Abstract
Atherosclerosis remains a leading cause of cardiovascular diseases. Although the mechanism for atherosclerosis is complex and has not been fully understood, inflammation and oxidative stress play a critical role in the development and progression of atherosclerosis. N-acetylcysteine (NAC) has been used as a mucolytic agent and an antidote for acetaminophen overdose with a well-established safety profile. NAC has antioxidant and anti-inflammatory effects through multiple mechanisms, including an increase in the intracellular glutathione level and an attenuation of the nuclear factor kappa-B mediated production of inflammatory cytokines like tumor necrosis factor-alpha and interleukins. Numerous animal studies have demonstrated that NAC significantly decreases the development and progression of atherosclerosis. However, the data on the outcomes of clinical studies in patients with atherosclerosis have been limited and inconsistent. The purpose of this review is to summarize the data on the effect of NAC on atherosclerosis from both pre-clinical and clinical studies and discuss the potential mechanisms of action of NAC on atherosclerosis, as well as challenges in the field.
1. Introduction
Atherosclerosis remains a leading cause of cardiovascular diseases (CVDs) globally and is considered a chronic inflammatory disease, with increased levels of reactive oxygen species (ROS) and oxidative stress [1,2]. Antioxidants, which inhibit oxidation, would be expected to have a favorable impact on patients with atherosclerosis. However, the Heart Outcomes Prevention Evaluation (HOPE) Study [3], a double-blind and randomized trial with patients at high risk for cardiovascular events, showed that treatment with antioxidant vitamin E had no beneficial effect over a mean follow-up of 4.5 years. Although there were no significant adverse effects of vitamin E, the primary outcome (a composite of myocardial infarction, stroke, and death from cardiovascular causes) and the secondary outcomes (including unstable angina, congestive heart failure, revascularization or amputation, death from any cause, complications of diabetes, and cancer) were the same in patients either on vitamin E or placebo [3]. Studies with antioxidant β-carotene treatment also failed to achieve significant clinical benefits in patients with CVDs, including atherosclerosis [4].
N-Acetylcysteine (NAC) is approved by the Food and Drug Administration (FDA) for the treatment of acetaminophen overdose. Although not approved for use as a dietary supplement, NAC has been widely used for acute respiratory distress syndrome, bronchitis, chemotherapy-induced toxicity, human immunodeficiency virus/acquired immune deficiency syndrome, radio-contrast-induced nephropathy, heavy metal toxicity, psychiatric disorders, and as an over-the-counter nutritional supplement [5,6]. In the cardiovascular area, NAC has been used off label for doxorubicin-induced cardiotoxicity, stable angina pectoris, and cardiac ischemia-reperfusion injury [6,7].
The primary mechanisms for the actions of NAC are considered to relate to its antioxidative effects via increasing intracellular glutathione (GSH) levels (crucial for cellular redox balance) and its anti-inflammatory effect through suppressing nuclear factor kappa B (NF-κB)-mediated expression of a variety of inflammatory mediators, including tumor necrosis factor-alpha (TNF-α) and interleukins (IL-6 and IL-1β) [5]. In this review, we summarize the data on the effects of NAC on atherosclerosis from both pre-clinical and clinical studies and discuss the potential mechanisms of the effects of NAC on atherosclerosis development and progression, as well as controversies and challenges concerning NAC and atherosclerosis. The actions and mechanisms of NAC on the modulation of lipid metabolism, homocysteine, and vascular endothelial cells will also be discussed.
2. Overview of NAC and Cardiovascular Diseases
As shown in Table 1, the intravenous administration of NAC significantly increases arterial vascular reactivity during reactive hyperemia in patients with chronic kidney disease following hemodialysis [8], reduces vasospasm in patients suffering from subarachnoid hemorrhage [9], and prevents ischemia-reperfusion syndrome following aortic clamping in patients during abdominal aortic aneurysmectomy [10]. NAC has been shown to decrease the frequency and severity of Raynaud’s phenomenon (RP) attacks and digital ulcers (DU) in patients with systemic sclerosis (SSc), with a significant reduction in plasma adrenomedullin concentrations [11,12,13]. Another study also demonstrated that NAC protected patients with RP secondary to SSc against DU, although NAC has no significant vasodilator effect on the microcirculation in hands [14].

Table 1.
Clinical studies with NAC in patients with peripheral vascular disease (PVD).
NAC has been reported to protect against coronary artery diseases (CAD), myocardial infarction (AMI), myocardial injuries, and cardiomyopathy [15,16,17,18,19,20] (Table 2). In patients with cardiac surgery, NAC decreases diabetes-associated cardiovascular, cardiopulmonary bypass, and cardiac surgery complications, including early reperfusion injury, pump-induced inflammatory response, and myocardial stress [21,22,23]. However, a systematic review has shown that NAC has no significant efficacy in improving major adverse outcomes, including mortality, acute renal failure, HF, length of stay and/or outcomes of care in intensive care unit, arrhythmia, and AMI, in patients following cardiac surgery [24]. Multiple clinical studies have demonstrated that NAC treatment effectively reduces the risk of atrial fibrillation (AF) (Table 3), a common arrhythmia post cardiac surgery [25,26,27,28,29], although one study reveals that a high dose of oral NAC treatment had no benefit on postoperative AF [30]. In addition, NAC has been reported to improve HF [31] however, no beneficial effect was observed in patients with doxorubicin-induced cardiomyopathy [32,33].

Table 2.
Clinical studies with NAC in atrial fibrillation (AF).

Table 3.
Clinical studies with NAC in coronary artery disease (CAD).
Animal studies have shown that NAC improves peripheral vascular diseases (PVD), with reduced muscular fatigue [40], restoration of redox balance and calcium retention capacity, as well as the suppression of reactive oxygen species (ROS) production in mice with hind limb ischemia [41]. NAC prevented excessive intracellular and extracellular ROS formation in mice with limb ischemia and enhanced the recovery of ischemic limb blood flow and function, in association with a selective increase in circulating endothelial progenitor cell (EPC) numbers (a group of cells critical for endothelial and vascular function) [42,43]. NAC treatment also attenuated C-reactive protein-induced ROS production in EPCs and apoptosis in vitro [44]. Animal studies also demonstrated that treating low-density lipoproteins (LDL) receptor deficient (LDLR KO) mice on a high-fat diet (HFD) with NAC in drinking water for 4 months significantly decreased ROS production and partially reversed the effects of hyperlipidemia on EPC populations [45]. Another animal study has revealed that NAC treatment effectively attenuated atherosclerosis progression following particulate matter (PM) exposure in LDLR KO mice with HFD, prevented excessive ROS generation, and reduced the levels of circulating oxidized LDL (ox-LDL) and inflammatory cytokines [46]. Preclinical animal studies have also shown that NAC normalized serum TNF-α level that was resistant to etanercept or infliximab, and improved HF in rats with cardiac injury [47,48,49]. A systematic review has demonstrated that NAC significantly decreased diabetes-associated cardiovascular complications including ischemia and non-ischemia cardiac damage through inhibition of oxidative stress in various animal models [23].
3. NAC and ROS
Excessive ROS plays a critical role in the development and progression of atherosclerosis. Many atherosclerogenic risk factors, including hypertension, diabetes, smoking, and dyslipidemia, increase ROS production, trigger inflammatory response, alter vascular function, and promote the growth of vascular smooth muscle [50,51,52]. NAC has been reported to prevent atherosclerosis formation in various animal models, as summarized in Table 4. Treatment of hypercholesterolemic rabbits with NAC significantly decreased the gelatinase expression, gelatinolytic activity, and matrix metalloproteinases (MMP)-9 expression in foam cells [53]. Similarly, it has been reported that treating atherosclerotic rabbits with NAC for 8 weeks significantly attenuated atherosclerotic formation, in association with a reduction in blood ox-LDL, MMP-9, MMP-2, and expression of MMP mRNA [54]. Using human THP-1 cells treated with phorbol 12-myristate 13-acetate for 48 h, followed by ox-LDL incubation for 4 days to induce foam cell formation, NAC treatment significantly reduced ROS production and ox-LDL uptake, leading to an inhibition of foam cell formation via a down-regulation of CD36 expression [55]. Another study reports that the treatment of apolipoprotein E knockout (ApoE KO) mice with NAC orally for 8 weeks significantly attenuated the progression of atherosclerosis, with decreased plaque collagen content and nitrotyrosine expression, probably via a reduction in oxidative stress [56]. In addition, aortic fatty streak plaque was effectively prevented in ApoE KO mice when treated with NAC via intraperitoneal injection for 8 weeks, with a reduction in aortic wall superoxide production [57]. A study shows that NAC treatment in drinking water for 12 weeks suppressed atherosclerotic development in ApoE KO mice with streptozotocin-induced type-1 diabetes, in association with improved GSH-dependent methylglyoxal elimination, decreased oxidative stress, and the restoration of phosphorylated Akt (p-Akt)/phosphorylated endothelial nitric-oxide synthase (p-eNOS) pathways in aortas [58].

Table 4.
Preclinical animal studies on NAC and atherosclerosis.
Native LDL per se is not atherogenic, and ox-LDL is one of the key components in hyperlipidemic states and a potent source of ROS [45,64,65]. NAC could inhibit the in vitro oxidation of LDL and prevent the depletion of antioxidant vitamins [66]. One study has shown that NAC treatment also effectively attenuated the in vivo biotransformation of human native LDL to ox-LDL in a mouse model [60]. In a small study with 10 patients with CAD and hyperlipidemia, NAC treatment for 7 days significantly decreased the serum ox-LDL level, while there was no significant change in the serum ox-LDL level in patients with placebo [60]. These data suggest that NAC decreases ROS levels through multiple mechanisms, including an inhibition of the in vivo biotransformation of native LDL to ox-LDL.
