Stability of Flavan-3-ols, Theaflavins, and Methylxanthines in 30 Industrial Green, Black, and White Tea (Camellia sinensis L.) Extracts Characterized via Liquid Chromatography Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Commercial Tea Extracts
2.3. Storage Conditions
2.4. Sample Preparation and Standard Solutions
2.5. Quantification via HPLC–DAD Analysis
2.6. Theaflavin Extraction from Black Tea Leaves
2.7. Identification via HPLC–ESI–QTOF-MS/MS Analysis
2.8. Quantification via HPTLC-UV/Vis Analysis
3. Results and Discussion
3.1. Outline and Focus of the Study
3.2. Stability of Bioactive Compounds in 26 Commercial Tea Extracts via HPLC–DAD
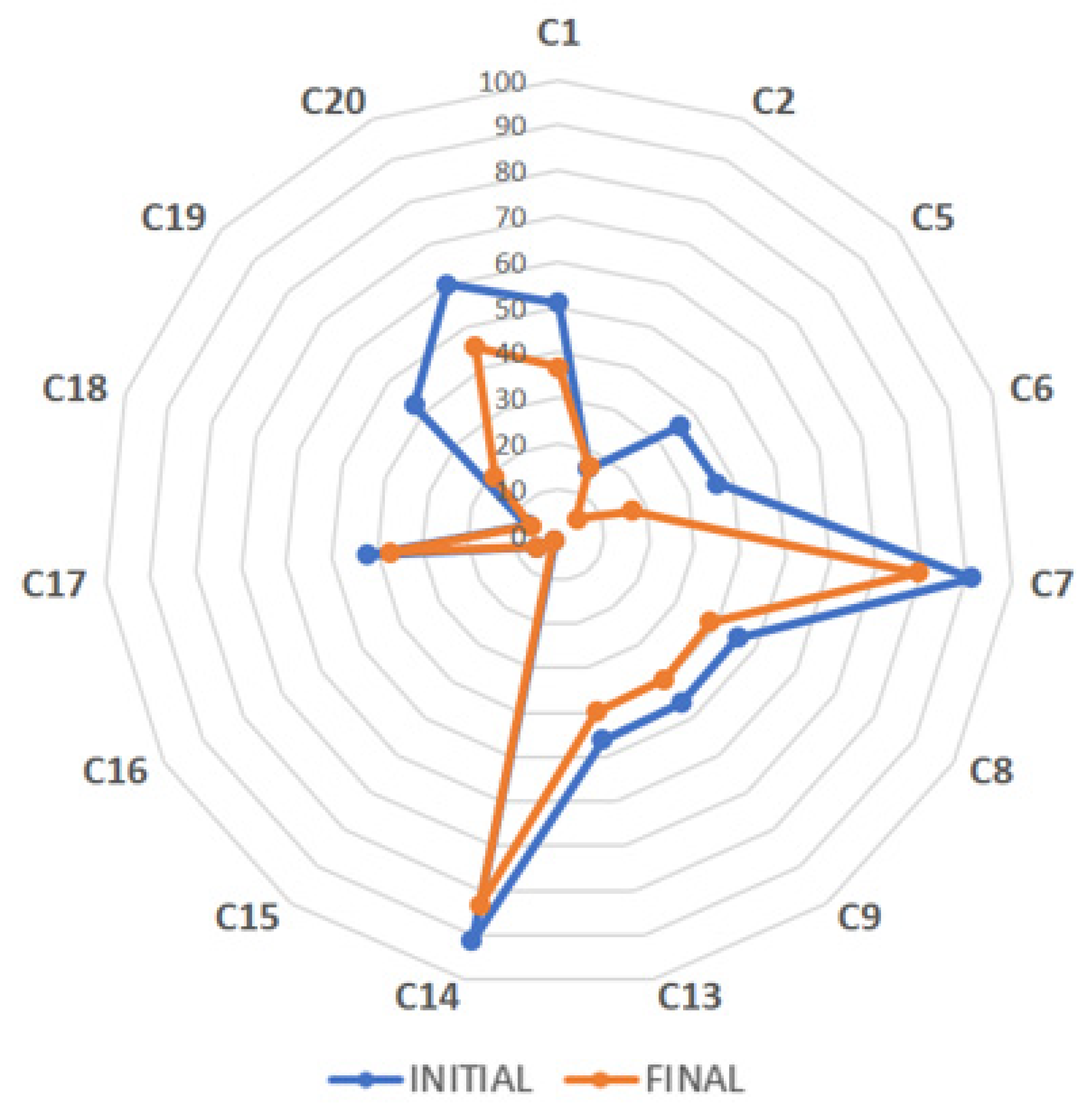
| Bioactive Compounds * | GA | TB | GC | TP | EGC | C | Caf | EC | EGCG | GCG | ECG | CG | Total Flavan-3-ols | Total Xanthines | Total Bioactives |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT (min) | 3.9 | 4.7 | 5.3 | 6.6 | 7.7 | 9.7 | 9.8 | 13.4 | 14.0 | 18.4 | 18.8 | 19.0 | |||
| C1 | 0.3 ± 0.0 | 0.1 ± 0.0 | 1.0 ± 0.2 | 0.0 ± 0.0 | 1.8 ± 0.2 | 0.2 ± 0.2 | 0.3 ± 0.0 | 2.8 ± 0.2 | 36.8 ± 0.7 | 6.1 ± 0.2 | 9.3 ± 0.3 | 1.1 ± 0.1 | 59.2 ± 2.4 | 0.4 ± 0.0 | 59.8 ± 2.4 |
| C2 | 2.2 ± 0.0 | 0.3 ± 0.0 | 1.3 ± 0.2 | 0.1 ± 0.0 | 3.9 ± 0.3 | 0.0 ± 0.0 | 2.3 ± 0.0 | 3.7 ± 0.2 | 16.4 ± 0.2 | 2.4 ± 0.2 | 2.7 ± 0.2 | 0.5 ± 0.2 | 30.9 ± 1.5 | 2.7 ± 0.0 | 35.8 ± 1.5 |
| C3 | 0.5 ± 0.1 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 2.6 ± 0.6 | 0.4 ± 0.0 | 3.0 ± 0.4 | 0.9 ± 0.2 | 1.3 ± 0.2 | 0.4 ± 0.0 | 7.2 ± 1.2 | 2.7 ± 0.6 | 10.3 ± 1.9 |
| C4 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.1 ± 0.0 | 0.4 ± 0.1 | 2.7 ± 0.1 | 0.7 ± 0.1 | 1.5 ± 0.1 | 0.4 ± 0.1 | 6.8 ± 0.8 | 0.1 ± 0.0 | 7.2 ± 0.8 |
