The Effects of Radix isatidis Raw Material on Egg Quality, Serum Biochemistry, Gut Morphology and Gut Flora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of R. isatidis Raw Materials
2.2. Determination of Routine Nutritional Components of RIHR
2.2.1. Determination of Amino Acids by High Performance Liquid Chromatography (HPLC)
2.2.2. Determination of Conventional Nutrients in Feed
2.3. Analysis of Chemical Constituents in the RIHR by LC-MS
2.4. Experimental Animals and Experimental Design
2.5. Feeding Management and Index Determination
2.5.1. Laying Hen Diet and Management
2.5.2. Determination of Production Performance of Laying Hens
- × 100%
- Egg weight: Measured with Egg Analyzer, accurate to 0.01 g.
2.5.3. Determination of Egg Quality
- Egg shape index: The transverse and longitudinal diameters of each egg were determined using electronic digital vernier calipers and then calculated.
- Eggshell strength: The intact eggs were tested on a THV-1DX dual indenter micro- Vickers hardness tester.
- Yolk color: The egg is broken and tested in the Egg Analyzer.
- Protein height and Haugh unit: The Egg is broken and tested in the Egg Analyzer.
- Eggshell thickness: The eggshell thickness meter is utilized to measure the eggshell thickness of the sharp end, blunt end and the middle part of the egg, with the average value being considered the overall eggshell thickness.
- Yolk index: Two egg yolks were selected for each replicate, and their height and diameter were measured using an electronic digital vernier caliper.
- Yolk ratio: The proteinaceous material covering the yolk surface, along with any adhering remnants, was eliminated, following which the yolk weight was recorded using an analytical balance.
2.5.4. Determination of Serum Biochemical Indexes
2.5.5. Determination of Antioxidant Index
2.5.6. Determination of Serum Immune Indexes
2.5.7. Histological Analysis
2.5.8. Fluorescent Quantitative PCR
2.5.9. Molecular Docking Simulation
2.6. Gut Microbiota Analysis
2.7. Statistical Analysis
3. Results
3.1. Determination of Conventional Nutritional Components of RIHR
3.2. Qualitative Results for Main Chemical Constituents in RIHR
3.3. Effect of RIHR on Production Performance of Laying Hens
3.4. Effect of RIHR on Egg Quality
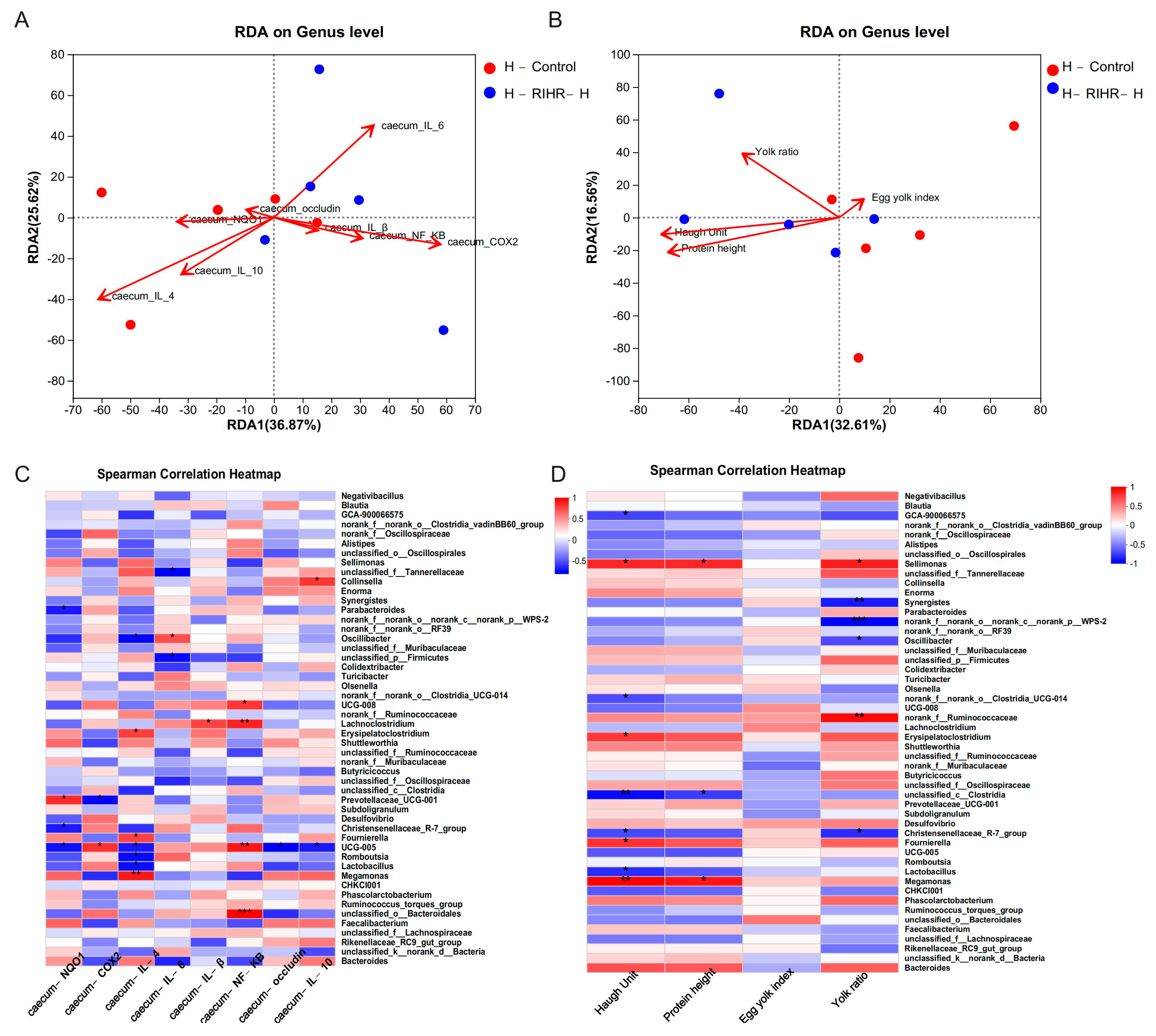
3.5. Effects of RIHR on Serum Biochemical Indices of Laying Hens
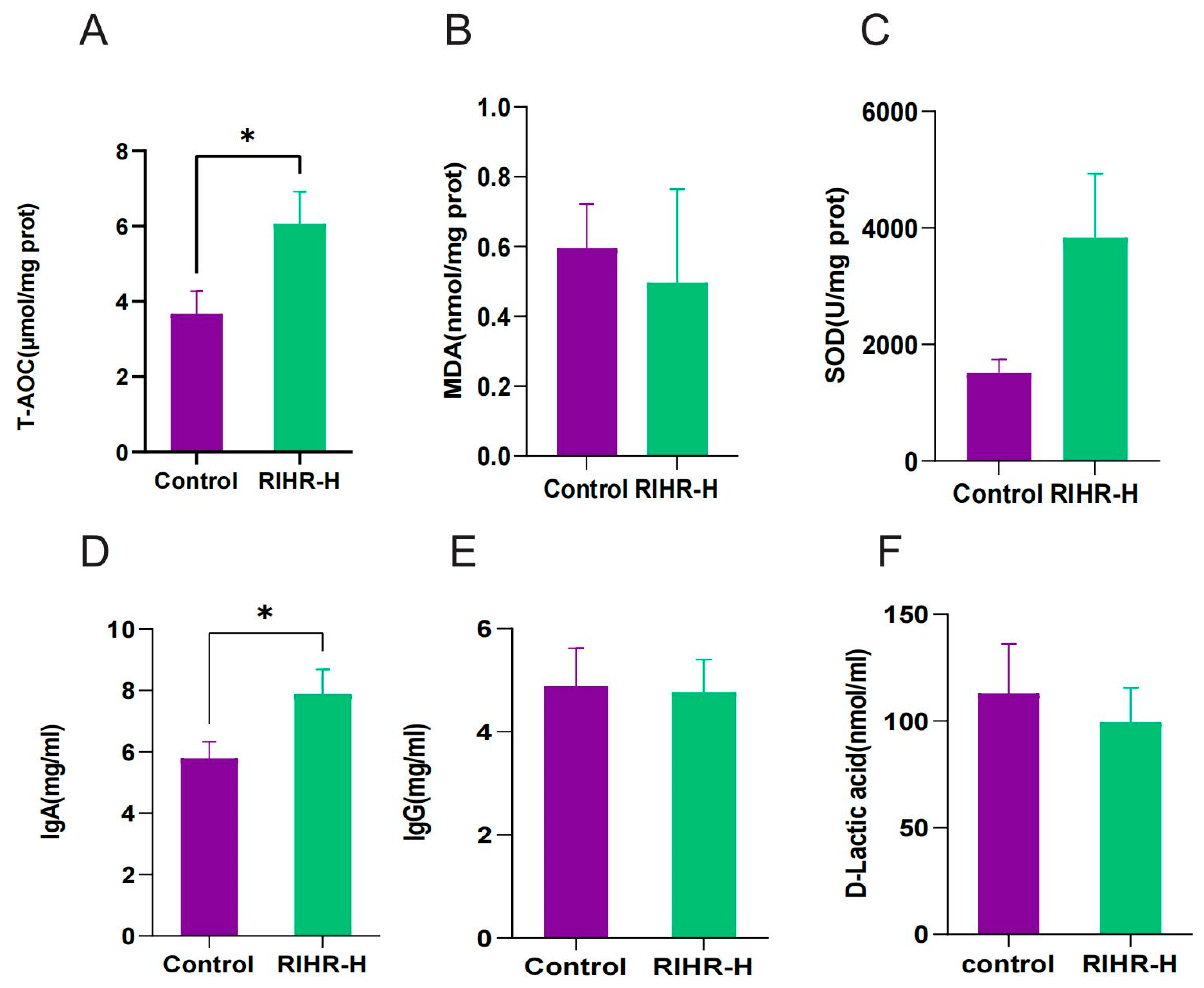