4. NAC, Inflammation, and Macrophages
Inflammation is closely related to the development and progression of CVDs, especially atherosclerosis. Myeloid cells-mediated innate immune responses significantly contribute to chronic vascular inflammation [67]. It has been reported that treatment of human umbilical vein endothelial cells (HUVEC) with NAC effectively blocks the interleukin (IL)-4-induced expression of vascular cell adhesion molecule-1, which stimulates the adhesion of lymphocytes and monocytes to the surface of the vascular endothelium during the early phase of atherosclerosis development [68,69]. IL-6 is known to increase inflammation and the development of vascular diseases, including atherosclerosis. NAC treatment inhibits the production of IL-6 in acetoacetate-treated human U937 monocytes [70]. Lysophosphatidylcholine is produced from the hydrolysis of phosphatidylcholine by secretory phospholipase A2 (sPLA2) and has proinflammatory and proatherogenic effects on the vasculature [71]. NAC treatment significantly reduced TNF-α-induced expressions of group V sPLA2 (sPLA2-V) mRNA and protein in HUVEC [72]. Intraperitoneal injection NAC significantly attenuated balloon-induced neointimal formation in the carotid artery in rats via the inhibition of NF-κB activity in the medial smooth muscle cells [73]. Treatment of hyperlipidemic rabbits with a combination of the anti-inflammatory drug colchicine with fenofibrate or NAC for 7 weeks significantly reduced aortic atherosclerotic plaque. However, the atherosclerotic burden was significantly lower in the hyperlipidemic rabbits treated with a combination of colchicine with NAC compared with that of colchicine plus fenofibrate. Serum IL-6 levels were also significantly decreased in animals treated with colchicine plus NAC [63].
Macrophages are one of the important sources for inflammatory cytokines [74] and play a critical role in the pathogenesis of atherosclerosis [75]. An increase in macrophage polarization to proinflammatory macrophages (M1), or a decrease to anti-inflammatory macrophages (M2), increases the level of inflammation and promotes atherosclerotic progression [76]. Thus, the M1/M2 ratio is an important determinant for the direction of inflammatory response [77]. Macrophages are also important for the stability of atherosclerotic plaques [78]. The data from a study using aging LDLR−/− mice showed that inflammatory markers (CRP, MCP-1, and IL-6) were significantly increased, while the anti-inflammatory cytokine IL-10 was significantly decreased in aging LDLR−/− mice, in association with a significantly increased aortic ROS level and an increased M1/M2 ratio, largely due to decreased M2 population in the aorta. Further studies using bone marrow transplants with GFP-labeled bone marrow cells showed that the increased M1/M2 ratio in the aorta of aging LDLR−/− mice was predominantly due to decreased M2 polarization, without a significant change in M1 polarization. NAC treatment effectively prevented changes in the expressions of pro-inflammatory and anti-inflammatory cytokines, the ROS level, and macrophage polarization in the aorta of aging LDLR−/− mice. Interestingly, NAC treatment has no effect on the migration of monocytes from circulation into the aorta in aging LDLR−/− mice or on M1 population in the aorta [59].
5. NAC and Atherosclerosis and CAD
Atherosclerosis and related CAD are a leading cause of mortality and morbidity in the world [79]. A multicenter clinical study, NAC in acute MI (NACIAM), with 112 patients, has shown that a combination of intravenous NAC treatment with a low dose of nitroglycerin (NTG) significantly shrinks the infarction size in patients with acute ST elevation MI undergoing primary percutaneous coronary intervention (PCI) [34]. Two more small studies (28 and 30 patients each) have reported that the combination of NAC with NTG and streptokinase reduced the levels of oxidative stress and plasma malondialdehyde (MDA), and improved left-ventricular function in patients with acute MI [35,36]. Similar results have been reported when NAC supplemented cold-blood cardioplegia [37] or when NAC was added to crystalloid cardioplegia in patients with CAD following a coronary artery bypass graft (CABG) [38]. NAC was also shown to potentiate the effects of NTG on the treatment of patients with unstable angina pectoris and other CAD patients [39,80]. A review of data from clinical studies has shown that NAC has cardioprotective effects in patients who had ischemic heart disease and underwent CABG and PCI [81]. However, a recent systematic review of 29 clinical trials with 2486 participants and a meta-analysis with 578 patients have demonstrated that NAC treatment does not significantly reduce major adverse events in patients undergoing cardiac surgery, including AMI and mortality [24,82] (Table 5).

Table 5.
Clinical studies with NAC in other cardiac diseases.
Animal studies have shown that the intraperitoneal injection of NAC prevented nonthyroidal illness syndrome-related thyroid hormone derangement and preserved cardiac function in male rats with acute ischemic myocardial injury via the restoration of the redox balance [84]. Intravenous injection of NAC decreased oxidative stress, infarct area, and apoptosis in a rat cardiac ischemia-reperfusion injury model [85]. However, intracoronary administration of NAC in a pig model that simulated a catheter-based reperfusion model for the therapy of acute ST-elevated MI (STEMI) showed no significant effect on reducing the infarction size [86]. A recent study, using an aging LDLR−/− mouse model with a regular diet, has demonstrated that NAC treatment significantly decreased aortic ROS levels and inflammatory cytokines in the serum and aortas of aging LDLR−/− mice. The same study has also shown that early and adequate NAC treatment could effectively attenuate atherosclerosis progression in aging LDLR−/− mice without extreme hyperlipidemia [59].
6. NAC and Lipid Metabolism
The disruption of the lipoprotein metabolism plays an important role in the development and progression of atherosclerosis [87]. Pretreatment with NAC significantly inhibited the differentiation of murine 3T3-L1 preadipocytes into adipocytes and decreased intracellular fat accumulation and the expressions of obesity-related proteins, including monoamine oxidase A, heat-shock protein 70, aminoacylase-1, and transketolase [88]. Similarly, NAC attenuates lipid accumulation and mitogen-activated protein kinases phosphorylation in murine embryonic fibroblasts during adipogenic differentiation [89]. Lipoprotein (Lp)(a) binds to apoprotein (a) and is considered an independent risk factor for premature atherosclerosis [90]. It has been shown that treating the serum from patients with a high concentration of Lp(a) with a high concentration of NAC (8 mg /mL or above) in vitro leads to dissociation of Lp(a) from apoprotein [91]. A small and yet significant reduction in Lp(a) concentration was observed in 12 subjects with a high serum Lp(a) level (87 mg/dL) following 6 weeks of NAC treatment [91]. However, another small clinical study of seven subjects with a median Lp(a) concentration of 14.3 mg/dL has demonstrated that NAC treatment for 6 weeks had no significant effect on plasma Lp(a) levels [91]. Similarly, no significant effect of NAC treatment on serum Lp(a) levels were found in 11 subjects with serum Lp(a) levels over 0.3 g/L [92]. An animal study has shown that treating LDLR KO mice on a HFD with NAC for 2 months or 4 months has no significant effect on the blood lipid profile, including triglycerides (TG), LDL, high-density lipoprotein (HDL), total cholesterol (TC), and non-HDL cholesterol [60]. Similarly, no significant effect of NAC treatment (250 mg/day, twice a day for 1 week) on the lipid profile was observed in human subjects with CAD and hyperlipidemia [60].
7. NAC and Homocysteine
An increased level of blood homocysteine is arguably considered a risk factor for atherosclerosis through increased oxidative stress, endothelial dysfunction, and thrombosis formation [93]. It has been reported that treating human subjects with NAC significantly reduced blood homocysteine levels by 45% over the placebo control [92]. The NAC treatment of patients with chronic renal failure led to a 16% reduction in plasma homocysteine levels [94]. Intravenous administration of NAC significantly decreased the level of plasma homocysteine in healthy subjects [95]. Data from controlled trials in middle-aged male subjects with unmedicated hyperlipidemia, with or without smoking, has shown that oral NAC treatment significantly reduced the level of total plasma homocysteine, regardless of lipid or smoking status [96]. However, Miner and colleagues have reported that treating cardiac transplant recipients with NAC had no significant impact on plasma homocysteine levels or brachial endothelial function [97].
8. Effects of NAC on Endothelial Cells
Endothelial dysfunction has been considered the first step of atherosclerosis development [98]. It was reported that treating endothelial cells from porcine pulmonary arteries with NAC significantly increased intracellular glutathione levels and partially prevented TNF-α-induced endothelial dysfunctions [99]. NAC also attenuated aortic endothelial damage in ApoE KO mice with streptozotocin-induced diabetes and HFD in association with increased levels of pAkt and -p-eNOS in aorta, as well as NO in serum [58]. Treatment of human aortic endothelial cells (HAEC) with NAC significantly attenuated TNF-α-induced ROS production and the DNA-binding activities of activator protein-1 and NF-κB, as well as p65 Ser276 phosphorylation [100]. Long-term treatment of endothelial cells (EC) from arterial segments of patients with severe CAD with NAC delayed senescence of diseased EC via the catalytic subunit of telomerase activation and transient telomere stabilization [101]. Intra-arterial infusion of NAC in healthy human subjects at a rate to achieve a blood concentration of 1 mM potentiated the effects of NTG on vasodilation and enhanced the biotransformation to an endothelium-derived relaxing factor equivalent nitrosothiol [102]. Intracoronary infusion of NAC in patients with or without coronary atherosclerosis significantly potentiated acetylcholine-induced coronary and femoral vasodilation and SNP-induced coronary vasodilation [103].