| C5 | 0.3 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.0 ± 0.0 | 1.9 ± 0.0 | 0.3 ± 0.1 | 2.3 ± 0.0 | 1.5 ± 0.0 | 5.2 ± 0.1 | 0.8 ± 0.0 | 1.6 ± 0.0 | 0.3 ± 0.1 | 12.1 ± 0.2 | 3.0 ± 0.0 | 15.4 ± 0.2 |
| C6 | 2.1 ± 0.0 | 0.3 ± 0.0 | 1.4 ± 0.2 | 0.1 ± 0.0 | 3.4 ± 0.3 | 0.8 ± 0.2 | 2.2 ± 0.0 | 3.3 ± 0.3 | 17.0 ± 0.5 | 4.3 ± 0.3 | 2.8 ± 0.3 | 0.7 ± 0.2 | 33.6 ± 2.1 | 2.5 ± 0.0 | 38.2 ± 2.1 |
| C7 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.6 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.1 | 79.2 ± 0.4 | 0.2 ± 0.2 | 1.7 ± 0.1 | 0.0 ± 0.0 | 82.8 ± 0.6 | 0.0 ± 0.0 | 82.9 ± 0.6 |
| C8 | 0.7 ± 0.0 | 0.1 ± 0.0 | 0.6 ± 0.2 | 0.0 ± 0.0 | 2.2 ± 0.6 | 0.7 ± 0.1 | 0.3 ± 0.0 | 2.9 ± 0.1 | 38.5 ± 1.7 | 2.1 ± 0.2 | 11.6 ± 0.0 | 0.7 ± 0.1 | 59.3 ± 3.1 | 0.4 ± 0.0 | 60.4 ± 3.2 |
| C9 | 0.9 ± 0.0 | 0.1 ± 0.0 | 0.7 ± 0.1 | 0.0 ± 0.0 | 2.3 ± 0.1 | 0.6 ± 0.1 | 0.6 ± 0.1 | 2.6 ± 0.1 | 39.3 ± 0.2 | 2.9 ± 0.1 | 10.9 ± 0.1 | 0.8 ± 0.1 | 60.2 ± 0.7 | 0.8 ± 0.0 | 61.9 ± 0.7 |
| C13 | 0.3 ± 0.0 | 0.0 ± 0.0 | 1.0 ± 0.1 | 0.0 ± 0.0 | 1.7 ± 0.1 | 0.0 ± 0.0 | 0.4 ± 0.0 | 3.8 ± 0.1 | 39.8 ± 0.3 | 5.9 ± 0.1 | 4.1 ± 0.2 | 0.7 ± 0.1 | 55.2 ± 3.2 | 0.5 ± 0.1 | 56.0 ± 3.2 |
| C14 | 0.1 ± 0.0 | - | 1.6 ± 0.0 | - | 1.6 ± 0.0 | - | - | - | 83.3 ± 0.5 | 1.6 ± 0.0 | 4.3 ± 0.0 | - | 92.4 ± 0.2 | 0.0 ± 0.0 | 92.5 ± 0.2 |
| C15 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.1 | 0.0 ± 0.0 | 0.7 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.1 | 1.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.4 ± 0.1 | 5.2 ± 2.1 | 0.2 ± 0.0 | 5.8 ± 2.1 |
| C16 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.2 | 0.0 ± 0.0 | 3.8 ± 0.1 | 0.6 ± 0.2 | 4.0 ± 0.0 | 1.3 ± 0.2 | 5.4 ± 0.2 | 0.4 ± 0.2 | 1.0 ± 0.2 | 0.5 ± 0.2 | 14.4 ± 1.2 | 4.6 ± 0.0 | 19.2 ± 1.2 |