3.6. Effects of RIHR on Antioxidant Capacity and Immunity of Laying Hens
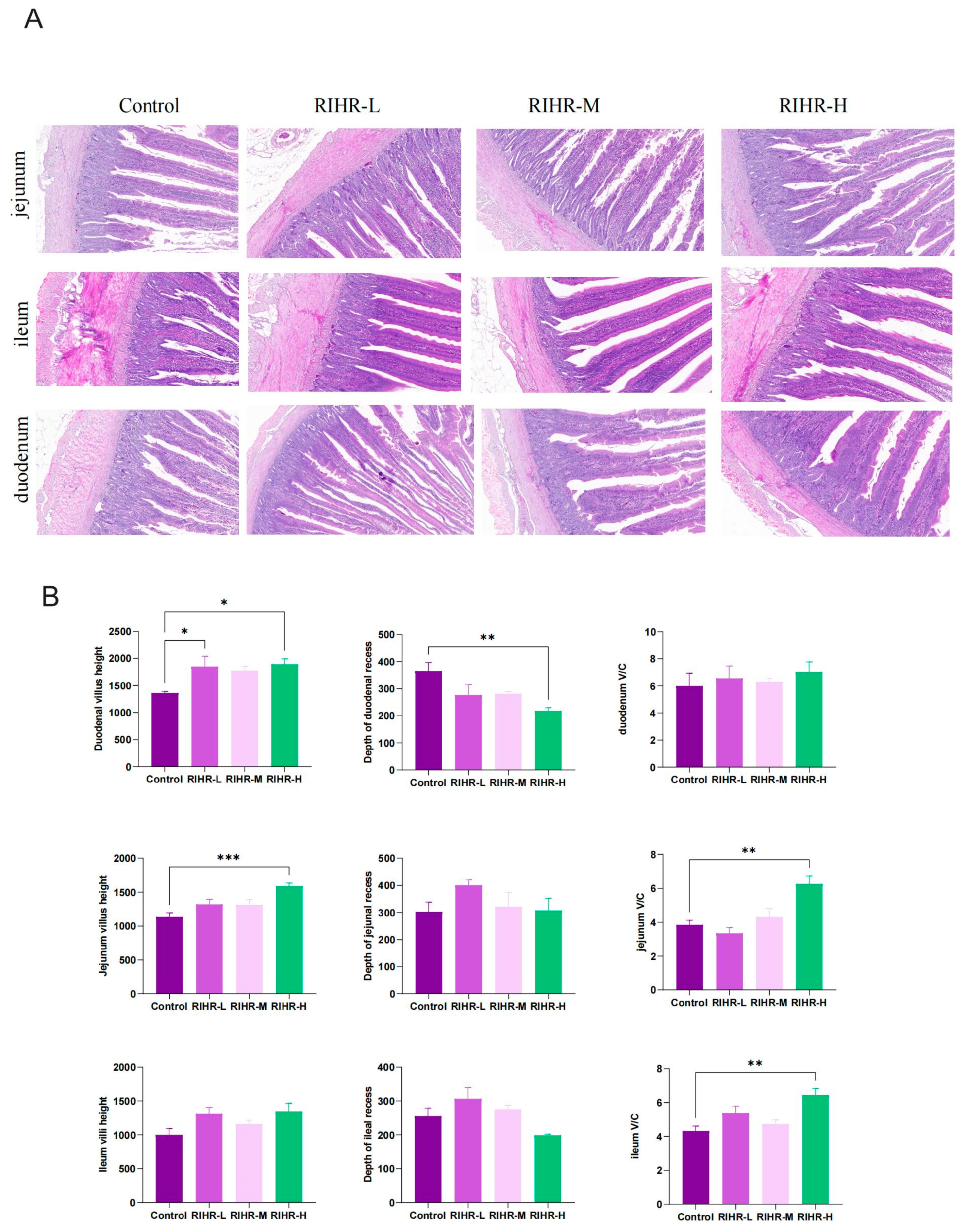
3.7. Effects of RIHR on the Intestinal Histomorphology of Laying Hens
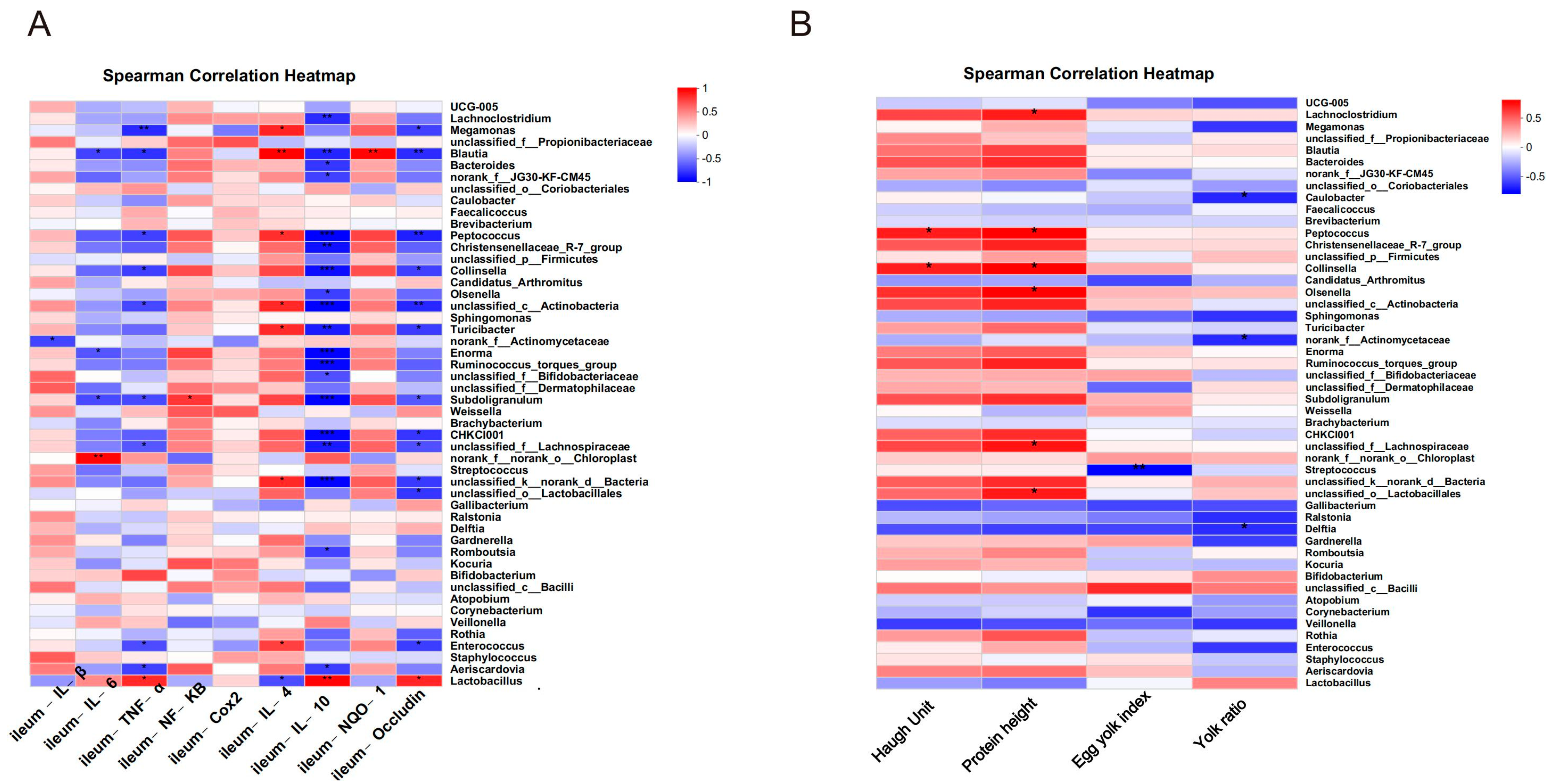
3.8. Effects of RIHR on the Expression of Related Genes in the Ileum and Cecum of Laying Hens
3.9. RIHR Docked with Related Protein Molecules
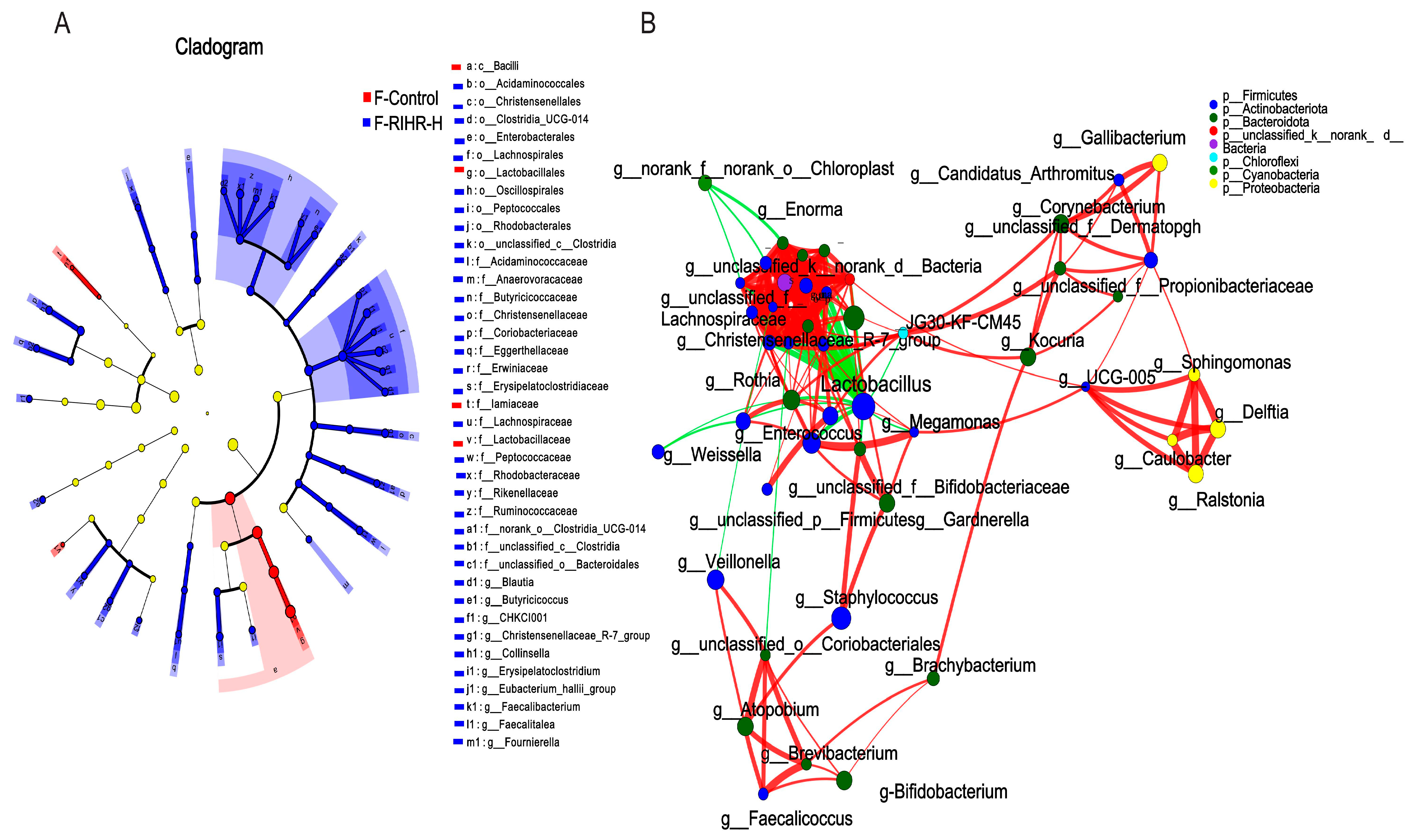
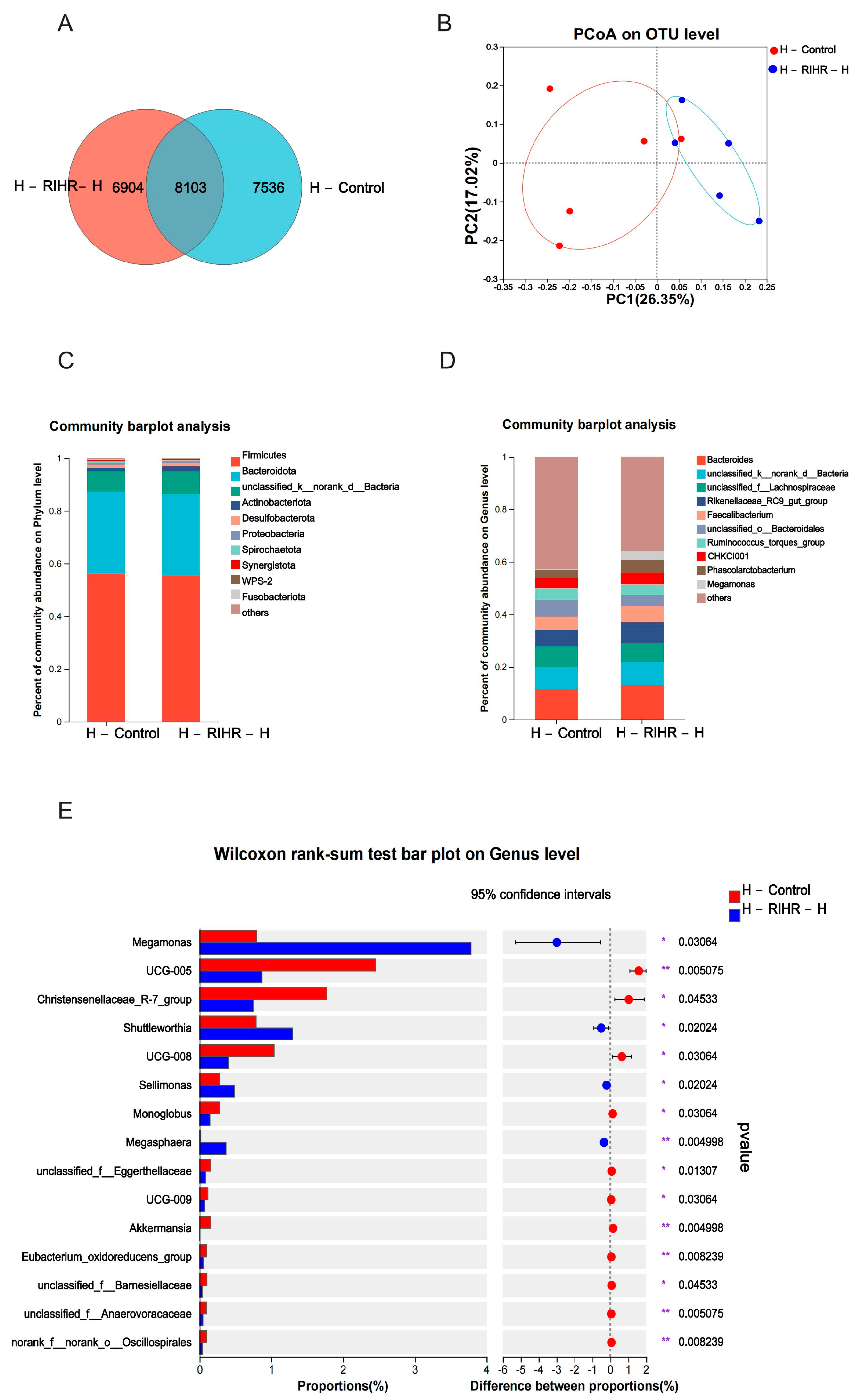
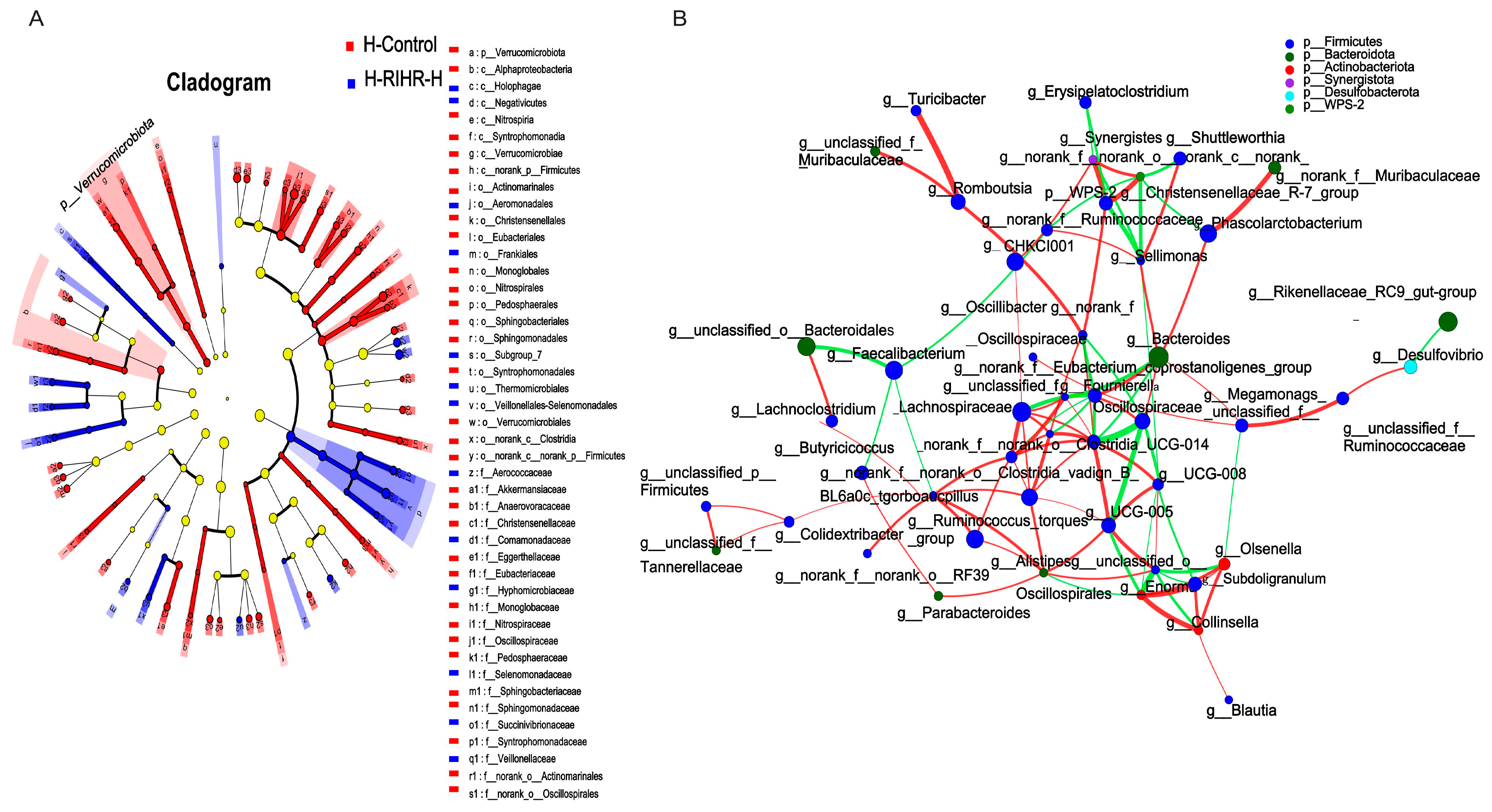
3.10. Effects of RIHR on Microorganisms in the Foregut of Laying Hens
3.10.1. Venn Diagram Analysis
3.10.2. Principal Component Analysis
3.10.3. Diversity Analysis
3.11. Effects of RIHR on Posterior Intestinal Microorganisms of Laying Hens
3.11.1. Venn Diagram Analysis
3.11.2. Principal Coordinates Analysis
3.11.3. Diversity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, C.; Li, Z.X.; Wu, Y.; Huang, Z.Y.; Hu, Y.; Gao, J. Treatment and bioresources utilization of traditional Chinese medicinal herb residues: Recent technological advances and industrial prospect. J. Environ. Manag. 2021, 299, 113607. [Google Scholar] [CrossRef]