9. Mechanisms for the Actions of NAC on Atherosclerosis
The mechanisms for the effects of NAC on ROS generation, inflammation and atherosclerosis are very complex and have not been fully defined. Traditionally, NAC is considered to function as an antioxidant through a reduction in disulfide bonds or the scavenging of ROS or replenishing intracellular GSH stores [104]. However, in many settings and situations, the mechanisms of actions of NAC have remained unclear. Accumulating data has supported the concept that NAC is more like an anti-inflammatory agent with immunomodulatory properties, through its ability to attenuate the activation of oxidant-sensitive pathways, including the NF-κB and p38 mitogen-activated protein kinase (MAPK) signaling pathways, and subsequent reductions in pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [5,105,106].
Due to the complex nature of atherosclerosis pathogenesis, the mechanisms for the effects of NAC on atherosclerosis are also complex, including (but not limited to) (1) modifications of lipid metabolism, (2) inhibition of the expressions of gelatinase, MMP-2, and MMP-9, (3) blocking the in vivo biotransformation of native LDL to ox-LDL, and directly suppressing ROS production from ox-LDL, (4) increasing intracellular glutathione levels, and thus protecting endothelial function, (5) attenuation of NF-κB and p38-MAPK signaling, thus decreasing inflammatory cytokine production, (6) the preservation of circulating endothelial cell progenitor cells, (7) reduction of homocysteine levels, (8) attenuation of endothelial senescence and damage, while enhancing endothelial function through multiple mechanisms, including activation of Akt signaling and eNOS, and (9) the preservation of M2 polarization in the hyperlipidemic condition, thus reducing inflammation and oxidative stress, as shown in Figure 1.
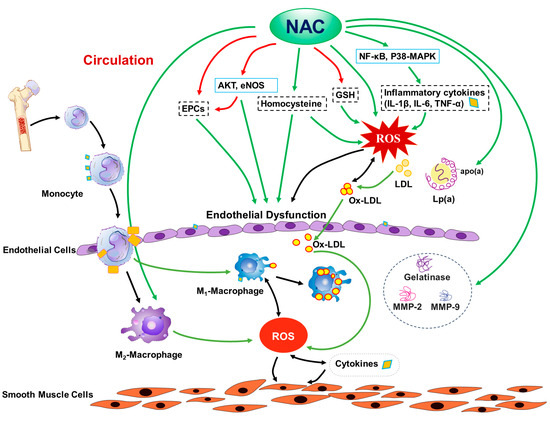
Figure 1.
Potential mechanisms for the effect of N-acetylcysteine (NAC) on atherosclerosis. EPCs: endothelial progenitor cells; AKT: serine-threonine protein kinase; eNOS: endothelial nitric-oxide synthase; GSH: glutathione; IL: interleukin; LDL: low-density lipoprotein cholesterol; Lp(a): lipoprotein-a; apo(a): apoprotein-a; Ox-LDL: oxidized LDL; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; p38-MAPK: p38 mitogen-activated protein kinase; ROS: reactive oxygen species; TNF-α: tumor necrosis factor-alpha; M1: proinflammatory macrophages; M2: anti-inflammatory macrophages; MMP: matrix metalloproteinases; red arrows: increase; green arrows: decrease.
10. Other Effects of NAC and Mechanisms
Ambient fine PM exposure increases the risk of cardiovascular diseases, including atherosclerosis [107,108]. It has been reported that NAC treatment can significantly inhibit the motorcycle exhaust particulates-induced proliferation of rat aortic vascular smooth muscle cells (VSMC) through an extracellular signal-regulated kinase 1/2-activated cyclooxygenase-2 signaling pathway [109]. Another study demonstrated that NAC prevented a PM-mediated reduction in NO production in HAEC [110]. Animal studies have shown that NAC treatment effectively attenuated the PM exposure-induced production of intracellular ROS and inflammatory cytokines TNF-α, IL-1β, and IL-6, and preserved EPC populations in bone marrow and blood in mice with PM exposure [111,112,113].
Oral NAC treatment has been reported to significantly decrease blood pressure in human subjects with or without hyperlipidemia [96]. Intravenous NAC administration in mice promoted arterial thrombolysis that is resistant to recombinant tissue-type plasminogen activators, direct thrombin inhibitors, and antiplatelet treatments through targeting vWF cross-link platelets in the thrombi [114]. Co-administration of NAC and a GpIIb/IIIa inhibitor significantly enhanced the thrombolytic efficacy via accelerating thrombus dissolution and preventing re-thrombosis without increasing hemorrhagic stroke risk [114]. Interestingly, a study demonstrated that the combined application of vitamin C and desferrioxamine did not exhibit significant beneficial effects against myocardial ischemia-reperfusion injury in pigs [115]. However, another study, using isolated ventricular cardiomyocytes and cardiac fibroblasts from neonatal rats in a simulated ischemia/reperfusion model, showed that the combination of vitamin C, desferrioxamine, and NAC protected cardiac fibroblasts with enhanced survival and improved function [116]. It was also reported that the administration of NAC and melatonin effectively preserved the expression of miR-142-3p in cardiomyocytes in response to endothelin 1 and isoproterenol induced stress [117].
DiNAC is a disulfide dimer of NAC and functions as an immunomodulating drug with potent anti-atherosclerotic effects [118,119]. It has been reported that a 3-month treatment of hyperlipidemic rabbits with DiNAC decreased thoracic aortic atherosclerotic lesions by 50% [61]. Endothelial function is also significantly improved after 3 weeks of treatment with the same dose of DiNAC in hyperlipidemic rabbits [120]. Treatment of hyperlipidemic male subjects with either 100 or 500 mg/day DiNAC for 24 weeks substantially increased brachial artery diameters at rest and during hyperemia without affecting blood lipid levels [121]. The mechanisms for the actions of DiNAC are considered to work mainly through the immunomodulation and attenuation of TNF-α-induced reduction in NO production [61,121]. Another new agent, S-Nitroso-N-acetylcysteine (SNAC), is a derivative of NAC and a water-soluble S-nitrosothiol, and is capable of releasing NO directly for a variety of vasoactive activities [122]. Treating LDLR KO mice on a HFD with SNAC decreased murine aortic plaque by 55% through a decrease in constitutive NO synthase expression but had no effects on vasomotor function, with minor changes in the plasma lipid profile [62].
11. Tolerability and Potential Toxicity and Side Effects of NAC
NAC can be administered orally, intravenously, or by inhalation [5,123]. The terminal half-life of NAC is estimated to be between 6 h to 18 h after single- or multiple-dose administrations intravenously or orally, with oral bioavailability of about 4.0–10% [5,123,124]. Oral dosage forms of NAC include capsules, granulate and effervescent, fast-dissolving, and slow-release tablets. Following both single- and multiple-dose administration, the plasma concentration of NAC increases rapidly and reaches a peak at approximately 1–2 h [5,124]. The maximum plasma concentration is higher after multiple doses than after a single dose, as expected. Oral NAC treatment has been well-tolerated by patients with a daily dose as high as 2 g and is associated with very few side effects, with an excellent safety profile based on multiple clinical studies [13,20,23,125,126].
Although NAC has a well-established safety profile, its toxic/side effects may occur at extremely high doses or upon overdosing [5]. An in vitro study has demonstrated that the viability of human THP-1 cells is significantly decreased when exposed to NAC at a concentration of 6 mM or higher [55]. Animal studies have shown that symptoms of acute toxicity including ataxia, hypoactivity, labored respiration, cyanosis, loss of righting reflex, and convulsions could be apparent following a single intravenous administration of NAC at a dose of 1000 mg/kg in mice, 2445 mg/kg in rats, 1500 mg/kg in guinea pigs, 1200 mg/kg in rabbits, and 500 mg/kg in dogs [5]. Although the toxicity of an NAC overdose has not yet been defined in humans, in a case report, a paracetamol overdose patient was accidently given a dose of 100 g NAC (instead of 10 g) and in a short period of time developed hemolysis, thrombocytopenia, acute renal failure, and subsequently died [127]. Potential side/toxic effects of NAC include anaphylactoid reactions after parenteral administration (can be more severe or even cause death in patients with asthma); skin rash, urticaria, pruritus, acute flushing and erythema after intravenous administration; chest tightness, bronchoconstriction, bronchospasm, and increased airway obstruction after oral inhalation; and gastrointestinal symptoms after oral administration [5].
12. Other Antioxidants and Atherosclerosis
Many pre-clinical studies have shown that a variety of antioxidants could prevent atherosclerosis. However, the data on the cardiovascular outcomes from clinical studies in human subjects have been inconsistent and sometimes controversial [128]. In vitro and in vivo animal studies have demonstrated that vitamin A could prevent atherosclerosis through inhibiting VSMC proliferation and inflammation, increasing NO production via increased eNOS phosphorylation, modulating angiogenesis via vascular endothelial growth factor production, and downregulating the angiotensin II type 1 receptor [129]. Data from human studies have revealed that treatment with a combination of Vitamin A with Vitamin D could attenuate atherosclerosis through a reduction in serum IL-1β levels [130], downregulation of IL-17 and retinoid-related orphan receptor-c [131], upregulation of forkhead box protein-3 gene expression, and an increased number of regulatory T cells in patients with atherosclerosis [132]. The Carotene and Retinol Efficacy Trial with 52 subjects (2 diabetic and 2 on lipid-lowering medications) has shown that a 5-year supplementation with β-carotene and vitamin A leads to a small, nonsignificant elevation of serum TG during treatment and a decrease in serum TG level after discontinuing the treatment, without changes in serum HDL, LDL or TC levels [133]. Treating patients with vitamin E could prevent cell aging and LDL oxidation, reduce serum TG level, and increase serum HDL level in association with enhanced plasma apolipoprotein A1 concentration and decreased cholesteryl-ester transfer protein activity [134,135,136]. However, high dose of vitamin E usage could have adverse effects on the cardiovascular system mainly through impairing endothelium-dependent vasodilation and potential paradoxical prooxidant effect [129].
