Metabolipidomic Analysis in Patients with Obstructive Sleep Apnea Discloses a Circulating Metabotype of Non-Dipping Blood Pressure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.1.1. Study Design
2.1.2. Baseline Clinical Evaluation
2.1.3. Polysomnography for OSA Diagnosis
2.1.4. Ambulatory BP Assessment
2.1.5. Sample Collection
2.1.6. Post-Treatment Evaluation
2.2. Metabolomic and Lipidomic Profiling
2.2.1. Metabolite/Lipid Isolation and Untargeted Analysis
2.2.2. Feature Identification and Pathway Enrichment Analysis
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Groups at Baseline
3.2. Untargeted Analysis of the Circulating Metabolipidome
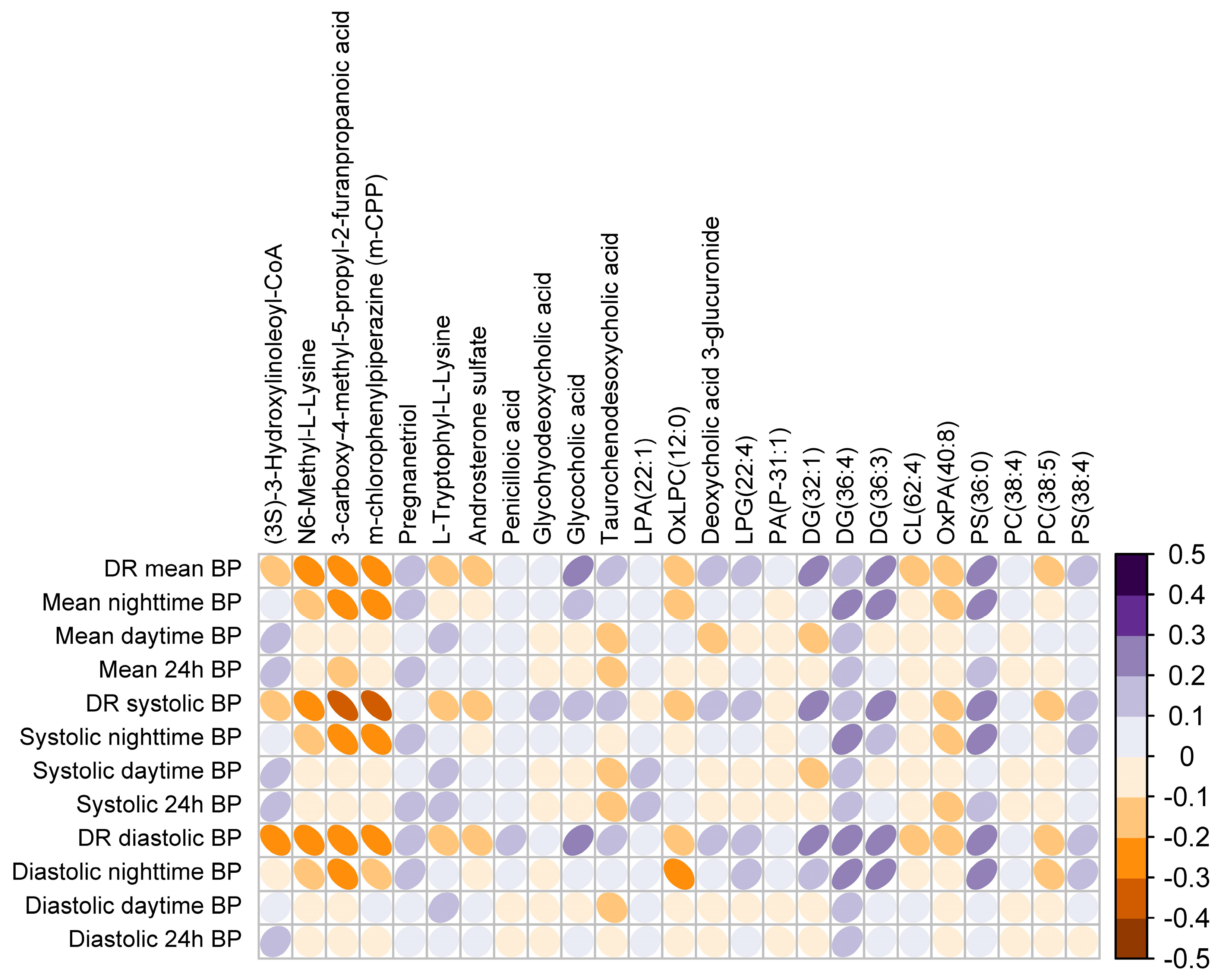
3.3. Plasma Metabotype Associated with Impaired BP Dipping in OSA
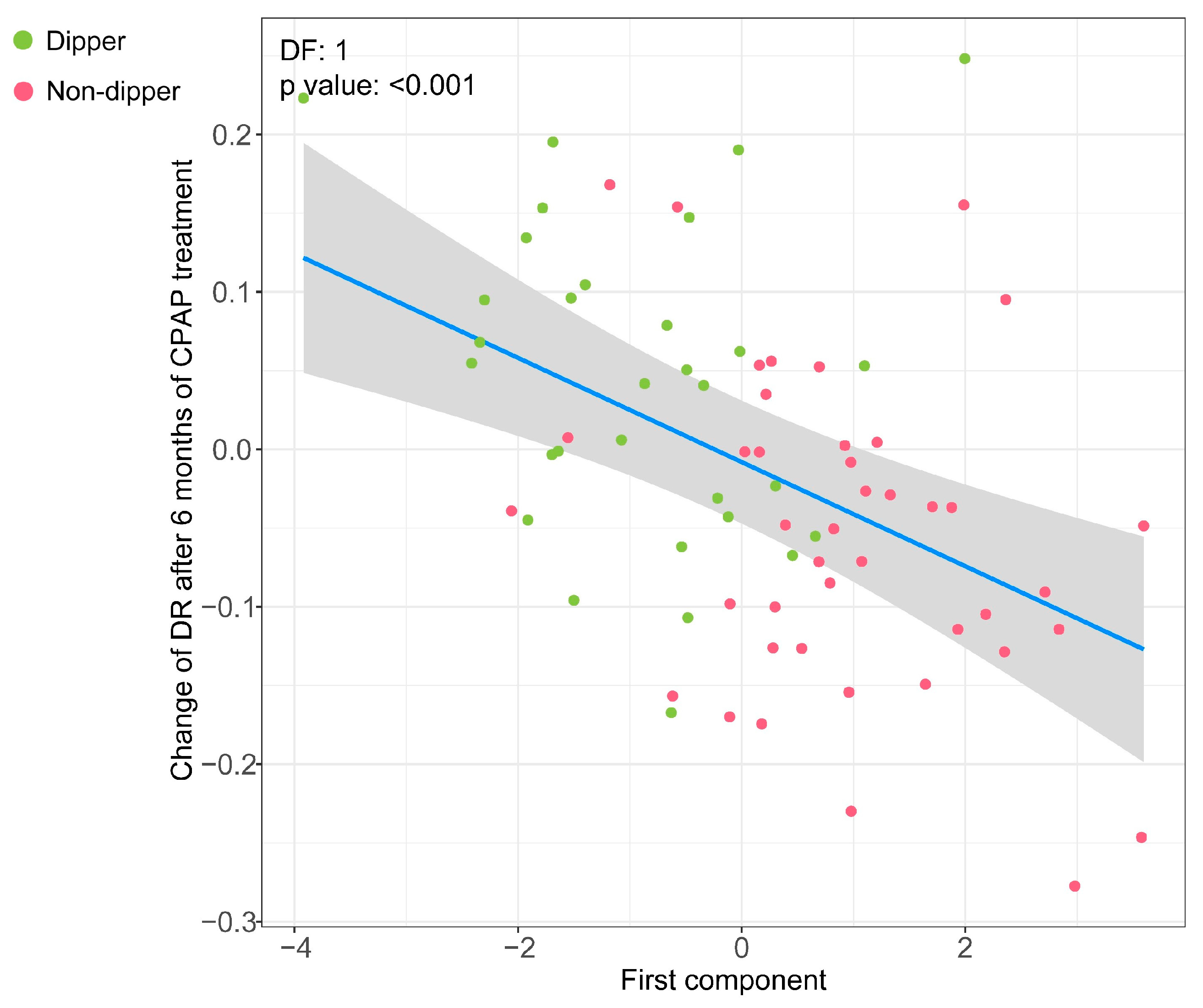
3.4. Association of the Metabolipidomic Fingerprint with Changes in the DR after OSA Treatment with CPAP
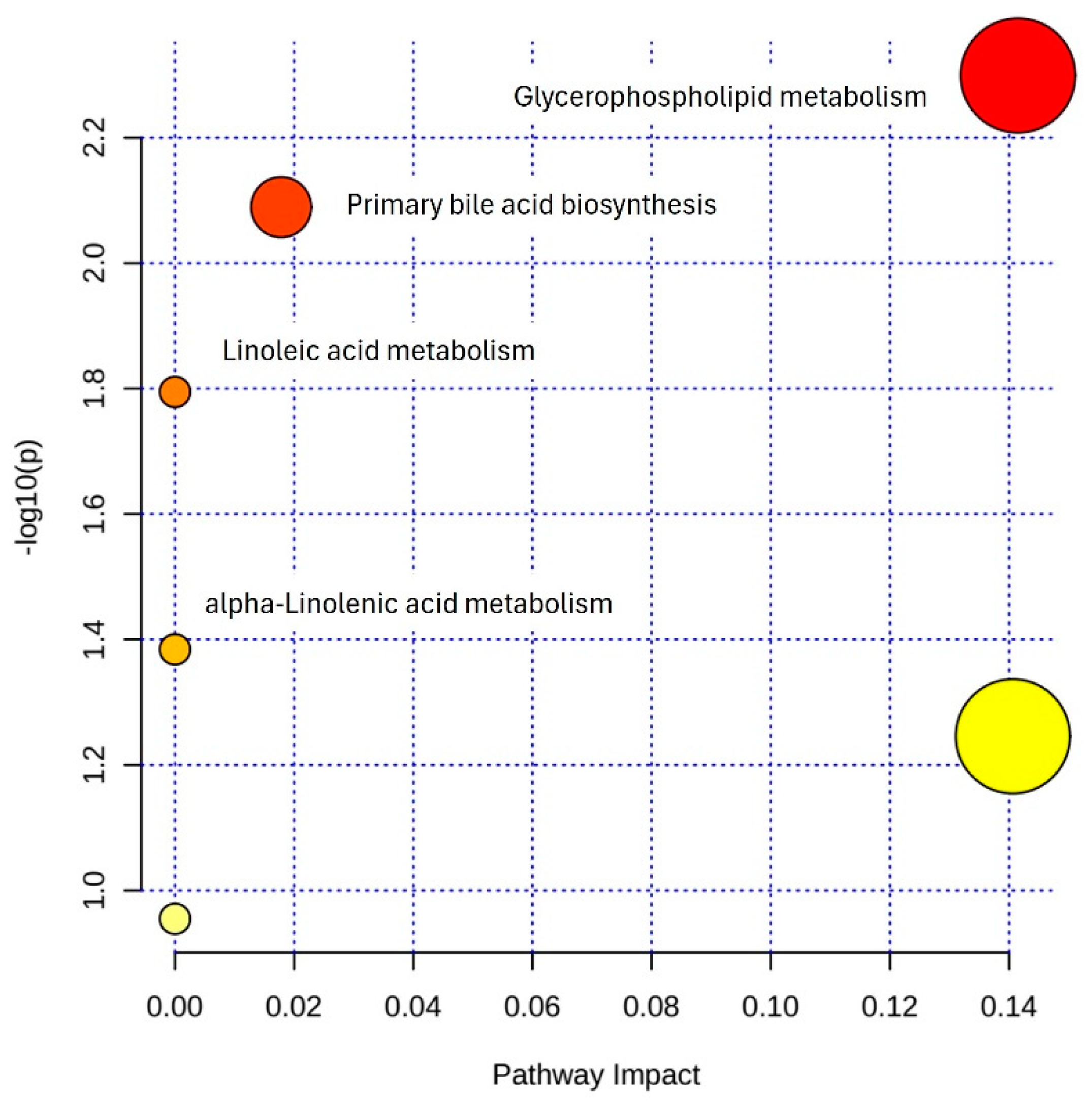
3.5. Pathway Enrichment Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.D.; et al. Estimation of the Global Prevalence and Burden of Obstructive Sleep Apnoea: A Literature-Based Analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Lévy, P.; Kohler, M.; McNicholas, W.T.; Barbé, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pépin, J.L. Obstructive Sleep Apnoea Syndrome. Nat. Rev. Dis. Prim. 2015, 1, 15015. [Google Scholar] [CrossRef]
- Jelic, S.; Le Jemtel, T.H. Inflammation, Oxidative Stress, and the Vascular Endothelium in Obstructive Sleep Apnea. Trends Cardiovasc. Med. 2008, 18, 253–260. [Google Scholar] [CrossRef]
- Lavie, L. Obstructive Sleep Apnoea Syndrome—An Oxidative Stress Disorder. Sleep Med. Rev. 2003, 7, 35–51. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Peres, B.U.; Allen, A.H.; Shah, A.; Fox, N.; Laher, I.; Almeida, F.; Jen, R.; Ayas, N. Obstructive Sleep Apnea and Circulating Biomarkers of Oxidative Stress: A Cross-Sectional Study. Antioxidants 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Tilkian, A.G.; Guilleminault, C.; Schroeder, J.S.; Lehrman, K.L.; Simmons, F.B.; Dement, W.C. Hemodynamics in Sleep Induced Apnea. Studies during Wakefulness and Sleep. Ann. Intern. Med. 1976, 85, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Alcántara, C.; Williams, N.; Bello, N.A.; Abdalla, M. Effect of Sleep Disturbances on Blood Pressure. Hypertension 2021, 77, 1036–1046. [Google Scholar] [CrossRef]
- O’Brien, E.; Sheridan, J.; O’Malley, K. Dippers and non-dippers. Lancet 1988, 332, 397. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, I.Z.; Kark, J.D.; Ben-Ishay, D.; Mekler, J.; Ben-Arie, L.; Bursztyn, M. Predictors of All-Cause Mortality in Clinical Ambulatory Monitoring: Unique Aspects of Blood Pressure during Sleep. Hypertension 2007, 49, 1235–1241. [Google Scholar] [CrossRef]
- Fagard, R.H. Dipping Pattern of Nocturnal Blood Pressure in Patients with Hypertension. Expert Rev. Cardiovasc. Ther. 2009, 7, 599–605. [Google Scholar] [CrossRef]
- Zweiker, R.; Eber, B.; Schumacher, M.; Toplak, H.; Klein, W. “Non-Dipping” Related to Cardiovascular Events in Essential Hypertensive Patients. Acta Med. Austriaca 1994, 21, 86—89. [Google Scholar]