| C17 | 0.6 ± 0.0 | 0.1 ± 0.0 | 0.7 ± 0.1 | 0.0 ± 0.0 | 3.2 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.0 | 2.8 ± 0.1 | 37.1 ± 0.1 | 1.7 ± 0.0 | 8.1 ± 0.0 | 0.6 ± 0.1 | 54.9 ± 0.6 | 0.7 ± 0.0 | 56.2 ± 0.7 |
| C18 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.8 ± 0.2 | 0.0 ± 0.0 | 1.2 ± 0.2 | 0.5 ± 0.2 | 6.7 ± 0.0 | 0.5 ± 0.1 | 6.3 ± 0.2 | 2.1 ± 0.2 | 1.2 ± 0.2 | 0.6 ± 0.2 | 13.3 ± 0.5 | 7.0 ± 0.0 | 20.3 ± 0.5 |
| C19 | 3.5 ± 0.0 | 0.2 ± 0.0 | 1.1 ± 0.1 | 0.0 ± 0.0 | 3.2 ± 0.2 | 0.8 ± 0.2 | 3.0 ± 0.0 | 3.5 ± 0.1 | 18.8 ± 0.0 | 2.3 ± 0.2 | 8.4 ± 0.1 | 0.8 ± 0.2 | 38.9 ± 0.9 | 3.2 ± 0.0 | 45.6 ± 0.9 |
| C20 | 0.8 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.1 | 0.0 ± 0.0 | 2.4 ± 0.1 | 0.8 ± 0.1 | 0.2 ± 0.0 | 3.0 ± 0.1 | 45.1 ± 0.5 | 3.6 ± 0.1 | 6.4 ± 0.1 | 0.6 ± 0.1 | 62.5 ± 1.3 | 0.3 ± 0.0 | 63.5 ± 1.3 |
| C21 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.0 ± 0.0 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 3.6 ± 0.0 | 0.2 ± 0.0 | 4.0 ± 0.0 |
| C22 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.1 | 0.2 ± 0.0 | 1.0 ± 0.1 |
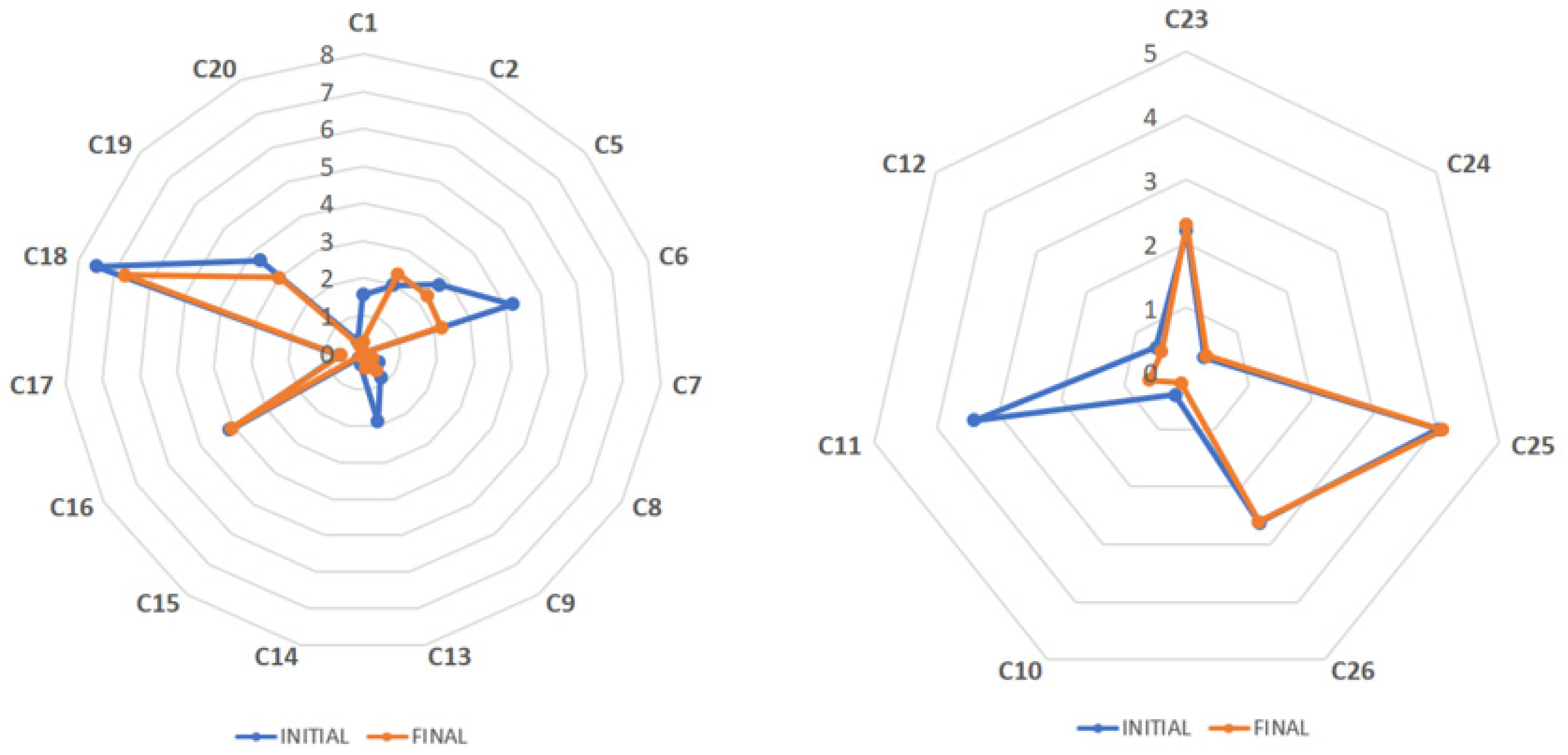
| Bioactive Components * | RT | C10 | C11 | C12 | C23 | C24 | C25 | C26 |
|---|---|---|---|---|---|---|---|---|
| GA | 3.9 | 7.8 ± 2.0 | 1.0 ± 0.0 | 1.2 ± 0.0 | 0.1 ± 0.0 | 1.2 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 |
| TB | 4.7 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.7 ± 0.0 | 0.1 ± 0.0 |
| GC | 5.3 | 3.1 ± 0.9 | 0.7 ± 0.0 | 0.9 ± 0.2 | 0.0 ± 0.0 | 0.7 ± 0.2 | 0.9 ± 0.0 | 0.7 ± 0.1 |
| TP | 6.6 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| EGC | 7.7 | 3.4 ± 0.9 | 2.4 ± 0.0 | 3.1 ± 0.2 | 0.0 ± 0.0 | 1.4 ± 0.2 | 1.8 ± 0.0 | 0.7 ± 0.1 |
| C | 9.7 | 3.3 ± 0.9 | 0.7 ± 0.0 | 0.8 ± 0.2 | 0.0 ± 0.0 | 0.8 ± 0.2 | 0.6 ± 0.0 | 0.7 ± 0.0 |