Another well-studied antioxidant, vitamin C, has also been reported to decrease the risk of CVDs by improving endothelial function via enhancing NO generation, preventing ox-LDL-induced cytotoxicity of VSMC, the expression of cell adhesion molecules, decreasing monocyte adhesion to the endothelium, and enhancing paraoxonase activity with vitamin E [129]. However, some studies have indicated an inverse relationship between vitamin C and CVDs, and raised concerns that the effects of vitamin C on CVDs in the studies using fruit and vegetables instead of pure vitamin C might be due to the atheroprotective effects of other nutrients in vitamin C-rich foods [129].
13. Unanswered Questions on NAC and Atherosclerosis and Challenges for Clinical Studies in Patients with Atherosclerosis
Abundant data supports the concept that NAC is a potent anti-inflammatory agent that attenuates the development and progression of atherosclerosis in pre-clinical animal studies. However, it is unclear if NAC could suppress the progression of atherosclerosis in humans. Many clinical studies were observational or included a small number of patients without randomization, with potential significant bias. A recent animal study showed that NAC treatment did not reverse the existing atherosclerotic lesions, and an extended period of treatment (6 months) was needed to show a beneficial effect of NAC on the progression of atherosclerosis in aging LDLR−/− mice [70]. Thus, it could take years to demonstrate a significant difference in patients with NAC treatment, since atherosclerotic lesions usually progress slowly, and NAC treatment only prevents new lesion formation. Thus, it is very challenging to determine the effect of NAC treatment on the progression of atherosclerosis in patients without a prolonged period of follow up. In the same study, it was reported that long-term NAC treatment (6 months) had no effect on atherosclerosis progression in aging LDLR−/− mice on HFD. No beneficial effect of NAC treatment on atherosclerosis progression was observed in aging LDLR−/− mice on a normal diet when advanced atherosclerotic lesions were present [71]. This data strongly suggests that the timing and duration of NAC treatment, as well as the serum lipid level and disease stage, are critical factors for NAC therapeutic outcomes on atherosclerosis. NAC treatment was only effective when applied early, over a long period of time, with a reasonable control of the serum lipid level.
Inflammatory cytokines and chemokines are important for atherosclerosis development and progression. The Cardiovascular Inflammation Reduction Trial (CIRT) has shown that there were no cardiovascular benefits in patients with MI or type 2 diabetes or metabolic syndrome when there were no reductions in CRP, IL-1β, or IL-6 levels [137,138,139]. Consistent with these observations, the data from the CANTOS study has revealed that the patients with the greatest reductions in IL-6 and hs-CRP levels benefited the most from canakinumab treatment, with reduced adverse cardiovascular events [140]. Interestingly, a recent study revealed a positive association between the area of atherosclerotic lesions and serum levels of CRP and IL-6 in aging LDLR−/− mice. Administration of NAC for six months effectively attenuated the levels of serum CRP and IL-6 in LDLR−/− mice at the age of 15 months [71]. Thus, a well-designed clinical study is also needed to determine the effect of NAC on the serum levels of inflammatory cytokines, especially IL-6, hs-CRP, and cardiovascular mortality and morbidity in patients with atherosclerosis.
Recently, a new concept in polytherapy has been proposed, namely, to use multiple antioxidants together as a combined therapy with different antioxidants due to (1) ROS generation being a very complex process that involves a wide spectrum of sources of ROS and a variety of enzymes and/or singling pathways, and (2) antioxidants exhibiting very diverse characteristics chemically, biologically, and pharmacologically [141]. Thus, the combination therapy of different antioxidants may potentially generate significant synergistic effects on ROS suppression, leading to a much better clinical outcome. Certainly, NAC could be an important part of polytherapy in future pre-clinical and clinical studies to determine the efficacy of combination therapy for disease conditions associated with excessive ROS, including atherosclerosis.
Accumulating data has shown that many microRNAs (miRNA), including (but not limited to) miR-126-5p, miR-155, miR-146a, MiR-125a, miR-22, and miR143/145, may play an important role in the development and progression of atherosclerosis, as nicely summarized in a recent review [142]. These miRNAs are critically involved in the regulation of endothelial function, inflammation, macrophage polarization, lipid metabolism, the function of VSMCs, and plaque stability, as well as vascular calcification, thus significantly contributing to a variety of pathophysiological events at different stages of atherosclerosis. In addition, there are extensive interactions between miRNAs and inflammatory cytokines (including TNF-α, IL-1β, and IL-6) and chemokines (such as CCL5, CCL8, CXCL2, and CXCL4) [143,144]; thus, they are closely associated with ROS formation and oxidative stress. It has been reported that NAC treatment significantly decreased the levels of miR146a and NF-κB p65 signaling in rats [145] and reduced the expression of miR-21 and miR-29b induced by C. parvum treatment in mice [146]. These data suggest that NAC could attenuate atherosclerosis by targeting inflammatory miRNAs. Further studies are needed to define the effect of NAC on the expression profiles of miRNAs in atherosclerosis.
14. Conclusions
NAC and NAC derivatives (DiNAC and SNAC) seem to be promising therapeutic options to attenuate the development and progression of atherosclerosis through multiple mechanisms with a well-established safety profile. The significant beneficial effects of NAC on CVDs, including atherosclerosis, have been reported in many clinical studies. However, these studies mostly included a small number of study subjects with short-term treatment with NAC and short periods of follow up time. Atherosclerosis is a slow and progressive condition; thus, a large, randomized double-blinded clinical trial with long-term treatment and follow-up periods is needed to determine the effect of NAC on the development and progression of atherosclerosis and the clinical outcomes of patients with atherosclerosis.
Author Contributions
Conceptualization, Z.L. and Y.C.; validation, Z.L., Y.C. and G.C.F.; resources, Z.L. and H.H.; data curation, Y.C. and Q.Z.; writing—original draft preparation, Y.C. and Q.Z.; writing—review and editing, Z.L. and G.C.F.; visualization, Q.Z.; supervision, Z.L.; funding acquisition, Z.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by US NIH grants R01 HL148196 (to Z.L.) and RF1 NS132279 (to Z.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors want to thank Jingshu Chi and Aiping Lin, for their assistance in the preparation of the figures. Their contributions to this paper are greatly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Engelen, S.E.; Robinson, A.J.B.; Zurke, Y.X.; Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: How to proceed? Nat. Rev. Cardiol. 2022, 19, 522–542. [Google Scholar] [CrossRef]
- El Hadri, K.; Smith, R.; Duplus, E.; El Amri, C. Inflammation, Oxidative Stress, Senescence in Atherosclerosis: Thioredoxine-1 as an Emerging Therapeutic Target. Int. J. Mol. Sci. 2021, 23, 77. [Google Scholar] [CrossRef] [PubMed]
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Dagenais, G.; Pogue, J.; Bosch, J.; Sleight, P. Vitamin E supplementation and cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 154–160. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2008, CD007176. [Google Scholar] [CrossRef]
- Tenorio, M.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Sochman, J. N-acetylcysteine in acute cardiology: 10 years later: What do we know and what would we like to know?! J. Am. Coll. Cardiol. 2002, 39, 1422–1428. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wittstock, A.; Burkert, M.; Zidek, W.; Tepel, M.; Scholze, A. N-acetylcysteine improves arterial vascular reactivity in patients with chronic kidney disease. Nephron Clin. Pract. 2009, 112, c184–c189. [Google Scholar] [CrossRef] [PubMed]