- Verdecchia, P.; Porcellati, C.; Schillaci, G.; Borgioni, C.; Ciucci, A.; Battistelli, M.; Guerrieri, M.; Gatteschi, C.; Zampi, I.; Santucci, A.; et al. Ambulatory Blood Pressure. An Independent Predictor of Prognosis in Essential Hypertension. Hypertension 1994, 24, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Akiguchi, I.; Oiwa, K.; Hayashi, M.; Kimura, J. Adverse Effect of Nighttime Blood Pressure on the Outcome of Lacunar Infarct Patients. Stroke 1998, 29, 570–576. [Google Scholar] [CrossRef]
- Hansen, T.W.; Li, Y.; Boggia, J.; Thijs, L.; Richart, T.; Staessen, J.A. Predictive Role of the Nighttime Blood Pressure. Hypertension 2011, 57, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.F.; Reboldi, G.; Fagard, R.H.; Cardoso, C.R.L.; Pierdomenico, S.D.; Verdecchia, P.; Eguchi, K.; Kario, K.; Hoshide, S.; Polonia, J.; et al. Prognostic Effect of the Nocturnal Blood Pressure Fall in Hypertensive Patients: The Ambulatory Blood Pressure Collaboration in Patients with Hypertension (ABC-H) Meta-Analysis. Hypertension 2016, 67, 693–700. [Google Scholar] [CrossRef]
- Verdecchia, P.; Angeli, F.; Mazzotta, G.; Garofoli, M.; Ramundo, E.; Gentile, G.; Ambrosio, G.; Reboldi, G. Day-Night Dip and Early-Morning Surge in Blood Pressure in Hypertension: Prognostic Implications. Hypertension 2012, 60, 34–42. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Cardoso, C.R.L.; Salles, G.F. Prognostic Value of Nocturnal Blood Pressure Reduction in Resistant Hypertension. Arch. Intern. Med. 2009, 169, 874–880. [Google Scholar] [CrossRef]
- Staplin, N.; de la Sierra, A.; Ruilope, L.M.; Emberson, J.R.; Vinyoles, E.; Gorostidi, M.; Ruiz-Hurtado, G.; Segura, J.; Baigent, C.; Williams, B. Relationship between Clinic and Ambulatory Blood Pressure and Mortality: An Observational Cohort Study in 59 124 Patients. Lancet 2023, 401, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Tadic, M.; Sala, C.; Gherbesi, E.; Grassi, G.; Mancia, G. Blood Pressure Non-Dipping and Obstructive Sleep Apnea Syndrome: A Meta-Analysis. J. Clin. Med. 2019, 8, 1367. [Google Scholar] [CrossRef]
- Hla, K.M.; Young, T.; Finn, L.; Peppard, P.E.; Szklo-Coxe, M.; Stubbs, M. Longitudinal Association of Sleep-Disordered Breathing and Nondipping of Nocturnal Blood Pressure in the Wisconsin Sleep Cohort Study. Sleep 2008, 31, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Ozono, R.; Edahiro, Y.; Ishii, K.; Seto, A.; Okita, T.; Teramen, K.; Fujiwara, S.; Kihara, Y. Impact of Non-Dipping on Cardiovascular Outcomes in Patients with Obstructive Sleep Apnea Syndrome. Clin. Exp. Hypertens. 2015, 37, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Crinion, S.J.; Ryan, S.; McNicholas, W.T. Obstructive Sleep Apnoea as a Cause of Nocturnal Nondipping Blood Pressure: Recent Evidence Regarding Clinical Importance and Underlying Mechanisms. Eur. Respir. J. 2017, 49, 1601818. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. “Metabonomics”: Understanding the Metabolic Responses of Living Systems to Pathophysiological Stimuli via Multivariate Statistical Analysis of Biological NMR Spectroscopic Data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Metabolomics: The Apogee of the Omics Trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Wilson, I.D.; Nicholson, J.K. Metabolic Phenotyping in Health and Disease. Cell 2008, 134, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Nordström, A.; Morita, H.; Siuzdak, G. From Exogenous to Endogenous: The Inevitable Imprint of Mass Spectrometry in Metabolomics. J. Proteome Res. 2007, 6, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics Activity Screening for Identifying Metabolites That Modulate Phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Ramautar, R.; Berger, R.; van der Greef, J.; Hankemeier, T. Human Metabolomics: Strategies to Understand Biology. Curr. Opin. Chem. Biol. 2013, 17, 841–846. [Google Scholar] [CrossRef]
- Johnson, C.H.; Ivanisevic, J.; Siuzdak, G. Metabolomics: Beyond Biomarkers and towards Mechanisms. Nat. Rev. Mol. Cell Biol. 2016, 17, 451–459. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Lloberes, P.; Durán-Cantolla, J.; Martínez-García, M.Á.; Marín, J.M.; Ferrer, A.; Corral, J.; Masa, J.F.; Parra, O.; Alonso-Álvarez, M.L.; Terán-Santos, J. Diagnóstico y Tratamiento Del Síndrome de Apneas-Hipopneas Del Sueño. Arch. Bronconeumol. 2011, 47, 143–156. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, L.; Benítez, I.D.; Gracia-Lavedan, E.; Torres, G.; Minguez, O.; Aguilà, M.; Targa, A.; Dalmases, M.; Mediano, O.; Masa, J.F.; et al. Polysomnographic Characterization of Circadian Blood Pressure Patterns in Patients with Obstructive Sleep Apnea. Sleep 2023, 46, zsad031. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; Jordan, A.S.; Merchia, P.; Malhotra, A. Central Sleep Apnea: Pathophysiology and Treatment. Chest 2007, 131, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. Documento Internacional de Consenso Sobre Apnea Obstructiva Del Sueño. Arch. Bronconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.; O’Brien, E.; Asmar, R.; Beilin, L.; Bilo, G.; Clement, D.; De La Sierra, A.; De Leeuw, P.; Dolan, E.; et al. European Society of Hypertension Practice Guidelines for Ambulatory Blood Pressure Monitoring. J. Hypertens. 2014, 32, 1359–1366. [Google Scholar] [CrossRef]
- Jové, M.; Mauri-Capdevila, G.; Suárez, I.; Cambray, S.; Sanahuja, J.; Quílez, A.; Farré, J.; Benabdelhak, I.; Pamplona, R.; Portero-Otín, M.; et al. Metabolomics Predicts Stroke Recurrence after Transient Ischemic Attack. Neurology 2015, 84, 36–45. [Google Scholar] [CrossRef]
- Pizarro, C.; Arenzana-Rámila, I.; Pérez-Del-Notario, N.; Pérez-Matute, P.; González-Sáiz, J.M. Plasma Lipidomic Profiling Method Based on Ultrasound Extraction and Liquid Chromatography Mass Spectrometry. Anal. Chem. 2013, 85, 12085–12092. [Google Scholar] [CrossRef]
- Purroy, F.; Cambray, S.; Mauri-Capdevila, G.; Jové, M.; Sanahuja, J.; Farré, J.; Benabdelhak, I.; Molina-Seguin, J.; Colàs-Campàs, L.; Begue, R.; et al. Metabolomics Predicts Neuroimaging Characteristics of Transient Ischemic Attack Patients. EBioMedicine 2016, 14, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Dakterzada, F.; Benítez, I.D.; Targa, A.; Carnes, A.; Pujol, M.; Jové, M.; Mínguez, O.; Vaca, R.; Sánchez-de-la-Torre, M.; Barbé, F.; et al. Cerebrospinal Fluid Lipidomic Fingerprint of Obstructive Sleep Apnoea in Alzheimer’s Disease. Alzheimers. Res. Ther. 2023, 15, 134. [Google Scholar] [CrossRef]