- Lv, Z.P.; Yan, S.J.; Li, G.; Liu, D.; Guo, Y.M. Genistein improves the reproductive performance and bone status of breeder hens during the late egg-laying period. Poult. Sci. 2019, 98, 7022–7029. [Google Scholar] [CrossRef]
- Liu, H.T.; Zhang, B.G.; Chen, J.M.; Xue, J. Advances in residues analysis of pesticide in Chinese herbal medicine and new technological development. Zhongguo Zhong Yao Za Zhi 2006, 31, 1841–1846. [Google Scholar]
- Gao, C.; Wu, L.; Zhao, W.; Chen, Y.; Deng, M.; Liu, G.; Guo, Y.; Sun, B. Effects of Fermented Herbal Tea Residue on Serum Indices and Fecal Microorganisms of Chuanzhong Black Goats. Microorganisms 2022, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Xue, Q.; Sun, M.; Liu, J.; Wong, M.H.; Wang, C.; Chen, S. Co-production of polysaccharides, ginsenosides and succinic acid from Panax ginseng residue: A typical industrial herbal waste. Bioresour. Technol. 2021, 331, 125073. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; He, W.L.; Yang, M.L.; Yan, Y.M.; Xue, Y.H.; Zhao, S.T. Effect of dietary supplementation of Ligustrum lucidum on performance, egg quality and blood biochemical parameters of Hy-Line Brown hens during the late laying period. Animal 2017, 11, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.Q.; Shao, D.; Sheng, Z.W.; Wang, Q.; Shi, S.R. A