- Pereira Filho Nde, A.; Pereira Filho Ade, A.; Soares, F.P.; Coutinho, L.M. Effect of N-acetylcysteine on vasospasm in subarachnoid hemorrhage. Arq. Neuro-Psiquiatr. 2010, 68, 918–922. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Klein, U.; Palutke, M.; Schirrmeister, W. Reduction of ischemia-reperfusion syndrome after abdominal aortic aneurysmectomy by N-acetylcysteine but not mannitol. Acta Anaesthesiol. Scand. 1996, 40, 657–664. [Google Scholar] [CrossRef]
- Sambo, P.; Amico, D.; Giacomelli, R.; Matucci-Cerinic, M.; Salsano, F.; Valentini, G.; Gabrielli, A. Intravenous N-acetylcysteine for treatment of Raynaud’s phenomenon secondary to systemic sclerosis: A pilot study. J. Rheumatol. 2001, 28, 2257–2262. [Google Scholar] [PubMed]
- Rosato, E.; Borghese, F.; Pisarri, S.; Salsano, F. The treatment with N-acetylcysteine of Raynaud’s phenomenon and ischemic ulcers therapy in sclerodermic patients: A prospective observational study of 50 patients. Clin. Rheumatol. 2009, 28, 1379–1384. [Google Scholar] [CrossRef]
- Salsano, F.; Letizia, C.; Proietti, M.; Rossi, C.; Proietti, A.R.; Rosato, E.; Pisarri, S. Significant changes of peripheral perfusion and plasma adrenomedullin levels in N-acetylcysteine long term treatment of patients with sclerodermic Raynauds phenomenon. Int. J. Immunopathol. Pharmacol. 2005, 18, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.J.; Mariz, H.A.; Andrade, L.E.; Kayser, C. Oral N-acetylcysteine in the treatment of Raynaud’s phenomenon secondary to systemic sclerosis: A randomized, double-blind, placebo-controlled clinical trial. Rev. Bras. Reumatol. 2014, 54, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef] [PubMed]
- Tepel, M.; van der Giet, M.; Statz, M.; Jankowski, J.; Zidek, W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: A randomized, controlled trial. Circulation 2003, 107, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, X.Z.; Shen, M.Z.; Xing, C.Y.; Ma, J.; Duan, Y.Y.; Yuan, L.J. N-Acetyl Cysteine improves the diabetic cardiac function: Possible role of fibrosis inhibition. BMC Cardiovasc. Disord. 2015, 15, 84. [Google Scholar] [CrossRef]
- Phaelante, A.; Rohde, L.E.; Lopes, A.; Olsen, V.; Tobar, S.A.; Cohen, C.; Martinelli, N.; Biolo, A.; Dal-Pizzol, F.; Clausell, N.; et al. N-acetylcysteine Plus Deferoxamine Improves Cardiac Function in Wistar Rats After Non-reperfused Acute Myocardial Infarction. J. Cardiovasc. Transl. Res. 2015, 8, 328–337. [Google Scholar] [CrossRef]
- Costa, C.R.M.; Seara, F.A.C.; Peixoto, M.S.; Ramos, I.P.; Barbosa, R.A.Q.; Carvalho, A.B.; Fortunato, R.S.; Silveira, A.L.B.; Olivares, E.L. Progression of heart failure is attenuated by antioxidant therapy with N-acetylcysteine in myocardial infarcted female rats. Mol. Biol. Rep. 2020, 47, 8645–8656. [Google Scholar] [CrossRef]
- Shafiei, E.; Bahtoei, M.; Raj, P.; Ostovar, A.; Iranpour, D.; Akbarzadeh, S.; Shahryari, H.; Anvaripour, A.; Tahmasebi, R.; Netticadan, T.; et al. Effects of N-acetyl cysteine and melatonin on early reperfusion injury in patients undergoing coronary artery bypass grafting: A randomized, open-labeled, placebo-controlled trial. Medicine 2018, 97, e11383. [Google Scholar] [CrossRef]
- Sucu, N.; Cinel, I.; Unlu, A.; Aytacoglu, B.; Tamer, L.; Kocak, Z.; Karaca, K.; Gul, A.; Dikmengil, M.; Atik, U.; et al. N-acetylcysteine for preventing pump-induced oxidoinflammatory response during cardiopulmonary bypass. Surg. Today 2004, 34, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Tossios, P.; Bloch, W.; Huebner, A.; Raji, M.R.; Dodos, F.; Klass, O.; Suedkamp, M.; Kasper, S.M.; Hellmich, M.; Mehlhorn, U. N-acetylcysteine prevents reactive oxygen species-mediated myocardial stress in patients undergoing cardiac surgery: Results of a randomized, double-blind, placebo-controlled clinical trial. J. Thorac. Cardiovasc. Surg. 2003, 126, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs 2018, 18, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.E.G.; El Dib, R.; Braz, L.G.; Escudero, J.; Hayes, J.; Johnston, B.C. N-acetylcysteine use among patients undergoing cardiac surgery: A systematic review and meta-analysis of randomized trials. PLoS ONE 2019, 14, e0213862. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, A.; Habibi, M.R.; Hasanzadeh Kiabi, F.; Alipour, A.; Habibi, V.; Azizi, S.; Emami Zeydi, A.; Sohrabi, F.B. The effect of intravenous N-acetylcysteine on prevention of atrial fibrillation after coronary artery bypass graft surgery: A double-blind, randomised, placebo-controlled trial. Kardiol. Pol. 2018, 76, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin, M.; Peker, O.; Erdogan, D.; Akcay, S.; Yucel, H.; Icli, A.; Ceyhan, B.M.; Sutcu, R.; Uysal, B.A.; Varol, E.; et al. Oxidative status, inflammation, and postoperative atrial fibrillation with metoprolol vs. carvedilol or carvedilol plus N-acetyl cysteine treatment. Clin. Cardiol. 2014, 37, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ozaydin, M.; Icli, A.; Yucel, H.; Akcay, S.; Peker, O.; Erdogan, D.; Varol, E.; Dogan, A.; Okutan, H. Metoprolol vs. carvedilol or carvedilol plus N-acetyl cysteine on post-operative atrial fibrillation: A randomized, double-blind, placebo-controlled study. Eur. Heart J. 2013, 34, 597–604. [Google Scholar] [CrossRef]
- Ozaydin, M.; Erdogan, D.; Yucel, H.; Peker, O.; Icli, A.; Akcay, S.; Etli, M.; Ceyhan, B.M.; Sutcu, R.; Varol, E.; et al. N-acetyl cysteine for the conversion of atrial fibrillation into sinus rhythm after cardiac surgery: A prospective, randomized, double-blind, placebo-controlled pilot study. Int. J. Cardiol. 2013, 165, 580–583. [Google Scholar] [CrossRef]
- Ozaydin, M.; Peker, O.; Erdogan, D.; Kapan, S.; Turker, Y.; Varol, E.; Ozguner, F.; Dogan, A.; Ibrisim, E. N-acetylcysteine for the prevention of postoperative atrial fibrillation: A prospective, randomized, placebo-controlled pilot study. Eur. Heart J. 2008, 29, 625–631. [Google Scholar] [CrossRef]
- Kazemi, B.; Akbarzadeh, F.; Safaei, N.; Yaghoubi, A.; Shadvar, K.; Ghasemi, K. Prophylactic high-dose oral-N-acetylcysteine does not prevent atrial fibrillation after heart surgery: A prospective double blind placebo-controlled randomized clinical trial. Pacing Clin. Electrophysiol. PACE 2013, 36, 1211–1219. [Google Scholar] [CrossRef]
- Mehra, A.; Shotan, A.; Ostrzega, E.; Hsueh, W.; Vasquez-Johnson, J.; Elkayam, U. Potentiation of isosorbide dinitrate effects with N-acetylcysteine in patients with chronic heart failure. Circulation 1994, 89, 2595–2600. [Google Scholar] [CrossRef]
- Dresdale, A.R.; Barr, L.H.; Bonow, R.O.; Mathisen, D.J.; Myers, C.E.; Schwartz, D.E.; d’Angelo, T.; Rosenberg, S.A. Prospective randomized study of the role of N-acetyl cysteine in reversing doxorubicin-induced cardiomyopathy. Am. J. Clin. Oncol. 1982, 5, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Unverferth, D.V.; Jagadeesh, J.M.; Unverferth, B.J.; Magorien, R.D.; Leier, C.V.; Balcerzak, S.P. Attempt to prevent doxorubicin-induced acute human myocardial morphologic damage with acetylcysteine. J. Natl. Cancer Inst. 1983, 71, 917–920. [Google Scholar] [PubMed]
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.K.; Du, Y.T.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early Use of N-acetylcysteine With Nitrate Therapy in Patients Undergoing Primary Percutaneous Coronary Intervention for ST-Segment-Elevation Myocardial Infarction Reduces Myocardial Infarct Size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation 2017, 136, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Arstall, M.A.; Yang, J.; Stafford, I.; Betts, W.H.; Horowitz, J.D. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction. Safety and biochemical effects. Circulation 1995, 92, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Yesilbursa, D.; Serdar, A.; Senturk, T.; Serdar, Z.; Sag, S.; Cordan, J. Effect of N-acetylcysteine on oxidative stress and ventricular function in patients with myocardial infarction. Heart Vessel. 2006, 21, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Koramaz, I.; Pulathan, Z.; Usta, S.; Karahan, S.C.; Alver, A.; Yaris, E.; Kalyoncu, N.I.; Ozcan, F. Cardioprotective effect of cold-blood cardioplegia enriched with N-acetylcysteine during coronary artery bypass grafting. Ann. Thorac. Surg. 2006, 81, 613–618. [Google Scholar] [CrossRef]
- Vento, A.E.; Nemlander, A.; Aittomaki, J.; Salo, J.; Karhunen, J.; Ramo, O.J. N-acetylcysteine as an additive to crystalloid cardioplegia increased oxidative stress capacity in CABG patients. Scand. Cardiovasc. J. SCJ 2003, 37, 349–355. [Google Scholar] [CrossRef]
- Horowitz, J.D.; Henry, C.A.; Syrjanen, M.L.; Louis, W.J.; Fish, R.D.; Smith, T.W.; Antman, E.M. Combined use of nitroglycerin and N-acetylcysteine in the management of unstable angina pectoris. Circulation 1988, 77, 787–794. [Google Scholar] [CrossRef]