- Pradas, I.; Huynh, K.; Cabré, R.; Ayala, V.; Meikle, P.J.; Jové, M.; Pamplona, R. Lipidomics Reveals a Tissue-Specific Fingerprint. Front. Physiol. 2018, 9, 387781. [Google Scholar] [CrossRef] [PubMed]
- Pradas, I.; Rovira-Llopis, S.; Naudí, A.; Bañuls, C.; Rocha, M.; Hernandez-Mijares, A.; Pamplona, R.; Victor, V.M.; Jové, M. Metformin Induces Lipid Changes on Sphingolipid Species and Oxidized Lipids in Polycystic Ovary Syndrome Women. Sci. Rep. 2019, 9, 16033. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global Metabolic Profiling of Animal and Human Tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-Mcintyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and Considerations for the Use of System Suitability and Quality Control Samples in Mass Spectrometry Assays Applied in Untargeted Clinical Metabolomic Studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.C.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed Minimum Reporting Standards for Chemical Analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinforma. 2019, 68, e86. [Google Scholar] [CrossRef]
- Pinilla, L.; Benítez, I.D.; Santamaria-Martos, F.; Targa, A.; Moncusí-Moix, A.; Dalmases, M.; Mínguez, O.; Aguilà, M.; Jové, M.; Sol, J.; et al. Plasma Profiling Reveals a Blood-Based Metabolic Fingerprint of Obstructive Sleep Apnea. Biomed. Pharmacother. 2022, 112425, 145. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- The R Foundation R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 19 September 2023).
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’Donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Claas, S.A. Omics of Blood Pressure and Hypertension. Circ. Res. 2018, 122, 1409–1419. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Graham, D.; Kastenmüller, G.; Alharbi, N.H.J.; Alsanosi, S.M.; Mcbride, M.; Mangino, M.; Titcombe, P.; Shin, S.Y.; Psatha, M.; et al. Metabolomic Identification of a Novel Pathway of Blood Pressure Regulation Involving Hexadecanedioate. Hypertension 2015, 66, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Au, A.; Cheng, K.-K.; Wei, L.K. Metabolomics, Lipidomics and Pharmacometabolomics of Human Hypertension. Adv. Exp. Med. Biol. 2017, 956, 599–613. [Google Scholar] [CrossRef]
- Ke, C.; Zhu, X.; Zhang, Y.; Shen, Y. Metabolomic Characterization of Hypertension and Dyslipidemia. Metabolomics 2018, 117, 14. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Deng, L.Y.; Larochelle, P. Effects of a β-Blocker or a Converting Enzyme Inhibitor on Resistance Arteries in Essential Hypertension. Hypertension 1994, 23, 83–91. [Google Scholar] [CrossRef]