| Caf | 9.9 | 0.2 ± 0.1 | 0.6 ± 0.0 | 0.5 ± 0.0 | 2.3 ± 0.0 | 0.4 ± 0.0 | 4.1 ± 0.0 | 2.6 ± 0.0 |
| EC | 13.4 | 3.7 ± 0.9 | 2.7 ± 0.0 | 0.6 ± 0.2 | 0.6 ± 0.1 | 2.5 ± 0.2 | 1.3 ± 0.1 | 0.7 ± 0.0 |
| EGCG | 14.0 | 7.6 ± 0.9 | 34.8 ± 0.1 | 33.8 ± 0.4 | 0.7 ± 0.1 | 26.5 ± 0.2 | 4.7 ± 0.0 | 1.0 ± 0.1 |
| GCG | 18.4 | 3.5 ± 0.9 | 2.5 ± 0.1 | 2.3 ± 0.2 | 0.6 ± 0.1 | 1.7 ± 0.2 | 1.3 ± 0.0 | 0.9 ± 0.0 |
| ECG | 18.8 | 4.8 ± 0.9 | 10.4 ± 0.0 | 7.5 ± 0.2 | 2.3 ± 0.0 | 11.0 ± 0.2 | 1.2 ± 0.5 | 0.8 ± 0.0 |
| CG | 19.0 | 3.3 ± 0.9 | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.2 | 1.3 ± 0.8 | 0.7 ± 0.0 |
| Theaflavin | 24.2 | 3.9 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 7.9 ± 0.9 | 2.2 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Theaflavin-3′-gallate | 24.7 | 6.4 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 14.7 ± 0.3 | 3.4 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Theaflavin-3,3′-digallate | 24.8 | 7.7 ± 0.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 21.6 ± 0.4 | 2.9 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Theaflavin-3-gallate | 24.9 | 3.5 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 9.1 ± 0.4 | 2.4 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Total flavan-3-ols | 32.8 ± 8.1 | 54.0 ± 0.2 | 49.7 ± 2.1 | 4.9 ± 1.2 | 45.5 ± 0.5 | 13.2 ± 1.3 | 6.1 ± 0.0 | |
| Total methylxanthines | 0.3 ± 0.1 | 0.7 ± 0.0 | 0.6 ± 0.0 | 2.3 ± 0.0 | 0.4 ± 0.0 | 4.8 ± 0.0 | 2.6 ± 0.2 | |
| Total theaflavins | 21.5 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 53.3 ± 0.0 | 10.9 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| Total bioactive components | 62.4 ± 6.4 | 56.6 ± 0.2 | 51.5 ± 2.1 | 60.5 ± 1.2 | 58.1 ± 0.5 | 18.4 ± 1.3 | 9.3 ± 0.0 | |


3.3. Stability of Bioactive Compounds in Self-Produced Tea Extracts via HPLC–DAD