- Roseguini, B.T.; Silva, L.M.; Polotow, T.G.; Barros, M.P.; Souccar, C.; Han, S.W. Effects of N-acetylcysteine on skeletal muscle structure and function in a mouse model of peripheral arterial insufficiency. J. Vasc. Surg. 2015, 61, 777–786. [Google Scholar] [CrossRef]
- Lejay, A.; Charles, A.L.; Georg, I.; Goupilleau, F.; Delay, C.; Talha, S.; Thaveau, F.; Chakfe, N.; Geny, B. Critical Limb Ischaemia Exacerbates Mitochondrial Dysfunction in ApoE-/- Mice Compared with ApoE+/+ Mice, but N-acetyl Cysteine still Confers Protection. Eur. J. Vasc. Endovasc. Surg. 2019, 58, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, L.; Xiao, Y.; Li, X.; Zhang, J.; Xie, X.; Tian, J.; Sen, C.K.; He, X.; Hao, H.; et al. N-acetylcysteine differentially regulates the populations of bone marrow and circulating endothelial progenitor cells in mice with limb ischemia. Eur. J. Pharmacol. 2020, 881, 173233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Hao, H.; Xu, H.; Fichman, Y.; Cui, Y.; Yang, C.; Wang, M.; Mittler, R.; Hill, M.A.; Cowan, P.J.; et al. Combination of Antioxidant Enzyme Overexpression and N-Acetylcysteine Treatment Enhances the Survival of Bone Marrow Mesenchymal Stromal Cells in Ischemic Limb in Mice With Type 2 Diabetes. J. Am. Heart Assoc. 2021, 10, e023491. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Li, S.H.; Szmitko, P.E.; Fedak, P.W.; Verma, S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2476–2482. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Narasimhulu, C.A.; Liu, L.; Li, X.; Xiao, Y.; Zhang, J.; Xie, X.; Hao, H.; Liu, J.Z.; He, G.; et al. Oxidized low-density lipoprotein alters endothelial progenitor cell populations. Front. Biosci. 2015, 20, 975–988. [Google Scholar] [CrossRef]
- Xu, Y.; Bu, H.; Jiang, Y.; Zhuo, X.; Hu, K.; Si, Z.; Chen, Y.; Liu, Q.; Gong, X.; Sun, H.; et al. N-acetyl cysteine prevents ambient fine particulate matter-potentiated atherosclerosis via inhibition of reactive oxygen species-induced oxidized low density lipoprotein elevation and decreased circulating endothelial progenitor cell. Mol. Med. Rep. 2022, 26, 236. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998. [Google Scholar] [CrossRef]
- Anker, S.D.; Coats, A.J. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. Int. J. Cardiol. 2002, 86, 123–130. [Google Scholar] [CrossRef]
- Cailleret, M.; Amadou, A.; Andrieu-Abadie, N.; Nawrocki, A.; Adamy, C.; Ait-Mamar, B.; Rocaries, F.; Best-Belpomme, M.; Levade, T.; Pavoine, C.; et al. N-acetylcysteine prevents the deleterious effect of tumor necrosis factor-(alpha) on calcium transients and contraction in adult rat cardiomyocytes. Circulation 2004, 109, 406–411. [Google Scholar] [CrossRef]
- Zhang, D.X.; Gutterman, D.D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2023–H2031. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Asanuma, K.; Godin, D.; Meng, X. N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage-derived foam cells: New target for antioxidant therapy? Circulation 1998, 97, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.P.; Yin, C.S.; Cui, J.H.; Li, Z.X.; Wang, L.; Wang, Y.W.; Li, Y.L. Inhibitory effect of N-acetylcysteine upon atherosclerotic processes in rabbit carotid. Zhonghua Yi Xue Za Zhi 2009, 89, 1850–1853. [Google Scholar] [PubMed]
- Sung, H.J.; Kim, J.; Kim, Y.; Jang, S.W.; Ko, J. N-acetyl cysteine suppresses the foam cell formation that is induced by oxidized low density lipoprotein via regulation of gene expression. Mol. Biol. Rep. 2012, 39, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, O.; Szumilak, D.; Nguyen-Khoa, T.; Ruellan, N.; Phan, O.; Lacour, B.; Descamps-Latscha, B.; Drueke, T.B.; Massy, Z.A. The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int. 2005, 67, 2288–2294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimada, K.; Murayama, T.; Yokode, M.; Kita, T.; Uzui, H.; Ueda, T.; Lee, J.D.; Kishimoto, C. N-acetylcysteine reduces the severity of atherosclerosis in apolipoprotein E-deficient mice by reducing superoxide production. Circ. J. 2009, 73, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liu, L.; Zhou, S.; Zhu, M.; Wang, B. Nacetylcysteine inhibits atherosclerosis by correcting glutathionedependent methylglyoxal elimination and dicarbonyl/oxidative stress in the aorta of diabetic mice. Mol. Med. Rep. 2021, 23, 201. [Google Scholar] [CrossRef]
- Zhu, Q.; Xiao, Y.; Jiang, M.; Liu, X.; Cui, Y.; Hao, H.; Flaker, G.C.; Liu, Q.; Zhou, S.; Liu, Z. N-acetylcysteine attenuates atherosclerosis progression in aging LDL receptor deficient mice with preserved M2 macrophages and increased CD146. Atherosclerosis 2022, 357, 41–50. [Google Scholar] [CrossRef]
- Cui, Y.; Narasimhulu, C.A.; Liu, L.; Zhang, Q.; Liu, P.Z.; Li, X.; Xiao, Y.; Zhang, J.; Hao, H.; Xie, X.; et al. N-acetylcysteine inhibits in vivo oxidation of native low-density lipoprotein. Sci. Rep. 2015, 5, 16339. [Google Scholar] [CrossRef]
- Wagberg, M.; Jansson, A.H.; Westerlund, C.; Ostlund-Lindqvist, A.M.; Sarnstrand, B.; Bergstrand, H.; Pettersson, K. N,N′-diacetyl-L-cystine (DiNAC), the disulphide dimer of N-acetylcysteine, inhibits atherosclerosis in WHHL rabbits: Evidence for immunomodulatory agents as a new approach to prevent atherosclerosis. J. Pharmacol. Exp. Ther. 2001, 299, 76–82. [Google Scholar] [PubMed]
- Krieger, M.H.; Santos, K.F.; Shishido, S.M.; Wanschel, A.C.; Estrela, H.F.; Santos, L.; De Oliveira, M.G.; Franchini, K.G.; Spadari-Bratfisch, R.C.; Laurindo, F.R. Antiatherogenic effects of S-nitroso-N-acetylcysteine in hypercholesterolemic LDL receptor knockout mice. Nitric Oxide Biol. Chem. 2006, 14, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Spartalis, M.; Siasos, G.; Mastrogeorgiou, M.; Spartalis, E.; Kaminiotis, V.V.; Mylonas, K.S.; Kapelouzou, A.; Kontogiannis, C.; Doulamis, I.P.; Toutouzas, K.; et al. The effect of per os colchicine administration in combination with fenofibrate and N-acetylcysteine on triglyceride levels and the development of atherosclerotic lesions in cholesterol-fed rabbits. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7765–7776. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Parthasarathy, S.; Hao, H.; Luo, M.; Ahmed, S.; Zhu, J.; Luo, S.; Kuppusamy, P.; Sen, C.K.; Verfaillie, C.M.; et al. Reactive oxygen species mediate oxidized low-density lipoprotein-induced inhibition of oct-4 expression and endothelial differentiation of bone marrow stem cells. Antioxid. Redox Signal. 2010, 13, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, L.; Si, Z.; Bu, H.; Narasimhulu, C.A.; Song, X.; Cui, M.Y.; Liu, H.; Lu, T.; He, G.; et al. Probucol Protects Endothelial Progenitor Cells Against Oxidized Low-Density Lipoprotein via Suppression of Reactive Oxygen Species Formation In Vivo. Cell. Physiol. Biochem. 2016, 39, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Rattan, A.K.; Arad, Y. Temporal and kinetic determinants of the inhibition of LDL oxidation by N-acetylcysteine (NAC). Atherosclerosis 1998, 138, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Libby, P.; Galis, Z.S. Cytokines regulate genes involved in atherogenesis. Ann. N. Y. Acad. Sci. 1995, 748, 158–168; discussion 168–170. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kuhn, H.; Hennig, B.; Neish, A.S.; Toborek, M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J. Mol. Cell. Cardiol. 2001, 33, 83–94. [Google Scholar] [CrossRef]
- Jain, S.K.; Kannan, K.; Lim, G.; Matthews-Greer, J.; McVie, R.; Bocchini, J.A., Jr. Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care 2003, 26, 2139–2143. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, K.; Iwase, M.; Ohdo, S.; Ieiri, I.; Matsuyama, N.; Takata, Y.; Kitazono, T. Telmisartan and N-acetylcysteine suppress group V secretory phospholipase A2 expression in TNFalpha-stimulated human endothelial cells and reduce associated atherogenicity. J. Cardiovasc. Pharmacol. 2012, 60, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Takahata, H.; Kitagawa, N.; Kitange, G.; Kaminogo, M.; Shibata, S. N-acetylcysteine inhibited nuclear factor-kappaB expression and the intimal hyperplasia in rat carotid arterial injury. Neurol. Res. 2001, 23, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.M.; Ward, L.J.; Forssell, C.; Siraj, N.; Li, W. Carotid Atheroma From Men Has Significantly Higher Levels of Inflammation and Iron Metabolism Enabled by Macrophages. Stroke 2018, 49, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Li, Q.; Evcimen, N.D.; Rask-Madsen, C.; Maeda, Y.; Maddaloni, E.; Yokomizo, H.; Shinjo, T.; St-Louis, R.; Fu, J.; et al. Exogenous Insulin Infusion Can Decrease Atherosclerosis in Diabetic Rodents by Improving Lipids, Inflammation, and Endothelial Function. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.L.; Dunning, J.; Kok, W.L.; Benam, K.H.; Benlahrech, A.; Repapi, E.; Martinez, F.O.; Drumright, L.; Powell, T.J.; Bennett, M.; et al. M1-like monocytes are a major immunological determinant of severity in previously healthy adults with life-threatening influenza. JCI Insight. 2017, 2, e91868. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Zernecke, A. Macrophages in vascular inflammation and atherosclerosis. Pflug. Arch. 2017, 469, 485–499. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Marchetti, G.; Lodola, E.; Licciardello, L.; Colombo, A. Use of N-acetylcysteine in the management of coronary artery diseases. Cardiologia 1999, 44, 633–637. [Google Scholar]
