; Islam, M.R.; Sharma, R. Perspectives on the Molecular Mediators of Oxidative Stress and Antioxidant Strategies in the Context of Neuroprotection and Neurolongevity: An Extensive Review. Oxidative Med. Cell. Longev. 2022, 2022, 7743705. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free. Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Cobley, J.N.; McHardy, H.; Morton, J.P.; Nikolaidis, M.G.; Close, G.L. Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations. Free. Radic. Biol. Med. 2015, 84, 65–76. [Google Scholar] [CrossRef]
- Masato, A.; Bubacco, L.; Greggio, E. Too much for your own good: Excessive dopamine damages neurons and contributes to Parkinson’s disease: An Editorial Highlight for “Enhanced tyrosine hydroxylase activity induces oxidative stress, causes accumulation of autotoxic catecholamine metabolites, and augments amphetamine effects in vivo”. J. Neurochem. 2021, 158, 833–836. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Das, N.; Raymick, J.; Sarkar, S. Role of metals in Alzheimer’s disease. Metab. Brain Dis. 2021, 36, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Morén, C.; de Souza, R.M.; Giraldo, D.M.; Uff, C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 9328. [Google Scholar] [CrossRef] [PubMed]
- Aborode, A.T.; Pustake, M.; Awuah, W.A.; Alwerdani, M.; Shah, P.; Yarlagadda, R.; Ahmad, S.; Silva Correia, I.F.; Chandra, A.; Nansubuga, E.P.; et al. Targeting Oxidative Stress Mechanisms to Treat Alzheimer’s and Parkinson’s Disease: A Critical Review. Oxidative Med. Cell. Longev. 2022, 2022, 7934442. [Google Scholar] [CrossRef]
- Leathem, A.; Ortiz-Cerda, T.; Dennis, J.M.; Witting, P.K. Evidence for Oxidative Pathways in the Pathogenesis of PD: Are Antioxidants Candidate Drugs to Ameliorate Disease Progression? Int. J. Mol. Sci. 2022, 23, 6923. [Google Scholar] [CrossRef] [PubMed]
- Mandil, R.; Prakash, A.; Rahal, A.; Singh, S.P.; Sharma, D.; Kumar, R.; Garg, S.K. In vitro and in vivo effects of flubendiamide and copper on cyto-genotoxicity, oxidative stress and spleen histology of rats and its modulation by resveratrol, catechin, curcumin and α-tocopherol. BMC Pharmacol. Toxicol. 2020, 21, 29. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Suzuki, K. The Integrative Role of Sulforaphane in Preventing Inflammation, Oxidative Stress and Fatigue: A Review of a Potential Protective Phytochemical. Antioxidants 2020, 9, 521. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Kaur, P.; Kaur, P.; Singh, H.; Batiha, G.E.; Verma, I. Exploring multifunctional antioxidants as potential agents for management of neurological disorders. Environ. Sci. Pollut. Res. 2022, 29, 24458–24477. [Google Scholar] [CrossRef]
- Mehta, K.J.; Farnaud, S.J.; Sharp, P.A. Iron and liver fibrosis: Mechanistic and clinical aspects. World J. Gastroenterol. 2019, 25, 521–538. [Google Scholar] [CrossRef]
- Behl, T.; Madaan, P.; Sehgal, A.; Singh, S.; Anwer, M.K.; Makeen, H.A.; Albratty, M.; Mohan, S.; Bungau, S. Mechanistic Insights Expatiating the Redox-Active-Metal-Mediated Neuronal Degeneration in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 678. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Dhorajia, V.V.; Kim, J.; Kim, Y. Mitochondrial iron metabolism and neurodegenerative diseases. Neurotoxicology 2022, 88, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Hershko, C. Control of disease by selective iron depletion: A novel therapeutic strategy utilizing iron chelators. Baillière’s Clin. Haematol. 1994, 7, 965–1000. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Khan, H.; Kanojia, N.; Singh, T.G.; Kaur, A.; Kaur, G. Therapeutic Insights on Ferroptosis in Parkinson’s disease. Eur. J. Pharmacol. 2022, 930, 175133. [Google Scholar] [CrossRef]