- Inoguchi, T.; Li, P.; Umeda, F.; Yu, H.Y.; Kakimoto, M.; Imamura, M.; Aoki, T.; Etoh, T.; Hashimoto, T.; Naruse, M.; et al. High Glucose Level and Free Fatty Acid Stimulate Reactive Oxygen Species Production through Protein Kinase C--Dependent Activation of NAD(P)H Oxidase in Cultured Vascular Cells. Diabetes 2000, 49, 1939–1945. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Liu, J.; Zhang, W.; Zhang, X.; Zha, L.; Liu, W.; Ling, Y.; Li, S.; Tang, S. High Glucose Induced Endothelial Cell Reactive Oxygen Species via OGG1/PKC/NADPH Oxidase Pathway. Life Sci. 2020, 256, 117886. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, D.; Li, D.; Huang, J.; Ma, F.; Zhang, H.; Sheng, Y.; Zhang, C.; Ha, X. Protein Kinase C: A Potential Therapeutic Target for Endothelial Dysfunction in Diabetes. J. Diabetes Complications 2023, 37, 108565. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Sun, H.; Du, Y.; Li, L.; Lv, Q.; Yu, H.; Li, F.; Wang, Y.; Jiao, X.; Hu, C.; et al. Comprehensive Metabolomics and Machine Learning Identify Profound Oxidative Stress and Inflammation Signatures in Hypertensive Patients with Obstructive Sleep Apnea. Antioxidants 2022, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.; Meikle, P.J.; Mamtani, M.; Weir, J.M.; Barlow, C.K.; Jowett, J.B.; Bellis, C.; Dyer, T.D.; Johnson, M.P.; Rainwater, D.L.; et al. Plasma Lipidomic Profile Signature of Hypertension in Mexican American Families: Specific Role of Diacylglycerols. Hypertension 2013, 62, 621–626. [Google Scholar] [CrossRef]
- Weljie, A.M.; Meerlo, P.; Goel, N.; Sengupta, A.; Kayser, M.S.; Abel, T.; Birnbaum, M.J.; Dinges, D.F.; Sehgal, A. Oxalic Acid and Diacylglycerol 36:3 Are Cross-Species Markers of Sleep Debt. Proc. Natl. Acad. Sci. USA 2015, 112, 2569–2574. [Google Scholar] [CrossRef]
- Vance, D.E. Phospholipid Methylation in Mammals: From Biochemistry to Physiological Function. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. MAM (Mitochondria-Associated Membranes) in Mammalian Cells: Lipids and Beyond. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2014, 1841, 595–609. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, Z.-X.; Cai, J.; Li, R.; Deng, K.-Q.; Ji, Y.-X.; Lei, F.; Li, H.-P.; Lu, Z.; Li, H. Energy Substrate Metabolism and Oxidative Stress in Metabolic Cardiomyopathy. J. Mol. Med. 2022, 100, 1721–1739. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Das, U.N. Ageing: Is There a Role for Arachidonic Acid and Other Bioactive Lipids? A Review. J. Adv. Res. 2018, 11, 67–79. [Google Scholar] [CrossRef]
- Bercea, C.; Cottrell, G.S.; Tamagnini, F.; McNeish, A.J. Omega-3 Polyunsaturated Fatty Acids and Hypertension: A Review of Vasodilatory Mechanisms of Docosahexaenoic Acid and Eicosapentaenoic Acid. Br. J. Pharmacol. 2021, 178, 860–877. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging Roles of Lysophospholipids in Health and Disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Suzuki, H.; Ogata, Y.; Imafuku, T.; Saruta, T. Bile Acids Are Able to Reduce Blood Pressure by Attenuating the Vascular Reactivity in Spontaneously Hypertensive Rats. Life Sci. 1988, 42, 1861–1868. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lulla, A.; Cheng, X.; Yeo, J.-Y.; Mandal, J.; Yang, T.; Mei, X.; Saha, P.; Golonka, R.M.; Yeoh, B.S.; et al. Conjugated Bile Acids Are Nutritionally Re-Programmable Antihypertensive Metabolites. J. Hypertens. 2023, 41, 979–994. [Google Scholar] [CrossRef]