- Talasaz, A.H.; Khalili, H.; Fahimi, F.; Mojtaba, S. Potential role of N-acetylcysteine in cardiovascular disorders. Therapy 2011, 8, 237–245. [Google Scholar] [CrossRef]
- Gu, W.J.; Wu, Z.J.; Wang, P.F.; Aung, L.H.; Yin, R.X. N-Acetylcysteine supplementation for the prevention of atrial fibrillation after cardiac surgery: A meta-analysis of eight randomized controlled trials. BMC Cardiovasc. Disord. 2012, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.J.; Nguyen, T.H.; Stansborough, J.; Surikow, S.; Mahadavan, G.; Worthley, M.; Horowitz, J. The N-AcetylCysteine and RAMipril in Takotsubo Syndrome Trial (NACRAM): Rationale and design of a randomised controlled trial of sequential N-Acetylcysteine and ramipril for the management of Takotsubo Syndrome. Contemp. Clin. Trials 2020, 90, 105894. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, T.E.; Santos, M.V.; Lima, A.; Maia, A.L.; Wajner, S.M. N-Acetylcysteine Prevents Low T3 Syndrome and Attenuates Cardiac Dysfunction in a Male Rat Model of Myocardial Infarction. Endocrinology 2017, 158, 1502–1510. [Google Scholar] [CrossRef][Green Version]
- Senturk, T.; Cavun, S.; Avci, B.; Yermezler, A.; Serdar, Z.; Savci, V. Effective inhibition of cardiomyocyte apoptosis through the combination of trimetazidine and N-acetylcysteine in a rat model of myocardial ischemia and reperfusion injury. Atherosclerosis 2014, 237, 760–766. [Google Scholar] [CrossRef]
- Meyer, M.; Bell, S.P.; Chen, Z.; Nyotowidjojo, I.; Lachapelle, R.R.; Christian, T.F.; Gibson, P.C.; Keating, F.F.; Dauerman, H.L.; LeWinter, M.M. High dose intracoronary N-acetylcysteine in a porcine model of ST-elevation myocardial infarction. J. Thromb. Thrombolysis 2013, 36, 433–441. [Google Scholar] [CrossRef]
- Lu, H.; Daugherty, A. Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 485–491. [Google Scholar] [CrossRef]
- Calzadilla, P.; Gomez-Serrano, M.; Garcia-Santos, E.; Schiappacasse, A.; Abalde, Y.; Calvo, J.C.; Peral, B.; Guerra, L.N. N-Acetylcysteine affects obesity-related protein expression in 3T3-L1 adipocytes. Redox Rep. 2013, 18, 210–218. [Google Scholar] [CrossRef]
- Pieralisi, A.; Martini, C.; Soto, D.; Vila, M.C.; Calvo, J.C.; Guerra, L.N. N-acetylcysteine inhibits lipid accumulation in mouse embryonic adipocytes. Redox Biol. 2016, 9, 39–44. [Google Scholar] [CrossRef]
- Scanu, A.M.; Fless, G.M. Lipoprotein(a). Heterogeneity and biological relevance. J. Clin. Investig. 1990, 85, 1709–1715. [Google Scholar] [CrossRef]
- Kroon, A.A.; Demacker, P.N.; Stalenhoef, A.F. N-acetylcysteine and serum concentrations of lipoprotein(a). J. Intern. Med. 1991, 230, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, O.; Fager, G.; Andersson, A.; Lundstam, U.; Masson, P.; Hultberg, B. N-acetylcysteine treatment lowers plasma homocysteine but not serum lipoprotein(a) levels. Atherosclerosis 1996, 119, 99–106. [Google Scholar] [CrossRef] [PubMed]
- McCully, K.S. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev. Clin. Pharmacol. 2015, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Bostom, A.G.; Shemin, D.; Yoburn, D.; Fisher, D.H.; Nadeau, M.R.; Selhub, J. Lack of effect of oral N-acetylcysteine on the acute dialysis-related lowering of total plasma homocysteine in hemodialysis patients. Atherosclerosis 1996, 120, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Ventura, P.; Panini, R.; Pasini, M.C.; Scarpetta, G.; Salvioli, G. N -Acetyl-cysteine reduces homocysteine plasma levels after single intravenous administration by increasing thiols urinary excretion. Pharmacol. Res. 1999, 40, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, W.; Sauer, R.; Bonaterra, G.; Dugi, K.A.; Edler, L.; Kinscherf, R. Oral N-acetylcysteine reduces plasma homocysteine concentrations regardless of lipid or smoking status. Am. J. Clin. Nutr. 2015, 102, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Miner, S.E.; Cole, D.E.; Evrovski, J.; Forrest, Q.; Hutchison, S.J.; Holmes, K.; Ross, H.J. N-acetylcysteine neither lowers plasma homocysteine concentrations nor improves brachial artery endothelial function in cardiac transplant recipients. Can. J. Cardiol. 2002, 18, 503–507. [Google Scholar]
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Toborek, M.; Barger, S.W.; Mattson, M.P.; McClain, C.J.; Hennig, B. Role of glutathione redox cycle in TNF-alpha-mediated endothelial cell dysfunction. Atherosclerosis 1995, 117, 179–188. [Google Scholar] [CrossRef]
- Yang, W.S.; Lee, J.M.; Han, N.J.; Kim, Y.J.; Chang, J.W.; Park, S.K. Mycophenolic acid attenuates tumor necrosis factor-alpha-induced endothelin-1 production in human aortic endothelial cells. Atherosclerosis 2010, 211, 48–54. [Google Scholar] [CrossRef]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Mamarbachi, A.M.; Villeneuve, L.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Chronic treatment with N-acetyl-cystein delays cellular senescence in endothelial cells isolated from a subgroup of atherosclerotic patients. Mech. Ageing Dev. 2008, 129, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Creager, M.A.; Roddy, M.A.; Boles, K.; Stamler, J.S. N-acetylcysteine does not influence the activity of endothelium-derived relaxing factor in vivo. Hypertension 1997, 29, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.P.; Prasad, A.; Quyyumi, A.A. N-acetylcysteine improves coronary and peripheral vascular function. J. Am. Coll. Cardiol. 2001, 37, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Pedre, B.; Barayeu, U.; Ezerina, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H(2)S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef] [PubMed]
- Tieu, S.; Charchoglyan, A.; Paulsen, L.; Wagter-Lesperance, L.C.; Shandilya, U.K.; Bridle, B.W.; Mallard, B.A.; Karrow, N.A. N-Acetylcysteine and Its Immunomodulatory Properties in Humans and Domesticated Animals. Antioxidants 2023, 12, 1867. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Yu, Z.; Taniguchi, M.; Picotin, R.; Oyama, N.; Stellwagen, D.; Ono, C.; Kikuchi, Y.; Matsui, K.; Nakanishi, M.; et al. N-Acetylcysteine Suppresses Microglial Inflammation and Induces Mortality Dose-Dependently via Tumor Necrosis Factor-alpha Signaling. Int. J. Mol. Sci. 2023, 24, 3798. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Sun, Q.; Liu, Z. Ambient particulate matter exposure and cardiovascular diseases: A focus on progenitor and stem cells. J. Cell. Mol. Med. 2016, 20, 782–793. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, A.; Jin, X.; Natanzon, A.; Duquaine, D.; Brook, R.D.; Aguinaldo, J.G.; Fayad, Z.A.; Fuster, V.; Lippmann, M.; et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005, 294, 3003–3010. [Google Scholar] [CrossRef]
- Tzeng, H.P.; Yang, R.S.; Ueng, T.H.; Liu, S.H. Upregulation of cyclooxygenase-2 by motorcycle exhaust particulate-induced reactive oxygen species enhances rat vascular smooth muscle cell proliferation. Chem. Res. Toxicol. 2007, 20, 1170–1176. [Google Scholar] [CrossRef]
- Du, Y.; Navab, M.; Shen, M.; Hill, J.; Pakbin, P.; Sioutas, C.; Hsiai, T.K.; Li, R. Ambient ultrafine particles reduce endothelial nitric oxide production via S-glutathionylation of eNOS. Biochem. Biophys. Res. Commun. 2013, 436, 462–466. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, Y.; Zhu, Q.; Cui, Y.; Hao, H.; Wang, M.; Cowan, P.J.; Korthuis, R.J.; Li, G.; Sun, Q.; et al. Circulating Endothelial Progenitor Cells Are Preserved in Female Mice Exposed to Ambient Fine Particulate Matter Independent of Estrogen. Int. J. Mol. Sci. 2021, 22, 7200. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jia, F.; He, J.; Xie, X.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, D.Z.; Cowan, P.J.; et al. Ambient Fine Particulate Matter Suppresses In Vivo Proliferation of Bone Marrow Stem Cells through Reactive Oxygen Species Formation. PLoS ONE 2015, 10, e0127309. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xie, X.; Jia, F.; He, J.; Li, Z.; Fu, M.; Hao, H.; Liu, Y.; Liu, J.Z.; Cowan, P.J.; et al. Ambient fine particulate matter induces apoptosis of endothelial progenitor cells through reactive oxygen species formation. Cell. Physiol. Biochem. 2015, 35, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Lizarrondo, S.; Gakuba, C.; Herbig, B.A.; Repesse, Y.; Ali, C.; Denis, C.V.; Lenting, P.J.; Touze, E.; Diamond, S.L.; Vivien, D.; et al. Potent Thrombolytic Effect of N-Acetylcysteine on Arterial Thrombi. Circulation 2017, 136, 646–660. [Google Scholar] [CrossRef]