- Farr, A.C.; Xiong, M.P. Challenges and Opportunities of Deferoxamine Delivery for Treatment of Alzheimer’s Disease, Parkinson’s Disease, and Intracerebral Hemorrhage. Mol. Pharm. 2021, 18, 593–609. [Google Scholar] [CrossRef]
- Nahon, P.; Ganne-Carrié, N.; Trinchet, J.C.; Beaugrand, M. Hepatic iron overload and risk of hepatocellular carcinoma in cirrhosis. Gastroentérologie Clin. Biol. 2010, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Bartosch, B.; Smirnova, O.A.; Isaguliants, M.G.; Kochetkov, S.N. HCV and oxidative stress in the liver. Viruses 2013, 5, 439–469. [Google Scholar] [CrossRef]
- Yasutake, K.; Kohjima, M.; Nakashima, M.; Kotoh, K.; Nakamuta, M.; Enjoji, M. Nutrition therapy for liver diseases based on the status of nutritional intake. Gastroenterol. Res. Pr. 2012, 2012, 859697. [Google Scholar] [CrossRef]
- Hino, K.; Nishina, S.; Sasaki, K.; Hara, Y. Mitochondrial damage and iron metabolic dysregulation in hepatitis C virus infection. Free. Radic. Biol. Med. 2019, 133, 193–199. [Google Scholar] [CrossRef]
- Tsuda, H.; Sekine, K.; Fujita, K.; Ligo, M. Cancer prevention by bovine lactoferrin and underlying mechanisms—A review of experimental and clinical studies. Biochem. Cell Biol. 2002, 80, 131–136. [Google Scholar] [CrossRef]
- Hakamata, W.; Nakanishi, I.; Masuda, Y.; Shimizu, T.; Higuchi, H.; Nakamura, Y.; Saito, S.; Urano, S.; Oku, T.; Ozawa, T.; et al. Planar catechin analogues with alkyl side chains: A potent antioxidant and an alpha-glucosidase inhibitor. J. Am. Chem. Soc. 2006, 128, 6524–6525. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, K.; Nakanishi, I.; Kansui, H.; Sugiyama, E.; Kimura, M.; Shimada, T.; Urano, S.; Yamaguchi, K.; Miyata, N. Enhanced radical-scavenging activity of a planar catechin analogue. J. Am. Chem. Soc. 2002, 124, 5952–5953. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.M.; Qi, D.H.; Zheng, Y.G.; Mu, Y.; Yan, G.L.; Yang, T.S.; Shen, J.C. ESR studies on reaction of saccharide with the free radicals generated from the xanthine oxidase/hypoxanthine system containing iron. FEBS Lett. 2001, 492, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855s–858s. [Google Scholar] [CrossRef]
- Ikeda, Y.; Funamoto, M.; Tsuchiya, K. The role of iron in obesity and diabetes. J. Med. Invest. 2022, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Interactions 2022, 367, 110173. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. A comparative study of the antioxidant power of flavonoid catechin and its planar analogue. J. Agric. Food Chem. 2007, 55, 7944–7949. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukuhara, K.; Nakanishi, I.; Imai, K.; Mizuno, M.; Matsumoto, K.-i.; Ohno, A. DTPA-Bound Planar Catechin with Potent Antioxidant Activity Triggered by Fe3+ Coordination. Antioxidants 2023, 12, 225. https://doi.org/10.3390/antiox12020225
Fukuhara K, Nakanishi I, Imai K, Mizuno M, Matsumoto K-i, Ohno A. DTPA-Bound Planar Catechin with Potent Antioxidant Activity Triggered by Fe3+ Coordination. Antioxidants. 2023; 12(2):225. https://doi.org/10.3390/antiox12020225
Chicago/Turabian StyleFukuhara, Kiyoshi, Ikuo Nakanishi, Kohei Imai, Mirei Mizuno, Ken-ichiro Matsumoto, and Akiko Ohno. 2023. "DTPA-Bound Planar Catechin with Potent Antioxidant Activity Triggered by Fe3+ Coordination" Antioxidants 12, no. 2: 225. https://doi.org/10.3390/antiox12020225
APA StyleFukuhara, K., Nakanishi, I., Imai, K., Mizuno, M., Matsumoto, K.-i., & Ohno, A. (2023). DTPA-Bound Planar Catechin with Potent Antioxidant Activity Triggered by Fe3+ Coordination. Antioxidants, 12(2), 225. https://doi.org/10.3390/antiox12020225