| ADM® Extract Tea Complex | Catechins as EGCG * eq. (% Dry Basis) | Xanthines as Caffeine eq. (% Dry Basis) | Theaflavins (% Dry Basis) |
|---|---|---|---|
| Control | 11.6 ± 0.0 | 6.4 ± 0.2 | 0.1 ± 0.0 |
| 6 Months | 13.0 ± 0.0 | 6.6 ± 0.4 | 0.1 ± 0.0 |
| 12 Months | 11.7 ± 0.2 | 7.7 ± 0.1 | 0.1 ± 0.0 |
| 24 Months | 12.3 ± 1.6 | 6.5 ± 0.0 | 0.1 ± 0.0 |
| White Tea | |||
| Control | 10.8 ± 0.8 | 6.1 ± 0.2 | - |
| 6 Months | 10.3 ± 0.2 | 5.4 ± 0.0 | - |
| 12 Months | 10.3 ± 1.2 | 6.5 ± 0.5 | - |
| 24 Months | 10.3 ± 0.1 | 5.8 ± 0.1 | - |
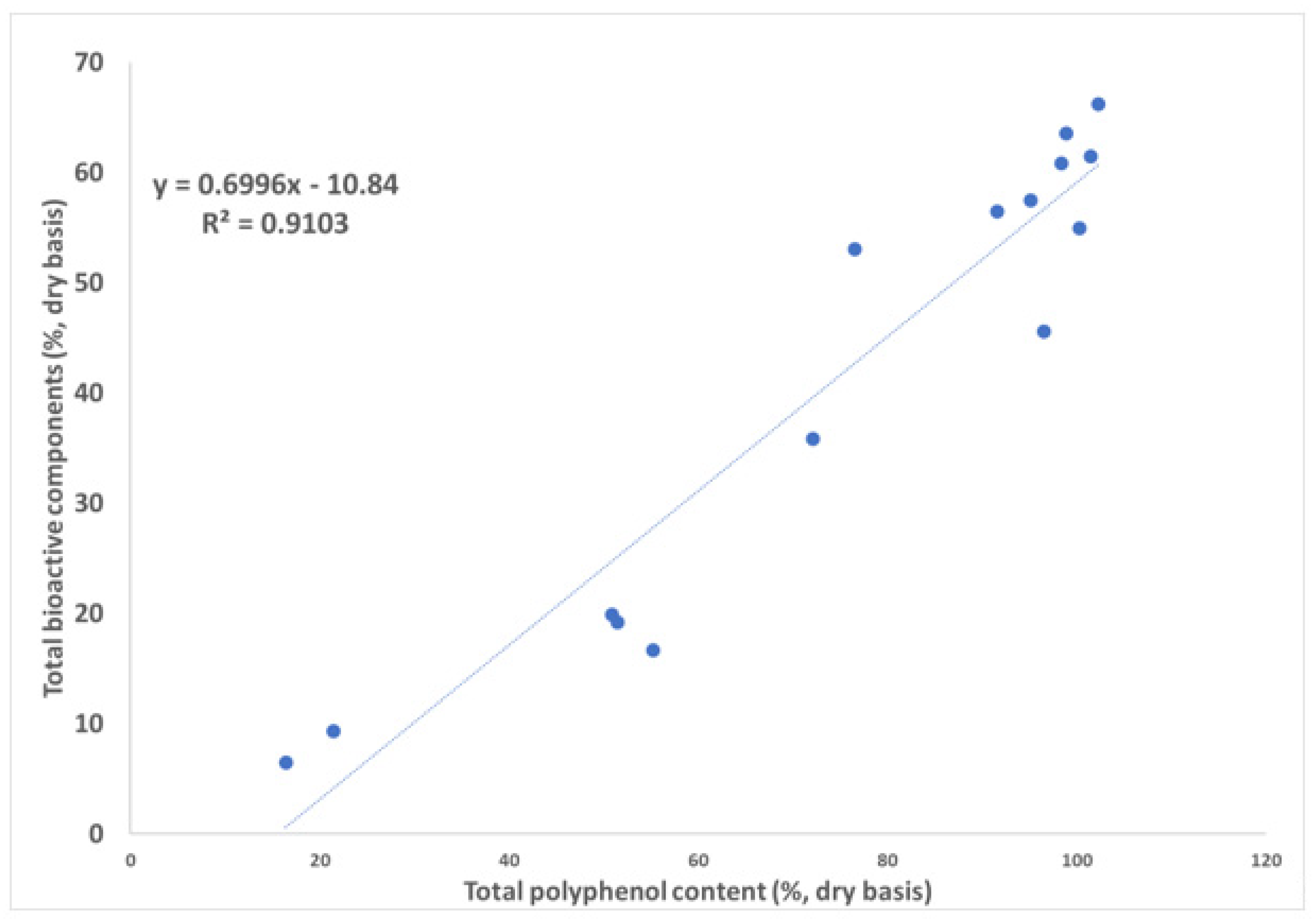
3.4. Correlation to the Total Polyphenol Content
| Bioactive Components * | Slope a x | Intercept b | Determination Coefficient R2 | Correlation Coefficient R |
|---|---|---|---|---|
| GA | 0.56 | 0.01 | 0.03 | 0.16 |
| TB | 0.00 | 0.46 | 0.17 | 0.41 |
| GC | 0.00 | 0.67 | 0.07 | 0.27 |
| TP | 0.00 | 0.00 | 0.00 | 0.02 |
| EGC | 0.02 | 1.08 | 0.30 | 0.54 |
| C | 0.00 | 0.50 | 0.02 | 0.15 |
| Caf | −0.02 | 3.03 | 0.18 | 0.43 |
| EC | 0.03 | 0.17 | 0.48 | 0.69 |
| EGCG | 0.49 | −15.37 | 0.76 | 0.87 |
| GCG | 0.03 | −0.22 | 0.46 | 0.68 |
| ECG | 0.12 | −3.64 | 0.78 | 0.88 |
| CG | 0.00 | 0.59 | 0.04 | 0.20 |
| Total catechins | 0.71 | −16.06 | 0.87 | 0.93 |
| Total methylxanthines | 86.28 | −6.43 | 0.17 | 0.41 |
| Total bioactive components | 0.07 | −10.84 | 0.91 | 0.95 |
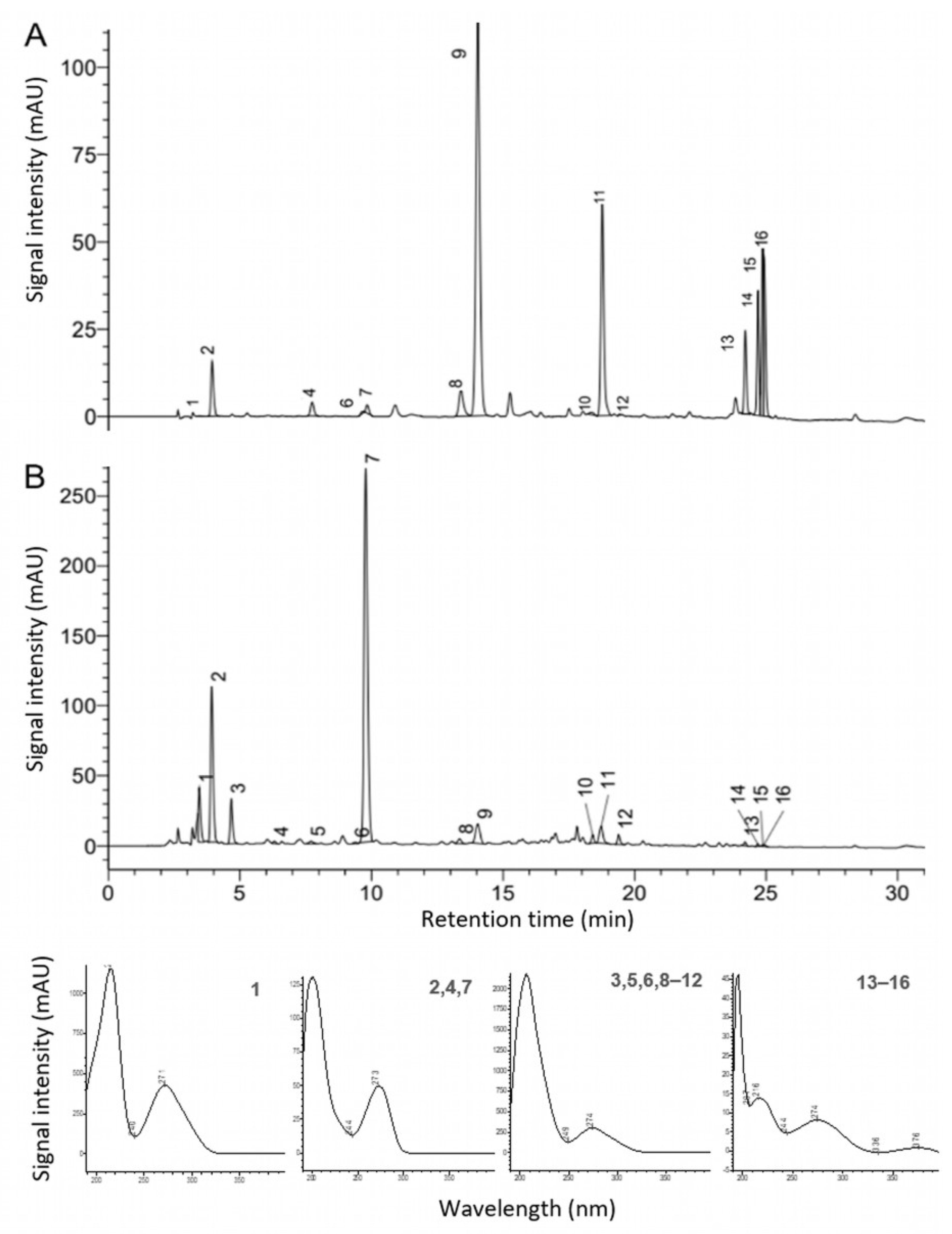
3.5. Characterization of Unidentified Components via HPLC–ESI–QTOF-MS/MS
| Compound Name | Score | Formula | Intensity | Expected m/z | Found m/z | Δ (ppm) | Fragment Observed m/z | RT (min) |
|---|---|---|---|---|---|---|---|---|
| 1-Caffeoylquinic acid | 86% | C16H18O9 | 9130 | 353.0878 | 353.0878 | 3.0 | - | 12.5 |