- Chatziathanasiou, G.N.; Nikas, D.N.; Katsouras, C.S.; Kazakos, N.D.; Bouba, V.; Vougiouklakis, T.; Naka, K.K.; Michalis, L.K. Combined intravenous treatment with ascorbic acid and desferrioxamine to reduce myocardial reperfusion injury in an experimental model resembling the clinical setting of primary PCI. Hell. J. Cardiol. 2012, 53, 195–204. [Google Scholar]
- Parra-Flores, P.; Riquelme, J.A.; Valenzuela-Bustamante, P.; Leiva-Navarrete, S.; Vivar, R.; Cayupi-Vivanco, J.; Castro, E.; Espinoza-Perez, C.; Ruz-Cortes, F.; Pedrozo, Z.; et al. The Association of Ascorbic Acid, Deferoxamine and N-Acetylcysteine Improves Cardiac Fibroblast Viability and Cellular Function Associated with Tissue Repair Damaged by Simulated Ischemia/Reperfusion. Antioxidants 2019, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Mushtaq, I.; Maryam, S.; Farhan, A.; Saba, K.; Jan, M.I.; Sultan, A.; Anees, M.; Duygu, B.; Hamera, S.; et al. Interplay of N acetyl cysteine and melatonin in regulating oxidative stress-induced cardiac hypertrophic factors and microRNAs. Arch. Biochem. Biophys. 2019, 661, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, K.; Bergstrand, H. The antiatherogenic effect of DiNAC: Experimental findings supporting immunomodulation as a new treatment for atherosclerosis related diseases. Cardiovasc. Drug Rev. 2003, 21, 119–132. [Google Scholar] [CrossRef]
- Doggrell, S.A. Immunomodulation with DiNAC—A new approach to the treatment of atherosclerosis? Expert Opin. Investig. Drugs 2002, 11, 717–720. [Google Scholar]
- Pettersson, K.S.; Eliasson, U.B.; Abrahamsson, T.; Wagberg, M.; Carrier, M.; Kengatharan, K.M. N,N-diacetyl-L-cystine improves endothelial function in atherosclerotic Watanabe heritable hyperlipidaemic rabbits. Basic Clin. Pharmacol. Toxicol. 2007, 100, 36–42. [Google Scholar] [CrossRef]
- Pettersson, K.; Kjerrulf, M.; Jungersten, L.; Johansson, K.; Langstrom, G.; Kalies, I.; Lenkei, R.; Walldius, G.; Lind, L. The new oral immunomodulating drug DiNAC induces brachial artery vasodilatation at rest and during hyperemia in hypercholesterolemic subjects, likely by a nitric oxide-dependent mechanism. Atherosclerosis 2008, 196, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Mazo, D.F.; de Oliveira, M.G.; Pereira, I.V.; Cogliati, B.; Stefano, J.T.; de Souza, G.F.; Rabelo, F.; Lima, F.R.; Ferreira Alves, V.A.; Carrilho, F.J.; et al. S-nitroso-N-acetylcysteine attenuates liver fibrosis in experimental nonalcoholic steatohepatitis. Drug Des. Dev. Ther. 2013, 7, 553–563. [Google Scholar] [CrossRef]
- Salamon, S.; Kramar, B.; Marolt, T.P.; Poljsak, B.; Milisav, I. Medical and Dietary Uses of N-Acetylcysteine. Antioxidants 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Di Stefano, A.F.D.; Radicioni, M. Pharmacokinetics and Safety of Single and Multiple Doses of Oral N-Acetylcysteine in Healthy Chinese and Caucasian Volunteers: An Open-Label, Phase I Clinical Study. Adv. Ther. 2021, 38, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Deepmala; Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar] [CrossRef] [PubMed]
- Renke, M.; Tylicki, L.; Rutkowski, P.; Larczynski, W.; Neuwelt, A.; Aleksandrowicz, E.; Lysiak-Szydlowska, W.; Rutkowski, B. The effect of N-acetylcysteine on blood pressure and markers of cardiovascular risk in non-diabetic patients with chronic kidney disease: A placebo-controlled, randomized, cross-over study. Med. Sci. Monit. 2010, 16, PI13–PI18. [Google Scholar] [PubMed]
- Mahmoudi, G.A.; Astaraki, P.; Mohtashami, A.Z.; Ahadi, M. N-acetylcysteine overdose after acetaminophen poisoning. Int. Med. Case Rep. J. 2015, 8, 65–69. [Google Scholar] [CrossRef]
- Steinhubl, S.R. Why have antioxidants failed in clinical trials? Am. J. Cardiol. 2008, 101, 14D–19D. [Google Scholar] [CrossRef]
- De Rosa, S.; Cirillo, P.; Paglia, A.; Sasso, L.; Di Palma, V.; Chiariello, M. Reactive oxygen species and antioxidants in the pathophysiology of cardiovascular disease: Does the actual knowledge justify a clinical approach? Curr. Vasc. Pharmacol. 2010, 8, 259–275. [Google Scholar] [CrossRef]
- Kadri, A.; Sjahrir, H.; Juwita Sembiring, R.; Ichwan, M. Combination of vitamin A and D supplementation for ischemic stroke: Effects on interleukin-1ss and clinical outcome. Med. Glas. 2020, 17, 425–432. [Google Scholar] [CrossRef]
- Mottaghi, A.; Ebrahimof, S.; Angoorani, P.; Saboor-Yaraghi, A.A. Vitamin A supplementation reduces IL-17 and RORc gene expression in atherosclerotic patients. Scand. J. Immunol. 2014, 80, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mottaghi, A.; Salehi, E.; Keshvarz, A.; Sezavar, H.; Saboor-Yaraghi, A.A. The influence of vitamin A supplementation on Foxp3 and TGF-beta gene expression in atherosclerotic patients. J. Nutr. Nutr. 2012, 5, 314–326. [Google Scholar] [CrossRef]
- Redlich, C.A.; Chung, J.S.; Cullen, M.R.; Blaner, W.S.; Van Bennekum, A.M.; Berglund, L. Effect of long-term beta-carotene and vitamin A on serum cholesterol and triglyceride levels among participants in the Carotene and Retinol Efficacy Trial (CARET). Atherosclerosis 1999, 143, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Baldi, S.; Innocenti, M.; Frascerra, S.; Nannipieri, M.; Lippi, A.; Rindi, P.; Ferrannini, E. Effects of hemodialysis and vitamin E supplementation on low-density lipoprotein oxidizability in end-stage renal failure. J. Nephrol. 2013, 26, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Daud, Z.A.; Tubie, B.; Sheyman, M.; Osia, R.; Adams, J.; Tubie, S.; Khosla, P. Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vasc. Health Risk Manag. 2013, 9, 747–761. [Google Scholar] [CrossRef] [PubMed]
- Corina, A.; Rangel-Zuniga, O.A.; Jimenez-Lucena, R.; Alcala-Diaz, J.F.; Quintana-Navarro, G.; Yubero-Serrano, E.M.; Lopez-Moreno, J.; Delgado-Lista, J.; Tinahones, F.; Ordovas, J.M.; et al. Low Intake of Vitamin E Accelerates Cellular Aging in Patients With Established Cardiovascular Disease: The CORDIOPREV Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E.; Atzler, D.; Doring, Y.; Duchene, J.; Steffens, S.; Weber, C. Immunotherapy for cardiovascular disease. Eur. Heart J. 2019, 40, 3937–3946. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Schwarz, L.; Luscher, T.F.; Camici, G.G. Inflammation and cardiovascular diseases: Lessons from seminal clinical trials. Cardiovasc. Res. 2021, 117, 411–422. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Orellana-Urzua, S.; Briones-Valdivieso, C.; Chichiarelli, S.; Saso, L.; Rodrigo, R. Potential Role of Natural Antioxidants in Countering Reperfusion Injury in Acute Myocardial Infarction and Ischemic Stroke. Antioxidants 2023, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Badacz, R.; Przewlocki, T.; Legutko, J.; Zmudka, K.; Kablak-Ziembicka, A. microRNAs Associated with Carotid Plaque Development and Vulnerability: The Clinician’s Perspective. Int. J. Mol. Sci. 2022, 23, 5645. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Aminizadeh, N.; Bodhale, N.; Sarkar, A.; Jafarzadeh, S.; Sharifi, I.; Saha, B. Bidirectional cytokine-microRNA control: A novel immunoregulatory framework in leishmaniasis. PLoS Pathog. 2022, 18, e1010696. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. The Interplay among miRNAs, Major Cytokines, and Cancer-Related Inflammation. Mol. Ther. Nucleic Acids 2020, 20, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Albeltagy, R.S.; Mumtaz, F.; Abdel Moneim, A.E.; El-Habit, O.H. N-Acetylcysteine Reduces miR-146a and NF-kappaB p65 Inflammatory Signaling Following Cadmium Hepatotoxicity in Rats. Biol. Trace Elem. Res. 2021, 199, 4657–4665. [Google Scholar] [CrossRef] [PubMed]
- Mathe, E.; Nguyen, G.H.; Funamizu, N.; He, P.; Moake, M.; Croce, C.M.; Hussain, S.P. Inflammation regulates microRNA expression in cooperation with p53 and nitric oxide. Int. J. Cancer 2012, 131, 760–765. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).