mixture of daidzein and Chinese herbs increases egg production and eggshell strength as well as blood plasma Ca, P, antioxidative enzymes, and luteinizing hormone levels in post-peak, brown laying hens. Poult. Sci. 2019, 98, 3298–3303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, X.Y. Research progress of Chinese herbal medicine Radix isatidis (banlangen). Am. J. Chin. Med. 2013, 41, 743–764. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Fu, T.; He, Z.J.; Zhou, H.P.; Hong, Y. Immunomodulatory effects of Radix isatidis polysaccharides in vitro and in vivo. Exp. Ther. Med. 2021, 22, 1405. [Google Scholar] [CrossRef]
- Du, Z.; Liu, H.; Zhang, Z.; Li, P. Antioxidant and anti-inflammatory activities of Radix isatidis polysaccharide in murine alveolar macrophages. Int. J. Biol. Macromol. 2013, 58, 329–335. [Google Scholar] [CrossRef]
- Wang, F.; Bi, J.; He, L.; Chen, J.; Zhang, Q.; Hou, X.; Xu, H. The indole alkaloids from the roots of Isatidis Radix. Fitoterapia 2021, 153, 104950. [Google Scholar] [CrossRef]
- He, L.; Fan, F.; Hou, X.; Wu, H.; Wang, J.; Xu, H.; Sun, Y. 4(3H)-Quinazolone regulates innate immune signaling upon respiratory syncytial virus infection by moderately inhibiting the RIG-1 pathway in RAW264.7 cell. Int. Immunopharmacol. 2017, 52, 245–252. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Huang, W.; Rao, X.; Lai, Y. Pharmacological properties of indirubin and its derivatives. Biomed. Pharmacother. 2022, 151, 113112. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Ye, W.; Chen, J.; Li, X. Antiviral activities against influenza virus (FM1) of bioactive fractions and representative compounds extracted from Banlangen (Radix isatidis). J. Tradit. Chin. Med. 2016, 36, 369–376. [Google Scholar]
- Luo, Z.; Liu, L.F.; Wang, X.H.; Li, W.; Jie, C.; Chen, H.; Wei, F.Q.; Lu, D.H.; Yan, C.Y.; Liu, B.; et al. Epigoitrin, an Alkaloid From Isatis indigotica, Reduces H1N1 Infection in Stress-Induced Susceptible Model in vivo and in vitro. Front. Pharmacol. 2019, 10, 78. [Google Scholar] [CrossRef]
- Thiex, N. Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles. J AOAC Int. 2009, 1, 61–73. [Google Scholar] [CrossRef]
- NY/T33-2004; Agricultural Industry Standard of the People’s Republic of China—Chicken Feeding Standard. National Standards of the People’s Republic of China: Beijing, China, 2004.