- Shin, D.-J.; Wang, L. Bile Acid-Activated Receptors: A Review on FXR and Other Nuclear Receptors. In Handbook of Experimental Pharmacology; Vallabh Prakashan: Delhi, India, Handb Exp Pharmacol; 2019; Volume 256, pp. 51–72. [Google Scholar]
- Moon, Y.; Choi, S.M.; Chang, S.; Park, B.; Lee, S.; Lee, M.O.; Choi, H.S.; Park, H. Chenodeoxycholic Acid Reduces Hypoxia Inducible Factor-1α Protein and Its Target Genes. PLoS ONE 2015, 10, e0130911. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M. Tauroursodeoxycholate—Bile Acid with Chaperoning Activity: Molecular and Cellular Effects and Therapeutic Perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef] [PubMed]



| All N = 164 | Dippers N = 76 | Non-Dippers N = 88 | p Value | |
|---|---|---|---|---|
| Clinical data | ||||
| Demographic/anthropometric | ||||
| Age (years) | 51.0 [44.8; 55.0] | 50.0 [44.8; 56.0] | 52.0 [44.8; 55.0] | 0.504 |
| Sex | 0.112 | |||
| Male | 114 (69.5%) | 58 (76.3%) | 56 (63.6%) | |
| Female | 50 (30.5%) | 18 (23.7%) | 32 (36.4%) | |
| BMI (kg/m2) | 29.3 [26.7; 33.4] | 28.5 [26.1; 31.8] | 30.2 [27.1; 34.6] | 0.029 |
| Smoking status | ||||
| Never | 67 (40.9%) | 27 (35.5%) | 40 (45.5%) | 0.433 |
| Former | 52 (31.7%) | 26 (34.2%) | 26 (29.5%) | |
| Current | 45 (27.4%) | 23 (30.3%) | 22 (25.0%) | |
| Comorbidities | ||||
| Diabetes | 17 (10.4%) | 4 (5.26%) | 13 (14.8%) | 0.083 |
| Hypertension | 56 (34.1%) | 21 (27.6%) | 35 (39.8%) | 0.142 |
| Dyslipidemia | 38 (23.5%) | 12 (16.2%) | 26 (29.5%) | 0.071 |
| Cardiovascular disease | 29 (17.7%) | 9 (11.8%) | 20 (22.7%) | 0.106 |
| Medication use | ||||
| Insulin | 7 (4.27%) | 1 (1.32%) | 6 (6.82%) | 0.124 |
| Any antihypertensive drug | 62 (37.8%) | 21 (27.6%) | 41 (46.6%) | 0.020 |
| ACE inhibitors | 41 (25.0%) | 13 (17.1%) | 28 (31.8%) | 0.047 |
| Beta-blockers | 29 (17.7%) | 12 (15.8%) | 17 (19.3%) | 0.700 |
| Diuretic agents | 23 (14.1%) | 6 (8.00%) | 17 (19.3%) | 0.065 |
| Calcium-channel blockers | 14 (8.59%) | 4 (5.26%) | 10 (11.5%) | 0.256 |
| Angiotensin II receptor blockers | 13 (8.02%) | 3 (3.95%) | 10 (11.6%) | 0.132 |
| Lipid-lowering drugs | 32 (19.6%) | 8 (10.5%) | 24 (27.6%) | 0.011 |
| ABPM data | ||||
| Dipping ratios | ||||
| 24 h DR | 0.91 [0.85; 0.97] | 0.85 [0.82; 0.87] | 0.97 [0.93; 1.00] | <0.001 |
| Systolic DR | 0.92 [0.86; 0.98] | 0.86 [0.83; 0.89] | 0.96 [0.93; 1.02] | <0.001 |
| Diastolic DR | 0.91 [0.87; 0.97] | 0.86 [0.84; 0.89] | 0.96 [0.92; 1.00] | <0.001 |
| Nighttime BP | ||||
| Mean (mmHg) | 88.8 [80.3; 97.5] | 83.2 [77.4; 91.3] | 94.0 [85.3; 106] | <0.001 |
| Systolic (mmHg) | 118 [106; 136] | 110 [103; 127] | 122 [112; 142] | <0.001 |
| Diastolic (mmHg) | 74.6 [68.9; 79.0] | 70.4 [67.0; 75.4] | 76.6 [72.8; 84.4] | <0.001 |
| Daytime BP | ||||
| Mean (mmHg) | 97.1 [90.5; 106] | 99.0 [92.3; 108] | 96.0 [89.0; 103] | 0.034 |
| Systolic (mmHg) | 128 [120; 143] | 128 [121; 145] | 127 [119; 141] | 0.292 |
| Diastolic (mmHg) | 81.3 [76.2; 85.4] | 82.1 [77.1; 87.2] | 79.6 [74.7; 84.8] | 0.029 |
| 24 h BP | ||||
| Mean (mmHg) | 95.0 [88.5; 105] | 94.6 [89.1; 105] | 95.8 [88.1; 104] | 0.924 |