| 4-Caffeoylquinic acid | 86% | C16H18O9 | 9130 | 353.0878 | 353.0878 | 3.0 | 179/191/195 | 12.5 |
| 5-Caffeoylquinic acid | 86% | C16H18O9 | 9130 | 353.0878 | 353.0878 | 3.0 | 169/191/151/173/187 | 12.5 |
| Chlorogenic acid | 86% | C16H18O9 | 9130 | 353.0878 | 353.0878 | 3.0 | 191/291 | 12.5 |
| (+)-Gallocatechin | 61% | C15H14O7 | 11,650 | 305.0667 | 305.0676 | 0.3 | - | 13.9 |
| (−)-Gallocatechin | 61% | C15H14O7 | 11,650 | 305.0667 | 305.0676 | 0.3 | - | 13.9 |
| Galloyl glucose | 71% | C13H16O10 | 25,124 | 331.0671 | 331.0685 | 0.3 | 169 | 8.1 |
| Galloylquinic acid | 74% | C14H16O10 | 111,763 | 343.0671 | 343.0687 | 0.9 | - | 5.6 |
3.6. Quantitative Verification via HPTLC–UV/Vis
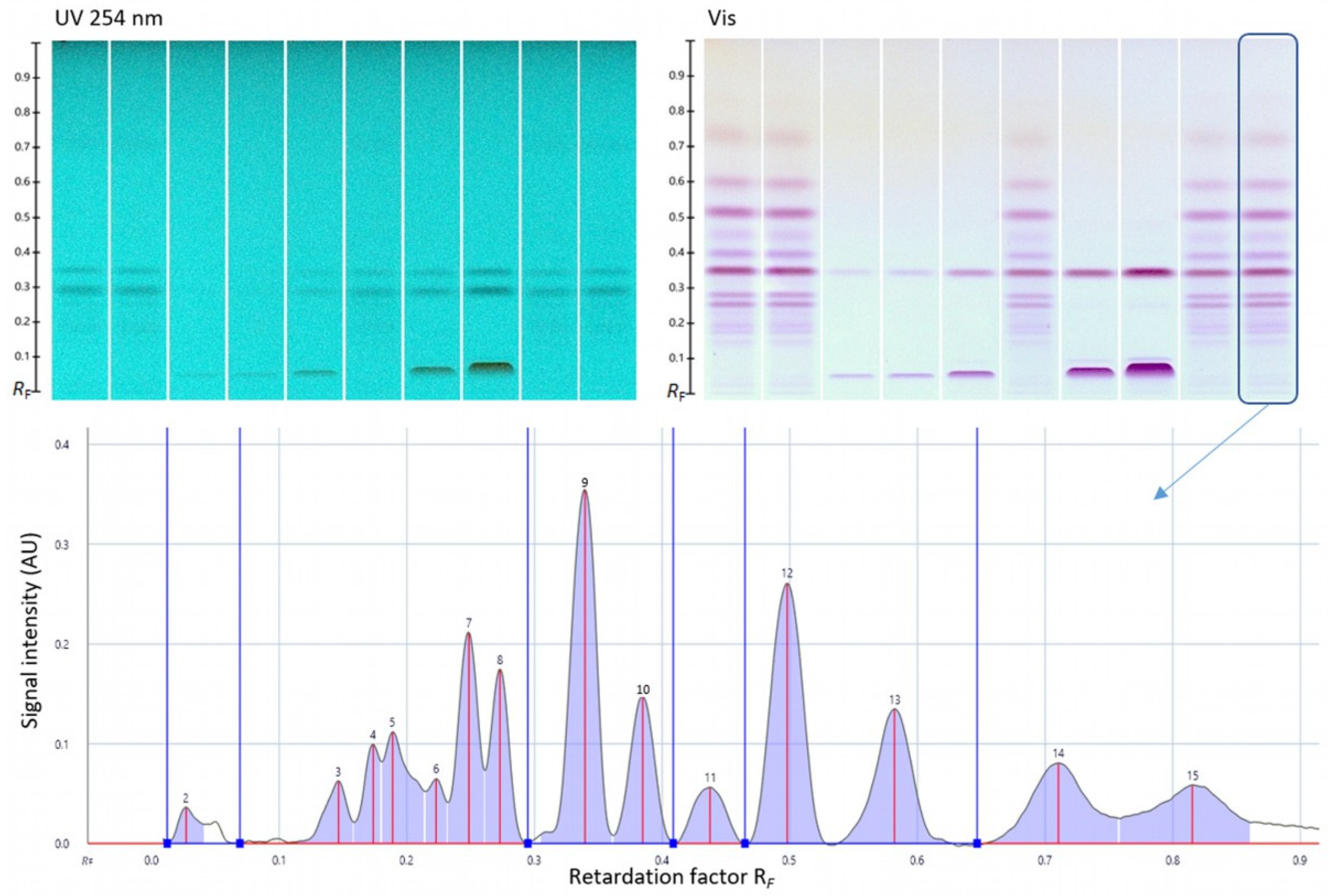
| Bioactive Compound Content (%) | One Sample | Mean of Five Batches (n = 5) | ||
|---|---|---|---|---|
| HPTLC–UV/Vis | HPLC–DAD | HPTLC–UV/Vis | HPLC–DAD | |
| EGCG * equivalents | 12.6 | 10.9 | 14.7 | 11.9 |
| Methylxanthines | 4.3 | 6.5 | 6.4 | 6.2 |
| Theaflavin equivalents | 0.1 | 0.1 | 0.3 | 0.2 |
| Total bioactive compounds | 17.0 | 17.5 | 21.3 | 18.1 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hilton, P.J.; Ellis, R.T. Estimation of the market value of Central African tea by theaflavin analysis. J. Sci. Food Agric. 1972, 23, 227–232. [Google Scholar] [CrossRef]
- Stodt, U.; Engelhardt, U.H. Progress in the analysis of selected tea constituents over the past 20 years. Food Res. Int. 2013, 53, 636–648. [Google Scholar] [CrossRef]
- Henning, S.M.; Fajardo-Lira, C.; Lee, H.W.; Youssefian, A.A.; Go, V.L.W.; Heber, D. Catechin Content of 18 Teas and a Green Tea Extract Supplement Correlates with the Antioxidant Capacity. Nutr. Cancer 2003, 45, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, N.; Venkatesh, P.; Ganguli, S.; Sinkar, V.P. Role of Polyphenol Oxidase and Peroxidase in the Generation of Black Tea Theaflavins. J. Agric. Food Chem. 1999, 47, 2571–2578. [Google Scholar] [CrossRef]