- Zhu, Z.; Wu, S.; Qi, B.; Wang, C.; Luo, J.; Wan, Y. High-solids enzymatic saccharification of starch-rich raw herbal biomass residues for producing high titers of glucose. Environ. Sci. Pollut. Res. Int. 2023, 30, 86232–86243. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.; Siegel, D. The diverse functionality of NQO1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Z.; Yao, B.Y. Progress in indirubin treatment of chronic myelocytic leukemia. J. Tradit. Chin. Med. 1983, 3, 245–248. [Google Scholar]
- Zhang, X.; Song, Y.; Wu, Y.; Dong, Y.; Lai, L.; Zhang, J.; Lu, B.; Dai, F.; He, L.; Liu, M.; et al. Indirubin inhibits tumor growth by antitumor angiogenesis via blocking VEGFR2-mediated JAK/STAT3 signaling in endothelial cell. Int. J. Cancer 2011, 129, 2502–2511. [Google Scholar] [CrossRef]
- Zhong, H.J.; Leung, K.H.; Lin, S.; Chan, D.S.; Han, Q.B.; Chan, S.L.; Ma, D.L.; Leung, C.H. Discovery of deoxyvasicinone derivatives as inhibitors of NEDD8-activating enzyme. Methods 2015, 71, 71–76. [Google Scholar] [CrossRef]
- Huo, X.; Liu, C.; Gao, L.; Xu, X.; Zhu, N.; Cao, L. Hepatoprotective Effect of Aqueous Extract from the Seeds of Orychophragmus violaceus against Liver Injury in Mice and HepG2 Cells. Int. J. Mol. Sci. 2017, 18, 1197. [Google Scholar] [CrossRef]
- Han, Y.H.; Kee, J.Y. Extract of Isatidis Radix Inhibits Lipid Accumulation in In Vitro and In Vivo by Regulating Oxidative Stress. Antioxidants 2023, 12, 1426. [Google Scholar] [CrossRef]
- Shin, E.K.; Kim, D.H.; Lim, H.; Shin, H.K.; Kim, J.K. The anti-inflammatory effects of a methanolic extract from Radix isatidis in murine macrophages and mice. Inflammation 2010, 33, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86 Pt 3, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Corr, S.C.; Hill, C.; Gahan, C.G. Understanding the mechanisms by which probiotics inhibit gastrointestinal pathogens. Adv. Food Nutr. Res. 2009, 56, 1–15. [Google Scholar]
- O’Callaghan, J.; O’Toole, P.W. Lactobacillus: Host-microbe relationships. Curr. Top. Microbiol. Immunol. 2013, 358, 119–154. [Google Scholar]
- Chen, X.; Qi, X.; Cao, Y.; Li, Y.; Li, H.; Wang, Q.; Ai, J. Indirubin relieves inflammatory injury of chondrocytes in a mouse model of osteoarthritis. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 1381–1388. [Google Scholar]
- Balmant, B.D.; Fonseca, D.C.; Prudêncio, A.P.A.; Rocha, I.M.; Callado, L.; Alves, J.T.M.; Torrinhas, R.; Borba, E.F.; Waitzberg, D.L. Megamonas funiformis, Plasma Zonulin, and Sodium Intake Affect C3 Complement Levels in Inactive Systemic Lupus Erythematosus. Nutrients 2023, 15, 1999. [Google Scholar] [CrossRef]
- Wongkuna, S.; Ghimire, S.; Antony, L.; Chankhamhaengdecha, S.; Janvilisri, T.; Scaria, J. Sellimonas caecigallum sp. nov., description and genome sequence of a new member of the Sellimonas genus isolated from the cecum of feral chicken. New Microbes New Infect. 2020, 33, 100626. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, L.; Gao, B.; Leclercq, S.; Pirlot, B.; Horsmans, Y.; De Timary, P.; Leclercq, I.; Fouts, D.; Schnabl, B.; Stärkel, P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020, 12, 1782157. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Dwibedi, C.; Sundh, D.; Pradhan, M.; Kraft, J.D.; Caesar, R.; Tremaroli, V.; Lorentzon, M.; Bäckhed, F. Synergy and oxygen adaptation for development of next-generation probiotics. Nature 2023, 620, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]



| Ingredient | Contents (%) | Nutrition Levels | Content (%) |
|---|---|---|---|
| Corn | 55 | ME/(MJ/kg) | 11.48 |
| Soybean | 25.1 | CP | 15.773 |
| Bran | 4 | EE | 2.56 |
| CaHPO4 | 1.5 | Lys | 0.76 |
| Limestone | 9 | Met | 0.35 |
| Coarse stone grain | 2.4 | Ca | 3.65 |
| a Mineral premix X | 3 | TP | 0.59 |
| Total | 100 | AP (%) | 0.37 |
| Name of the Compound (μg/g) | Mean ± SEM |
|---|---|
| L-arginine | 1.19 ± 0.19 |
| guanine | 1.02 ± 0.39 |
| L-phenylalanine | 0.19 ± 0.06 |
| Epigoitrin | 0.39 ± 0.05 |
| deoxyvasicinone | 0.18 ± 0.05 |
| 3-indole acetonitrile | 0.52 ± 0.12 |
| indigo | 1.90 ± 0.55 |
| indirubin | 0.21 ± 0.06 |