| Systolic (mmHg) | 125 [117; 140] | 123 [117; 139] | 128 [117; 142] | 0.498 |
| Diastolic (mmHg) | 79.4 [74.0; 84.2] | 79.4 [74.8; 84.4] | 79.4 [73.5; 84.2] | 0.568 |
| Polysomnography data | ||||
| Respiratory disturbances | ||||
| AHI (events/h) | 28.6 [14.0; 50.4] | 22.4 [11.8; 41.8] | 34.9 [21.4; 60.5] | 0.001 |
| Obstructive apnea index (events/h) | 5.23 [1.59; 14.5] | 3.26 [1.30; 12.4] | 6.46 [1.92; 26.2] | 0.043 |
| Hypopnea index (events/h) | 18.0 [9.89; 27.2] | 13.0 [8.50; 25.1] | 20.8 [12.0; 30.4] | 0.011 |
| Nocturnal hypoxemia | ||||
| Mean SaO2 (%) | 94.0 [92.0; 95.0] | 94.0 [93.0; 95.0] | 93.0 [91.0; 95.0] | 0.052 |
| Minimum SaO2 (%) | 83.0 [76.0; 88.0] | 85.0 [78.2; 89.0] | 82.0 [73.5; 87.0] | 0.027 |
| TSat90 (%) | 1.97 [0.22; 8.30] | 1.33 [0.16; 4.50] | 2.40 [0.29; 15.9] | 0.028 |
| Desaturation index (events/h) | 4.47 [1.27; 19.4] | 4.34 [1.19; 10.4] | 7.01 [1.46; 23.8] | 0.268 |
| Sleep fragmentation | ||||
| Respiratory arousal index (events/h) | 20.1 [8.92; 37.2] | 15.0 [7.14; 24.2] | 25.8 [14.5; 46.8] | <0.001 |
| Movement arousal index (events/h) | 3.49 [1.33; 6.59] | 3.67 [1.71; 5.50] | 3.49 [1.31; 7.42] | 0.564 |
| Unspecific arousal index (events/h) | 6.32 [3.04; 10.6] | 6.46 [2.61; 9.90] | 6.21 [3.19; 11.9] | 0.927 |
| Sleep architecture | ||||
| Stage N1 (%) | 11.4 [7.09; 16.9] | 11.3 [6.50; 16.8] | 11.8 [7.44; 17.2] | 0.737 |
| Stage N2 (%) | 43.7 [37.6; 53.2] | 47.0 [40.5; 53.8] | 40.4 [34.2; 52.1] | 0.014 |
| Stage N3 (%) | 26.3 [17.6; 37.2] | 24.2 [15.9; 35.2] | 27.8 [19.1; 38.9] | 0.130 |
| Stage R (%) | 14.1 [9.59; 18.3] | 14.1 [11.0; 17.2] | 14.1 [9.00; 19.3] | 0.992 |
| Sleep quality | ||||
| Total sleep time (min) | 346 [318; 375] | 344 [322; 372] | 347 [311; 376] | 0.786 |
| Sleep latency (min) | 14.6 [7.80; 29.0] | 12.5 [7.03; 20.1] | 18.0 [8.00; 32.4] | 0.085 |
| Sleep efficiency (%) | 86.0 [77.9; 90.6] | 87.2 [79.9; 91.7] | 83.2 [76.9; 89.4] | 0.025 |
| Total wake time (min) | 58.0 [36.2; 89.7] | 51.4 [30.2; 84.0] | 68.1 [48.0; 96.0] | 0.021 |
| WASO (min) | 40.0 [24.5; 68.7] | 35.7 [21.3; 52.0] | 47.8 [29.3; 72.5] | 0.062 |
| Somnolence (ESS) | 11.0 [7.00; 14.5] | 11.0 [7.00; 14.0] | 11.0 [7.00; 15.0] | 0.736 |
| Mass | RT (min) | Method | Regulation Dipper vs. Non-Dipper | Putative Identification | Class | Reliability |
|---|---|---|---|---|---|---|
| 791.6017 | 11.42984 | M | Up | PC(38:4) | GP | b |
| 598.2597 | 8.905328 | M | Up | LysoPG(22:4) | GP | b |
| 791.5651 | 10.62096 | M | Up | PS(36:0) | GP | b |
| 598.4338 | 11.2998 | M | Up | PA(P-31:1) | GP | b |
| 811.5371 | 14.02962 | M | Up | PS(38:4) | GP | a |
| 514.31245 | 10.82806 | M | Up | LysoPA(22:1) | GP | a |
| 760.4527 | 11.71748 | M | Down | OxPA(40:8) | GP | b |
| 658.4229 | 11.58019 | M | Down | CL(62:4) | GP | a |
| 515.2295 | 11.71635 | M | Down | OxLysoPC(12:0) | GP | b |
| 807.5784 | 7.231465 | L | Down | PC(38:5) | GP | a |
| 465.3101 | 8.988933 | M | Up | Glycocholic acid | ST | a |
| 568.3252 | 9.260691 | M | Up | Deoxycholic acid 3-glucuronide | ST | b |
| 449.3152 | 8.721428 | M | Up | Glycohyodeoxycholic acid | ST | b |