- Teixeira, S.; Siquet, C.; Alves, C.; Boal, I.; Marques, M.P.; Borges, F.; Lima, J.L.F.C.; Reis, S. Structure-property studies on the antioxidant activity of flavonoids present in diet. Free Radic. Biol. Med. 2005, 39, 1099–1108. [Google Scholar] [CrossRef]
- Fernández, P.L.; Martín, M.J.; González, A.G.; Pablos, F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst 2000, 125, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.C.; Hildreth, J.; Bannister, S. Determination of Catechins and Caffeine in Camillia sinensis Raw Materials, Extracts, and Dietary Supplements by HPLC-UV: Single-Laboratory Validation. J. AOAC Int. 2013, 96, 933–941. [Google Scholar] [CrossRef]
- Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Off. J. Eur. Union. 2006, 404, 9–25.
- Dalluge, J.J.; Nelson, B.C. Determination of tea catechins. J. Chromatogr. A 2000, 881, 411–424. [Google Scholar] [CrossRef]
- Dias, F.D.S.; Lovillo, M.P.; Barroso, C.G.; David, J.M. Optimization and validation of a method for the direct determination of catechin and epicatechin in red wines by HPLC/fluorescence. Microchem. J. 2010, 96, 17–20. [Google Scholar] [CrossRef]
- Morlock, G.E.; Heil, J.; Inarejos-Garcia, A.M.; Maeder, J. Effect-Directed Profiling of Powdered Tea Extracts for Catechins, Theaflavins, Flavonols and Caffeine. Antioxidants 2021, 10, 117. [Google Scholar] [CrossRef]
- Commision Regulation (EU) 2022/2340 of 30 November 2022 amending annex III to regulation (EC) no 1925/2006 of the European Parliament and of the Council as regards green tea extracts containing (−)-epigallocatechin-3-gallate. Off. J. Eur. Union. 2022, 310, 7–11.