| Week | Item | Mean ± SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | RIHR-L | RIHR-M | RIHR-H | |||
| first week | Laying rate, % | 90.38 ± 2.09 | 91.13 ± 1.37 | 89.38 ± 1.83 | 87.88 ± 1.56 | 0.586 |
| Average daily feed intake, g/d | 112.36 ± 0.29 bc | 108.38 ± 0.42 a | 113.01 ± 0.11 c | 111.40 ± 0.90 b | 0.000 | |
| Feed–egg ratio | 1.85 ± 0.01 b | 1.76 ± 0.01 a | 1.83 ± 0.02 b | 1.83 ± 0.01 b | 0.001 | |
| Egg weight, g | 60.90 ± 0.53 | 61.55 ± 0.38 | 61.83 ± 0.61 | 60.85 ± 0.49 | 0.450 | |
| eighth week | Laying rate, % | 91.63 ± 0.73 | 90.25 ± 1.22 | 91.50 ± 0.70 | 89.38 ± 1.81 | 0.508 |
| Average daily feed intake, g/d | 113.50 ± 0.53 | 115.83 ± 2.21 | 113.80 ± 0.88 | 113.01 ± 0.67 | 0.425 | |
| Feed–egg ratio | 2.01 ± 0.23 | 2.07 ± 0.07 | 1.99 ± 0.027 | 2.05 ± 0.043 | 0.602 | |
| Egg weight, g | 59.43 ± 0.71 b | 64.38 ± 1.00 a | 64.05 ± 0.83 a | 62.65 ± 1.32 a | 0.006 | |
| Item | Mean ± SEM | p-Value | |||
|---|---|---|---|---|---|
| Control | RIHR-L | RIHR-M | RIHR-H | ||
| Egg shape index, % | 73.24 ± 0.60 b | 74.86 ± 0.52 ab | 74.76 ± 0.79 ab | 76.93 ± 1.06 a | 0.020 |
| Eggshell strength, kg f | 2.30 ± 0.14 | 3.32 ± 0.14 | 3.45 ± 0.07 | 3.16 ± 0.18 | 0.120 |
| Yolk color | 4.38 ± 0.18 a | 4.88 ± 0.13 b | 4.88 ± 0.13 b | 5.38 ± 0.18 c | 0.001 |
| Haugh unit | 65.63 ± 2.49 b | 74.64 ± 1.99 a | 79.68 ± 1.48 a | 78.00 ± 1.88 a | 0.000 |
| Protein height, mm | 6.70 ± 0.26 b | 7.83 ± 0.21 a | 8.32 ± 0.15 a | 8.08 ± 0.20 a | 0.000 |
| Egg yolk index | 0.34 ± 0.01 | 0.34 ± 0.01 | 0.38 ± 0.03 | 0.38 ± 0.01 | 0.294 |
| Yolk ratio | 0.28 ± 0.00 | 0.29 ± 0.00 | 0.30 ± 0.00 | 0.29 ± 0.01 | 0.272 |
| Eggshell thickness, mm | 0.29 ± 0.01 c | 0.37 ± 0.01 a | 0.34 ± 0.01 ab | 0.33 ± 0.01 b | 0.000 |
| Item | Mean ± SEM | p-Value | |||
|---|---|---|---|---|---|
| Control | RIHR-L | RIHR-M | RIHR-H | ||
| lg M, g/L | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.06 ± 0.05 | 0.05 ± 0.03 | 0.579 |
| lg A, g/L | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.02 | 0.04 ± 0.04 | 0.524 |
| lg G, g/L | 0.01 ± 0.00 b | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 ab | 0.055 |
| IP, mmol/L | 3.02 ± 0.17 | 2.79 ± 0.20 | 3.28 ± 0.27 | 3.30 ± 0.45 | 0.579 |
| Ca, mmol/L | 5.46 ± 0.32 | 5.35 ± 0.13 | 5.56 ± 0.25 | 5.80 ± 0.22 | 0.606 |
| ALT, U/L | 6.86 ± 1.57 | 8.10 ± 1.89 | 8.09 ± 1.94 | 3.50 ± 1.39 | 0.243 |
| AST, U/L | 279.73 ± 16.33 | 272.61 ± 13.76 | 251.61 ± 7.44 | 273.19 ± 10.86 | 0.431 |
| ALP, U/L | 565.04 ± 68.02 | 769.44 ± 311.20 | 889.81 ± 355.88 | 612.03 ± 106.52 | 0.775 |
| ALB, g/L | 25.21 ± 1.35 ab | 23.66 ± 0.46 b | 25.58 ± 0.98 ab | 27.10 ± 1.05 a | 0.145 |
| GLU, mmol/L | 11.56 ± 0.27 | 11.34 ± 0.25 | 11.49 ± 0.20 | 11.94 ± 0.20 | 0.319 |
| TG, mmol/L | 19.97 ± 2.08 ab | 16.24 ± 2.01 b | 21.71 ± 1.65 ab | 23.74 ± 1.83 a | 0.059 |
| TC, mmol/L | 4.20 ± 0.58 | 3.22 ± 0.28 | 3.71 ± 0.37 | 4.49 ± 0.44 | 0.192 |
| HDL-C, mmol/L | 1.43 ± 0.10 b | 1.42 ± 0.11 b | 1.57 ± 0.13 ab | 1.92 ± 0.17 a | 0.036 |
| LDL-C, mmol/L | 0.89 ± 0.08 b | 0.83 ± 0.10 b | 1.13 ± 0.21 ab | 1.45 ± 0.27 a | 0.090 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Yan, Z.; Shi, P.; Wang, D.; Liu, Z.; Lu, M.; Li, C.; Yin, Y.; Huang, P. The Effects of Radix isatidis Raw Material on Egg Quality, Serum Biochemistry, Gut Morphology and Gut Flora. Antioxidants 2023, 12, 2084. https://doi.org/10.3390/antiox12122084
Li P, Yan Z, Shi P, Wang D, Liu Z, Lu M, Li C, Yin Y, Huang P. The Effects of Radix isatidis Raw Material on Egg Quality, Serum Biochemistry, Gut Morphology and Gut Flora. Antioxidants. 2023; 12(12):2084. https://doi.org/10.3390/antiox12122084
Chicago/Turabian StyleLi, Pingping, Zenghao Yan, Panpan Shi, Deqin Wang, Zhenhui Liu, Mengting Lu, Chuyuan Li, Yulong Yin, and Peng Huang. 2023. "The Effects of Radix isatidis Raw Material on Egg Quality, Serum Biochemistry, Gut Morphology and Gut Flora" Antioxidants 12, no. 12: 2084. https://doi.org/10.3390/antiox12122084
APA StyleLi, P., Yan, Z., Shi, P., Wang, D., Liu, Z., Lu, M., Li, C., Yin, Y., & Huang, P. (2023). The Effects of Radix isatidis Raw Material on Egg Quality, Serum Biochemistry, Gut Morphology and Gut Flora. Antioxidants, 12(12), 2084. https://doi.org/10.3390/antiox12122084