| 499.2975 | 10.32784 | M | Up | Taurochenodesoxycholic acid | ST | a |
| 318.26 | 11.57911 | M | Up | Pregnanetriol | ST | b |
| 370.1825 | 8.089659 | M | Down | Androsterone sulfate | ST | a |
| 602.4646 | 11.71269 | M | Up | DG(32:1) | GL | b |
| 616.5038 | 8.262866 | L | Up | DG(36:4) | GL | b |
| 635.5499 | 7.994549 | L | Up | DG(36:3) | GL | a |
| 240.1008 | 7.275254 | M | Down | 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid | FA | b |
| 1079.255 | 7.688919 | L | Down | (3S)-3-Hydroxylinoleoyl-CoA | FA | b |
| 160.1219 | 0.5020314 | M | Down | N6-Methyl-L-Lysine | AA | a |
| 368.1674 | 8.3971 | M | Down | L-Tryptophyl-L-Lysine | AA | a |
| 412.1364 | 11.15013 | M | Up | Penicilloic acid | Drug | a |
| 256.0955 | 4.755068 | M | Down | m-chlorophenylpiperazine (m-CPP) | Drug | b |
| 827.1027 | 0.5837527 | M | Up | Unknown | ||
| 822.2788 | 11.38875 | M | Up | Unknown | ||
| 816.2971 | 11.38298 | M | Up | Unknown | ||
| 1108.853 | 11.57688 | M | Up | Unknown | ||
| 764.4844 | 12.07795 | M | Up | Unknown | ||
| 1212.914 | 11.70673 | M | Up | Unknown | ||
| 1249.356 | 9.835346 | L | Up | Unknown | ||
| 1338.199 | 10.34988 | L | Up | Unknown | ||
| 1171.264 | 7.82933 | L | Up | Unknown | ||
| 1266.198 | 10.65473 | L | Up | Unknown | ||
| 1384.454 | 9.951343 | L | Up | Unknown | ||
| 474.2918 | 12.98659 | M | Down | Unknown | ||
| 606.0906 | 12.08467 | M | Down | Unknown | ||
| 1025.551 | 13.29278 | M | Down | Unknown | ||
| 542.1265 | 8.394325 | M | Down | Unknown | ||
| 166.9866 | 0.9055392 | L | Down | Unknown | ||
| 665.0966 | 5.214503 | L | Down | Unknown | ||
| 1428.382 | 10.15159 | L | Down | Unknown | ||
| 305.3186 | 2.950193 | L | Down | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinilla, L.; Benítez, I.D.; Gracia-Lavedan, E.; Torres, G.; Mínguez, O.; Vaca, R.; Jové, M.; Sol, J.; Pamplona, R.; Barbé, F.; et al. Metabolipidomic Analysis in Patients with Obstructive Sleep Apnea Discloses a Circulating Metabotype of Non-Dipping Blood Pressure. Antioxidants 2023, 12, 2047. https://doi.org/10.3390/antiox12122047
Pinilla L, Benítez ID, Gracia-Lavedan E, Torres G, Mínguez O, Vaca R, Jové M, Sol J, Pamplona R, Barbé F, et al. Metabolipidomic Analysis in Patients with Obstructive Sleep Apnea Discloses a Circulating Metabotype of Non-Dipping Blood Pressure. Antioxidants. 2023; 12(12):2047. https://doi.org/10.3390/antiox12122047
Chicago/Turabian StylePinilla, Lucía, Iván D. Benítez, Esther Gracia-Lavedan, Gerard Torres, Olga Mínguez, Rafaela Vaca, Mariona Jové, Joaquim Sol, Reinald Pamplona, Ferran Barbé, and et al. 2023. "Metabolipidomic Analysis in Patients with Obstructive Sleep Apnea Discloses a Circulating Metabotype of Non-Dipping Blood Pressure" Antioxidants 12, no. 12: 2047. https://doi.org/10.3390/antiox12122047
APA StylePinilla, L., Benítez, I. D., Gracia-Lavedan, E., Torres, G., Mínguez, O., Vaca, R., Jové, M., Sol, J., Pamplona, R., Barbé, F., & Sánchez-de-la-Torre, M. (2023). Metabolipidomic Analysis in Patients with Obstructive Sleep Apnea Discloses a Circulating Metabotype of Non-Dipping Blood Pressure. Antioxidants, 12(12), 2047. https://doi.org/10.3390/antiox12122047