- Kosińska, A.; Andlauer, W. Antioxidant capacity of tea: Effect of processing and storage. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 109–120. [Google Scholar]
- Friedman, M.; Levin, C.E.; Lee, S.-U.; Kozukue, N. Stability of Green Tea Catechins in Commercial Tea Leaves during Storage for 6 Months. J Food Sci. 2009, 74, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.L.; Ong, C.N. Comparative analysis of tea catechins and theaflavins by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2000, 881, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Colon, M.; Nerin, C. Molecular Interactions between Caffeine and Catechins in Green Tea. J. Agric. Food Chem. 2014, 62, 6777–6783. [Google Scholar] [CrossRef] [PubMed]
- Jhoo, J.W.; Lo, C.Y.; LI, S.; Sang, S.; Ang, C.Y.W.; Heinze, T.M.; HO, C.T. Stability of Black Tea Polyphenol, Theaflavin, and Identification of Theanaphthoquinone as Its Major Radical Reaction Product. J. Agric. Food Chem. 2005, 53, 6146–6150. [Google Scholar] [CrossRef]
- Su, Y.L.; Leung, L.K.; Huang, Y.; Chen, Z.Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Morlock, G.E. Planar chromatographic super-hyphenations for rapid dereplication. Phytochem. Rev. 2023. [Google Scholar] [CrossRef]
- Morlock, G.E. High-performance thin-layer chromatography combined with effect-directed assays and high-resolution mass spectrometry as an emerging hyphenated technology: A tutorial review. Anal. Chim. Acta 2021, 1180, 338644. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.; Morlock, G.E. Investigation of the estrogenic potential of 15 rosé, white and red wines via effect-directed ten-dimensional hyphenation. J. Chromatogr. A 2023, 1690, 463775. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.; Sauter, D.; Friz, M.; Heil, J.; Morlock, G.E. Is our Natural Food our Homeostasis? Array of A Thousand Effect-Directed Profiles of 68 Herbs and Spices. Front. Pharmacol. 2021, 12, 755941. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, T.; Ronzheimer, A.; Friz, M.; Morlock, G.E. Multiplex planar bioassay with reduced diffusion on normal phase, identifying androgens, verified antiandrogens and synergists in botanicals via 12D hyphenation. Food Chem. 2022, 395, 133610. [Google Scholar] [CrossRef]
| 19 Green Tea Extracts | Standardization | Analysis |
|---|---|---|
| C1 | Polyphenols (98%), catechins (85%), EGCG * (50%) | UV, HPLC |
| C2 | Polyphenols (70%), catechins (35%), EGCG (15%) | UV, HPLC |
| C3 | L-Theanine 20% | HPLC |
| C4 | L-Theanine 40% | HPLC |
| C5 | Polyphenols (50%) | UV |
| C6 | Catechins (≥50%) | HPLC |
| C7 | EGCG (≥90%) | HPLC |
| C8 | Polyphenols (98%), catechins (75%), EGCG (45%) | UV, HPLC |
| C9 | Polyphenols (95%), catechins (60%), EGCG (45%) | UV, HPLC |
| C13 | EGCG (≥45%) | HPLC |
| C14 | EGCG (≥90%) | HPLC |
| C15 | Polyphenols (15%) | UV |
| C16 | Polyphenols (50%) | UV |
| C17 | Polyphenols (50%) | UV |
| C18 | Catechins (≥15%) | UV |
| C19 | Polyphenols (90%), catechins (60%), EGCG (40%) | UV, HPLC |
| C20 | Polyphenols (95%), catechins (80%), EGCG (55%) | UV, HPLC |
| C21 | L-Theanine 20% | HPLC |
| C22 | L-Theanine 60% | HPLC |
| 7 Black Tea Extracts | ||
| C10 | Polyphenols (70%), theaflavins (40%) | HPLC |
| C11 | Polyphenols (95%), catechins (70%), EGCG (40%) | HPLC |
| C12 | Polyphenols (98%), catechins (70%), EGCG (40%) | HPLC |
| C23 | Theaflavins 60% | HPLC |
| C24 | Theaflavins 10% | HPLC |
| C25 | Polyphenols 50% | UV |
| C26 | Polyphenols 20% | UV |
| 4 Self-Produced ADM® Extracts | ||
| Green Tea | 20% flavan-3-ols, 8% EGCG, 40–60% polyphenols | HPLC |
| White Tea | 7% flavan-3-ols (monomeric), 3% xanthines | HPLC |
| Black Tea | 10% total polyphenols, 3% caffeine | HPLC |
| Tea Complex | 10% flavan-3-ols (monomeric and theaflavins), 5% xanthines | HPLC, HPTLC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inarejos-Garcia, A.M.; Heil, J.; Guilera Bermell, S.; Morlock, G.E. Stability of Flavan-3-ols, Theaflavins, and Methylxanthines in 30 Industrial Green, Black, and White Tea (Camellia sinensis L.) Extracts Characterized via Liquid Chromatography Techniques. Antioxidants 2023, 12, 2121. https://doi.org/10.3390/antiox12122121
Inarejos-Garcia AM, Heil J, Guilera Bermell S, Morlock GE. Stability of Flavan-3-ols, Theaflavins, and Methylxanthines in 30 Industrial Green, Black, and White Tea (Camellia sinensis L.) Extracts Characterized via Liquid Chromatography Techniques. Antioxidants. 2023; 12(12):2121. https://doi.org/10.3390/antiox12122121
Chicago/Turabian StyleInarejos-Garcia, Antonio M., Julia Heil, Sonia Guilera Bermell, and Gertrud E. Morlock. 2023. "Stability of Flavan-3-ols, Theaflavins, and Methylxanthines in 30 Industrial Green, Black, and White Tea (Camellia sinensis L.) Extracts Characterized via Liquid Chromatography Techniques" Antioxidants 12, no. 12: 2121. https://doi.org/10.3390/antiox12122121
APA StyleInarejos-Garcia, A. M., Heil, J., Guilera Bermell, S., & Morlock, G. E. (2023). Stability of Flavan-3-ols, Theaflavins, and Methylxanthines in 30 Industrial Green, Black, and White Tea (Camellia sinensis L.) Extracts Characterized via Liquid Chromatography Techniques. Antioxidants, 12(12), 2121. https://doi.org/10.3390/antiox12122121








